mTOR-Mediated Autophagy Regulates Cadmium-Induced Kidney Injury via Pyroptosis
Abstract
1. Introduction
2. Results
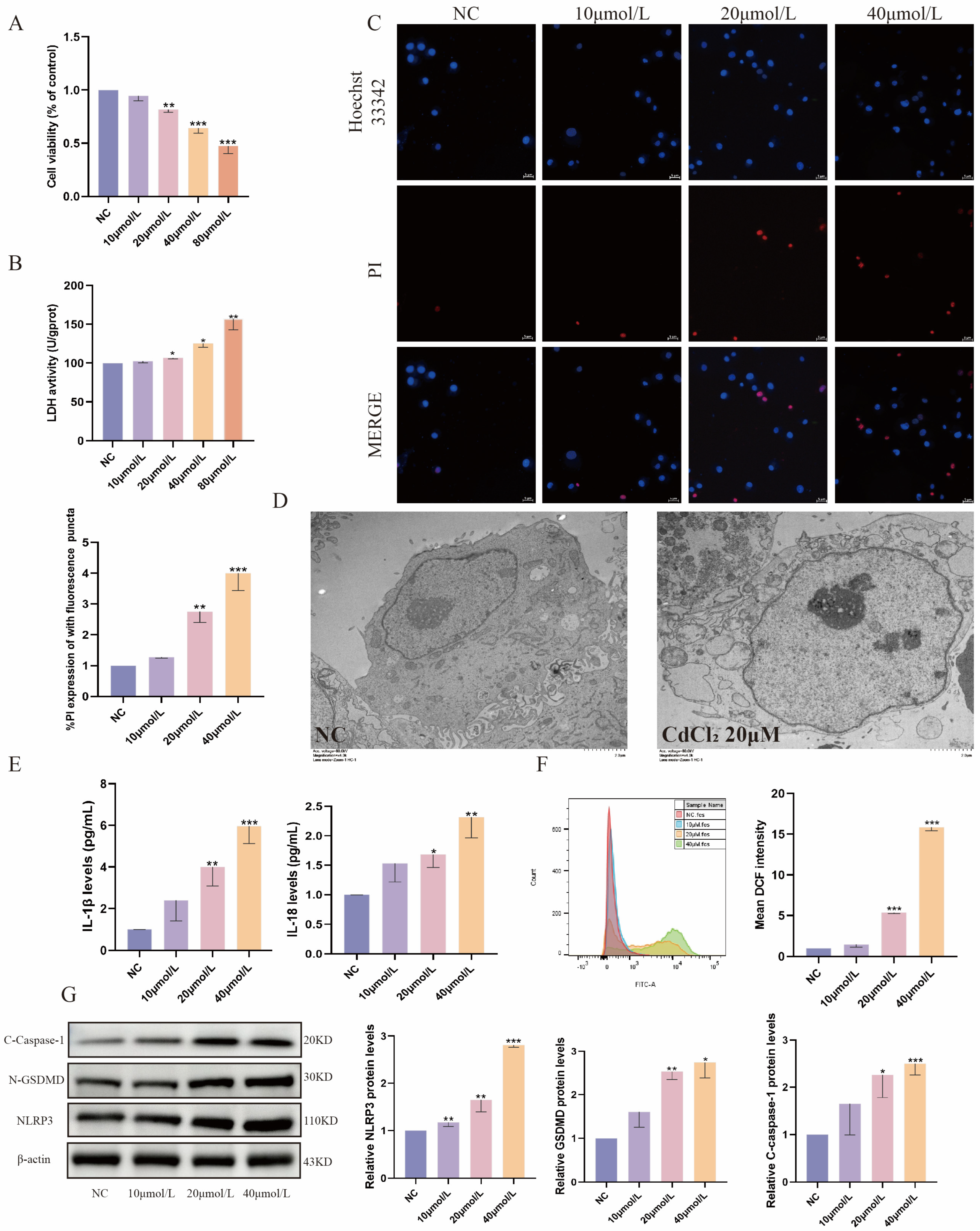
2.1. Cd Exposure Induces Pyroptosis in MDBK Cells
2.2. Inhibition of NLRP3 Alleviates Cd-Induced Pyroptosis in MDBK Cells
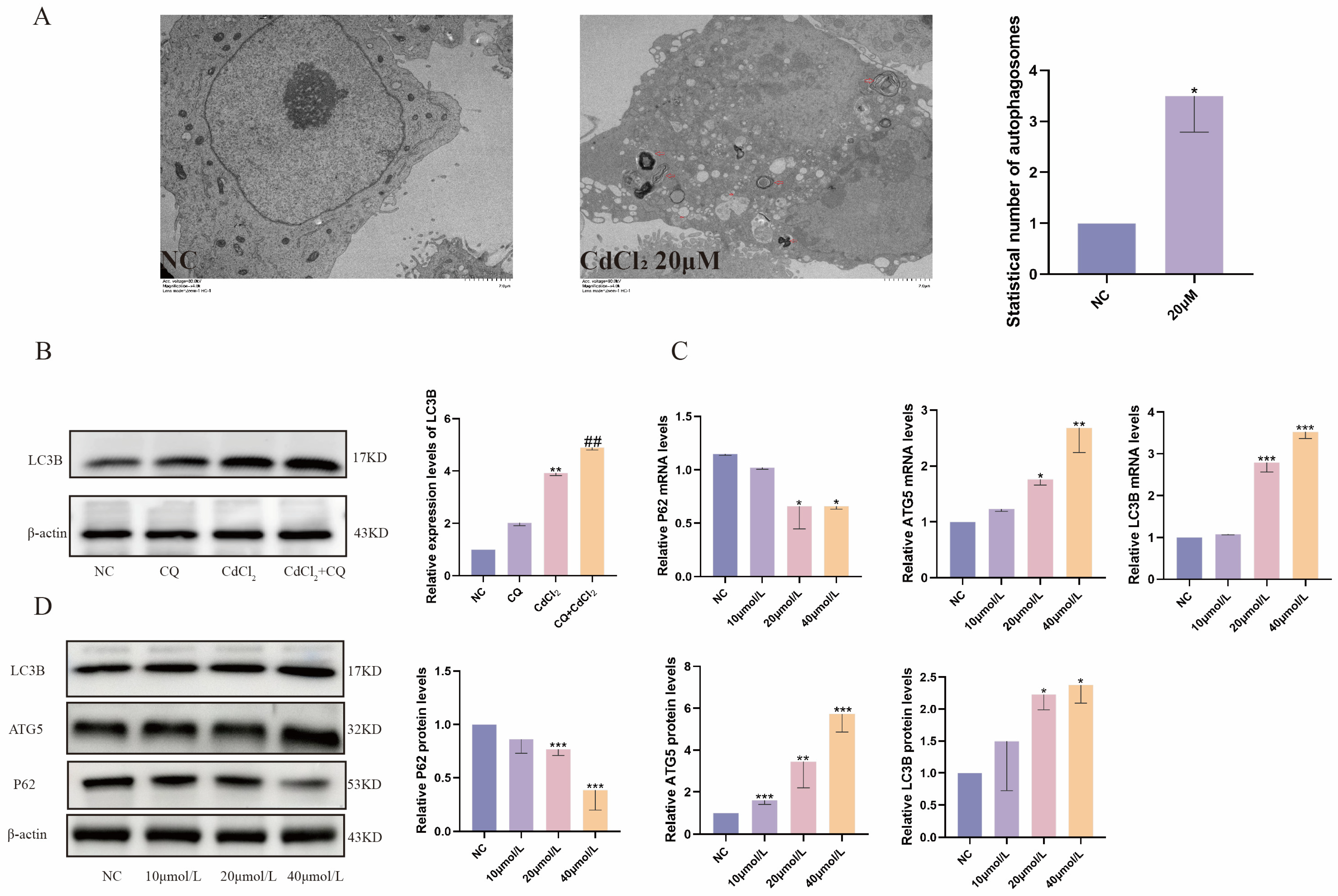
2.3. Cd Exposure Promotes Autophagy in MDBK Cells
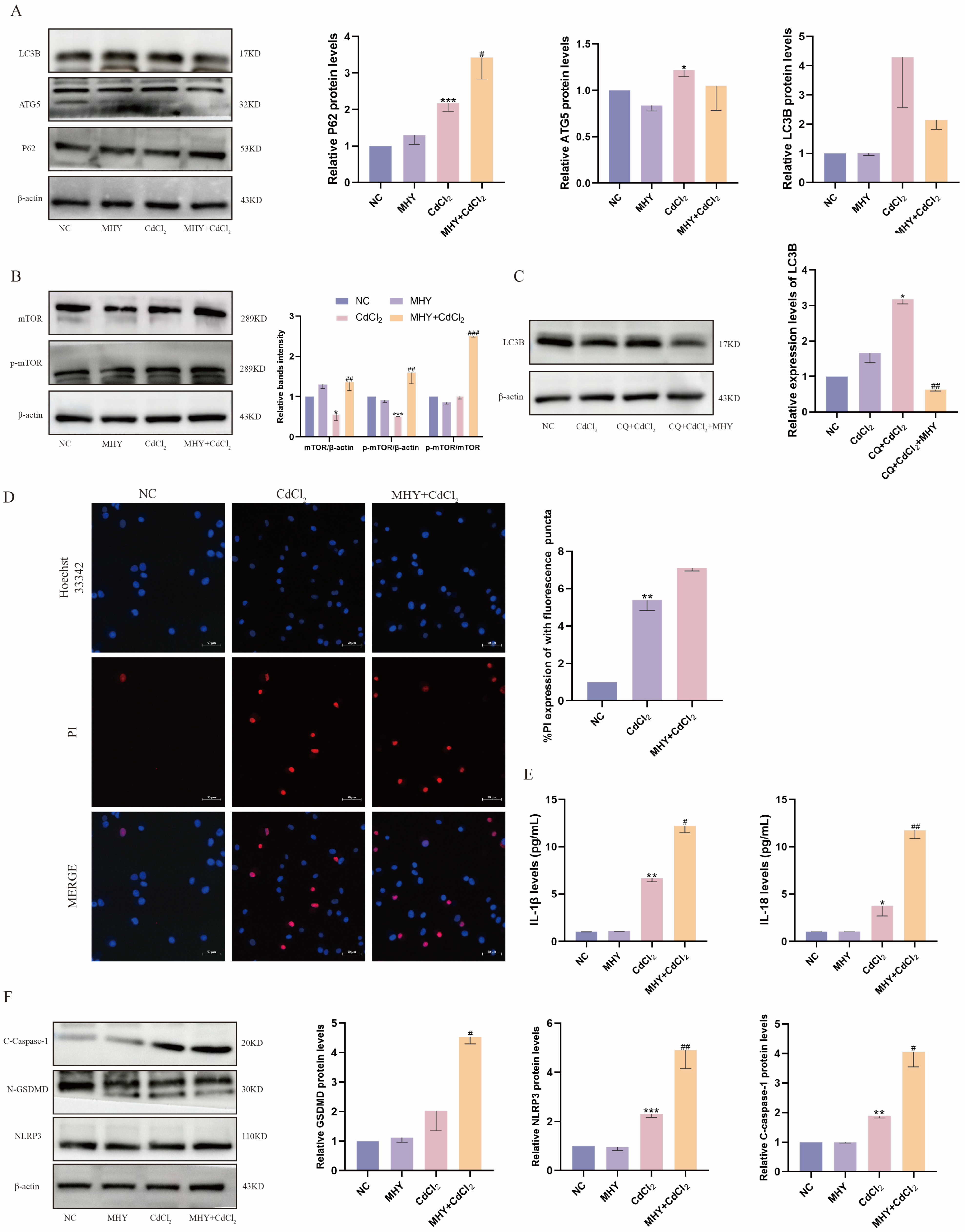
2.4. Activation of mTOR Exacerbates Cd-Induced Pyroptosis
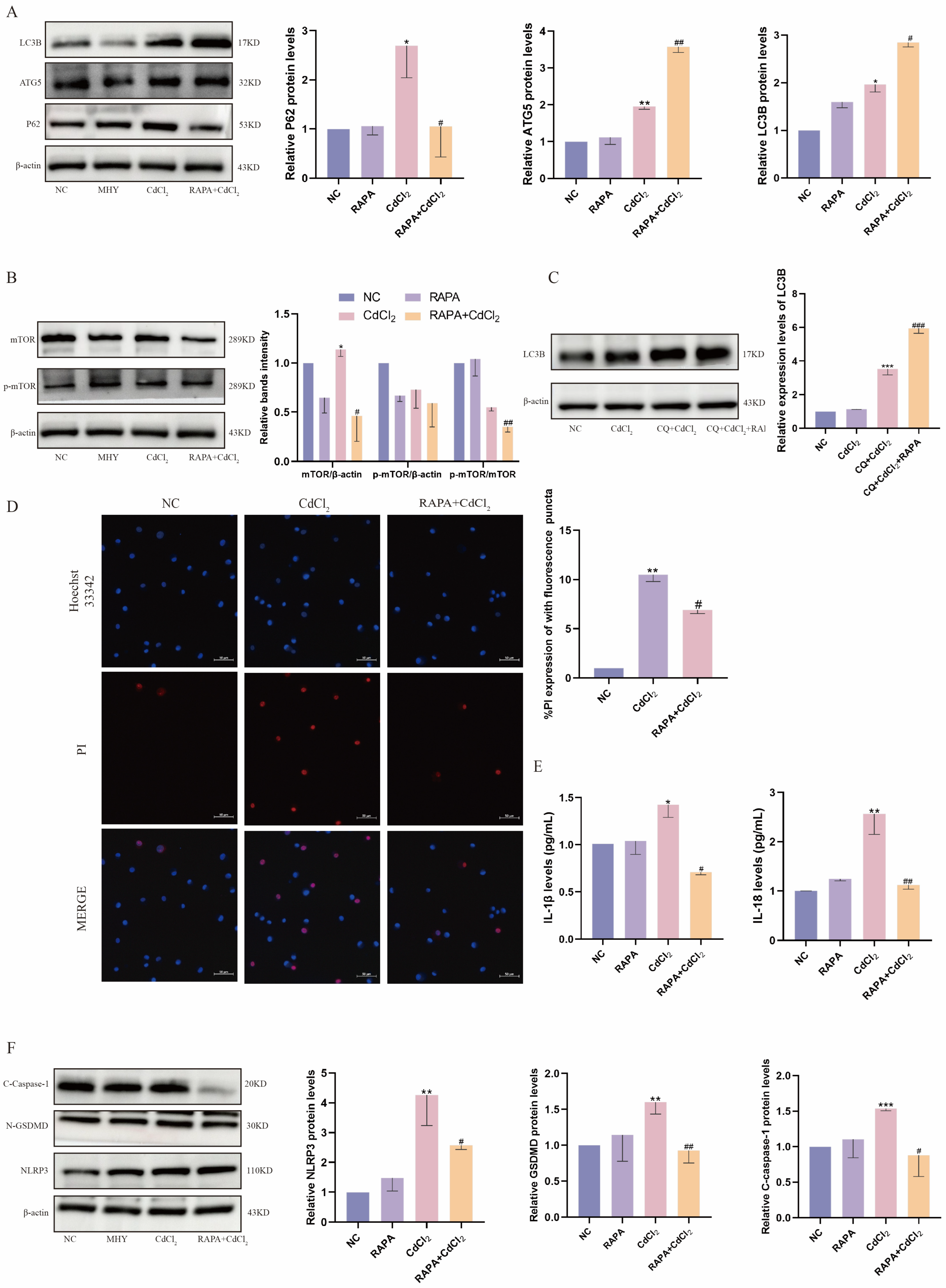
2.5. Inhibition of mTOR Attenuates Cd-Induced Pyroptosis in MDBK Cells
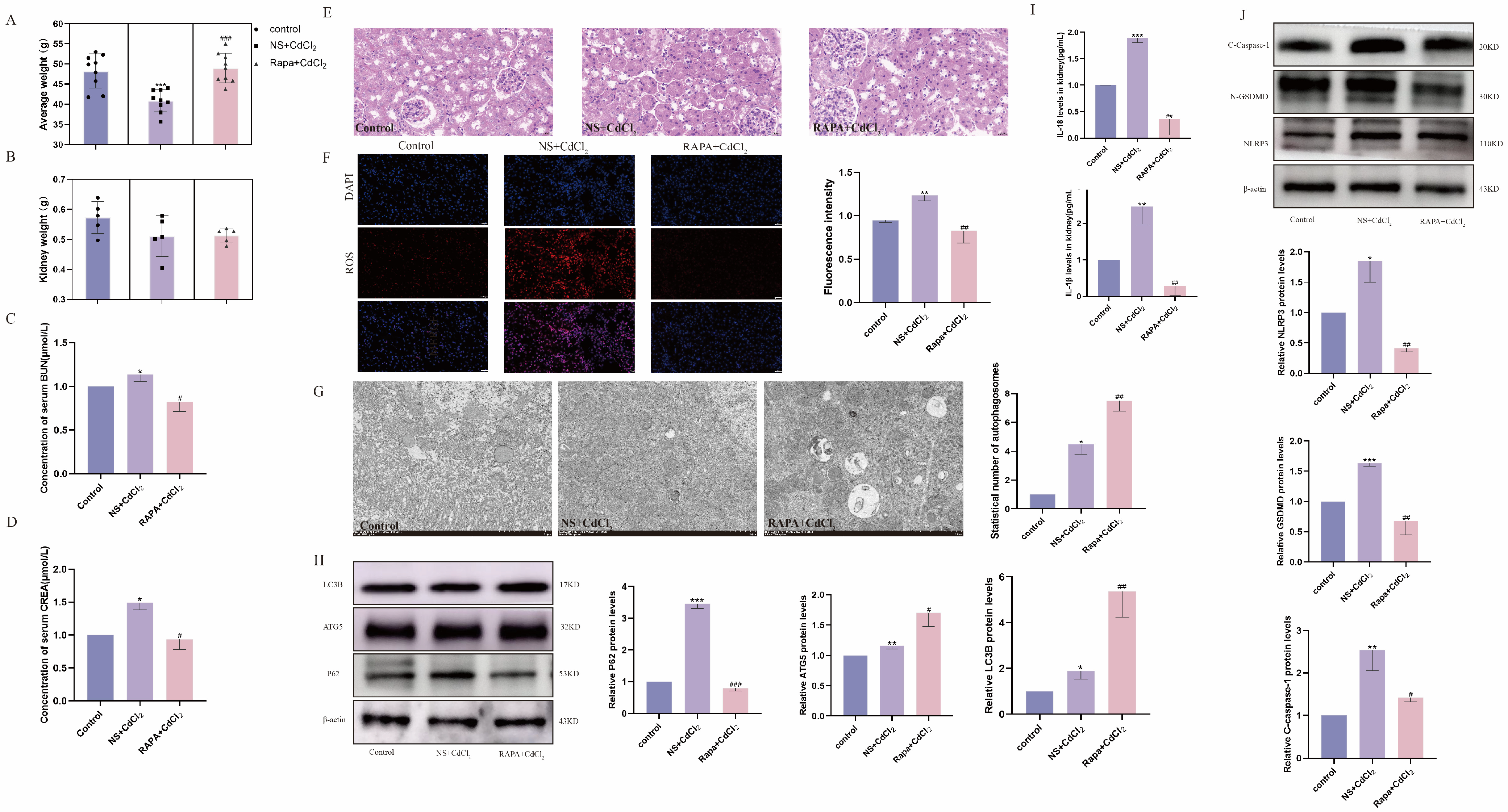
2.6. Inhibition of mTOR Alleviates Cd-Induced Kidney Injury in Mice
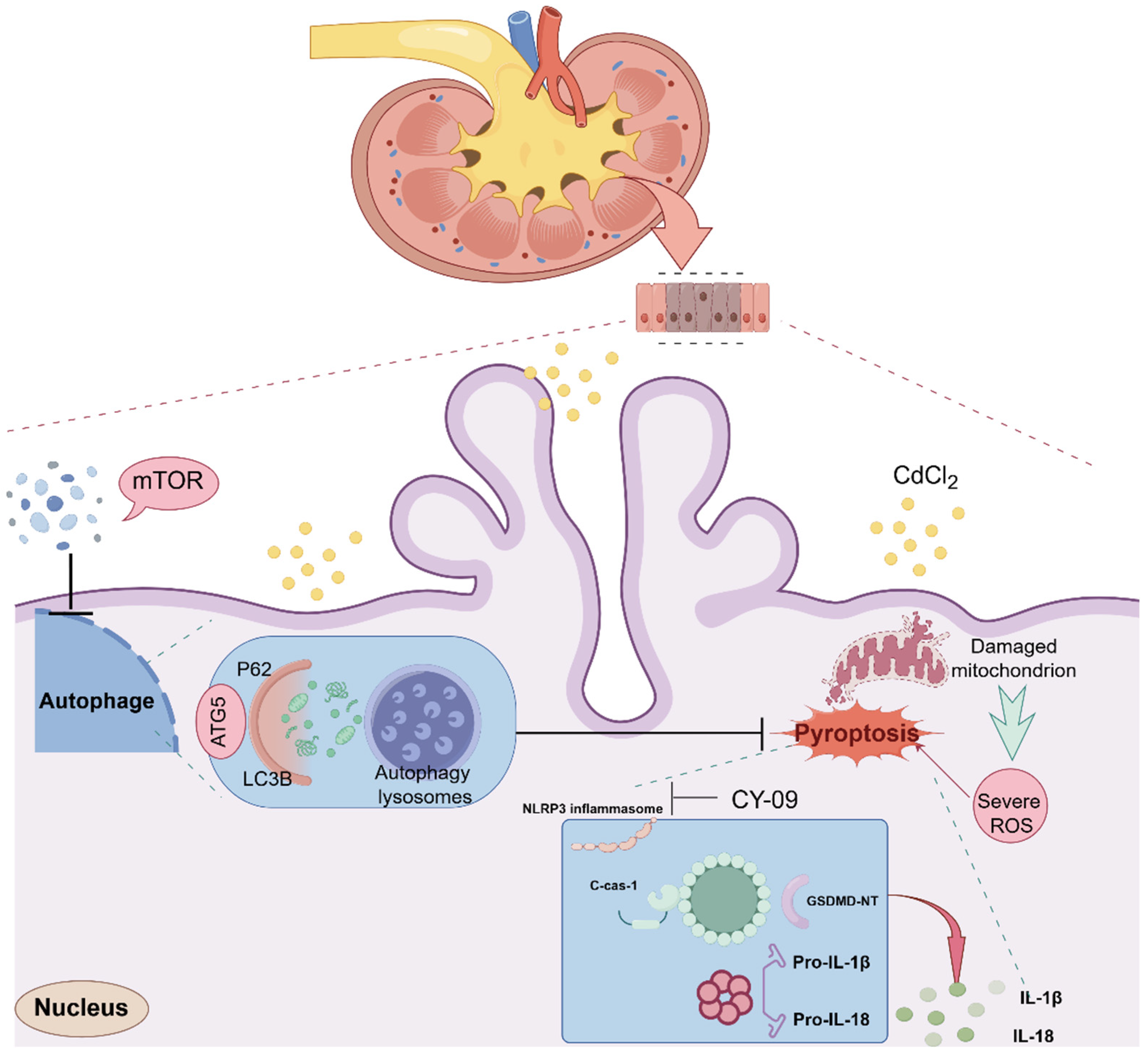
3. Discussion
4. Materials and Methods
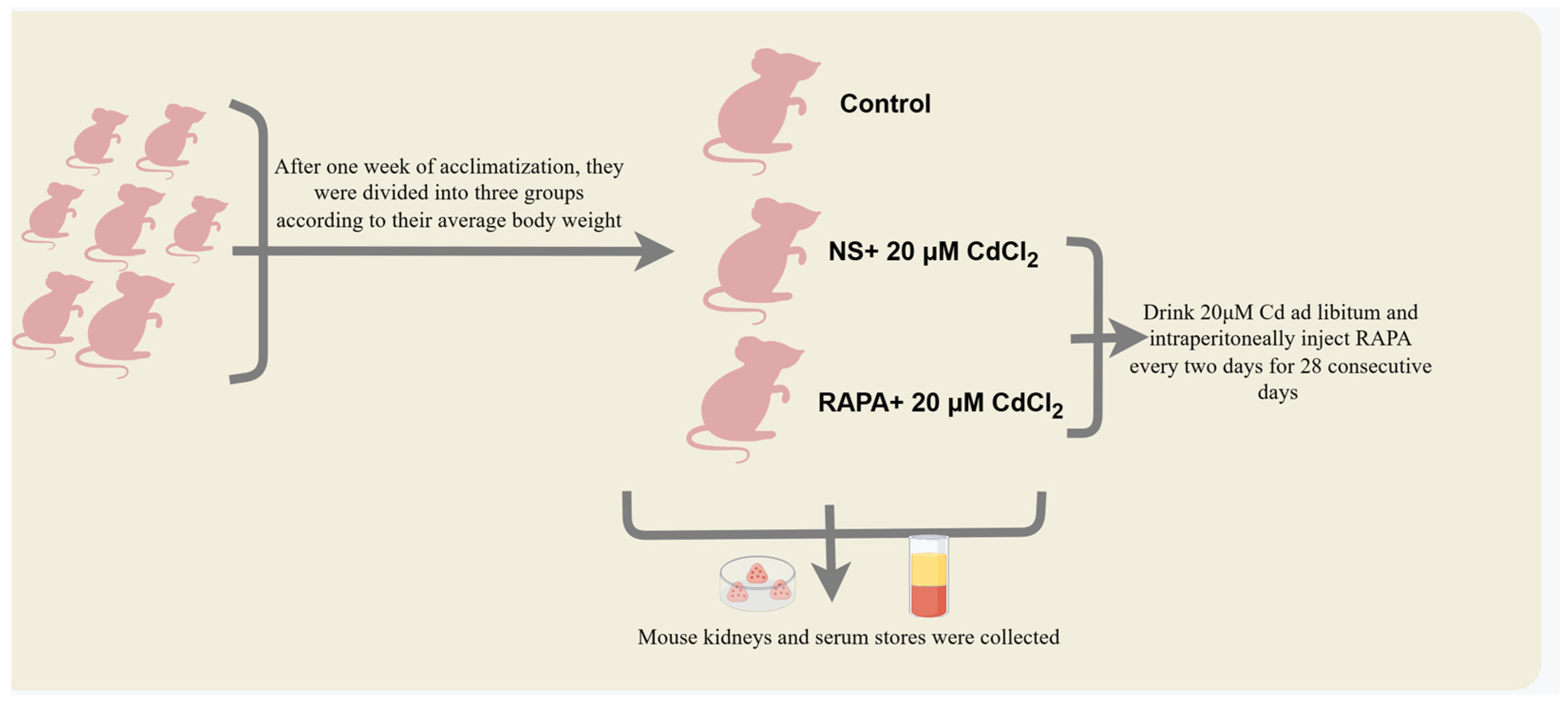
4.1. Experimental Animals and Treatments
4.2. Determination of Biochemical Indices of Serum Renal Function in Mice
4.3. Hematoxylin and Eosin Staining and Tissue ROS Staining
4.4. Cell Culture and Treatments
4.5. Cell Viability Analysis
4.6. Cellular Lactate Dehydrogenase Activity Assay
4.7. Extraction of Total RNA and Real-Time Quantitative Reverse Transcription PCR (RT-qPCR)
4.8. Western Blotting
4.9. Flow Cytometry
4.10. Transmission Electron Microscopy
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zhang, S.; Lai, M.; Gao, Z.; Li, H.; Yang, X.; Wei, Y.; Luo, F.; Yu, Z.; Zhang, D.; Ji, X. An ICT-based ratiometric fluorescent probe for tracking and imaging SO2 during Cd-induced stress in plants. Sens. Actuators B Chem. 2023, 395, 134499. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Current health risk assessment practice for dietary cadmium: Data from different countries. Food Chem. Toxicol. 2017, 106, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Gobe, G.C.; Vesey, D.A.; Phelps, K.R. Cadmium and Lead Exposure, Nephrotoxicity, and Mortality. Toxics 2020, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S. Cadmium Sources and Toxicity. Toxics 2019, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, Z.; Song, W.; Hong, D.; Huang, L.; Li, Y. A review on Cadmium Exposure in the Population and Intervention Strategies Against Cadmium Toxicity. Bull. Environ. Contam. Toxicol. 