Jasonia glutinosa (L.) DC.: Back in Our Pantries? A Review of Its Pharmacological Activity and Mechanisms of Action
Abstract
:1. Introduction
2. Ethnomedicinal/Traditional Uses
3. Phytochemical Composition
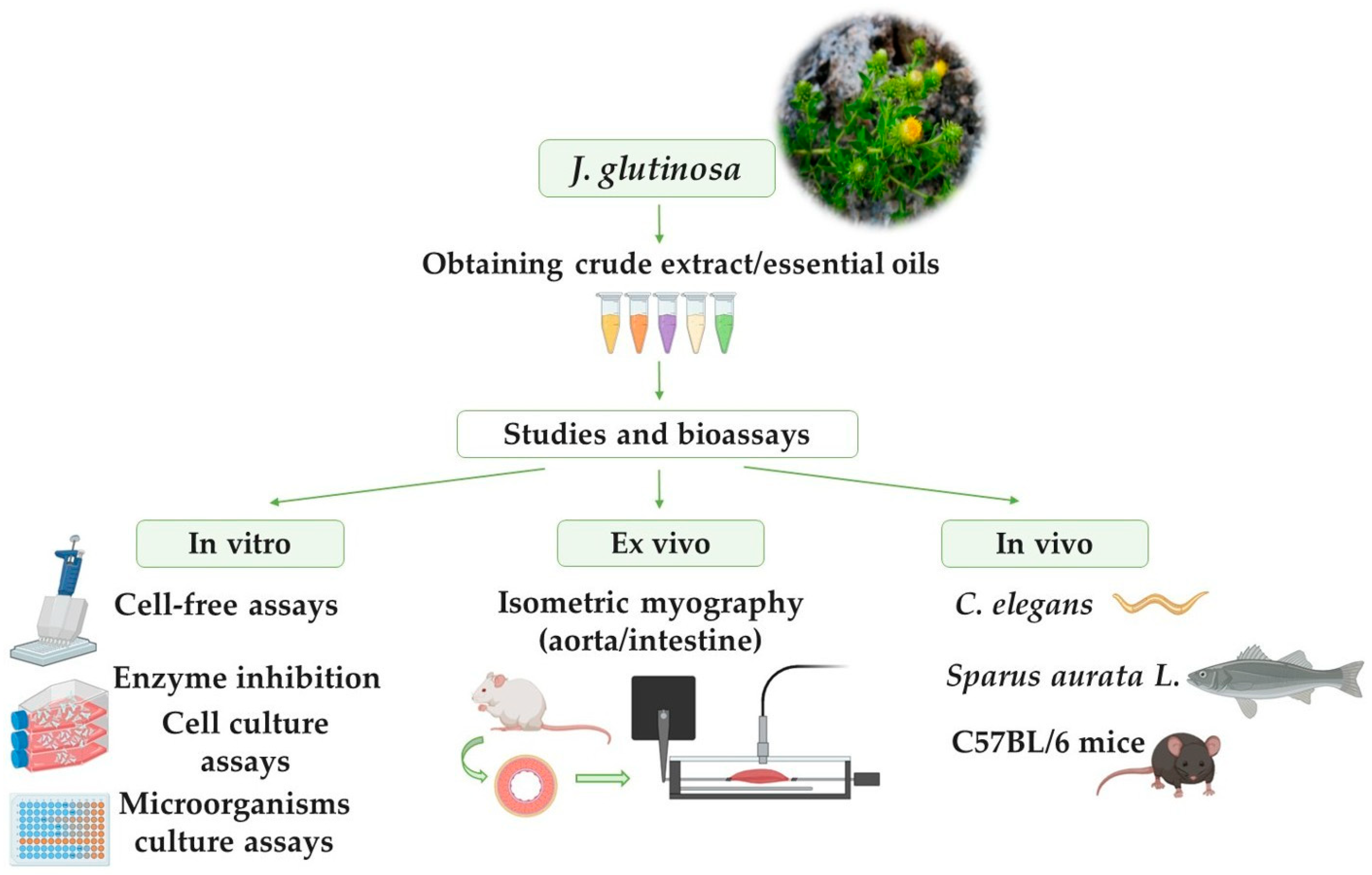
4. Pharmacological Activities and Possible Molecular Mechanisms of Action
4.1. In Vitro Studies
4.1.1. Antimicrobial Activity
4.1.2. Antioxidant Activity
4.1.3. Anti-Inflammatory Activity
4.1.4. Neuroprotective Properties
4.1.5. Antihypertensive Effects
4.1.6. Anti-Obesity and Antidiabetic Activity
4.2. Ex Vivo Studies
4.2.1. Antihypertensive Effect
4.2.2. Antispasmodic Activity
4.3. In Vivo Studies
4.3.1. Antioxidant Activity
4.3.2. Anti-Inflammatory Activity
4.3.3. Digestive Activity
Antispasmodic Activity
Improvement of Inflammatory Bowel Disease
4.3.4. Neuroprotective Properties
5. Current Situation and Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | Acetylcholinesatase |
| CAT | Catalase |
| CNS | Central Nervous System |
| DSS | Dextran Sulphate Sodium |
| FAAH | Fatty Acid Amide Hydrolase |
| HSP 70 | Heat Shock Protein 70 |
| HK | Head and Kidney |
| iNOS | Inducible Nitric Oxide Synthase |
| IgM | Immunoglobulin M |
| IL | Interleukin |
| LPS | Lipopolysaccharide |
| LTC4 | Leukotriene C4 |
| MAO | Monoamine Oxidase |
| MHCII | Major Histocompatibility Complex |
| NF-κβ | Factor Nuclear Kappa β |
| NO | Nitric Oxide |
| Nrf2 | Nuclear Factor Erythroid 2 |
| PGE2 | Prostaglandin E2 |
| ROS | Reactive oxygen Species |
| SOD | Superoxide Dismutase |
| SSZ | Sulfasalazine |
| TG | Triglyceride |
| TNF-α | Tumor Necrosis Factor alpha |
| TYR | Tyrosinase |
| TBX2 | Thromboxane B2 |
| ZO-1 | Zonula Occludens-1 |
| α-GLU | α-Glucosidase |
| βDEF | β-Defensines |
References
- Pardo Santayana, M.; Morales, R. Consideraciones sobre el género Jasonia (Compositae, Inuleae). Sistemática y usos. Acta Bot. Malacit. 2004, 29, 221–232. [Google Scholar] [CrossRef]
- Pardo de Santayana, M.; Morales, R.; Aceituno-Mata, L.; Molina, M. Inventario Español de Los Conocimientos Tradicionales Relativos a La Biodiversidad; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2014. [Google Scholar]
- Castillo, L.V.; Zaragozá García, F.Z.; Arnáez, C.Z. The Genus Chiliadenus: A Comprehensive Review of Taxonomic Aspects, Traditional Uses, Phytochemistry and Pharmacological Activities. Plants 2025, 14, 205. [Google Scholar] [CrossRef]
- Pardo de Santayana, M.; Blanco, E.; Morales, R. Plants known as té in Spain: An ethno-pharmaco-botanical review. J. Ethnopharmacol. 2005, 98, 1–19. [Google Scholar] [CrossRef]
- Lacadena, A.; López, V. Plantas medicinales en la comarca de la Jacetania (Huesca): Estudio etnobotánico preliminar. Rev. Fitoter. 2018, 18, 71–81. [Google Scholar]
