Effect of Free Long-Chain Fatty Acids on Anagen Induction: Metabolic or Inflammatory Aspect?
Abstract
1. Introduction
2. Results
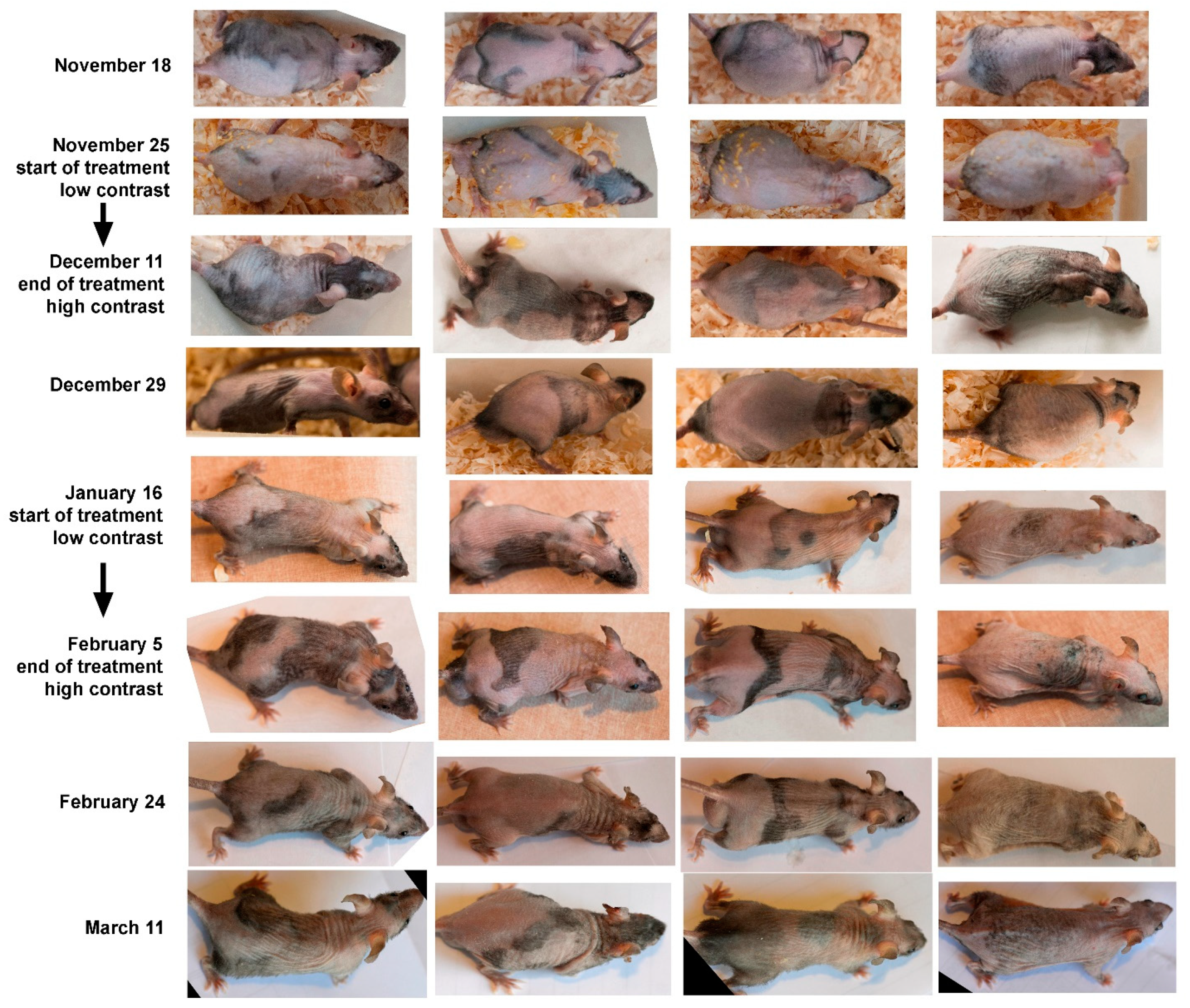
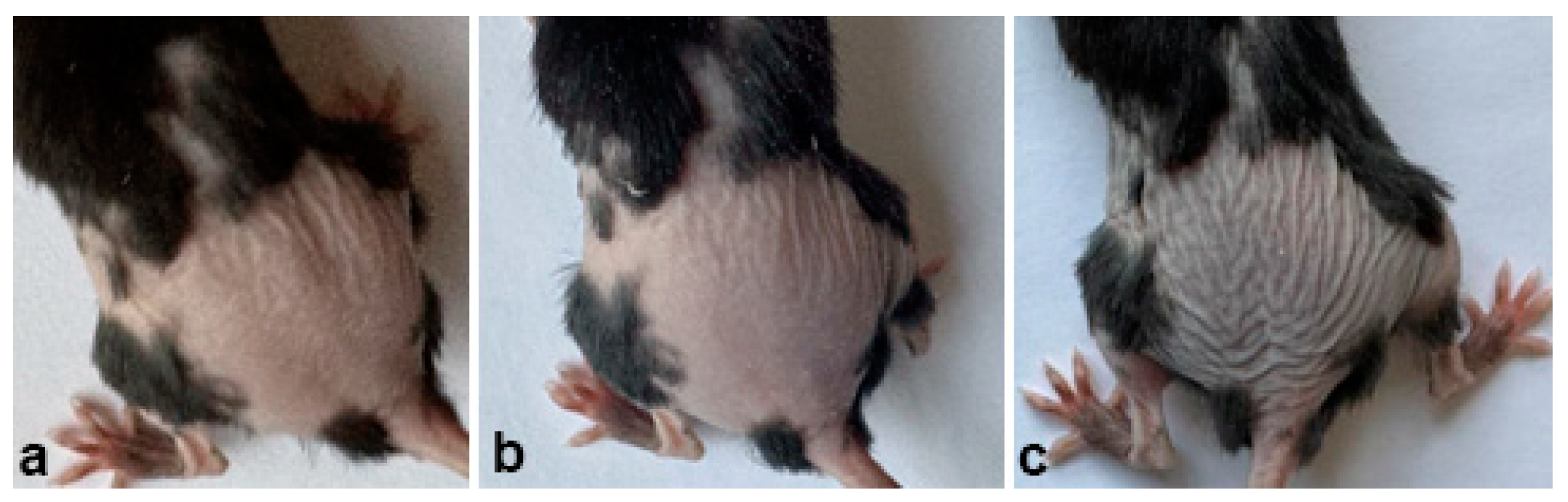
2.1. Experiments with we/we wal/wal Mice
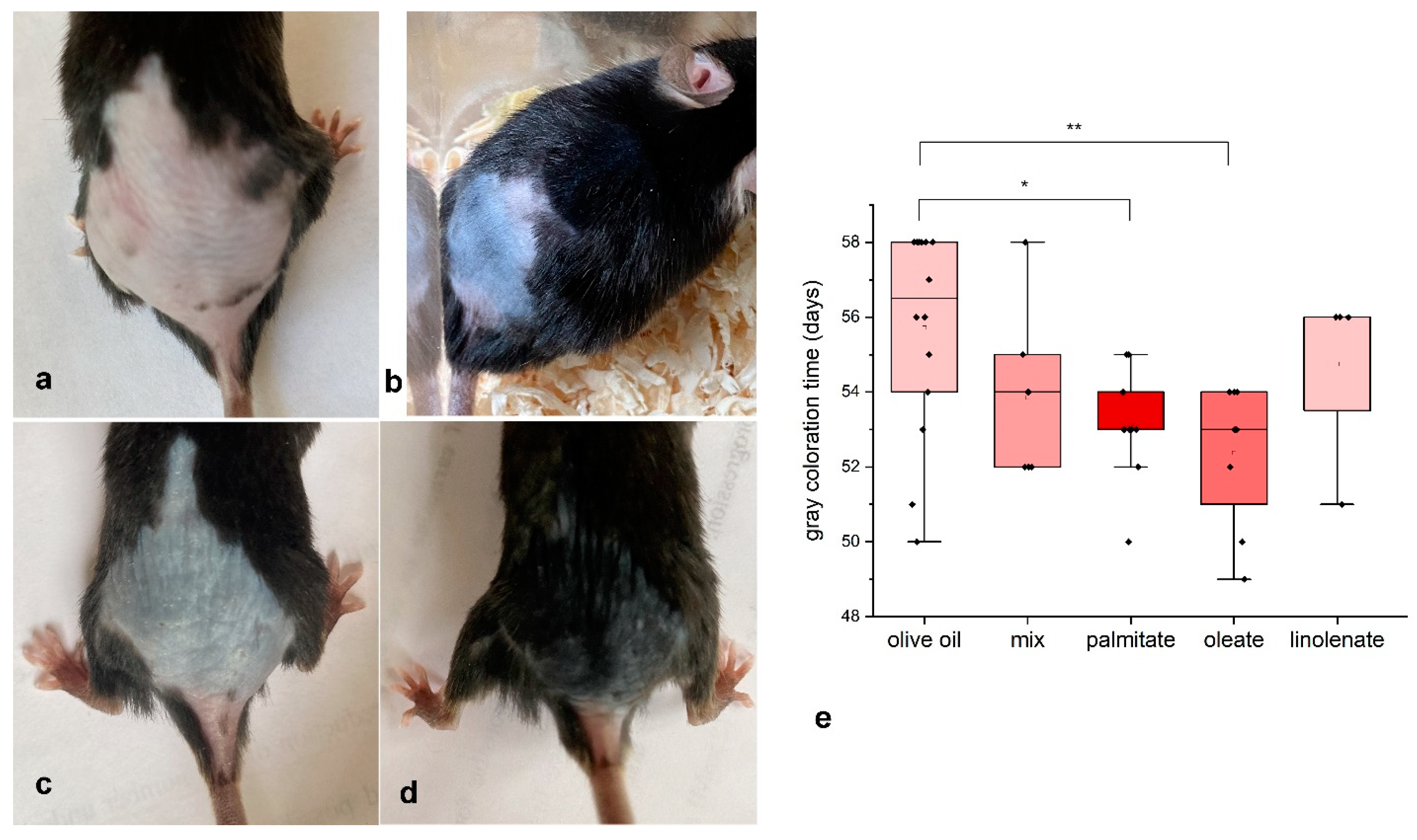
2.2. Experiments with Young C57Bl/6 Mice
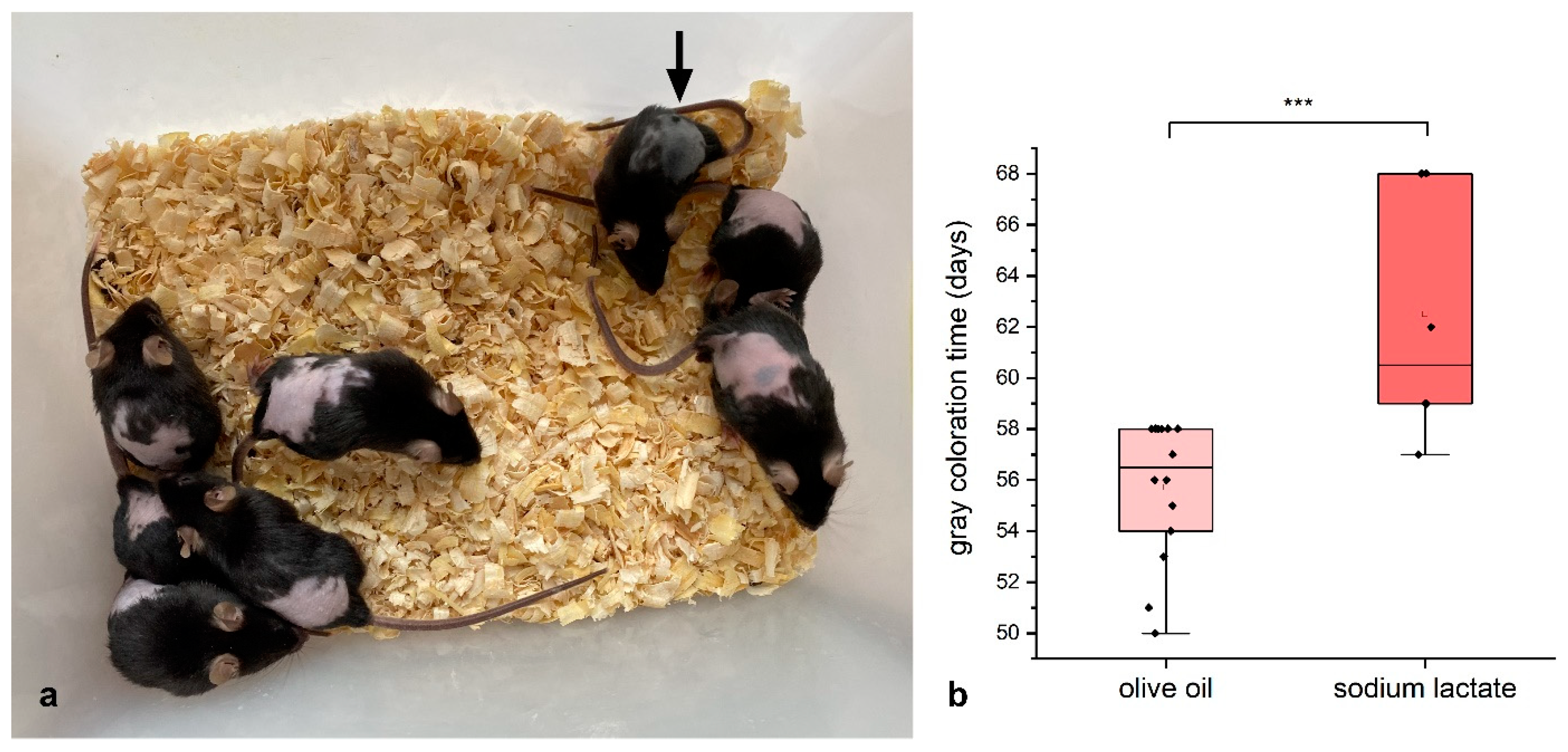
2.3. Lactate Experiments
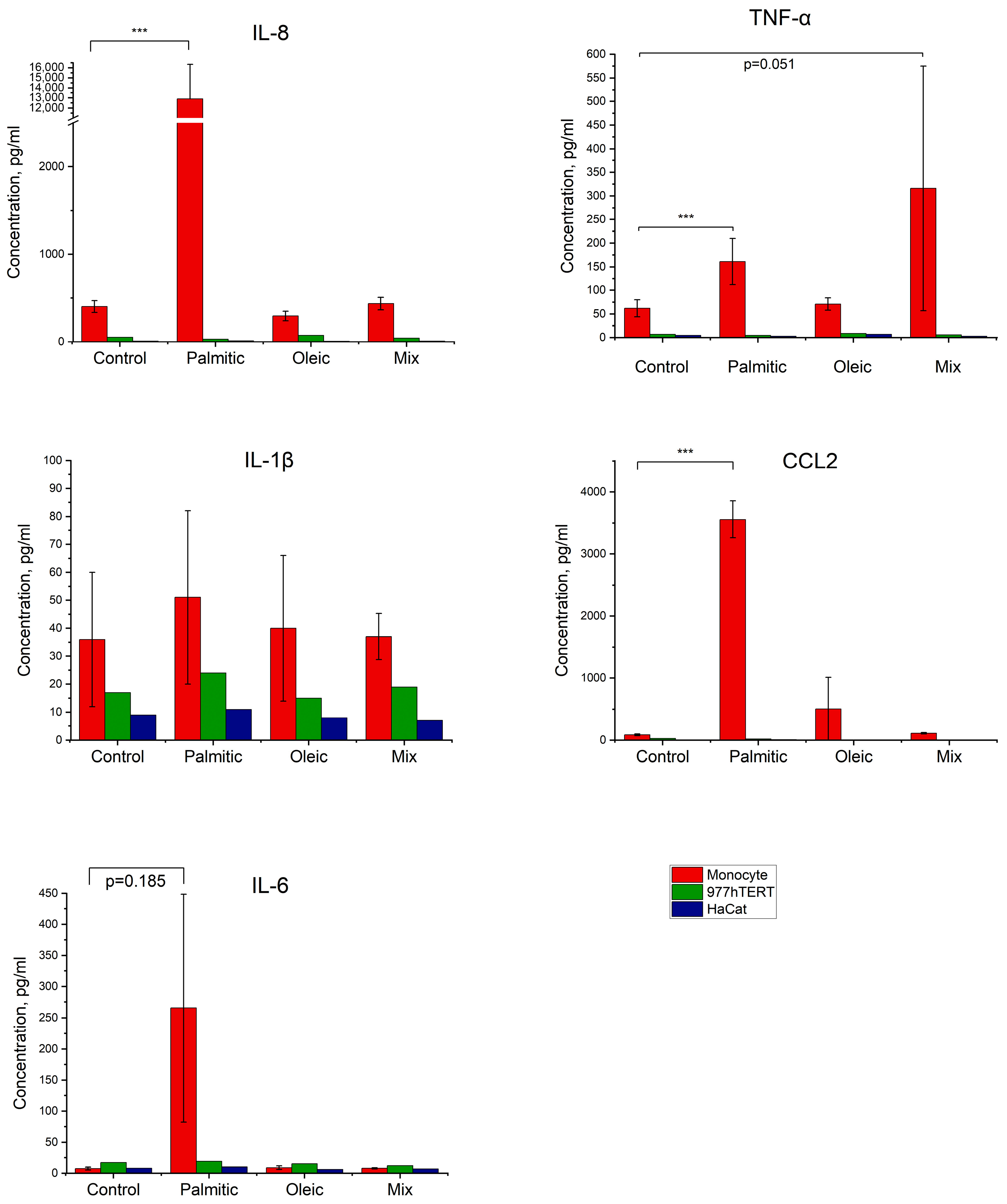
2.4. Effect of Fatty Acids on the Production of Inflammatory Factors by Cells in Culture
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Experiments on C57BL/6 Mice
4.3. Fatty Acids
4.4. Cells
4.5. Evaluation of Cytokine Secretion
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.H.; Choi, S. Deciphering the molecular mechanisms of stem cell dynamics in hair follicle regeneration. Exp. Mol. Med. 2024, 56, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Sennett, R.; Rendl, M. Mesenchymal–epithelial interactions during hair follicle morphogenesis and cycling. Semin. Cell Dev. Biol. 2012, 23, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, T. Local and systemic mechanisms that control the hair follicle stem cell niche. Nat. Rev. Mol. Cell Biol. 2024, 25, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Wang, L.; Plikus, M.V.; Jiang, T.X.; Murray, P.J.; Ramos, R.; Guerrero-Juarez, C.F.; Hughes, M.W.; Lee, O.K.; Shi, S.; et al. Organ-Level Quorum Sensing Directs Regeneration in Hair Stem Cell Populations. Cell 2015, 161, 277–290. [Google Scholar] [CrossRef]
