Recent Insights into Bioactive Dichalcogen Derivatives: From Small Molecules to Complex Materials
Abstract
1. Introduction
1.1. Chalcogens and Bioactivity
1.2. Diorganochalcogenides and Bioactivity
2. Diselenide Derivatives with Biological Activity
2.1. Diselenides with Anticancer Activity
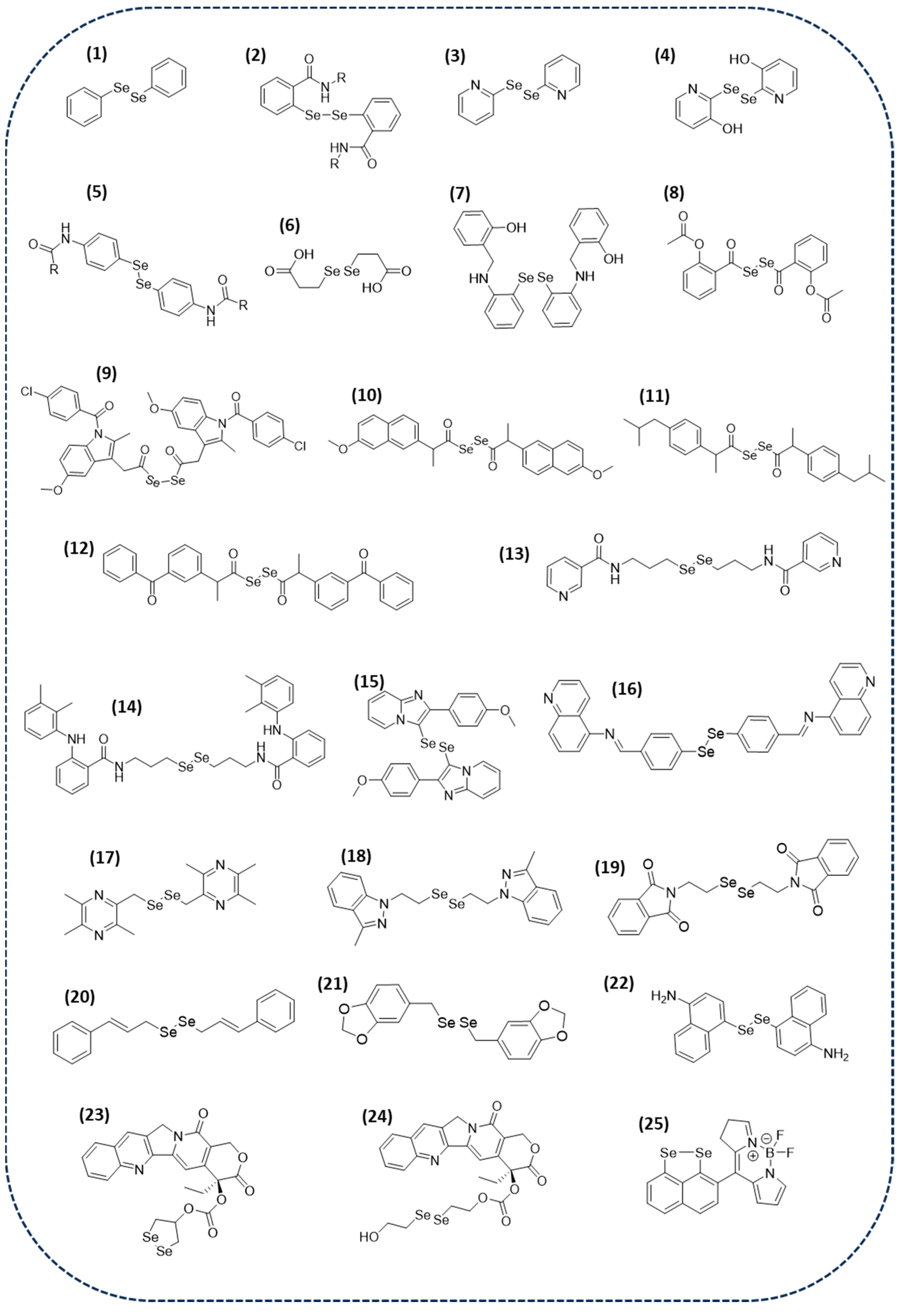
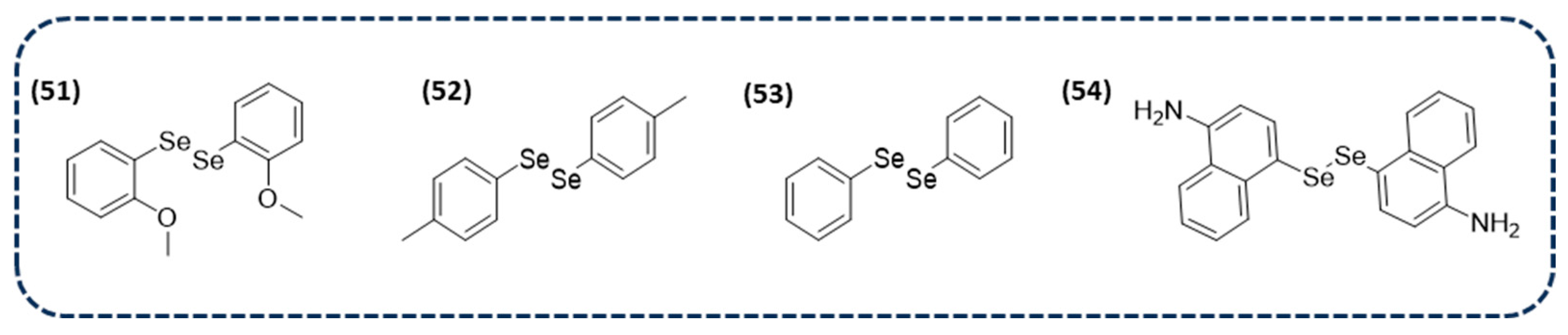
2.1.1. Small Molecules
2.1.2. Metal Complexes
2.1.3. Complex Materials
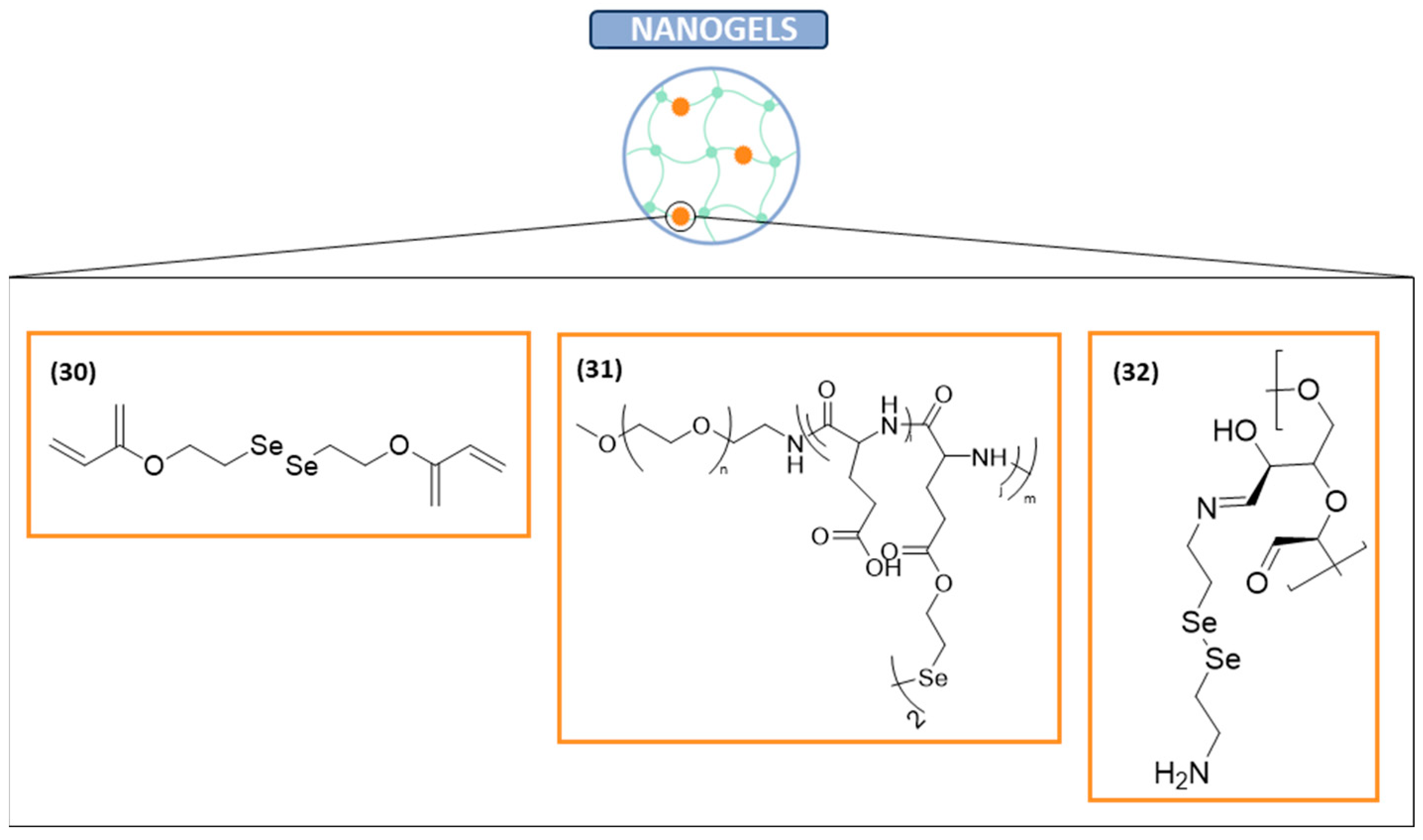
Nanogels
Nanophotosensitizers
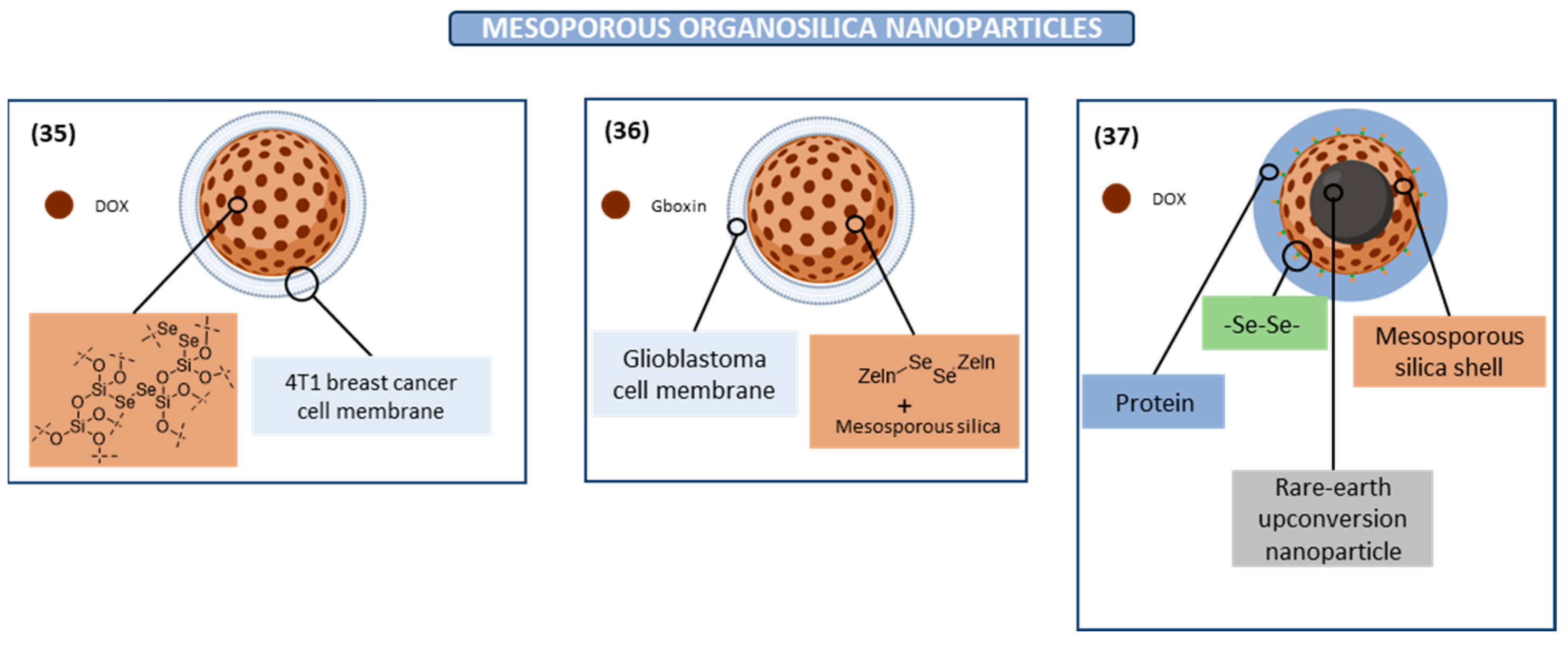
Mesoporous Organosilica Nanoparticle
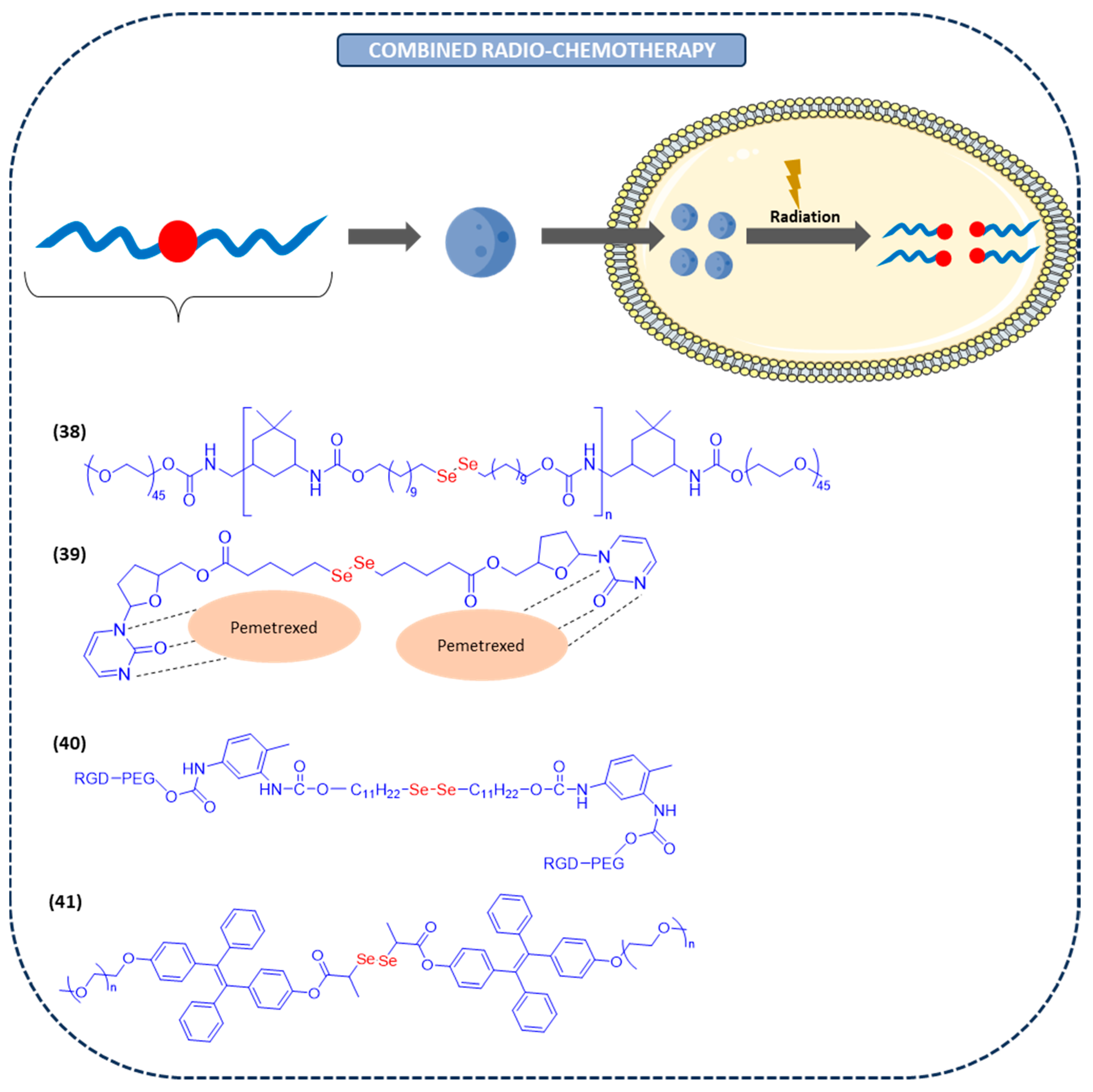
Nano-Assemblies for Combined Chemoradiotherapy
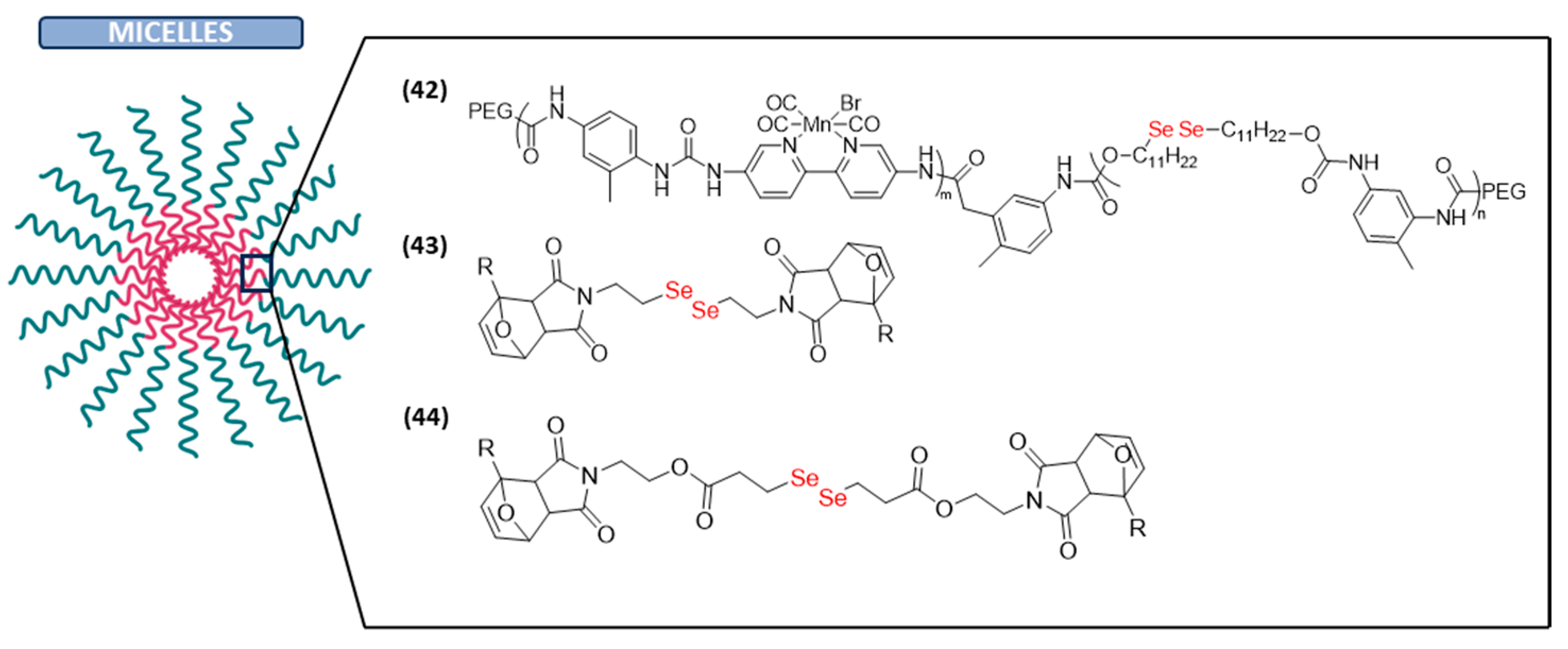
Micelles
2D Nanomaterials
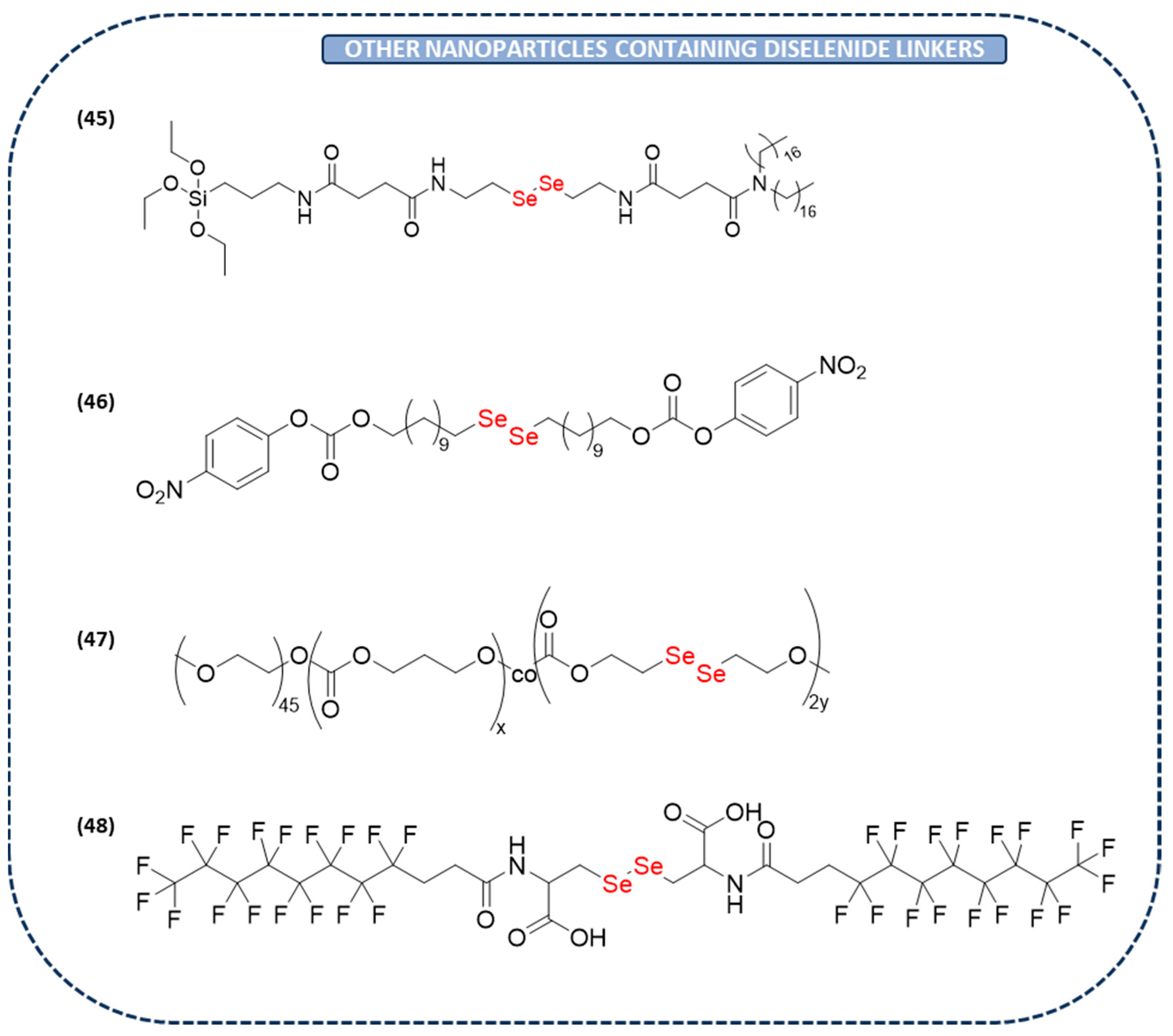
Other Diselenide Nanoparticles
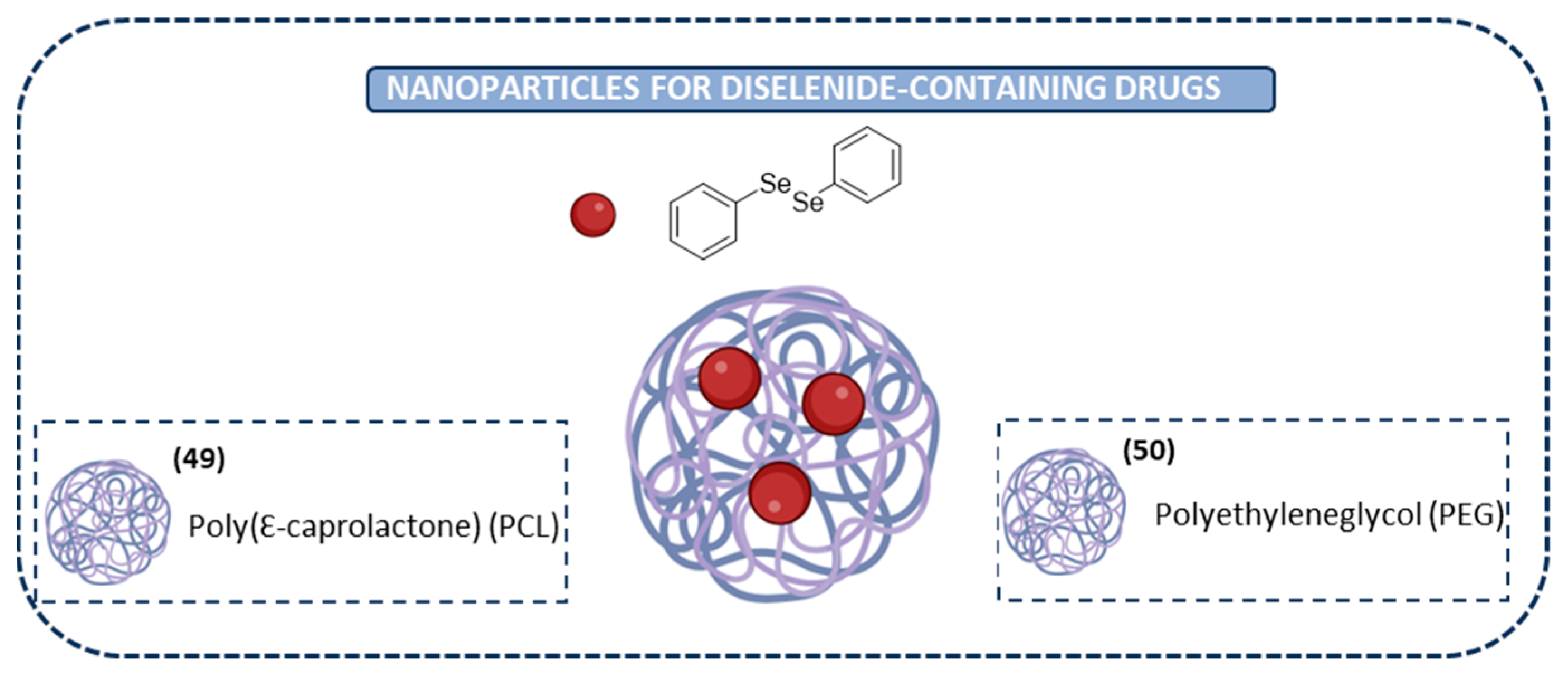
Nanoparticles for Diselenide-Containing Derivatives
2.2. Diselenides with Antibacterial Activity
2.3. Diselenides with Antiparasitic Activity
2.4. Diselenides with Antiviral Activity
2.5. Diselenides for Neurodegenerative Diseases
3. Ditelluride Derivatives with Biological Activity
3.1. Bioactive Small Molecules Containing Ditelluride Bond
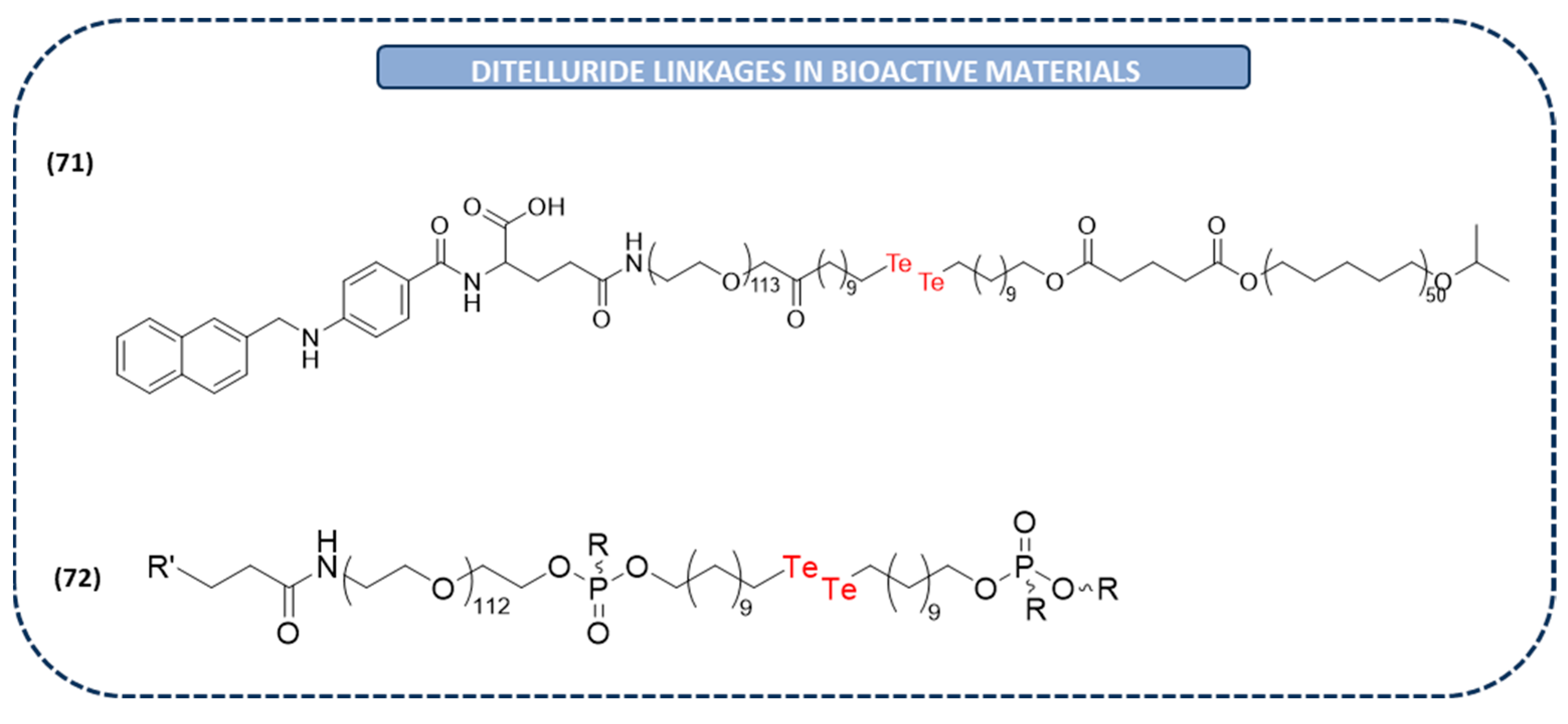
3.2. Bioactive Complex Materials Containing Ditelluride Bond
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aakeroy, C.B.; Bryce, D.L.; Desiraju, G.R.; Frontera, A.; Legon, A.C.; Nicotra, F.; Rissanen, K.; Scheiner, S.; Terraneo, G.; Metrangolo, P.; et al. Definition of the chalcogen bond (iupac recommendations 2019). Pure Appl. Chem. 2019, 91, 1889–1892. [Google Scholar] [CrossRef]