- Akerreta, S.; Cavero, R.Y.; López, V.; Calvo, M.I. Analyzing factors that influence the folk use and phytonomy of 18 medicinal plants in Navarra. J. Ethnobiol. Ethnomed. 2007, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Cavero, R.Y.; Akerreta, S.; Calvo, M.I. Pharmaceutical ethnobotany in the Middle Navarra (Iberian Peninsula). J. Ethnopharmacol. 2011, 137, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Rigat, M.; Vallès, J.; Iglésias, J.; Garnatje, T. Traditional and alternative natural therapeutic products used in the treatment of respiratory tract infectious diseases in the eastern Catalan Pyrenees (Iberian Peninsula). J. Ethnopharmacol. 2013, 148, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Peris, J.B.; Stubing, G.; Romo, A. Plantas medicinales De La Penınsula Iberica E Islas Baleares; Ediciones Jaguar: Madrid, Spain, 2001. [Google Scholar]
- Guillén, M.D.; Ibargoitia, M.L. Volatile components obtained from the leaves of Jasonia glutinosa. Food Chem. 1996, 56, 155–158. [Google Scholar] [CrossRef]
- González Romero, M.A.; Villaescusa Castillo, L.; Díaz Lanza, A.M.; Arribas Bricio, J.M.; Soria Monzón, C.A.; Sanz Perucha, J. Volatile Composition of Jasonia glutinosa D. C. Z. Für Naturforschung C 2003, 58, 804–806. [Google Scholar] [CrossRef]
- Pascual Teresa, J.; Barrero, A.F.; San Feliciano, A.; Grande, M.; Medarde, M. Kudtdiol, new sesquiterpene alcohol from Jasonia glutinosa D.C. Tetrahedron Lett. 1978, 19, 4141–4144. [Google Scholar] [CrossRef]
- Pascual Teresa, J.; Barrero, A.F.; San Feliciano, A.; Medarde, M. Eudesmane alcohols from Jasonia glutinosa. Phytochemistry 1980, 19, 2155–2157. [Google Scholar] [CrossRef]
- Villaescusa-Castillo, L.; Díaz-Lanza, A.M.; Faure, R.; Debrauwer, L.; Elias, R.; Balansar, G. Two sesquiterpenoids, lucinone and glutinone, from Jasonia glutinosa. Phytochemistry 1995, 40, 1193–1195. [Google Scholar] [CrossRef]
- Sanchez-Martinez, R.; Villaescusa-Castillo, L.; Bernabe, M.; Díaz-Lanza, A.M. Two new eudesmane alcohols from Jasonia glutinosa. Z. Naturforsch C. 2000, 55, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Villaescusa, L.; Díaz, A.; Bartolomé, C. Methoxylated flavonoids from Jasonia glutinosa, D.C., and their relation with others species of Jasonia. Pharmazie 1995, 50, 639–640. [Google Scholar]
- Rubio, B.; Villaescusa, L.; Diaz, A.; Fernandez, L.; Martin, T. Flavonol Glycosides from Scolymus hispanicus and Jasonia glutinosa. Planta Med. 1995, 61, 583. [Google Scholar] [CrossRef]
- Ortega-Vidal, J.; Ruiz-Riaguas, A.; Fernández-de Córdova, M.L.; Ortega-Barrales, P.; Llorent-Martínez, E.J. Phenolic profile and antioxidant activity of Jasonia glutinosa herbal tea. Influence of simulated gastrointestinal in vitro digestion. Food Chem. 2019, 287, 258–264. [Google Scholar] [CrossRef]