- Morgun, E.I.; Vorotelyak, E.A. Epidermal Stem Cells in Hair Follicle Cycling and Skin Regeneration: A View From the Perspective of Inflammation. Front. Cell Dev. Biol. 2020, 8, 581697. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, X.; Liang, Y.; Yu, J.; Li, H.; Shokhirev, M.N.; Zheng, Y. Glucocorticoid signaling and regulatory T cells cooperate to maintain the hair-follicle stem-cell niche. Nat. Immunol. 2022, 23, 1086–1097. [Google Scholar] [CrossRef]
- Xu, X.-G.; Chen, H.-D. Prostanoids and Hair Follicles: Implications for Therapy of Hair Disorders. Acta Derm. Venereol. 2018, 98, 318–323. [Google Scholar] [CrossRef]
- Castellana, D.; Paus, R.; Perez-Moreno, M. Macrophages Contribute to the Cyclic Activation of Adult Hair Follicle Stem Cells. PLoS Biol. 2014, 12, e1002002. [Google Scholar] [CrossRef]
- Alshoubaki, Y.K.; Nayer, B.; Das, S.; Martino, M.M. Modulation of the Activity of Stem and Progenitor Cells by Immune Cells. Stem Cells Transl. Med. 2022, 11, 248–258. [Google Scholar] [CrossRef]
- Hao, J.; Jin, R.; Zeng, J.; Hua, Y.; Yorek, M.S.; Liu, L.; Mandal, A.; Li, J.; Zheng, H.; Sun, Y.; et al. Consumption of fish oil high-fat diet induces murine hair loss via epidermal fatty acid binding protein in skin macrophages in skin macrophages. Cell Rep. 2022, 41, 111804. [Google Scholar] [CrossRef]
- Rahmani, W.; Sinha, S.; Biernaskie, J. Immune modulation of hair follicle regeneration. NPJ Regen. Med. 2020, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Nic, C.; O’Sullivan, J.D.B.; Ramos, R.; Timperi, L.; Lai, T.; Farjo, N.; Farjo, B.; Pople, J.; Bhogal, R.; Hardman, J.A.; et al. Dermal Adipose Tissue Secretes HGF to Promote Human Hair Growth and Pigmentation. J. Investig. Dermatol. 2021, 141, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Correa-Gallegos, D.; Jiang, D.; Rinkevich, Y. Fibroblasts as confederates of the immune system. Immunol. Rev. 2021, 302, 147–162. [Google Scholar] [CrossRef]