2021, 106, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, H.R.; Dennis, S.; Fitzpatrick, S. Cadmium: Mitigation strategies to reduce dietary exposure. J. Food Sci. 2020, 85, 260–267. [Google Scholar] [CrossRef]
- Bovio, F.; Melchioretto, P.; Forcella, M.; Fusi, P.; Urani, C. Cadmium promotes glycolysis upregulation and glutamine dependency in human neuronal cells. Neurochem. Int. 2021, 149, 105144. [Google Scholar] [CrossRef]
- Chen, J.; He, W.; Zhu, X.; Yang, S.; Yu, T.; Ma, W. Epidemiological study of kidney health in an area with high levels of soil cadmium and selenium: Does selenium protect against cadmium-induced kidney injury? Sci. Total Environ. 2020, 698, 134106. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Jiang, T.; Yuan, X.; Ren, Z.; Tuffour, A.; Liu, H.; Zhou, Y.; Gu, J.; Shi, H. Nephrotoxicity Profile of Cadmium Revealed by Proteomics in Mouse Kidney. Biol. Trace Elem. Res. 2020, 199, 1929–1940. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.; Liu, Y.; Huang, W.; Yang, W. A novel electrochemical ion imprinting sensor modified with carbon nanocomposites and its high selectivity for cadmium ion detection in real samples. Polym. Int. 2023, 72, 1096–1103. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Six, L.; Smolders, E. Future trends in soil cadmium concentration under current cadmium fluxes to European agricultural soils. Sci. Total Environ. 2014, 485, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Dudley, R.E.; Gammal, L.M.; Klaassen, C.D. Cadmium-induced hepatic and renal injury in chronically exposed rats: Likely role of hepatic cadmium-metallothionein in nephrotoxicity. Toxicol. Appl. Pharmacol. 1985, 77, 414–426. [Google Scholar] [CrossRef]