- Carugo, O.I. Chalcogen bonds formed by protein sulfur atoms in proteins. A survey of high-resolution structures deposited in the protein data bank. J. Biomol. Struct. Dyn. 2023, 41, 9576–9582. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Li, B. How nature incorporates sulfur and selenium into bioactive natural products. Curr. Res. Chem. Biol. 2023, 76, 102377. [Google Scholar] [CrossRef]
- Moriarty, R.M.; Naithani, R.; Surve, B. Organosulfur compounds in cancer chemoprevention. Mini Rev. Med. Chem. 2007, 7, 827–838. [Google Scholar] [CrossRef]
- Wong, W.W.L.; MacDonald, S.; Langler, R.F.; Penn, L.Z. Novel synthetic organosulfur compounds induce apoptosis of human leukemic cells. Anticancer Res. 2000, 20, 1367–1374. [Google Scholar] [CrossRef]
- Chiu, C.K.; Chen, T.Y.; Lin, J.H.; Wang, C.Y.; Wang, B.S. Protective effects of five allium derived organosulfur compounds against mutation and oxidation. Food Chem. 2016, 197, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Ruhee, R.T.; Roberts, L.A.; Ma, S.H.; Suzuki, K. Organosulfur compounds: A review of their anti-inflammatory effects in human health. Front. Nutr. 2020, 7, 64. [Google Scholar] [CrossRef]
- You, S.X.; Nakanishi, E.; Kuwata, H.; Chen, J.H.; Nakasone, Y.; He, X.; He, J.H.; Liu, X.X.; Zhang, S.R.; Zhang, B.; et al. Inhibitory effects and molecular mechanisms of garlic organosulfur compounds on the production of inflammatory mediators. Mol. Nutr. Food Res. 2013, 57, 2049–2060. [Google Scholar] [CrossRef]
- Reddy, B.S.; Rao, C.V. Chemoprevention of colon carcinogenesis by organosulfur compounds. Cancer Res. 1994, 546, 164–172. [Google Scholar]
- Xiao, D.H.; Pinto, J.T.; Gundersen, G.G.; Weinstein, I.B. Effects of a series of organosulfur compounds on mitotic arrest and induction of apoptosis in colon cancer cells. Mol. Cancer Ther. 2005, 4, 1388–1398. [Google Scholar] [CrossRef]