- Bertolini, M.; McElwee, K.; Gilhar, A.; Bulfone-Paus, S.; Paus, R. Hair follicle immune privilege and its collapse in alopecia areata. Exp. Dermatol. 2020, 29, 703–725. [Google Scholar] [CrossRef] [PubMed]
- Stenn, K.S.; Paus, R. Controls of Hair Hollicle Cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar] [CrossRef]
- Plikus, M.V.; Chuong, C.-M. Complex Hair Cycle Domain Patterns and Regenerative Hair Waves in Living Rodents. J. Investig. Dermatol. 2008, 128, 1071–1080. [Google Scholar] [CrossRef]
- Wei, P.; Dove, K.K.; Bensard, C.; Schell, J.C.; Rutter, J. The Force Is Strong with This One: Metabolism (Over)powers Stem Cell Fate. Trends Cell Biol. 2018, 28, 551–559. [Google Scholar] [CrossRef]
- Vishniakova, K.S.; Vetkova, L.G.; Aliper, A.M.; Zhavoronkov, A.A.; Snezhkina, A.V.; Kudriavtseva, A.V.; Popov, K.V.; Egorov, E.E. Preparation stimulating hair growth possibly acts by inhibiting hair follicle ageing experiments on mice and transcriptome analysis. Adv. Gerontol. 2014, 27, 631–636, [Article in Russian]. [Google Scholar] [PubMed]
- Vishniakova, K.S.; Vetkova, L.G.; Jasko, M.V.; Aliper, A.M.; Buzdin, A.A.; Popov, K.V.; Kudryavtseva, A.V.; Yegorov, Y.E. Hair growth stimulation by a Natural Remedy: Animal Studies. Madridge J. Dermatol. Res. 2018, 3, 37–44. [Google Scholar] [CrossRef]
- Vishnyakova, K.S.; Popov, K.V.; Pan, X.; Jasko, M.V.; Yegorov, Y.E. Long-Chain Free Fatty Acids Influence Lipid Accumulation, Lysosome Activation and Glycolytic Shift in Various Cells In Vitro. Mol. Biol. 2021, 55, 683–696. [Google Scholar] [CrossRef]
