Biopolymeric Scaffolds with Melatonin for Tissue Engineering—A Review
Abstract
1. Introduction
2. Biofunctionalization of Scaffolds for Tissue Engineering
3. Melatonin in Tissue Engineering
4. Preparation of Scaffolds Containing Melatonin
| Processing Method | Matrix | Application | References |
|---|---|---|---|
| 3D multilayer molding method | Polycaprolactone | sciatic nerve regeneration | [81] |
| Solvent casting and particulate leaching method with MNP entrapment | Polycaprolactone | cartilage regeneration | [82] |
| 3D printing | Magnesium/polycaprolactone | inhibition the development of osteosarcoma | [84] |
| Polycaprolactone/β-TCP/ PLGA NPs | diabetic bone defect repairing | [83] | |
| Electrospinning | Polycaprolactone/alginate | sciatic nerve regeneration | [92] |
| Polycaprolactone/ sodium alginate | tendon regeneration | [20] | |
| Polycaprolactone/gelatin | nerve tissue engineering | [86] | |
| Gelatin/polylactide | vascularized bone regeneration | [85] | |
| Lyophilization | Alginate/chitosan/β-TCP | tibia bone defect regeneration | [89] |
| Alginate/chitosan/β-TCP | periodontal regeneration | [88] | |
| Chitosan/hydroxyapatite | bone formation and inhibiting osteoclast activity | [87] | |
| Chitosan/hydroxyapatite | osteosarcoma therapy | [93] | |
| Chitosan/ hydroxyapatite/PLGA NPs | bone formation and inhibiting osteoclast activity | [94] | |
| Chitosan/collagen | wound treatment | [22,23] | |
| Combined method: foaming and lyophilization | Gelatine | skin tissue engineering | [91] |
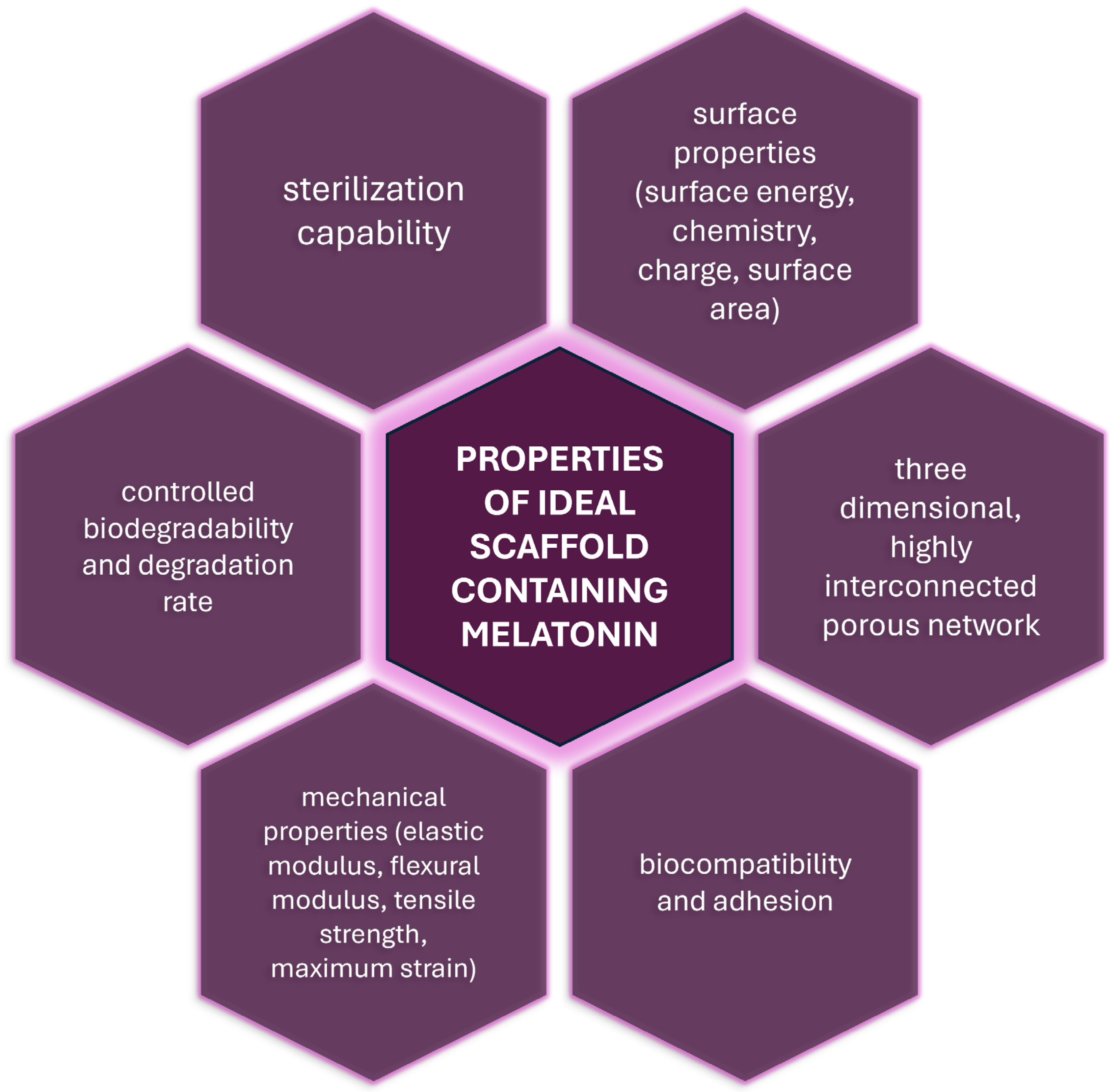
5. Properties of Scaffolds’ Containing Melatonin
- (1)
- appropriate surface properties facilitating cell attachment, proliferation and differentiation (surface energy, chemistry, charge, surface area);
- (2)
- a three-dimensional, highly interconnected porous network, together with an appropriate porosity, pore size and pore structure for cell growth and the transport of nutrients and metabolic waste;
- (3)
- biocompatibility and adhesion to ensure that scaffolds integrate seamlessly with native tissues without eliciting adverse immune responses;
- (4)
- appropriate mechanical properties that are similar to the tissue in the immediate surroundings of the defect (elastic modulus, flexural modulus, tensile strength, maximum strain);
- (5)
- a controlled biodegradability and degradation rate that matches the rate of tissue regeneration, minimizing the risk of inflammation or structural failure;
- (6)
- sterilization capability—compatibility with sterilization methods such as ethylene oxide, gamma radiation, UV irradiation, or ethanol, ensuring the scaffold is free from contaminants before use.
| Matrix | (1) Appropriate Surface Properties | (2) Porous Structure | (3) Biocompatibility | (4) Appropriate Mechanical Properties | (5) Controlled Biodegradability and Degradation Rate with Controlled Drug Release | (6) Sterilization Capability | References |
|---|---|---|---|---|---|---|---|
| Polycaprolactone | n/a | ✓ | ✓ | ✓ | ✓ | n/a | [81,82] |
| Gelatine | n/a | ✓ | ✓ | ✓ | ✓ | ✓ | [91] |
| Magnesium/ polycaprolactone | ✓ | ✓ | ✓ | ✓ | ✓ | n/a | [84] |
| Polycaprolactone/ β-TCP/PLGA NPs | n/a | ✓ | ✓ | ✓ | ✓ | n/a | [83] |
| polycaprolactone/sodium alginate | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | [20,92] |
| Polycaprolactone/gelatin | ✓ | ✓ | ✓ | ✓ | ✓ | n/a | [86] |
