Vesicular Glutamate Transporter 3 Is Involved in Glutamatergic Signalling in Podocytes
Abstract
1. Introduction
2. Results
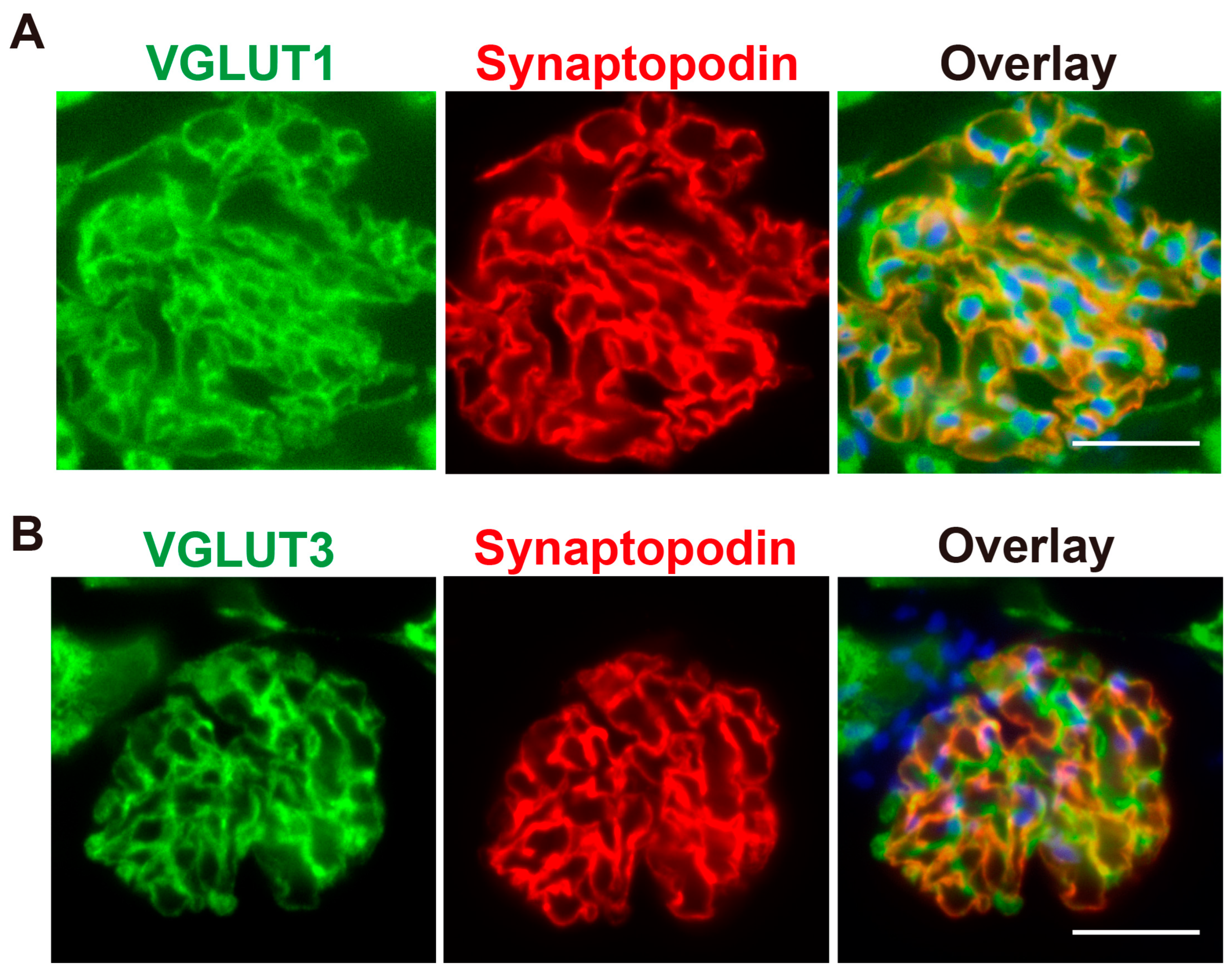
2.1. VGLUT3 Is Present in Glomerular Podocytes
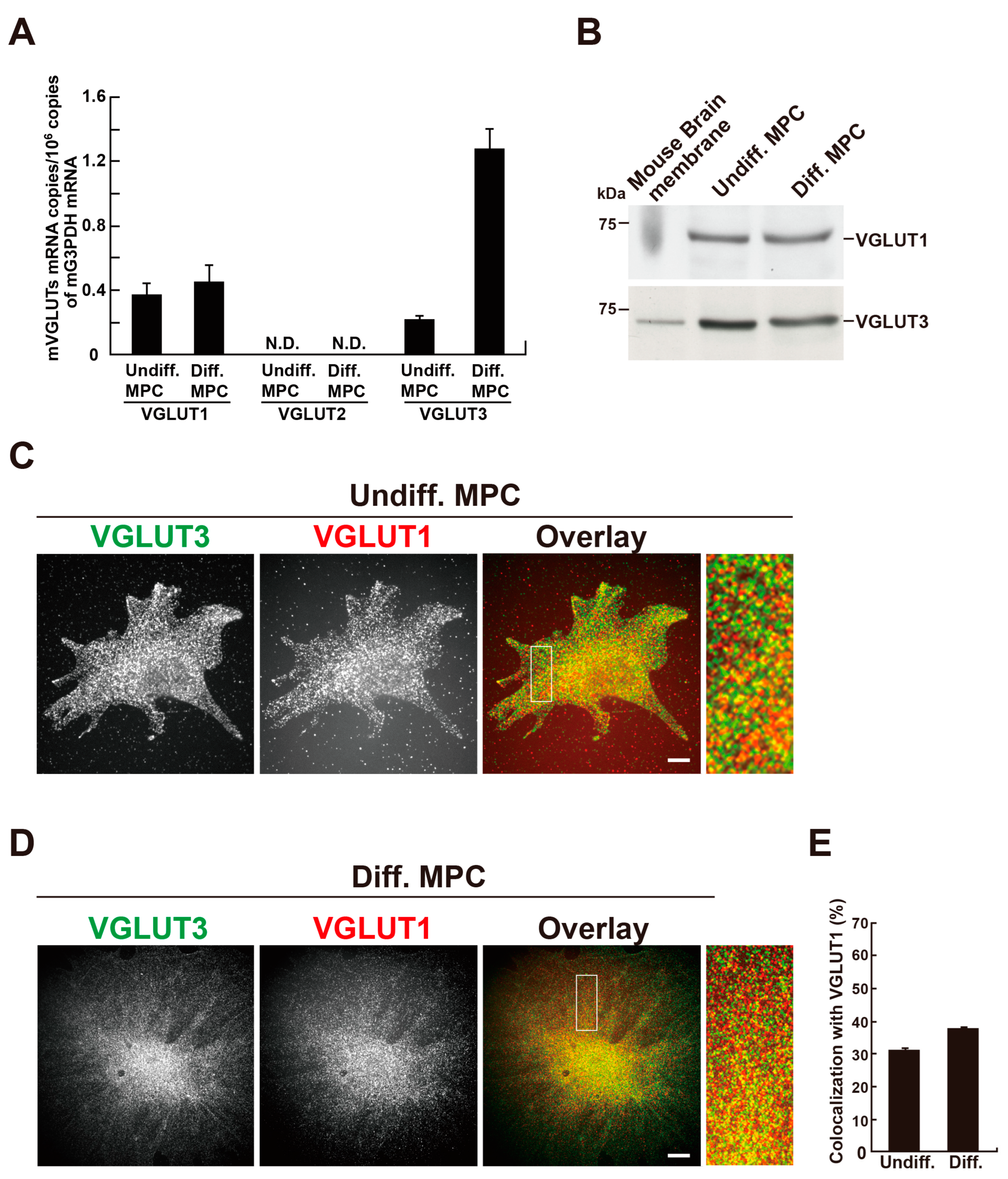
2.2. VGLUT1 and VGLUT3 Are Co-Expressed in Differentiated MPCs
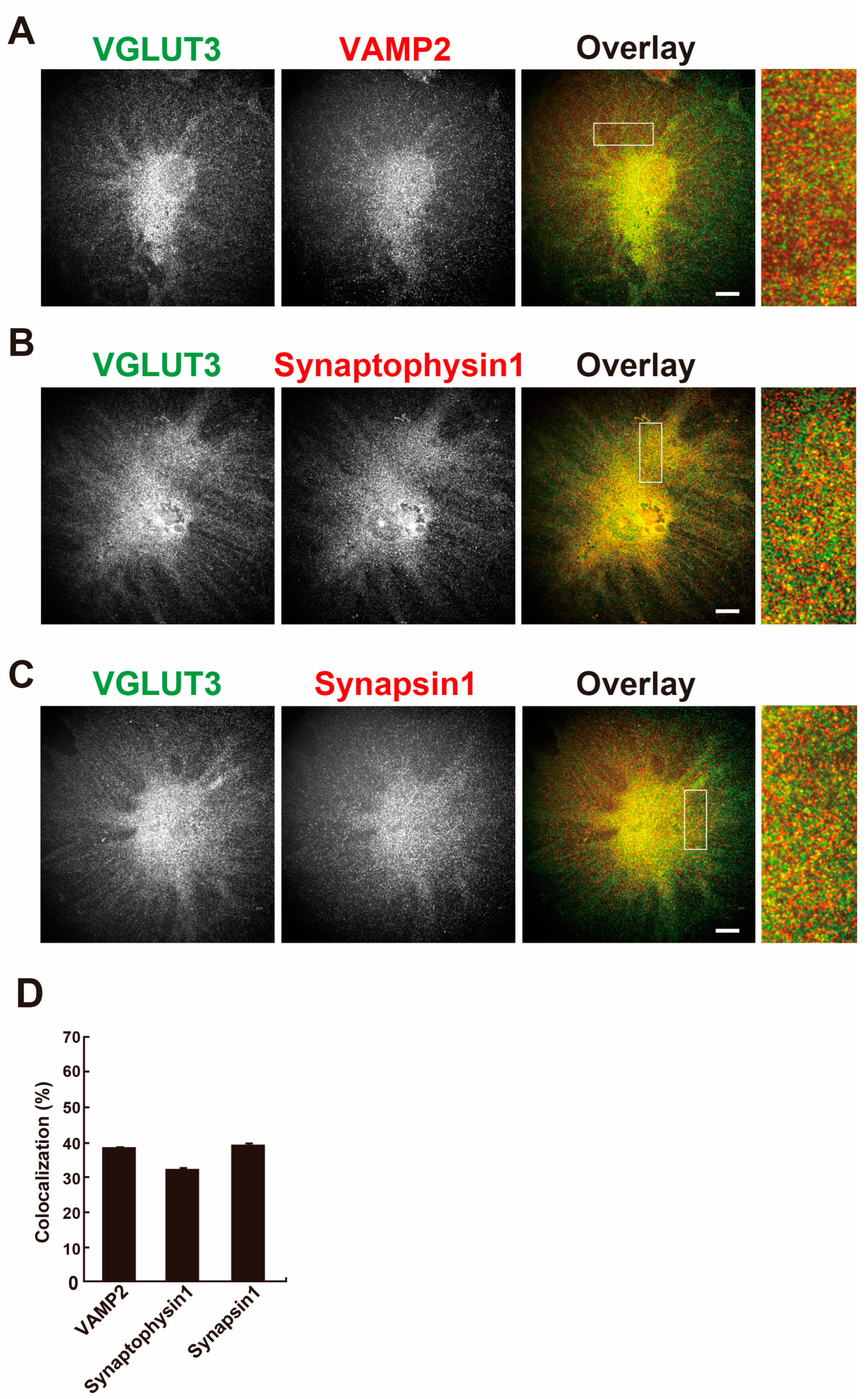
2.3. VGLUT3 Colocalizes with Synaptic Vesicle Marker Proteins in Differentiated MPCs
2.4. VGLUT3 Associates with Synaptic-like Vesicles in Differentiated MPCs
2.5. VGLUT3 Depletion in Differentiated MPCs Reduces Glutamate Release into the Intracellular Space
3. Discussion
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. Cell Culture
4.3. siRNA-Mediated Interference and Transfection
4.4. Quantitative PCR
4.5. Immunohistochemistry
4.6. Electron Microscopy
4.7. Fluorescent Microscopy
4.8. Determination of Glutamate Release
4.9. Morphometry
4.10. Ethics and Animal Use Statement
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pavenstädt, H.; Kriz, W.; Kretzler, M. Cell Biology of the Glomerular Podocyte. Physiol. Rev. 2003, 83, 253–307. [Google Scholar] [CrossRef] [PubMed]
- Flavell, S.W.; Greenberg, M.E. Signaling Mechanisms Linking Neuronal Activity to Gene Expression and Plasticity of the Nervous System. Annu. Rev. Neurosci. 2008, 31, 563–590. [Google Scholar] [CrossRef]
- Morimoto, R.; Hayashi, M.; Yatsushiro, S.; Otsuka, M.; Yamamoto, A.; Moriyama, Y. Co-expression of vesicular glutamate transporters (VGLUT1 and VGLUT2) and their association with synaptic-like microvesicles in rat pinealocytes. J. Neurochem. 2003, 84, 382–391. [Google Scholar] [CrossRef]