- Brennan, B.M.; Huynh, M.T.; Rabah, M.A.; Shaw, H.E.; Bisaillon, J.J.; Radden, L.A., II; Nguyen, T.V.; King, T.R. The mouse wellhaarig (we) mutations result from defects in epidermal-type transglutaminase 3 (Tgm3). Mol. Genet. Metab. 2015, 116, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Sorokina, J.D.; Blandova, Z.K. Waved alopecia. Mouse News Lett. 1985, 73, 23. [Google Scholar]
- Chermnykh, E.S.; Schepetov, D.M.; Vorotelyak, E.A. Wal Mutant Mice Have a Mutation Associated with Autism Spectrum Disorders. Dokl. Biol. Sci. 2021, 497, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Konyukhov, B.V.; Malinina, N.A.; Martynova, M.Y. The we gene is amodifier of the wal gene in mice. Russ. J. Genet. 2004, 40, 968–974. [Google Scholar] [CrossRef]
- Nesterova, A.P.; Nizamutdinov, I.I.; Koniukhov, B.V. Interaction of mutant genes Fgf5(go-Y), we, and wal changes the duration of hair growth cycles in mice. Ontogenez 2012, 43, 60–65, [Article in Russian]. [Google Scholar] [CrossRef] [PubMed]
- Rippa, A.; Leonova, O.; Popenko, V.; Vasiliev, A.; Terskikh, V.; Vorotelyak, E. Early Stages of we/we wal/wal Mouse Hair Morphogenesis: Light and Fluorescent Microscopy of the Whole-Mount Epidermis. Biomed. Res. Int. 2014, 2014, 856978. [Google Scholar] [CrossRef]
- Flores, A.; Schell, J.; Krall, A.S.; Jelinek, D.; Miranda, M.; Grigorian, M.; Braas, D.; White, A.C.; Zhou, J.L.; Graham, N.A.; et al. Lactate dehydrogenase activity drives hair follicle stem cell activation. Nat. Cell Biol. 2017, 19, 1017–1026. [Google Scholar] [CrossRef]