| Gelatin/ polylactide | n/a | ✓ | ✓ | ✓ | ✓ | n/a | [85] |
| Alginate/ chitosan/β-TCP | n/a | ✓ | ✓ | ✓ | n/a | ✓ | [88,89] |
| Chitosan/ hydroxyapatite | n/a | ✓ | ✓ | ✓ | ✓ | n/a | [87,93] |
| Chitosan/ hydroxyapatite/ PLGA NPs | n/a | ✓ | ✓ | ✓ | ✓ | ✓ | [94] |
| Chitosan/ collagen | n/a | ✓ | ✓ | ✓ | ✓ | n/a | [22,23] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ebhodaghe, S.O. Natural Polymeric Scaffolds for Tissue Engineering Applications. J. Biomater. Sci. Polym. Ed. 2021, 32, 2144–2194. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Seidi, F.; Azarfam, M.Y.; Yazdi, M.K.; Erfani, A.; Barani, M.; Chauhan, N.P.S.; Rabiee, N.; Kuang, T.; Kucinska-Lipka, J.; et al. Biopolymer-based composites for tissue engineering applications: A basis for future opportunities. Compos. B Eng. 2023, 258, 110701. [Google Scholar] [CrossRef]
- Behrens, M.R.; Ruder, W.C. Biopolymers in Regenerative Medicine: Overview, Current Advances, and Future Trends. In Biopolymers for Biomedical and Biotechnological Applications; Wiley: Hoboken, NJ, USA, 2021; pp. 357–380. [Google Scholar] [CrossRef]
- Veeman, D.; Sai, M.S.; Sureshkumar, P.; Jagadeesha, T.; Natrayan, L.; Ravichandran, M.; Mammo, W.D. Additive Manufacturing of Biopolymers for Tissue Engineering and Regenerative Medicine: An Overview, Potential Applications, Advancements, and Trends. Int. J. Polym. Sci. 2021, 2021, 4907027. [Google Scholar] [CrossRef]
- Danglad-Flores, J.; Sletten, E.T.; Reuber, E.E.; Bienert, K.; Riegler, H.; Seeberger, P.H. Optimized platform for automated glycan assembly. Device 2024, 2, 100499. [Google Scholar] [CrossRef]
- Guo, B.; Lei, B.; Li, P.; Ma, P.X. Functionalized scaffolds to enhance tissue regeneration. Regen. Biomater. 2015, 2, 47–57. [Google Scholar] [CrossRef]
- John, J.V.; McCarthy, A.; Karan, A.; Xie, J. Electrospun Nanofibers for Wound Management. ChemNanoMat 2022, 8, e202100349. [Google Scholar] [CrossRef]
- Grayson, W.L.; Martens, T.P.; Eng, G.M.; Radisic, M.; Vunjak-Novakovic, G. Biomimetic approach to tissue engineering. Semin. Cell Dev. Biol. 2009, 20, 665–673. [Google Scholar] [CrossRef]
- Xia, P.; Luo, Y. Vascularization in tissue engineering: The architecture cues of pores in scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1206–1214. [Google Scholar] [CrossRef]
- Lekhavadhani, S.; Shanmugavadivu, A.; Selvamurugan, N. Role and architectural significance of porous chitosan-based scaffolds in bone tissue engineering. Int. J. Biol. Macromol. 2023, 251, 126238. [Google Scholar] [CrossRef]
- Farazin, A.; Zhang, C.; Gheisizadeh, A.; Shahbazi, A. 3D bio-printing for use as bone replacement tissues: A review of biomedical application. Biomed. Eng. Adv. 2023, 5, 100075. [Google Scholar] [CrossRef]
- Mukasheva, F.; Adilova, L.; Dyussenbinov, A.; Yernaimanova, B.; Abilev, M.; Akilbekova, D. Optimizing scaffold pore size for tissue engineering: Insights across various tissue types. Front. Bioeng. Biotechnol. 2024, 12, 1444986. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.; Busari, H.; Seims, K.B.; Schwarzenberg, P.; Dailey, H.L.; Chow, L.W. 3D printing with peptide–polymer conjugates for single-step fabrication of spatially functionalized scaffolds. Biomater. Sci. 2019, 7, 4237–4247. [Google Scholar] [CrossRef] [PubMed]
- Rahmany, M.B.; Van Dyke, M. Biomimetic approaches to modulate cellular adhesion in biomaterials: A review. Acta Biomater. 2013, 9, 5431–5437. [Google Scholar] [CrossRef]