- Reich, H.J.; Hondal, R.J. Why nature chose selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef] [PubMed]
- Barchielli, G.; Capperucci, A.; Tanini, D. The role of selenium in pathologies: An updated review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta 2015, 1850, 1642–1660. [Google Scholar] [CrossRef]
- Wessjohann, L.A.; Schneider, A.; Abbas, M.; Brandt, W. Selenium in chemistry and biochemistry in comparison to sulfur. Biol. Chem. 2007, 388, 997–1006. [Google Scholar] [CrossRef]
- Nauser, T.; Steinmann, D.; Koppenol, W.H. Why do proteins use selenocysteine instead of cysteine? Amino Acids 2012, 42, 39–44. [Google Scholar] [CrossRef]
- Sanmartín, C.; Plano, D.; Palop, J.A. Selenium compounds and apoptotic modulation: A new perspective in cancer therapy. Mini Rev. Med. Chem. 2008, 8, 1020–1031. [Google Scholar] [CrossRef]
- Wallenberg, M.; Misra, S.; Björnstedt, M. Selenium cytotoxicity in cancer. Basic Clin. Pharmacol. Toxicol. 2014, 114, 377–386. [Google Scholar] [CrossRef]
- Huang, S.; Sheng, X.; Bian, M.; Yang, Z.; Lu, Y.; Liu, W. Synthesis and in vitro anticancer activities of selenium n-heterocyclic carbene compounds. Chem. Biol. Drug Des. 2021, 98, 435–444. [Google Scholar] [CrossRef]
- Chivers, T.; Laitinen, R.S. Tellurium: A maverick among the chalcogens. Chem. Soc. Rev. 2015, 44, 1725–1739. [Google Scholar] [CrossRef]
- Ba, L.A.; Döring, M.; Jamier, V.; Jacob, C. Tellurium: An element with great biological potency and potential. Org. Biomol. Chem. 2010, 8, 4203–4216. [Google Scholar] [CrossRef]