- Valero, M.S.; Oliván-Viguera, A.; Garrido, I.; Langa, E.; Berzosa, C.; López, V.; Gómez-Rincón, C.; Murillo, M.D.; Köhler, R. Rock Tea extract (Jasonia glutinosa) relaxes rat aortic smooth muscle by inhibition of L-type Ca2+ channels. J. Physiol. Biochem. 2015, 71, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Valero, M.S.; González, M.; Ramón-Gimenez, M.; Andrade, P.B.; Moreo, E.; Les, F.; Fernandes, F.; Gómez-Rincón, C.; Berzosa, C.; García de Jalón, J.A.; et al. Jasonia glutinosa (L.) DC., a traditional herbal medicine, reduces inflammation, oxidative stress and protects the intestinal barrier in a murine model of colitis. Inflammopharmacology 2020, 28, 1717–1734. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Abdulkarim, A.K.; Alamami, A.D.; Elshibani, F.A. Phytochemical Constituents and Biological Activities of Jasonia glutinosa L.: The First Report for the Plant Growing in North Africa. J. Chem. 2022, 2022, 4510176. [Google Scholar] [CrossRef]
- Castro, M.; Ramón Giménez, M.; Les Pereda, F.; Trejo, L.; Plaza, M.Á.; López, V.; Murillo, M.D.; Valero Gracia, M.S. Spasmolytic effect of Jasonia glutinosa on rodent intestine. Rev. Española Enfermedades Dig. 2016, 108, 785–789. [Google Scholar] [CrossRef]
- Villaescusa-Castillo, L.; Díaz-Lanza, A.M.; Gasquet, M.; Delmas, F.; Ollivier, E.; Bernabé, M.; Faure, R.; Elias, R.; Balansard, G. Antiprotozoal activity of sesquiterpenes from Jasonia glutinosa. Pharm. Biol. 2000, 38, 176–180. [Google Scholar] [CrossRef]
- López, V.; Akerreta, S.; Casanova, E.; García-Mina, J.; Cavero, R.; Calvo, M. Screening of spanish medicinal plants for antioxidant and antifungal activities. Pharm. Biol. 2008, 46, 602–609. [Google Scholar] [CrossRef]
- Espinosa, C.; García Beltrán, J.M.; Messina, C.M.; Esteban, M.Á. Effect of Jasonia glutinosa on immune and oxidative status of gilthead seabream (Sparus aurata L.). Fish. Shellfish Immunol. 2020, 100, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Cásedas, G.; Valero, M.S.; Arbonés-Mainar, J.M.; López, V. Rock tea (Jasonia glutinosa (L.) DC.) polyphenolic extract inhibits triglyceride accumulation in 3T3-L1 adipocyte-like cells and obesity related enzymes in vitro. Food Funct. 2020, 11, 8931–8938. [Google Scholar] [CrossRef]
- Bermejo, B.P.; Abad, M.J.; Díaz, A.M.; Villaescusa, L.; González, M.A.; Silván, A.M. Sesquiterpenes from Jasonia glutinosa: In vitro anti-inflammatory activity. Biol. Pharm. Bull. 2002, 25, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Valero, M.S.; Moliner, C.; Weinkove, D.; López, V.; Gómez-Rincón, C. Jasonia glutinosa (L.) DC., a Traditional Herbal Tea, Exerts Antioxidant and Neuroprotective Properties in Different In Vitro and In Vivo Systems. Biology 2021, 10, 443. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current advances in naturally occurring caffeoylquinic acids: Structure, bioactivity, and synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef]