- Tai, Y.; Banerjee, A.; Goodrich, R.; Jin, L.; Nam, J. Development and Utilization of Multifunctional Polymeric Scaffolds for the Regulation of Physical Cellular Microenvironments. Polymers 2021, 13, 3880. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, M.; Wang, X.; Liu, S.; Luo, L.; Zeng, Q.; Wu, Y.; Zeng, Y.; Yang, Z.; Sheng, G.; et al. Emerging Nanochitosan for Sustainable Agriculture. Int. J. Mol. Sci. 2024, 25, 12261. [Google Scholar] [CrossRef]
- Sohn, E.-H.; Kim, S.-N.; Lee, S.-R. Melatonin’s Impact on Wound Healing. Antioxidants 2024, 13, 1197. [Google Scholar] [CrossRef]
- Murali, R.; Thanikaivelan, P.; Cheirmadurai, K. Melatonin in functionalized biomimetic constructs promotes rapid tissue regeneration in Wistar albino rats. J. Mater. Chem. B 2016, 4, 5850–5862. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, X.; Qian, Y.; Tang, H.; Song, J.; Qu, X.; Yue, B.; Yuan, W. Melatonin-Based and Biomimetic Scaffold as Muscle–ECM Implant for Guiding Myogenic Differentiation of Volumetric Muscle Loss. Adv. Funct. Mater. 2020, 30, 2002378. [Google Scholar] [CrossRef]
- Yao, Z.; Qian, Y.; Jin, Y.; Wang, S.; Li, J.; Yuan, W.-E.; Fan, C. Biomimetic multilayer polycaprolactone/sodium alginate hydrogel scaffolds loaded with melatonin facilitate tendon regeneration. Carbohydr. Polym. 2022, 277, 118865. [Google Scholar] [CrossRef]
- Aykora, D.; Oral, A.; Aydeğer, C.; Uzun, M. 3D Bioprinting Strategies for Melatonin-Loaded Polymers in Bone Tissue Engineering. Macromol. Mater. Eng. 2025, 310, 2400263. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepańska, B.; Pin, J.M.; Zasada, L.; Sonne, M.M.; Reiter, R.J.; Slominski, A.T.; Steinbrink, K.; Kleszczyński, K. Assessment of Melatonin-Cultured Collagen/Chitosan Scaffolds Cross-Linked by a Glyoxal Solution as Biomaterials for Wound Healing. Antioxidants 2022, 11, 570. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek-Szczepańska, B.; Ostrowska, J.; Kozłowska, J.; Szota, Z.; Brożyna, A.A.; Dreier, R.; Reiter, R.J.; Slominski, A.T.; Steinbrink, K.; Kleszczyński, K. Evaluation of Polymeric Matrix Loaded with Melatonin for Wound Dressing. Int. J. Mol. Sci. 2021, 22, 5658. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.I.R.; do Carmo Neto, J.R.; Rocha, V.L.; Machado, J.R.; Amaral, A.C.; Miguel, M.P. A Revision of Polymeric Nanoparticles as a Strategy to Improve the Biological Activity of Melatonin. Curr. Med. Chem. 2023, 30, 3315–3334. [Google Scholar] [CrossRef]
- Chuffa, L.G.d.A.; Seiva, F.R.F.; Novais, A.A.; Simão, V.A.; Giménez, V.M.M.; Manucha, W.; Zuccari, D.A.P.d.C.; Reiter, R.J. Melatonin-Loaded Nanocarriers: New Horizons for Therapeutic Applications. Molecules 2021, 26, 3562. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, K.; Gaida, V.A.; Schäfer, J.; Bosse, L.; Diemer, C.; Reiter, R.J.; Slominski, A.T.; Steinbrink, K.; Sionkowska, A.; Kleszczyński, K. Melatonin/Sericin Wound Healing Patches: Implications for Melanoma Therapy. Int. J. Mol. Sci. 2024, 25, 4858. [Google Scholar] [CrossRef]
- Biswal, T. Biopolymers for tissue engineering applications: A review. Mater. Today Proc. 2021, 41, 397–402. [Google Scholar] [CrossRef]
- Ullah, S.; Chen, X. Fabrication, applications and challenges of natural biomaterials in tissue engineering. Appl. Mater. Today 2020, 20, 100656. [Google Scholar] [CrossRef]