- Fujishiro, H.; Yano, Y.; Takada, Y.; Tanihara, M.; Himeno, S. Roles of ZIP8, ZIP14, and DMT1 in transport of cadmium and manganese in mouse kidney proximal tubule cells. Metallomics 2012, 4, 700. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.; Zheng, J. Nephrotoxic metals of cadmium, lead, mercury and arsenic and the odds of kidney stones in adults: An exposure-response analysis of NHANES 2007–2016. Environ. Int. 2019, 132, 105115. [Google Scholar] [CrossRef]
- Matović, V.; Buha, A.; Ðukić-Ćosić, D.; Bulat, Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015, 78, 130–140. [Google Scholar] [CrossRef]
- Babaknejad, N.; Moshtaghie, A.A.; Nayeri, H.; Hani, M.; Bahrami, S. Protective Role of Zinc and Magnesium against Cadmium Nephrotoxicity in Male Wistar Rats. Biol. Trace Elem. Res. 2016, 174, 112. [Google Scholar] [CrossRef]
- Hellström, L.; Elinder, C.G.; Dahlberg, B.; Lundberg, M.; Järup, L.; Persson, B.; Axelson, O. Cadmium exposure and end-stage renal disease. Am. J. Kidney Dis. 2001, 38, 1001–1008. [Google Scholar] [CrossRef]
- Battaglioni, S.; Benjamin, D.; Wälchli, M.; Maier, T.; Hall, M.N. mTOR substrate phosphorylation in growth control. Cell 2022, 185, 1814–1836. [Google Scholar] [CrossRef]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target. Ther. 2023, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Jhanwar-Uniyal, M.; Zeller, S.L.; Spirollari, E.; Das, M.; Hanft, S.J.; Gandhi, C.D. Discrete Mechanistic Target of Rapamycin Signaling Pathways, Stem Cells, and Therapeutic Targets. Cells 2024, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Fantus, D.; Rogers, N.M.; Grahammer, F.; Huber, T.B.; Thomson, A.W. Roles of mTOR complexes in the kidney: Implications for renal disease and transplantation. Nat. Rev. Nephrol. 2016, 12, 587–609. [Google Scholar] [CrossRef]
- Diekmann, F.; Rovira, J.; Carreras, J.; Arellano, E.M.; Ban, E.; Gutie, A.; Campistol, J.M. Mammalian Target of Rapamycin Inhibition Halts the Progression of Proteinuria in a Rat Model of Reduced Renal Mass. J. Am. Soc. Nephrol. 2007, 18, 2653–2660. [Google Scholar] [CrossRef] [PubMed]