- Jucá, M.M.; Cysne Filho, F.M.S.; de Almeida, J.C.; Mesquita, D.d.S.; Barriga, J.R.M.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.K.A.M.; Ribeiro, J.E.; et al. Flavonoids: Biological activities and therapeutic potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Anca Badarau, I.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef]
- Surowiak, A.K.; Balcerzak, L.; Lochyński, S.; Strub, D.J. Biological Activity of Selected Natural and Synthetic Terpenoid Lactones. Int. J. Mol. Sci. 2021, 22, 5036. [Google Scholar] [CrossRef]
- Li, H.-Y.; Yang, W.-Q.; Zhou, X.-Z.; Shao, F.; Shen, T.; Guan, H.-Y.; Zheng, J.; Zhang, L.-M. Antibacterial and Antifungal Sesquiterpenoids: Chemistry, Resource, and Activity. Biomolecules 2022, 12, 1271. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Teixeira, P.; Cerca, N.; Azeredo, J.; Oliveira, R. Effect of farnesol on structure and composition of Staphylococcus epidermidis biofilm matrix. Curr. Microbiol. 2011, 63, 354–359. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.J.; Kim, H. Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants 2021, 10, 1448. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Sayahi, M.; Sayahi, M. The antidiabetic and antioxidant properties of some phenolic phytochemicals: A review study. Diabetes Metab. Syndr. 2019, 13, 854–857. [Google Scholar] [CrossRef]
- Sinha, S.; Doble, M.; Manju, S.L. 5-Lipoxygenase as a drug target: A review on trends in inhibitors structural design, SAR and mechanism based approach. Bioorg. Med. Chem. 2019, 27, 3745–3759. [Google Scholar] [CrossRef]
- Mukhopadhyay, N.; Shukla, A.; Makhal, P.N.; Kaki, V.R. Natural product-driven dual COX-LOX inhibitors: Overview of recent studies on the development of novel anti-inflammatory agents. Heliyon 2023, 9, e14569. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Moliner, C.; Barros, L.; Dias, M.I.; López, V.; Langa, E.; Ferreira, I.C.F.R.; Gómez-Rincón, C. Edible flowers of Tagetes erecta L. As functional ingredients: Phenolic composition, antioxidant and protective effects on caenorhabditis elegans. Nutrients 2018, 10, 2002. [Google Scholar] [CrossRef]
- Les, F.; Prieto, J.M.; Arbonés-Mainar, J.M.; Valero, M.S.; López, V. Bioactive properties of commercialised pomegranate (Punica granatum) juice: Antioxidant, antiproliferative and enzyme inhibiting activities. Food Funct. 2015, 6, 2049–2057. [Google Scholar] [CrossRef]
- Moliner, C.; Barros, L.; Dias, M.I.; Reigada, I.; Ferreira, I.C.F.R.; López, V.; Langa, E.; Rincón, C.G. Viola cornuta and Viola x wittrockiana: Phenolic compounds, antioxidant and neuroprotective activities on Caenorhabditis elegans. J. Food Drug Anal. 2019, 27, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.; Johnson, D.S.; Cravatt, B.F. Fatty acid amide hydrolase as a potential therapeutic target for the treatment of pain and CNS disorders. Expert Opin. Drug Discov. 2009, 4, 763–784. [Google Scholar] [CrossRef]