- Jabbari, E. Challenges for Natural Hydrogels in Tissue Engineering. Gels 2019, 5, 30. [Google Scholar] [CrossRef]
- Asghar, M.S.; Li, J.; Ahmed, I.; Ghazanfar, U.; Irshad, M.S.; Idrees, M.; Haq, Z.; Rizwan, M.; Sheikh, F.; Yasmeen, F. Antioxidant, and enhanced flexible nano porous scaffolds for bone tissue engineering applications. Nano Sel. 2021, 2, 1356–1367. [Google Scholar] [CrossRef]
- Selvakumar, G.; Lonchin, S. A bio-polymeric scaffold incorporated with p-Coumaric acid enhances diabetic wound healing by modulating MMP-9 and TGF-β3 expression. Colloids Surf. B Biointerfaces 2023, 225, 113280. [Google Scholar] [CrossRef]
- Karan, A.; Sharma, N.S.; Darder, M.; Su, Y.; Andrabi, S.M.; Shahriar, S.M.S.; John, J.V.; Luo, Z.; DeCoster, M.A.; Zhang, Y.S.; et al. Copper–Cystine Biohybrid-Embedded Nanofiber Aerogels Show Antibacterial and Angiogenic Properties. ACS Omega 2024, 9, 9765–9781. [Google Scholar] [CrossRef] [PubMed]
- John, J.V.; Sharma, N.S.; Tang, G.; Luo, Z.; Su, Y.; Weihs, S.; Shahriar, S.M.S.; Wang, G.; McCarthy, A.; Dyke, J.; et al. Nanofiber Aerogels with Precision Macrochannels and LL-37-Mimic Peptides Synergistically Promote Diabetic Wound Healing. Adv. Funct. Mater. 2023, 33, 2206936. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.J.; Martino, M.M.; De Laporte, L.; Tortelli, F.; Briquez, P.S.; Hubbell, J.A. Engineering the Regenerative Microenvironment with Biomaterials. Adv. Healthc. Mater. 2013, 2, 57–71. [Google Scholar] [CrossRef]
- López-Gutierrez, J.; Ramos-Payán, R.; Ayala-Ham, A.; Romero-Quintana, J.G.; Castillo-Ureta, H.; Villegas-Mercado, C.; Bermúdez, M.; Sanchez-Schmitz, G.; Aguilar-Medina, M. Biofunctionalization of hydrogel-based scaffolds for vascular tissue regeneration. Front. Mater. 2023, 10, 1168616. [Google Scholar] [CrossRef]
- Casanova, M.R.; Reis, R.L.; Martins, A.; Neves, N.M. Surface biofunctionalization to improve the efficacy of biomaterial substrates to be used in regenerative medicine. Mater. Horiz. 2020, 7, 2258–2275. [Google Scholar] [CrossRef]
- Boddepalli, Y.; Chava, N.; Gadiraju, S.; Sri Pachchava, K.; Kotikalapudi, K.; Siddiqui, N. Review on Growth Factor Loaded Scaffolds for Rapid Healing of Bone Tissue. Int. J. Life Sci. Pharma Res. 2023, 13, L176–L198. [Google Scholar] [CrossRef]
- Safari, B.; Davaran, S.; Aghanejad, A. Osteogenic potential of the growth factors and bioactive molecules in bone regeneration. Int. J. Biol. Macromol. 2021, 175, 544–557. [Google Scholar] [CrossRef]
- De Witte, T.-M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef]
- Barabaschi, G.D.G.; Manoharan, V.; Li, Q.; Bertassoni, L.E. Engineering Pre-Vascularized Scaffolds for Bone Regeneration; Springer: Berlin/Heidelberg, Germany, 2015; pp. 79–94. [Google Scholar] [CrossRef]
- Davies, N.H.; Schmidt, C.; Bezuidenhout, D.; Zilla, P. Sustaining Neovascularization of a Scaffold Through Staged Release of Vascular Endothelial Growth Factor-A and Platelet-Derived Growth Factor-BB. Tissue Eng. Part A 2012, 18, 26–34. [Google Scholar] [CrossRef]
- Karpov, T.E.; Peltek, O.O.; Muslimov, A.R.; Tarakanchikova, Y.V.; Grunina, T.M.; Poponova, M.S.; Karyagina, A.S.; Chernozem, R.V.; Pariy, I.O.; Mukhortova, Y.R.; et al. Development of Optimized Strategies for Growth Factor Incorporation onto Electrospun Fibrous Scaffolds to Promote Prolonged Release. ACS Appl. Mater. Interfaces 2020, 12, 5578–5592. [Google Scholar] [CrossRef]