- Domínguez-Álvarez, E.; Rácz, B.; Marć, M.A.; Nasim, M.J.; Szemerédi, N.; Viktorová, J.; Jacob, C.; Spengler, G. Selenium and tellurium in the development of novel small molecules and nanoparticles as cancer multidrug resistance reversal agents. Drug Resist. Updates 2022, 63, 100844. [Google Scholar] [CrossRef] [PubMed]
- Sredni, B. Immunomodulating tellurium compounds as anti-cancer agents. Semin. Cancer Biol. 2012, 22, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Saidu, N.E.; Intemann, J.; Jacob, C.; Montenarh, M. A new tellurium-containing amphiphilic molecule induces apoptosis in hct116 colon cancer cells. Biochim. Biophys. Acta 2014, 1840, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Dörsam, B.; Fahrer, J. The disulfide compound α-lipoic acid and its derivatives: A novel class of anticancer agents targeting mitochondria. Cancer Lett. 2016, 371, 12–19. [Google Scholar] [CrossRef]
- Mitra, S.; Das, R.; Emran, T.B.; Labib, R.K.; Noor, E.T.; Islam, F.; Sharma, R.; Ahmad, I.; Nainu, F.; Chidambaram, K.; et al. Diallyl disulfide: A bioactive garlic compound with anticancer potential. Front. Pharmacol. 2022, 13, 943967. [Google Scholar] [CrossRef]
- Gunasekaran, K.; Vasamsetti, B.M.K.; Thangavelu, P.; Natesan, K.; Mujyambere, B.; Sundaram, V.; Jayaraj, R.; Kim, Y.J.; Samiappan, S.; Choi, J.W. Cytotoxic effects of nanoliposomal cisplatin and diallyl disulfide on breast cancer and lung cancer cell lines. Biomedicines 2023, 11, 1021. [Google Scholar] [CrossRef]
- Eirich, J.; Braig, S.; Schyschka, L.; Servatius, P.; Hoffmann, J.; Hecht, S.; Fulda, S.; Zahler, S.; Antes, I.; Kazmaier, U.; et al. A small molecule inhibits protein disulfide isomerase and triggers the chemosensitization of cancer cells. Angew. Chem. Int. Ed. Engl. 2014, 53, 12960–12965. [Google Scholar] [CrossRef]