- Silva, H. The Vascular Effects of Isolated Isoflavones-A Focus on the Determinants of Blood Pressure Regulation. Biology 2021, 10, 49. [Google Scholar] [CrossRef]
- Hajizadeh-Sharafabad, F.; Ghoreishi, Z.; Maleki, V.; Tarighat-Esfanjani, A. Mechanistic insights into the effect of lutein on atherosclerosis, vascular dysfunction, and related risk factors: A systematic review of in vivo, ex vivo and in vitro studies. Pharmacol. Res. 2019, 149, 104477. [Google Scholar] [CrossRef]
- Nigusse, T.; Zhang, L.; Wang, R.; Wang, X.N.; Li, J.; Liu, C. Flavonoids in a crude extract of Catha edulis inhibit rat intestinal contraction via blocking Ca2+ channels. Neurogastroenterol. Motil. 2019, 31, e13602. [Google Scholar] [CrossRef] [PubMed]
- Cardeal dos Santos, A.N.; da Cruz Freire, J.E.; Rodrigues, B.F.; Ferreira, W.; Júnior, J.E.R.H.; Leal-Cardoso, J.H.; Coelho de Souza, A. Translational Perspectives on the Therapeutic Potential of Hyptis Crenata Essential Oil Terpenes in Smooth Muscle Function. Planta Med. 2024, 90, 1005–1014. [Google Scholar] [CrossRef]
- Surco-Laos, F.; Cabello, J.; Gómez-Orte, E.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C.; Dueñas, M. Effects of O-methylated metabolites of quercetin on oxidative stress, thermotolerance, lifespan and bioavailability on Caenorhabditis elegans. Food Funct. 2011, 2, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Bruner, L.P.; White, A.M.; Proksell, S. Inflammatory Bowel Disease. Prim. Care 2023, 50, 411–427. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Yang, M.F.; Song, Y.; Xu, H.M.; Xu, J.; Yue, N.N.; Zhang, Y.; Tian, C.M.; Shi, R.Y.; Liang, Y.J.; et al. Exploring the efficacy of herbal medicinal products as oral therapy for inflammatory bowel disease. Biomed. Pharmacother. 2023, 165, 115266. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.G.; Mandal, P.K.; Maroon, J.C. Oxidative Stress Occurs Prior to Amyloid Aβ Plaque Formation and Tau Phosphorylation in Alzheimer’s Disease: Role of Glutathione and Metal Ions. ACS Chem. Neurosci. 2023, 14, 2944–2954. [Google Scholar] [CrossRef]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative stress and inflammation in the pathogenesis of neurological disorders: Mechanisms and implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Lei, M.; Chen, Y.; Tian, S.; Li, C.; Zhang, B. How Oxidative Stress Induces Depression? ASN Neuro 2023, 15, 17590914231181037. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wen, J.; Wang, N.; Wang, C.; Xu, Q.; Yang, Y. Ion Channels and Vascular Diseases. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e146–e156. [Google Scholar] [CrossRef]
- Ortner, N.J.; Striessnig, J. L-type calcium channels as drug targets in CNS disorders. Channels 2016, 10, 7–13. [Google Scholar] [CrossRef]