- Seims, K.B.; Hunt, N.K.; Chow, L.W. Strategies to Control or Mimic Growth Factor Activity for Bone, Cartilage, and Osteochondral Tissue Engineering. Bioconjug. Chem. 2021, 32, 861–878. [Google Scholar] [CrossRef]
- Bayer, E.A.; Gottardi, R.; Fedorchak, M.V.; Little, S.R. The scope and sequence of growth factor delivery for vascularized bone tissue regeneration. J. Control. Release 2015, 219, 129–140. [Google Scholar] [CrossRef]
- Kass, L.E.; Nguyen, J. Nanocarrier-hydrogel composite delivery systems for precision drug release. WIREs Nanomed. Nanobiotechnol. 2021, 14, e1756. [Google Scholar] [CrossRef]
- Rahman, S.U.; Nagrath, M.; Ponnusamy, S.; Arany, P.R. Nanoscale and Macroscale Scaffolds with Controlled-Release Polymeric Systems for Dental Craniomaxillofacial Tissue Engineering. Materials 2018, 11, 1478. [Google Scholar] [CrossRef]
- Ali, F.; Khan, I.; Chen, J.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Emerging Fabrication Strategies of Hydrogels and Its Applications. Gels 2022, 8, 205. [Google Scholar] [CrossRef]
- Yanez, M.; Blanchette, J.; Jabbarzadeh, E. Modulation of Inflammatory Response to Implanted Biomaterials Using Natural Compounds. Curr. Pharm. Des. 2018, 23, 6347–6357. [Google Scholar] [CrossRef]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef]
- Batool, F.; Özçelik, H.; Stutz, C.; Gegout, P.-Y.; Benkirane-Jessel, N.; Petit, C.; Huck, O. Modulation of immune-inflammatory responses through surface modifications of biomaterials to promote bone healing and regeneration. J. Tissue Eng. 2021, 12, 20417314211041428. [Google Scholar] [CrossRef]
- Muzzio, N.; Moya, S.; Romero, G. Multifunctional Scaffolds and Synergistic Strategies in Tissue Engineering and Regenerative Medicine. Pharmaceutics 2021, 13, 792. [Google Scholar] [CrossRef]
- Todd, E.A.; Mirsky, N.A.; Silva, B.L.G.; Shinde, A.R.; Arakelians, A.R.L.; Nayak, V.V.; Marcantonio, R.A.C.; Gupta, N.; Witek, L.; Coelho, P.G. Functional Scaffolds for Bone Tissue Regeneration: A Comprehensive Review of Materials, Methods, and Future Directions. J. Funct. Biomater. 2024, 15, 280. [Google Scholar] [CrossRef]
- Hunziker, R.; Lumelsky, N.; Wang, F. Editorial: Scaffolds for Regenerative Medicine: A Special Issue of the Annals of Biomedical Engineering. Ann. Biomed. Eng. 2015, 43, 487–488. [Google Scholar] [CrossRef][Green Version]
- Ali Zahid, A.; Chakraborty, A.; Shamiya, Y.; Ravi, S.P.; Paul, A. Leveraging the advancements in functional biomaterials and scaffold fabrication technologies for chronic wound healing applications. Mater. Horiz. 2022, 9, 1850–1865. [Google Scholar] [CrossRef]
- Oliveira, R.L.M.S.; Barbosa, L.; Hurtado, C.R.; Ramos, L.d.P.; Montanheiro, T.L.A.; Oliveira, L.D.; Tada, D.B.; Trichês, E.d.S. Bioglass-based scaffolds coated with silver nanoparticles: Synthesis, processing and antimicrobial activity. J. Biomed. Mater. Res. A 2020, 108, 2447–2459. [Google Scholar] [CrossRef]
- Kalyan, K.S.D.R.; Vinay, C.; Arunbhupathi; Uloopi, K.S.; Chandrasekhar, R.; RojaRamya, K.S. Preclinical Evaluation and Clinical Trial of Chlorhexidine Polymer Scaffold for Vital Pulp Therapy. J. Clin. Pediatr. Dent. 2019, 43, 109–115. [Google Scholar] [CrossRef]