- Kronick, J.; Gabril, M.Y.; House, A.A. House Microscopic Kidney Disease in Tuberous Sclerosis Complex and Treatment With mTOR Inhibition. Am. J. Kidney Dis. 2023, 82, 772–775. [Google Scholar] [CrossRef]
- Yasuda-Yamahara, M.; Kume, S.; Maegawa, H. Roles of mTOR in Diabetic Kidney Disease. Antioxidants 2021, 10, 321. [Google Scholar] [CrossRef]
- Lin, H.; Chen, H.; Qian, R.; Tang, G.; Ding, Y.; Jiang, Y.; Chen, C.; Wang, D.; Chu, M.; Guo, X. Integrated single-cell RNA-seq analysis revealed podocyte injury through activation of the BMP7/AMPK/mTOR mediated autophagy pathway. Chem. Biol. Interact. 2023, 82, 110559. [Google Scholar] [CrossRef]
- Nakamura, S.; Izumi, M. Chlorophagy is ATG gene-dependent microautophagy process. Plant Signal. Behav. 2018, 14, 1554469. [Google Scholar] [CrossRef]
- Radomski, N.; Rebbig, A.; Leonhardt, R.M.; Knittler, M.R. Xenophagic pathways and their bacterial subversion in cellular self-defense–πατα ρει–everything is in flux. Int. J. Med. Microbiol. 2018, 308, 185–196. [Google Scholar] [CrossRef]
- Al-Bari, M.A.A.; Xu, P. Molecular regulation of autophagy machinery by mTOR-dependent and -independent pathways. Ann. N. Y. Acad. Sci. 2020, 1467, 3–20. [Google Scholar] [CrossRef]
- Spitz, D.; Comas, M.; Gerstner, L.; Kayser, S.; Helmstädter, M.; Walz, G.; Hermle, T. mTOR-Dependent Autophagy Regulates Slit Diaphragm Density in Podocyte-like Drosophila Nephrocytes. Cells 2022, 11, 2103. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, S.B.; Miao, E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, Z.; Hou, Q.; Chen, W.; Mu, D.; Zhang, Y.; Liu, Q.; Liu, Z.; Yang, D. Zebrafish GSDMEb Cleavage-Gated Pyroptosis Drives Septic Acute Kidney Injury In Vivo. J. Immunol. 2020, 204, 1929–1942. [Google Scholar] [CrossRef]
- Miao, N.; Yin, F.; Xie, H.; Wang, Y.; Xu, Y.; Shen, Y.; Xu, D.; Yin, J.; Wang, B.; Zhou, Z.; et al. The cleavage of gasdermin D by caspase-11 promotes tubular epithelial cell pyroptosis and urinary IL-18 excretion in acute kidney injury. Kidney Int. 2019, 96, 1105–1120. [Google Scholar] [CrossRef]