- Li, B.; Wever, W.J.; Walsh, C.T.; Bowers, A.A. Dithiolopyrrolones: Biosynthesis, synthesis, and activity of a unique class of disulfide-containing antibiotics. Nat. Prod. Rep. 2014, 31, 905–923. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Y.; Long, X.; Wang, C.; Ouyang, G.; Wang, Z. Antibacterial activity of allicin-inspired disulfide derivatives against Xanthomonas axonopodis pv. Citri. Int. J. Mol. Sci. 2022, 23, 11947. [Google Scholar] [CrossRef]
- Dilem Doğan, Ş.; Buran, S.; Gözde Gündüz, M.; Özkul, C.; Siva Krishna, V.; Sriram, D. Synthesis of disulfide-bridged n-phenyl-n’-(alkyl/aryl/heteroaryl)urea derivatives and evaluation of their antimicrobial activities. Chem. Biodivers. 2019, 16, e1900461. [Google Scholar] [CrossRef]
- Khan, M.R.; Chonhenchob, V.; Huang, C.; Suwanamornlert, P. Antifungal activity of propyl disulfide from neem (Azadirachta indica) in vapor and agar diffusion assays against anthracnose pathogens (Colletotrichum gloeosporioides and Colletotrichum acutatum) in mango fruit. Microorganisms 2021, 9, 839. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H. Disulfide and multisulfide antitumor agents and their modes of action. Arch. Pharm. Res. 2009, 32, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Krasowska, D.; Iraci, N.; Santi, C.; Drabowicz, J.; Cieslak, M.; Kaźmierczak-Barańska, J.; Palomba, M.; Królewska-Golińska, K.; Magiera, J.; Sancineto, L.; et al. Diselenides and benzisoselenazolones as antiproliferative agents and glutathione-s-transferase inhibitors. Molecules 2019, 24, 2914. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdallah, B.; Al-Faiyz, Y.S.; Shaaban, S. Anticancer, antimicrobial, and antioxidant activities of organodiselenide-tethered methyl anthranilates. Biomolecules 2022, 12, 1765. [Google Scholar] [CrossRef]