- Osorio, D.; Pinzón, A.; Martín-Jiménez, C.; Barreto, G.E.; González, J. Multiple Pathways Involved in Palmitic Acid-Induced Toxicity: A System Biology Approach. Front. Neurosci. 2020, 13, 1410. [Google Scholar] [CrossRef]
- Dludla, P.V.; Silvestri, S.; Orlando, P.; Mazibuko-Mbeje, S.E.; Johnson, R.; Marcheggiani, F.; Cirilli, I.; Muller, C.J.F.; Louw, J.; Chellan, N.; et al. Palmitate-induced toxicity is associated with impaired mitochondrial respiration and accelerated oxidative stress in cultured cardiomyocytes: The critical role of coenzyme Q9/10. Toxicol. In Vitro 2020, 68, 104948. [Google Scholar] [CrossRef]
- Ishaq, A.; Tchkonia, T.; Kirkland, J.L.; Siervo, M.; Saretzki, G. Palmitate induces DNA damage and senescence in human adipocytes in vitro that can be alleviated by oleic acid but not inorganic nitrate. Exp. Gerontol. 2022, 163, 111798. [Google Scholar] [CrossRef]
- Vázquez-Mosquera, M.E.; Fernández-Moreno, M.; Cortés-Pereira, E.; Relaño, S.; Dalmao-Fernández, A.; Ramos-Louro, P.; Durán Sotuela, A.; Rego-Pérez, I.; Blanco, F.J. Oleate Prevents Palmitate-Induced Mitochondrial Dysfunction in Chondrocytes. Front. Physiol. 2021, 12, 670753. [Google Scholar] [CrossRef] [PubMed]
- Tse, E.K.; Belsham, D.D. Palmitate induces neuroinflammation, ER stress, and Pomc mRNA expression in hypothalamic mHypoA-POMC/GFP neurons through novel mechanisms that are prevented by oleate. Mol. Cell Endocrinol. 2018, 5, 40–49. [Google Scholar] [CrossRef]
- Kakimoto, P.A.; Serna, J.D.C.; de Miranda Ramos, V.; Zorzano, A.; Kowaltowski, A.J. Increased glycolysis is an early consequence of palmitate lipotoxicity mediated by redox signaling. Redox Biol. 2021, 45, 102026. [Google Scholar] [CrossRef]