- Yamakage, M.; Chen, X.; Tsujiguchi, N.; Kamada, Y.; Namiki, A. Different inhibitory effects of volatile anesthetics on T- and L-type voltage-dependent Ca2+ channels in porcine tracheal and bronchial smooth muscles. Anesthesiology 2001, 94, 683–693. [Google Scholar] [CrossRef]
- Dwivedi, R.; Drumm, B.T.; Griffin, C.S.; Dudem, S.; Bradley, E.; Alkawadri, T.; Martin, S.L.; Sergeant, G.P.; Hollywood, M.A.; Thornbury, K.D. Excitatory cholinergic responses in mouse primary bronchial smooth muscle require both Ca2+ entry via L-type Ca2+ channels and store operated Ca2+ entry via Orai channels. Cell Calcium 2023, 112, 102721. [Google Scholar] [CrossRef]
- Manvi; Khan, M.I.; Badruddeen; Akhtar, J.; Ahmad, M.; Siddiqui, Z.; Fatima, G. Role of Plant Bioactive as Diuretics: General Considerations and Mechanism of Diuresis. Curr. Hypertens. Rev. 2023, 19, 79–92. [Google Scholar] [CrossRef]
- Jiménez-Ferrer, E.; Alarcón-Alonso, J.; Aguilar-Rojas, A.; Zamilpa, A.; Jiménez-Ferrer, C.I.; Tortoriello, J.; Herrera-Ruiz, M. Diuretic effect of compounds from Hibiscus sabdariffa by modulation of the aldosterone activity. Planta Med. 2012, 78, 1893–1898. [Google Scholar] [CrossRef] [PubMed]
- Nirumand, M.C.; Hajialyani, M.; Rahimi, R.; Farzaei, M.H.; Zingue, S.; Nabavi, S.M.; Bishayee, A. Dietary Plants for the Prevention and Management of Kidney Stones: Preclinical and Clinical Evidence and Molecular Mechanisms. Int. J. Mol. Sci. 2018, 19, 765. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Xi, Y.; Jiang, W. Protective roles of flavonoids and flavonoid-rich plant extracts against urolithiasis: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2125–2135. [Google Scholar] [CrossRef] [PubMed]
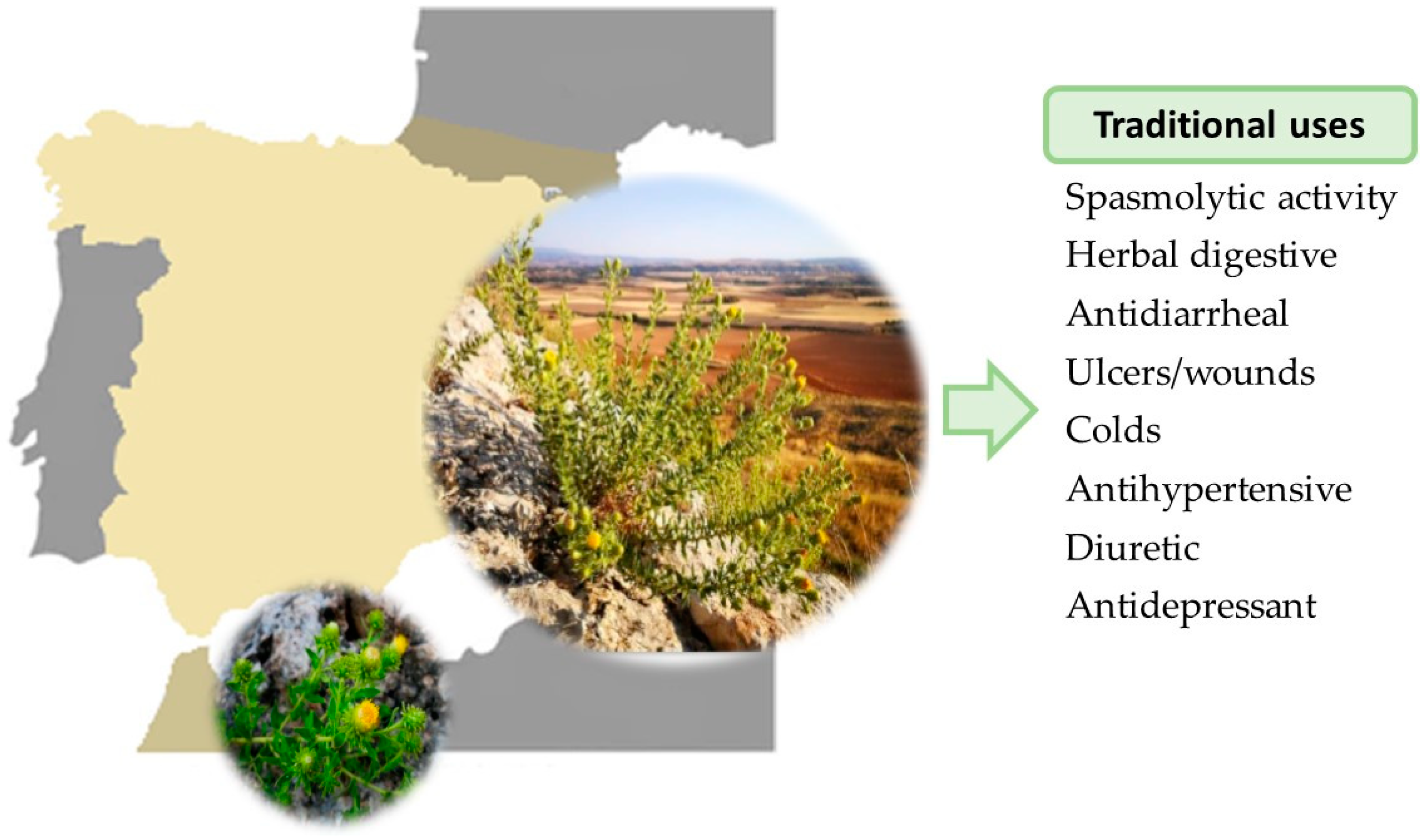
| System | Traditional Uses | Form of Plant Used | Reference |
|---|---|---|---|
| Digestive | Digestive, stomach ulcer, stomach pain, colic, appendicitis, emetics, antidiarrheal, whets the appetite, carminative, antispasmodic | Infusion and maceration in anisette | [1,2,4,5,6,7] |
| Genitourinary | Diuretic, kidney pain, depurative, kidney stones | Infusion | [1,2] |
| Respiratory | Colds, flu | Infusion | [1,2,8] |
| Bronchitis, asthma | Infusion, vapors released during its cooking | [1,2,8] | |
| Endocrine-metabolic | Slimming, lipolytic | Infusion | [1,2] |
| Musculoskeletal | Rheumatic pain, muscle inflammation, bruises | Applying a cloth soaked in the plant decoction | [1,2,9] |
| Integumentary | Heal and wash wounds, ulcers, and burns Disinfectant, healing agent, and anti-inflammatory for wounds | Applying a cloth soaked in the plant decoction, or macerated in alcohol, poultices from the fried or cooked leaves that are applied to wounds Ointment | [1,2] |