- Sancineto, L.; Piccioni, M.; De Marco, S.; Pagiotti, R.; Nascimento, V.; Braga, A.L.; Santi, C.; Pietrella, D. Diphenyl diselenide derivatives inhibit microbial biofilm formation involved in wound infection. BMC Microbiol. 2016, 16, 220. [Google Scholar] [CrossRef]
- Pesarico, A.P.; Sartori, G.; dos Santos, C.F.; Neto, J.S.; Bortolotto, V.; Santos, R.C.; Nogueira, C.W.; Prigol, M. 2,2′-dithienyl diselenide pro-oxidant activity accounts for antibacterial and antifungal activities. Microbiol. Res. 2013, 168, 563–568. [Google Scholar] [CrossRef]
- Plano, D.; Baquedano, Y.; Moreno-Mateos, D.; Font, M.; Jiménez-Ruiz, A.; Palop, J.A.; Sanmartín, C. Selenocyanates and diselenides: A new class of potent antileishmanial agents. Eur. J. Med. Chem. 2011, 46, 3315–3323. [Google Scholar] [CrossRef]
- Pinton, S.; Sampaio, T.B.; Ramalho, R.M.; Rodrigues, C.M.; Nogueira, C.W. p,p′-methoxyl-diphenyl diselenide prevents neurodegeneration and glial cell activation induced by streptozotocin in rats. J. Alzheimer’s Dis. 2013, 33, 133–144. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, J.; Tian, L.; Li, J.; Gao, Y.; Xing, Y.; Yan, W.; Hua, C.; Xie, X.; Liu, C.; et al. Insights into stimuli-responsive diselenide bonds utilized in drug delivery systems for cancer therapy. Biomed. Pharmacother. 2022, 155, 113707. [Google Scholar] [CrossRef]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawski, K. Selenium compounds as novel potential anticancer agents. Int. J. Mol. Sci. 2021, 22, 1009. [Google Scholar] [CrossRef]