- Sun, P.; Wang, Z.; Li, S.; Yin, J.; Gan, Y.; Liu, S.; Lin, Z.; Wang, H.; Fan, Z.; Qu, Q.; et al. Autophagy induces hair follicle stem cell activation and hair follicle regeneration by regulating glycolysis. Cell Biosci. 2024, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Christofk, H.; Jones, D.L.; Lowry, W.E. Topical Inhibition of the Electron Transport Chain Can Stimulate the Hair Cycle. J. Investig. Dermatol. 2018, 138, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Son, M.J.; Jeong, J.K.; Kwon, Y.; Ryu, J.S.; Mun, S.J.; Kim, H.J.; Kim, S.W.; Yoo, S.; Kook, J.; Lee, H.; et al. A novel and safe small molecule enhances hair follicle regeneration by facilitating metabolic reprogramming. Exp. Mol. Med. 2018, 6, 1–15. [Google Scholar] [CrossRef]
- Williams, R.; Philpott, M.P.; Kealey, T. Metabolism of freshly isolated human hair follicles capable of hair elongation: A glutaminolytic, aerobic glycolytic tissue. J. Investig. Dermatol. 1993, 100, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Lemasters, J.J.; Ramshesh, V.K.; Lovelace, G.L.; Lim, J.; Wright, G.D.; Harland, D.; Dawson, T.L., Jr. Compartmentation of Mitochondrial and Oxidative Metabolism in Growing Hair Follicles: A Ring of Fire. J. Investig. Dermatol. 2017, 137, 1434–1444. [Google Scholar] [CrossRef]
- Figlak, K.; Williams, G.; Bertolini, M.; Paus, R.; Philpott, M.P. Human hair follicles operate an internal Cori cycle and modulate their growth via glycogen phosphorylase. Sci. Rep. 2021, 11, 20761. [Google Scholar] [CrossRef]
- Rovetto, M.J.; Lamberton, W.F.; Neely, J.R. Mechanisms of glycolytic inhibition in ischemic rat hearts. Circ. Res. 1975, 37, 742–751. [Google Scholar] [CrossRef]