| Nervous | Headache, antidepressant, tranquilizer, nerve calmer, analgesic, dizziness | Infusion | [1,2,6,7] |
| Cardiovascular | Hypotensive, venotonic, and antianemic | Infusion | [1,2] |
| Plant Drug | Extract | Extraction Method | Phytochemical Assay | Main Phytochemical Composition | Reference |
|---|---|---|---|---|---|
| Dried leaves | The essential oil and pentane extract | Distillation and ultrasound assisted maceration | GC-MS analysis | Camphor, endo-borneol, α-terpineol, nerolidol and T-cadinol | [10] |
| Fresh Leaves | The essential oil | Steam distillation | GC-MS analysis | Camphor, borneol, caryophyllene oxide, farnesol, bornyl formate, β-pinene, eucalyptol, linalool, cadinol, and spatulenol | [11] |
| Air-dried aerial parts | Fraction of a benzene extract | Refluxing C6H6 and chromatography of the neutral fraction on a drySi02 column | IR spectra, H NMR, and TMS analysis | Kudtdiol, α-epoxy kudtdiol, 5-epi-kudtriol, and kudtriol | [12,13] |
| Dried aerial parts | CH2C12 extract | MeOH maceration, partitioning between n-hexane and 5% aq. MeOH. The aq. MeOH portion was further extracted with CH2C12 | 1D and 2D NMR analysis | Lucinone and glutinone | [14] |
| Aerial parts | Acetone/water extract | Maceration | GC-MS analysis and TLC-UV | Eudesmane alcohols | [15] |
| Aerial parts | MeOH/H2O extract | Maceration | TLC-UV, HNMR, 3C-NMR and 13C-NMR-DEP | Patuletin and quercetin glucosides derivatives | [16] |
| Aerial parts | Butanolic extract | Maceration | TLC-UV, HNMR, 3C-NMR and 13C-NMR-DEP | Kaempferol and quercetin glucosides derivatives | [17] |
| Dry plant material of commercial Rock teas | MeOH and water extracts | Ultrasonic-bath maceration for MeOH extracts, and infusion for water extracts | HPLC-MS analysis | Caffeoylquinic acids, citric acid, benzoic acid, mearnsetin, quercetin, vicenin-2, rutin, kaempferol, isorhamnetin | [18] |
| Dried aerial parts | Ethanolic extract | Maceration | HPLC–MS analysis | Patuletin glucopyranoside | [19] |
| Dried leaves | Ethanolic extract | Maceration | HPLC–DAD analysis | Caffeoylquinic acids, isoferulic acid, quercetin glycosides, kaempferol, isorhamnetin, lutein, β-carotene, chlorophyll b. | [20] |
| Aerial parts | MeOH (70%) extract | Soxhlet | LC-MS Analysis | Caffeoylquinic acids, quercetin glycosides, kaempferol, isorhamnetin, lutein. | [21] |
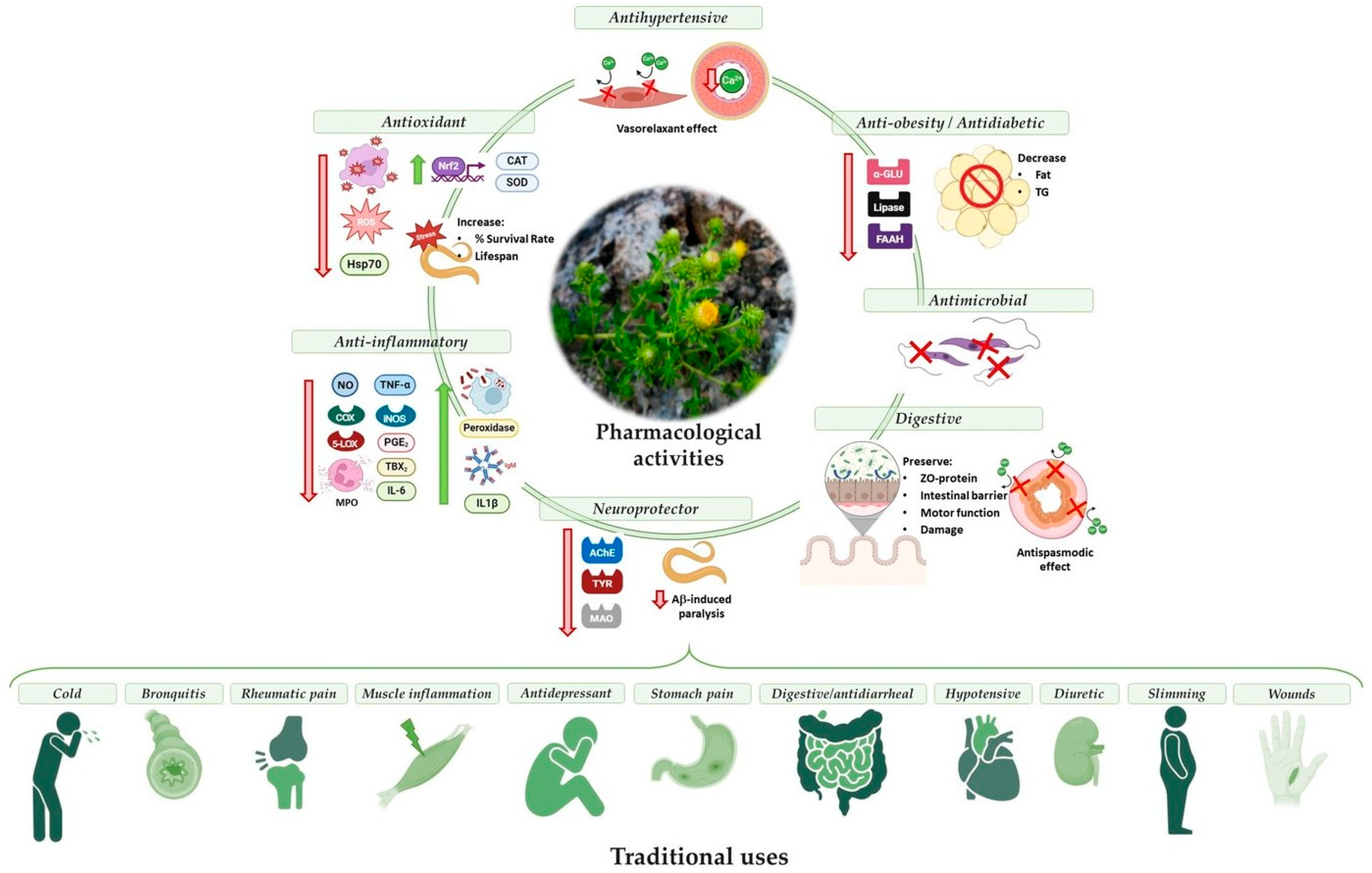
| Biological Activity | Observed Effects/Action Mechanism | Responsible Molecule |
|---|---|---|
| Antimicrobial Antiprotozoal Antifungal | In vitro: inhibition of Entamoeba histolytica, Leishmania donovani, Plasmodium falciparum [23]. In vitro: inhibition of Rhizopus stolonifera [24]. | Sesquiterpenoids (5-epi-kudtriol) Not analyzed |