- Hayashi, M.; Morimoto, R.; Yamamoto, A.; Moriyama, Y. Expression and Localization of Vesicular Glutamate Transporters in Pancreatic Islets, Upper Gastrointestinal Tract, and Testis. J. Histochem. Cytochem. 2003, 51, 1375–1390. [Google Scholar] [CrossRef]
- Gu, L.; Liang, X.; Wang, L.; Yan, Y.; Ni, Z.; Dai, H.; Gao, J.; Mou, S.; Wang, Q.; Chen, X.; et al. Functional metabotropic glutamate receptors 1 and 5 are expressed in murine podocytes. Kidney Int. 2012, 81, 458–468. [Google Scholar] [CrossRef][Green Version]
- Puliti, A.; Rossi, P.I.A.; Caridi, G.; Corbelli, A.; Ikehata, M.; Armelloni, S.; Li, M.; Zennaro, C.; Conti, V.; Vaccari, C.M.; et al. Albuminuria and Glomerular Damage in Mice Lacking the Metabotropic Glutamate Receptor. Am. J. Pathol. 2011, 178, 1257–1269. [Google Scholar] [CrossRef]
- Anderson, M.; Suh, J.M.; Kim, E.Y.; Dryer, S.E. Functional NMDA receptors with atypical properties are expressed in podocytes. Am. J. Physiol. Physiol. 2011, 300, C22–C32. [Google Scholar] [CrossRef]
- Roshanravan, H.; Kim, E.Y.; Dryer, S.E. NMDA Receptors as Potential Therapeutic Targets in Diabetic Nephropathy: Increased Renal NMDA Receptor Subunit Expression in Akita Mice and Reduced Nephropathy Following Sustained Treatment with Memantine or MK-801. Diabetes 2016, 65, 3139–3150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, D.; Shibata, S.; Ji, T.; Zhang, L.; Zhang, R.; Yang, H.; Ma, L.; Jiao, J. Group I metabotropic glutamate receptor activation induces TRPC6-dependent calcium influx and RhoA activation in cultured human kidney podocytes. Biochem. Biophys. Res. Commun. 2019, 511, 374–380. [Google Scholar] [CrossRef]