- Jiang, J.; Huang, D.; Jiang, Y.; Hou, J.; Tian, M.; Li, J.; Sun, L.; Zhang, Y.; Zhang, T.; Li, Z.; et al. Lactate Modulates Cellular Metabolism Through Histone Lactylation-Mediated Gene Expression in Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 647559. [Google Scholar] [CrossRef]
- Cai, X.; Ng, C.P.; Jones, O.; Fung, T.S.; Ryu, K.W.; Li, D.; Thompson, C.B. Lactate activates the mitochondrial electron transport chain independently of its metabolism. Mol. Cell. 2023, 83, 3904–3920. [Google Scholar] [CrossRef]
- Li, S.; Yu, J.; Zhang, J.; Li, X.; Yu, J. LSD1 interacting with HSP90 promotes skin wound healing by inducing metabolic reprogramming of hair follicle stem cells through the c-MYC/LDHA axis. FASEB J. 2023, 37, e23031. [Google Scholar] [CrossRef]
- Schell, J.C.; Wisidagama, D.R.; Bensard, C.; Zhao, H.; Wei, P.; Tanner, J.; Flores, A.; Mohlman, J.; Sorensen, L.K.; Earl, C.S.; et al. Control of intestinal stem cell function and proliferation by mitochondrial pyruvate metabolism. Nat. Cell Biol. 2017, 19, 1027–1036. [Google Scholar] [CrossRef]
- Jackson, B.T.; Finley, L.W.S. Metabolic regulation of the hallmarks of stem cell biology. Cell Stem Cell. 2024, 31, 161–180. [Google Scholar] [CrossRef]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef]
- Miwa, H. High-performance liquid chromatographic determination of free fatty acids and esterified fatty acids in biological materials as their 2-nitrophenylhydrazides. Anal. Chim. Acta 2002, 465, 237–255. [Google Scholar] [CrossRef]