- Xiong, L.; Zhou, B.; Young, J.L.; Wintergerst, K.; Cai, L. Exposure to low-dose cadmium induces testicular ferroptosis. Ecotoxicol. Environ. Saf. 2022, 234, 113373. [Google Scholar] [CrossRef]
- Guo, Y.; Lundebye, A.K.; Li, N.; Ergon, Å.; Pang, S.; Jiang, Y.; Zhu, W.; Zhao, Y.; Li, X.; Yao, L.; et al. Comparative assessment of food safety regulations and standards for arsenic, cadmium, lead, mercury and iodine in macroalgae used as food and feed in China and Europe. Trends Food Sci. Technol. 2023, 141, 104204. [Google Scholar] [CrossRef]
- Yan, L.J.; Allen, D.C. Cadmium-Induced Kidney Injury: Oxidative Damage as a Unifying Mechanism. Biomolecules 2021, 11, 1575. [Google Scholar] [CrossRef]
- Ma, Y.; Su, Q.; Yue, C.; Zou, H.; Zhu, J.; Zhao, H.; Song, R.; Liu, Z. The Effect of Oxidative Stress-Induced Autophagy by Cadmium Exposure in Kidney, Liver, and Bone Damage, and Neurotoxicity. Int. J. Mol. Sci. 2022, 23, 13491. [Google Scholar] [CrossRef]
- Karami, E.; Goodarzi, Z.; Ghanbari, A.; Dehdashti, A.; Bandegi, A.R.; Yosefi, S. Atorvastatin prevents cadmium-induced renal toxicity in a rat model. Toxicol. Ind. Health 2023, 39, 218–228. [Google Scholar] [CrossRef]
- Wei, Z.; Nie, G.; Yang, F.; Pi, S.; Wang, C.; Cao, H.; Guo, X.; Liu, P.; Li, G.; Hu, G.; et al. Inhibition of ROS/NLRP3/Caspase-1 mediated pyroptosis attenuates cadmium-induced apoptosis in duck renal tubular epithelial cells. Environ. Pollut. 2021, 273, 115919. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.F.; Li, Z.F.; Lian, C.Y.; Wang, Z.Y.; Wang, L. Inhibited transcription factor EB function induces reactive oxygen species overproduction to promote pyroptosis in cadmium-exposed renal tubular epithelial cells. Chem.-Biol. Interact. 2022, 368, 110249. [Google Scholar] [CrossRef]