- Pietrancosta, N.; Djibo, M.; Daumas, S.; El Mestikawy, S.; Erickson, J.D. Molecular, Structural, Functional, and Pharmacological Sites for Vesicular Glutamate Transporter Regulation. Mol. Neurobiol. 2020, 57, 3118–3142. [Google Scholar] [CrossRef]
- Rastaldi, M.P.; Armelloni, S.; Berra, S.; Calvaresi, N.; Corbelli, A.; Giardino, L.A.; Li, M.; Wang, G.Q.; Fornasieri, A.; Villa, A.; et al. Glomerular podocytes contain neuron-like functional synaptic vesicles. FASEB J. 2006, 20, 976–978. [Google Scholar] [CrossRef] [PubMed]
- Fremeau, R.T.; Burman, J.; Qureshi, T.; Tran, C.H.; Proctor, J.; Johnson, J.; Zhang, H.; Sulzer, D.; Copenhagen, D.R.; Storm-Mathisen, J.; et al. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc. Natl. Acad. Sci. USA 2002, 99, 14488–14493. [Google Scholar] [CrossRef] [PubMed]
- Giardino, L.; Armelloni, S.; Corbelli, A.; Mattinzoli, D.; Zennaro, C.; Guerrot, D.; Tourrel, F.; Ikehata, M.; Li, M.; Berra, S.; et al. Podocyte Glutamatergic Signaling Contributes to the Function of the Glomerular Filtration Barrier. J. Am. Soc. Nephrol. 2009, 20, 1929–1940. [Google Scholar] [CrossRef]
- Mundel, P.; Reiser, J.; Kriz, W. Induction of differentiation in cultured rat and human podocytes. J. Am. Soc. Nephrol. 1997, 8, 697–705. [Google Scholar] [CrossRef]
- Schäfer, M.K.-H.; Varoqui, H.; Defamie, N.; Weihe, E.; Erickson, J.D. Molecular Cloning and Functional Identification of Mouse Vesicular Glutamate Transporter 3 and Its Expression in Subsets of Novel Excitatory Neurons. J. Biol. Chem. 2002, 277, 50734–50748. [Google Scholar] [CrossRef]