- Felice, B.; Sánchez, M.A.; Socci, M.C.; Sappia, L.D.; Gómez, M.I.; Cruz, M.K.; Felice, C.J.; Martí, M.; Pividori, M.I.; Simonelli, G.; et al. Controlled degradability of PCL-ZnO nanofibrous scaffolds for bone tissue engineering and their antibacterial activity. Mater. Sci. Eng. C 2018, 93, 724–738. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Q.; Wang, Y.; Meng, J.; Wu, M.; Xu, H.; Du, L.; Yang, X. Fabrication and Characterization of Electrospun Poly(Caprolactone)/Tannic Acid Scaffold as an Antibacterial Wound Dressing. Polymers 2023, 15, 593. [Google Scholar] [CrossRef]
- Jiménez-Gastélum, G.R.; Aguilar-Medina, E.M.; Soto-Sainz, E.; Ramos-Payán, R.; Silva-Benítez, E.L. Antimicrobial Properties of Extracellular Matrix Scaffolds for Tissue Engineering. Biomed. Res. Int. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Sarkar, N.; Bose, S. Liposome-Encapsulated Curcumin-Loaded 3D Printed Scaffold for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 17184–17192. [Google Scholar] [CrossRef]
- Murphy, K.P.; Hendley, M.A.; Isely, C.; Annamalai, P.; Peña, E.; Gower, R.M. Resveratrol Delivery from Porous Poly(lactide- co -glycolide) Scaffolds Promotes an Anti-Inflammatory Environment within Visceral Adipose Tissue. ACS Appl. Mater. Interfaces 2018, 10, 43363–43374. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Wang, Z.; Qi, Y.; Li, L.; Zhang, P.; Chen, X.; Huang, Y. Composite PLA/PEG/nHA/Dexamethasone Scaffold Prepared by 3D Printing for Bone Regeneration. Macromol. Biosci. 2018, 18, e1800068. [Google Scholar] [CrossRef]
- Marrazzo, P.; O’Leary, C. Repositioning Natural Antioxidants for Therapeutic Applications in Tissue Engineering. Bioengineering 2020, 7, 104. [Google Scholar] [CrossRef]
- Jayasinghe, S.N. Thoughts on Scaffolds. Adv. Biosyst. 2017, 1, e1700067. [Google Scholar] [CrossRef]
- Luchetti, F.; Canonico, B.; Bartolini, D.; Arcangeletti, M.; Ciffolilli, S.; Murdolo, G.; Piroddi, M.; Papa, S.; Reiter, R.J.; Galli, F. Melatonin regulates mesenchymal stem cell differentiation: A review. J. Pineal Res. 2014, 56, 382–397. [Google Scholar] [CrossRef]
- Dalgic, A.D.; Atila, D.; Tezcaner, A.; Gürses, S.; Keskin, D. Diatom silica frustules-doped fibers for controlled release of melatonin for bone regeneration. Eur. Polym. J. 2023, 186, 111858. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and inflammation—Story of a double-edged blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef]
- Favero, G.; Franceschetti, L.; Bonomini, F.; Rodella, L.F.; Rezzani, R. Melatonin as an Anti-Inflammatory Agent Modulating Inflammasome Activation. Int. J. Endocrinol. 2017, 2017, 1835195. [Google Scholar] [CrossRef]
- Radogna, F.; Diederich, M.; Ghibelli, L. Melatonin: A pleiotropic molecule regulating inflammation. Biochem. Pharmacol. 2010, 80, 1844–1852. [Google Scholar] [CrossRef]
- Sánchez-Barceló, E.J.; Mediavilla, M.D.; Tan, D.X.; Reiter, R.J. Scientific Basis for the Potential Use of Melatonin in Bone Diseases: Osteoporosis and Adolescent Idiopathic Scoliosis. J. Osteoporos. 2010, 2010, 830231. [Google Scholar] [CrossRef]
- Maria, S.; Samsonraj, R.M.; Munmun, F.; Glas, J.; Silvestros, M.; Kotlarczyk, M.P.; Rylands, R.; Dudakovic, A.; van Wijnen, A.J.; Enderby, L.T.; et al. Biological effects of melatonin on osteoblast/osteoclast cocultures, bone, and quality of life: Implications of a role for MT2 melatonin receptors, MEK1/2, and MEK5 in melatonin-mediated osteoblastogenesis. J. Pineal Res. 2018, 64, e12465. [Google Scholar] [CrossRef]