- Samir, P.; Kesavardhana, S.; Patmore, D.M.; Gingras, S.; Malireddi, R.S.; Karki, R.; Guy, C.S.; Briard, B.; Place, D.E.; Bhattacharya, A.; et al. DDX3X acts as a live-or-die checkpoint in stressed cells by regulating NLRP3 inflammasome. Nature 2019, 573, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Q.; Yin, H.; Li, S. Cadmium exposure induces pyroptosis of lymphocytes in carp pronephros and spleens by activating NLRP3. Ecotoxicol. Environ. Saf. 2020, 202, 110903. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.W.; Li, C.I.; Huang, K.C.; Liu, C.S.; Chen, H.L.; Lee, C.C.; Chiou, Y.Y.; Chen, R.J. 3-MCPD and glycidol coexposure induces systemic toxicity and synergistic nephrotoxicity via NLRP3 inflammasome activation, necroptosis, and autophagic cell death. J. Hazard. Mater. 2021, 405, 124241. [Google Scholar] [CrossRef]
- Ka, S.M.; Chao, L.K.; Lin, J.C.; Chen, S.T.; Li, W.T.; Lin, C.N.; Cheng, J.C.; Jheng, H.L.; Chen, A.; Hua, K.F. A low toxicity synthetic cinnamaldehyde derivative ameliorates renal inflammation in mice by inhibiting NLRP3 inflammasome and its related signaling pathways. Free Radic. Biol. Med. 2016, 91, 10–24. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the Pathogenesis of Disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef]
- Choi, M.E. Autophagy in Kidney Disease. Annu. Rev. Physiol. 2020, 82, 297–322. [Google Scholar] [CrossRef]
- Tang, C.; Livingston, M.J.; Liu, Z.; Dong, Z. Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 2020, 16, 489–508. [Google Scholar] [CrossRef]
- Peng, H.; Yang, J.; Li, G.; You, Q.; Han, W.; Li, T.; Gao, D.; Xie, X.; Lee, B.H.; Du, J.; et al. Ubiquitylation of p62/sequestosome1 activates its autophagy receptor function and controls selective autophagy upon ubiquitin stress. Cell Res. 2017, 27, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Li, H.; Hu, H.; Li, Y.; Wang, T. The Role of HDAC6 in Autophagy and NLRP3 Inflammasome. Front. Immunol. 2021, 12, 763831. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chen, Z.; Bu, X.; Chen, S.; Guan, S. Elaidic acid induced hepatocyte pyroptosis via autophagy-CTSB-NLRP3 pathway. Food Chem. Toxicol. 2023, 181, 114060. [Google Scholar] [CrossRef]