- Gras, C.; Herzog, E.; Bellenchi, G.C.; Bernard, V.; Ravassard, P.; Pohl, M.; Gasnier, B.; Giros, B.; El Mestikawy, S. A Third Vesicular Glutamate Transporter Expressed by Cholinergic and Serotoninergic Neurons. J. Neurosci. 2002, 22, 5442–5451. [Google Scholar] [CrossRef]
- Takamori, S.; Malherbe, P.; Broger, C.; Jahn, R. Molecular cloning and functional characterization of human vesicular glutamate transporter 3. Embo Rep. 2002, 3, 798–803. [Google Scholar] [CrossRef]
- Rastaldi, M.P.; Armelloni, S.; Berra, S.; Li, M.; Pesaresi, M.; Poczewski, H.; Langer, B.; Kerjaschki, D.; Henger, A.; Blattner, S.M.; et al. Glomerular Podocytes Possess the Synaptic Vesicle Molecule Rab3A and Its Specific Effector Rabphilin-3a. Am. J. Pathol. 2003, 163, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, N.B.; Attwell, D. Do astrocytes really exocytose neurotransmitters? Nat. Rev. Neurosci. 2010, 11, 227–238. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Huesgen, P.F.; Koch, R.E. The podocyte protease web: Uncovering the gatekeepers of glomerular disease. Am. J. Physiol. Physiol. 2018, 315, F1812–F1816. [Google Scholar] [CrossRef]
- Rossi, D.J.; Oshima, T.; Attwell, D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature 2000, 403, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Gloy, J.; Reitinger, S.; Fischer, K.-G.; Schreiber, R.; Boucherot, A.; Kunzelmann, K.; Mundel, P.; Pavenstädt, H. Amino acid transport in podocytes. Am. J. Physiol. Physiol. 2000, 278, F999–F1005. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Anderson, C.M.; Keung, E.C.; Chen, Y.; Chen, Y.; Swanson, R.A. P2X7Receptor-Mediated Release of Excitatory Amino Acids from Astrocytes. J. Neurosci. 2003, 23, 1320–1328. [Google Scholar] [CrossRef]