| Antioxidant | In vitro: positive results in DPPH [18,24,25], ABTS [18], NADH/PMS system, X/XO system [20], FRAP, ORAC [26]. Inhibition of xanthine oxidase [20]. In vivo: reduce HSP 70, increase CAT and SOD, reduce oxidation proteins [25]. Improved the survival rate and the lifespan of C.elegans [28]. | Not analyzed Not analyzed |
| Anti-inflammatory | In vitro: inhibit NO and TNF-α production in LPS-stimulated murine macrophages (J774.2). Inhibit LOX-5 [20]. Increase phagocytic capacity and respiratory burst activity in leukocytes [25]. Inhibit TBX2-release in mouse peritoneal cells [27]. Inhibit COX-1 and PGE2 in mouse peritoneal cells [27]. In vivo: reduce inflammation in a murine model of colitis. Inhibit MPO, IL-6, and the expression of iNOS and COX-2 [20]. Increase peroxidase activity, IgM levels, and Il1β gene expression in fish [25]. | Not analyzed Glutinone Lucinone, glutinone, 5-epikutdtriol and kutdtriol Not analyzed |
| Neuroprotective | In vitro: Inhibition of CNS enzymes as AChE, MAO and TYR [28]. In vivo: Decreased Aβ-induced paralysis in C. elegans [28]. | Not analyzed Not analyzed |
| Antihypertensive | In vitro: Antagonist of L-type Ca2+ channel in A7r5 cells [19]. Ex vivo: vasorelaxant effect in aortic rings. Prevents the increase in cytosolic calcium [19]. | Not analyzed Not analyzed |
| Anti-obesity and antidiabetic | In vitro: inhibition of α-GLU and lipase. Anti-adipogenic activity and delipidating in murine 3T3-L1 preadipocytes [26]. | Not analyzed |
| Digestive | Ex vivo: improve dysmotility [20] and gastrointestinal transit time in a murine model of colitis [22]. Antispasmodic effect. Inhibition of L-type Ca2+ channels [22]. In vivo: protective effect in a murine model of colitis. Prevent symptomatology, damage, and tissue function [20]. | Not analyzed Not analyzed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valero, M.S.; Gómez-Rincón, C.; López, V.; Les, F. Jasonia glutinosa (L.) DC.: Back in Our Pantries? A Review of Its Pharmacological Activity and Mechanisms of Action. Int. J. Mol. Sci. 2025, 26, 2536. https://doi.org/10.3390/ijms26062536
Valero MS, Gómez-Rincón C, López V, Les F. Jasonia glutinosa (L.) DC.: Back in Our Pantries? A Review of Its Pharmacological Activity and Mechanisms of Action. International Journal of Molecular Sciences. 2025; 26(6):2536. https://doi.org/10.3390/ijms26062536
Chicago/Turabian StyleValero, Marta Sofía, Carlota Gómez-Rincón, Víctor López, and Francisco Les. 2025. "Jasonia glutinosa (L.) DC.: Back in Our Pantries? A Review of Its Pharmacological Activity and Mechanisms of Action" International Journal of Molecular Sciences 26, no. 6: 2536. https://doi.org/10.3390/ijms26062536
APA StyleValero, M. S., Gómez-Rincón, C., López, V., & Les, F. (2025). Jasonia glutinosa (L.) DC.: Back in Our Pantries? A Review of Its Pharmacological Activity and Mechanisms of Action. International Journal of Molecular Sciences, 26(6), 2536. https://doi.org/10.3390/ijms26062536