- Narayan, S.; Malaiappan, S. Additive Benefits of Melatonin on Osteogenic Differentiation Rate and Osteogenic Potential Quantified by Alkaline Phosphatase—A Systematic Review. Int. J. Biomed. Investig. 2023, 6, 1–10. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Y.; Liu, X.; Diao, J.; Zhao, N.; Shi, X.; Wang, Y. Melatonin decorated 3D-printed beta-tricalcium phosphate scaffolds promoting bone regeneration in a rat calvarial defect model. J. Mater. Chem. B 2019, 7, 3250–3259. [Google Scholar] [CrossRef]
- Majidinia, M.; Reiter, R.J.; Shakouri, S.K.; Mohebbi, I.; Rastegar, M.; Kaviani, M.; Darband, S.G.; Jahanban-Esfahlan, R.; Nabavi, S.M.; Yousefi, B. The multiple functions of melatonin in regenerative medicine. Ageing Res. Rev. 2018, 45, 33–52. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Etemad, L.; Yari, D.; Hashemi, M.; Salmasi, Z. Evaluation of Melatonin and its Nanostructures Effects on Skin Disorders Focused on Wound Healing. Mini-Rev. Med. Chem. 2024, 24, 1856–1881. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D.; Collin, F. Melatonin: Action as antioxidant and potential applications in human disease and aging. Toxicology 2010, 278, 55–67. [Google Scholar] [CrossRef]
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; González-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14. [Google Scholar] [CrossRef]
- Goradel, N.H.; Asghari, M.H.; Moloudizargari, M.; Negahdari, B.; Haghi-Aminjan, H.; Abdollahi, M. Melatonin as an angiogenesis inhibitor to combat cancer: Mechanistic evidence. Toxicol. Appl. Pharmacol. 2017, 335, 56–63. [Google Scholar] [CrossRef]
- Qian, Y.; Han, Q.; Zhao, X.; Song, J.; Cheng, Y.; Fang, Z.; Ouyang, Y.; Yuan, W.; Fan, C. 3D melatonin nerve scaffold reduces oxidative stress and inflammation and increases autophagy in peripheral nerve regeneration. J. Pineal Res. 2018, 65, e12516. [Google Scholar] [CrossRef]
- Rao, S.K.; Jaison, D.; Sridhar, K.; Kasthuri, N.; Gopinath, V.; Sivaperumal, P.; Patil, S. Melatonin delivery from PCL scaffold enhances glycosaminoglycans deposition in human chondrocytes—Bioactive scaffold model for cartilage regeneration. Process Biochem. 2020, 99, 36–47. [Google Scholar] [CrossRef]
- Chen, T.; Wu, Z.; Hou, Q.; Mei, Y.; Yang, K.; Xu, J.; Wang, L. The Dual Angiogenesis Effects via Nrf2/HO-1 Signaling Pathway of Melatonin Nanocomposite Scaffold on Promoting Diabetic Bone Defect Repair. Int. J. Nanomed. 2024, 19, 2709–2732. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, W.; Li, Q.; Zhao, D.; Qu, J.; Yuan, Z.; Cheng, Z.; Zhu, X.; Zhuang, X.; Zhang, Z. 3D-printing magnesium–polycaprolactone loaded with melatonin inhibits the development of osteosarcoma by regulating cell-in-cell structures. J. Nanobiotechnol. 2021, 19, 263. [Google Scholar] [CrossRef]
- Lv, N.; Hou, M.; Deng, L.; Hua, X.; Zhou, X.; Liu, H.; Zhu, X.; Xu, Y.; Qian, Z.; Li, Q.; et al. A sponge-like nanofiber melatonin-loaded scaffold accelerates vascularized bone regeneration via improving mitochondrial energy metabolism. Mater. Today Bio 2024, 26, 101078. [Google Scholar] [CrossRef]
- Chen, T.; Jiang, H.; Li, X.; Zhang, D.; Zhu, Y.; Chen, X.; Yang, H.; Shen, F.; Xia, H.; Zheng, J.; et al. Proliferation and differentiation study of melatonin functionalized polycaprolactone/gelatin electrospun fibrous scaffolds for nerve tissue engineering. Int. J. Biol. Macromol. 2022, 197, 103–110. [Google Scholar] [CrossRef]