- Virginio, C.; MacKenzie, A.; Rassendren, F.A.; North, R.A.; Surprenant, A. Pore dilation of neuronal P2X receptor channels. Nat. Neurosci. 1999, 2, 315–321. [Google Scholar] [CrossRef]
- Vonend, O.; Turner, C.M.; Chan, C.M.; Loesch, A.; Dell’Anna, G.C.; Srai, K.S.; Burnstock, G.; Unwin, R.J. Glomerular expression of the ATP-sensitive P2X7 receptor in diabetic and hypertensive rat models. Kidney Int. 2004, 66, 157–166. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dehaye, J.P.; Winand, J.; Poloczek, P.; Christophe, J. Characterization of muscarinic cholinergic receptors on rat pancreatic acini by N-[3H] methylscopolamine binding. Their relationship with calcium 45 efflux and amylase secretion. J. Biol. Chem. 1984, 259, 294–300. [Google Scholar] [CrossRef]
- Bai, L.; Sun, S.; Sun, Y.; Wang, F.; Nishiyama, A. N-type calcium channel and renal injury. Int. Urol. Nephrol. 2022, 54, 2871–2879. [Google Scholar] [CrossRef]
- Makino, S.-I.; Shirata, N.; Trejo, J.A.O.; Yamamoto-Nonaka, K.; Yamada, H.; Miyake, T.; Mori, K.; Nakagawa, T.; Tashiro, Y.; Yamashita, H.; et al. Impairment of Proteasome Function in Podocytes Leads to CKD. J. Am. Soc. Nephrol. 2021, 32, 597–613. [Google Scholar] [CrossRef]
- Yamada, H.; Abe, T.; Satoh, A.; Okazaki, N.; Tago, S.; Kobayashi, K.; Yoshida, Y.; Oda, Y.; Watanabe, M.; Tomizawa, K.; et al. Stabilization of Actin Bundles by a Dynamin 1/Cortactin Ring Complex Is Necessary for Growth Cone Filopodia. J. Neurosci. 2013, 33, 4514–4526. [Google Scholar] [CrossRef]