- Guo, X.; Cheng, Y.; Zhao, X.; Luo, Y.; Chen, J.; Yuan, W.E. Advances in redox-responsive drug delivery systems of tumor microenvironment. J. Nanobiotechnol. 2018, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Cupp-Sutton, K.A.; Ashby, M.T. Biological chemistry of hydrogen selenide. Antioxidants 2016, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Morán-Serradilla, C.; Angulo-Elizari, E.; Henriquez-Figuereo, A.; Sanmartín, C.; Sharma, A.K.; Plano, D. Seleno-metabolites and their precursors: A new dawn for several illnesses? Metabolites 2022, 12, 874. [Google Scholar] [CrossRef] [PubMed]
- Pinton, S.; Luchese, C.; Nogueira, C.W. Comparison of the antioxidant properties and the toxicity of p,p′-dichlorodiphenyl ditelluride with the parent compound, diphenyl ditelluride. Biol. Trace Elem. Res. 2011, 139, 204–216. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B. Organoselenium and organotellurium compounds: Toxicology and pharmacology. Chem. Rev. 2004, 104, 6255–6285. [Google Scholar] [CrossRef]
- Cao, W.; Wang, L.; Xu, H. Selenium/tellurium containing polymer materials in nanobiotechnology. Nano Today 2015, 10, 717–736. [Google Scholar] [CrossRef]
- Sohail, M.; Guo, W.; Li, Z.; Xu, H.; Zhao, F.; Chen, D.; Fu, F. Nanocarrier-based drug delivery system for cancer therapeutics: A review of the last decade. Curr. Med. Chem. 2021, 28, 3753–3772. [Google Scholar] [CrossRef]
- Zhu, R.; He, Q.; Li, Z.; Ren, Y.; Liao, Y.; Zhang, Z.; Dai, Q.; Wan, C.; Long, S.; Kong, L.; et al. Ros-cleavable diselenide nanomedicine for nir-controlled drug release and on-demand synergistic chemo-photodynamic therapy. Acta Biomater. 2022, 153, 442–452. [Google Scholar] [CrossRef]
- Wei, C.; Zhang, Y.; Xu, H.; Xu, Y.; Xu, Y.; Lang, M. Well-defined labile diselenide-centered poly(ε-caprolactone)-based micelles for activated intracellular drug release. J. Mater. Chem. B 2016, 4, 5059–5067. [Google Scholar] [CrossRef]