- Jarrar, H.; Çetin Altındal, D.; Gümüşderelioğlu, M. Effect of melatonin/BMP-2 co-delivery scaffolds on the osteoclast activity. J. Mater. Sci. Mater. Med. 2021, 32, 32. [Google Scholar] [CrossRef]
- Abdelrasoul, M.; El-Fattah, A.A.; Kotry, G.; Ramadan, O.; Essawy, M.; Kamaldin, J.; Kandil, S. Regeneration of critical-sized grade II furcation using a novel injectable melatonin-loaded scaffold. Oral. Dis. 2023, 29, 3583–3598. [Google Scholar] [CrossRef]
- Abdelrasoul, M.R.; Kamaldin, J.; Ooi, J.P.; El-Fattah, A.A.; Kotry, G.S.; Ramadan, O.; Kandil, S. An Eight-Week In Vivo Study on the Clinical Signs of Systemic Toxicity and Bone Regenerative Performance of Composites Containing Beta Tricalcium Phosphate, Hydrogel and Melatonin in Adult New Zealand Rabbit (Oryctolagus cuniculus). Malays. J. Med. Health Sci. 2020, 16, 38–45. [Google Scholar]
- Perić Kačarević, Ž.; Rider, P.; Alkildani, S.; Retnasingh, S.; Pejakić, M.; Schnettler, R.; Gosau, M.; Smeets, R.; Jung, O.; Barbeck, M. An introduction to bone tissue engineering. Int. J. Artif. Organs 2020, 43, 69–86. [Google Scholar] [CrossRef]
- Chanu, N.R.; Bhattacharya, K.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Evaluation of a novel melatonin-loaded gelatin sponge as a wound dressing. J. Vasc. Nurs. 2022, 40, 2–10. [Google Scholar] [CrossRef]
- Wang, X.; Yao, X.; Sun, Z.; Jin, Y.; Yan, Z.; Jiang, H.; Ouyang, Y.; Yuan, W.-E.; Wang, C.; Fan, C. An extracellular matrix mimicking alginate hydrogel scaffold manipulates an inflammatory microenvironment and improves peripheral nerve regeneration by controlled melatonin release. J. Mater. Chem. B 2023, 11, 11552–11561. [Google Scholar] [CrossRef]
- Çetin Altındal, D.; Gümüşderelioğlu, M. Dual-functional melatonin releasing device loaded with PLGA microparticles and cyclodextrin inclusion complex for osteosarcoma therapy. J. Drug Deliv. Sci. Technol. 2019, 52, 586–596. [Google Scholar] [CrossRef]
- Jarrar, H.; Çetin Altındal, D.; Gümüşderelioğlu, M. Scaffold-based osteogenic dual delivery system with melatonin and BMP-2 releasing PLGA microparticles. Int. J. Pharm. 2021, 600, 120489. [Google Scholar] [CrossRef] [PubMed]
- Suamte, L.; Tirkey, A.; Barman, J.; Jayasekhar Babu, P. Various manufacturing methods and ideal properties of scaffolds for tissue engineering applications. Smart Mater. Manuf. 2023, 1, 100011. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 1–19. [Google Scholar] [CrossRef]
- Sultana, N. Scaffolds for Tissue Engineering; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–17. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarek-Szczepańska, B.; Grabska-Zielińska, S. Biopolymeric Scaffolds with Melatonin for Tissue Engineering—A Review. Int. J. Mol. Sci. 2025, 26, 2520. https://doi.org/10.3390/ijms26062520
Kaczmarek-Szczepańska B, Grabska-Zielińska S. Biopolymeric Scaffolds with Melatonin for Tissue Engineering—A Review. International Journal of Molecular Sciences. 2025; 26(6):2520. https://doi.org/10.3390/ijms26062520
Chicago/Turabian StyleKaczmarek-Szczepańska, Beata, and Sylwia Grabska-Zielińska. 2025. "Biopolymeric Scaffolds with Melatonin for Tissue Engineering—A Review" International Journal of Molecular Sciences 26, no. 6: 2520. https://doi.org/10.3390/ijms26062520
APA StyleKaczmarek-Szczepańska, B., & Grabska-Zielińska, S. (2025). Biopolymeric Scaffolds with Melatonin for Tissue Engineering—A Review. International Journal of Molecular Sciences, 26(6), 2520. https://doi.org/10.3390/ijms26062520