- Nikiforov, N.G.; Kirichenko, T.V.; Kubekina, M.V.; Chegodaev, Y.S.; Zhuravlev, A.D.; Ilchuk, L.A.; Nikolaeva, M.A.; Arefieva, A.S.; Popov, M.A.; Verkhova, S.S.; et al. Macrophages derived from LPS-stimulated monocytes from individuals with subclinical atherosclerosis were characterized by increased pro-inflammatory activity. Cytokine 2023, 172, 156411. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Vishnyakova, K.S.; Chermnykh, E.S.; Jasko, M.V.; Zhuravlev, A.D.; Verkhova, S.S.; Chegodaev, Y.S.; Popov, M.A.; Nikiforov, N.G.; Yegorov, Y.E. Effect of Free Long-Chain Fatty Acids on Anagen Induction: Metabolic or Inflammatory Aspect? Int. J. Mol. Sci. 2025, 26, 2567. https://doi.org/10.3390/ijms26062567
Pan X, Vishnyakova KS, Chermnykh ES, Jasko MV, Zhuravlev AD, Verkhova SS, Chegodaev YS, Popov MA, Nikiforov NG, Yegorov YE. Effect of Free Long-Chain Fatty Acids on Anagen Induction: Metabolic or Inflammatory Aspect? International Journal of Molecular Sciences. 2025; 26(6):2567. https://doi.org/10.3390/ijms26062567
Chicago/Turabian StylePan, Xiaowen, Khava S. Vishnyakova, Elina S. Chermnykh, Maxim V. Jasko, Alexander D. Zhuravlev, Svetlana S. Verkhova, Yegor S. Chegodaev, Mikhail A. Popov, Nikita G. Nikiforov, and Yegor E. Yegorov. 2025. "Effect of Free Long-Chain Fatty Acids on Anagen Induction: Metabolic or Inflammatory Aspect?" International Journal of Molecular Sciences 26, no. 6: 2567. https://doi.org/10.3390/ijms26062567
APA StylePan, X., Vishnyakova, K. S., Chermnykh, E. S., Jasko, M. V., Zhuravlev, A. D., Verkhova, S. S., Chegodaev, Y. S., Popov, M. A., Nikiforov, N. G., & Yegorov, Y. E. (2025). Effect of Free Long-Chain Fatty Acids on Anagen Induction: Metabolic or Inflammatory Aspect? International Journal of Molecular Sciences, 26(6), 2567. https://doi.org/10.3390/ijms26062567