- Zhong, Z.; Umemura, A.; Sanchez-Lopez, E.; Liang, S.; Shalapour, S.; Wong, J.; He, F.; Boassa, D.; Perkins, G.; Ali, S.R.; et al. NF-κB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell 2016, 16, 896–910. [Google Scholar] [CrossRef] [PubMed]
- Wipperman, M.F.; Montrose, D.C.; Gotto, A.M., Jr.; Hajjar, D.P. Mammalian Target of Rapamycin. Am. J. Pathol. 2019, 189, 492–501. [Google Scholar] [CrossRef]
- El Aggan, H.; Helmy, M.; Younis, L.; Hassona, E.; Lashen, S. Mammalian target of rapamycin (mtor) and autophagy in hepatitis c virus-related hepatocellular carcinoma: Relation to tumor progression. HPB 2019, 21, S298. [Google Scholar] [CrossRef]
- Abou Daher, A.; Alkhansa, S.; Azar, W.S.; Rafeh, R.; Ghadieh, H.E.; Eid, A.A. Translational Aspects of the Mammalian Target of Rapamycin Complexes in Diabetic Nephropathy. Antioxid. Redox Signal. 2022, 37, 802–819. [Google Scholar] [CrossRef]
- Panda, P.K.; Fahrner, A.; Vats, S.; Seranova, E.; Sharma, V.; Chipara, M.; Desai, P.; Torresi, J.; Rosenstock, T.; Kumar, D.; et al. Chemical Screening Approaches Enabling Drug Discovery of Autophagy Modulators for Biomedical Applications in Human Diseases. Front. Cell Dev. Biol. 2019, 7, 38. [Google Scholar] [CrossRef]
- Saresella, M.; Zoia, C.P.; La Rosa, F.; Bazzini, C.; Sala, G.; Grassenis, E.; Marventano, I.; Hernis, A.; Piancone, F.; Conti, E.; et al. Glibenclamide-Loaded Engineered Nanovectors (GNVs) Modulate Autophagy and NLRP3-Inflammasome Activation. Pharmaceuticals 2023, 16, 1725. [Google Scholar] [CrossRef]
- Guo, R.; Zhao, G.; Bai, G.; Chen, J.; Han, W.; Cui, N.; Wang, H. Depletion of mTOR ameliorates CD4+ T cell pyroptosis by promoting autophagy activity in septic mice. Int. Immunopharmacol. 2023, 124, 110964. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Y.; Liu, C.; Cheng, J.; He, F. Taohong Siwu decoction reduces acute myocardial ischemia–reperfusion injury by promoting autophagy to inhibit pyroptosis. J. Ethnopharmacol. 2024, 321, 117515. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Z.; Hu, J.; Liu, G.; Hu, H.; Ouyang, F.; Yang, J. Hydrogen sulfide ameliorates abdominal aorta coarctation-induced myocardial fibrosis by inhibiting pyroptosis through regulating eukaryotic translation initiation factor 2α phosphorylation and activating PI3K/AKT1 pathway. Korean J. Physiol. Pharmacol. 2023, 27, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Dupont, N.; Jiang, S.; Pilli, M.; Ornatowski, W.; Bhattacharya, D.; Deretic, V. Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1β. EMBO J. 2011, 30, 4701–4711. [Google Scholar] [CrossRef] [PubMed]

| Gene | Sequence (5′–3′) | Size |
|---|---|---|
| Cattle: β-actin: | F: GCAGGAGTACGATGAGTCCG | 103 |
| R: GCAGGAGTACGATGAGTCCG | ||
| IL-1β: | F: AAAAATCCCTGGTGCTGGCT | 138 |
| R: CATGCAGAACACCACTTCTCG | ||
| IL-18: | F: CCTTTGAGGCATCCAGGACA | 116 |
| R: CACACCACAGGGGAGAAGTG | ||
| Mouse: β-actin: | F: CCAACCGTGAGAAGATGAC | 220 |
| R: AGGCATACAGGGACAGCACA | ||
| IL-1β: | F: AACCTGCTGGTGTGTGACGTTC | 128 |
| R: CAGCACGAGGCTTTTTTGTTGT | ||
| IL-18: | F: GTGAACCCCAGACCAGACTG | 114 |
| R: CCTGGAACACGTTTCTGAAAGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Wang, K.; Xu, J.; Wan, G.; Zhao, Y.; Chen, Y.; Jiang, K.; Li, X. mTOR-Mediated Autophagy Regulates Cadmium-Induced Kidney Injury via Pyroptosis. Int. J. Mol. Sci. 2025, 26, 2589. https://doi.org/10.3390/ijms26062589
Hu Y, Wang K, Xu J, Wan G, Zhao Y, Chen Y, Jiang K, Li X. mTOR-Mediated Autophagy Regulates Cadmium-Induced Kidney Injury via Pyroptosis. International Journal of Molecular Sciences. 2025; 26(6):2589. https://doi.org/10.3390/ijms26062589
Chicago/Turabian StyleHu, Yuan, Kui Wang, Jie Xu, Guohuan Wan, Yiyi Zhao, Yajing Chen, Kangfeng Jiang, and Xiaobing Li. 2025. "mTOR-Mediated Autophagy Regulates Cadmium-Induced Kidney Injury via Pyroptosis" International Journal of Molecular Sciences 26, no. 6: 2589. https://doi.org/10.3390/ijms26062589
APA StyleHu, Y., Wang, K., Xu, J., Wan, G., Zhao, Y., Chen, Y., Jiang, K., & Li, X. (2025). mTOR-Mediated Autophagy Regulates Cadmium-Induced Kidney Injury via Pyroptosis. International Journal of Molecular Sciences, 26(6), 2589. https://doi.org/10.3390/ijms26062589