- Godel, H.; Graser, T.; Földi, P.; Pfaender, P.; Fürst, P. Measurement of free amino acids in human biological fluids by high-performance liquid chromatography. J. Chromatogr. A 1984, 297, 49–61. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishii, N.; Kawai, T.; Yasuoka, H.; Abe, T.; Tatsumi, N.; Harada, Y.; Miyaji, T.; Li, S.; Tsukano, M.; Watanabe, M.; et al. Vesicular Glutamate Transporter 3 Is Involved in Glutamatergic Signalling in Podocytes. Int. J. Mol. Sci. 2025, 26, 2485. https://doi.org/10.3390/ijms26062485
Nishii N, Kawai T, Yasuoka H, Abe T, Tatsumi N, Harada Y, Miyaji T, Li S, Tsukano M, Watanabe M, et al. Vesicular Glutamate Transporter 3 Is Involved in Glutamatergic Signalling in Podocytes. International Journal of Molecular Sciences. 2025; 26(6):2485. https://doi.org/10.3390/ijms26062485
Chicago/Turabian StyleNishii, Naoko, Tomoko Kawai, Hiroki Yasuoka, Tadashi Abe, Nanami Tatsumi, Yuika Harada, Takaaki Miyaji, Shunai Li, Moemi Tsukano, Masami Watanabe, and et al. 2025. "Vesicular Glutamate Transporter 3 Is Involved in Glutamatergic Signalling in Podocytes" International Journal of Molecular Sciences 26, no. 6: 2485. https://doi.org/10.3390/ijms26062485
APA StyleNishii, N., Kawai, T., Yasuoka, H., Abe, T., Tatsumi, N., Harada, Y., Miyaji, T., Li, S., Tsukano, M., Watanabe, M., Ogawa, D., Wada, J., Takei, K., & Yamada, H. (2025). Vesicular Glutamate Transporter 3 Is Involved in Glutamatergic Signalling in Podocytes. International Journal of Molecular Sciences, 26(6), 2485. https://doi.org/10.3390/ijms26062485