- Shao, D.; Li, M.; Wang, Z.; Zheng, X.; Lao, Y.H.; Chang, Z.; Zhang, F.; Lu, M.; Yue, J.; Hu, H.; et al. Bioinspired diselenide-bridged mesoporous silica nanoparticles for dual-responsive protein delivery. Adv. Mater. 2018, 30, e1801198. [Google Scholar] [CrossRef]
- Choi, Y.S.; Huh, K.M.; Shim, M.S.; Park, I.S.; Cho, Y.-Y.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Disrupting the redox balance with a diselenide drug delivery system: Synergistic or antagonistic? Adv. Funct. Mater. 2021, 31, 2007275. [Google Scholar] [CrossRef]
- Yadav, S.; Ramesh, K.; Reddy, O.S.; Karthika, V.; Kumar, P.; Jo, S.H.; Yoo, S.I.; Park, S.H.; Lim, K.T. Redox-responsive comparison of diselenide and disulfide core-cross-linked micelles for drug delivery application. Pharmaceutics 2023, 15, 1159. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Cheng, G.; He, Y.; Nie, Y.; Jiang, Q.; Cai, X.; Gu, Z. Influence of reduction-sensitive diselenide bonds and disulfide bonds on oligoethylenimine conjugates for gene delivery. J. Mater. Chem. B 2014, 2, 7210–7221. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, L.; Wang, Y.; Li, L.; Lu, Y.; Shen, L.; Zhang, L.W. Ultrasensitive gsh-responsive ditelluride-containing poly(ether-urethane) nanoparticles for controlled drug release. ACS Appl. Mater. Interfaces 2016, 8, 35106–35113. [Google Scholar] [CrossRef]
- Kuršvietienė, L.; Mongirdienė, A.; Bernatonienė, J.; Šulinskienė, J.; Stanevičienė, I. Selenium anticancer properties and impact on cellular redox status. Antioxidants 2020, 9, 80. [Google Scholar] [CrossRef]
- Tinggi, U. Selenium: Its role as antioxidant in human health. Environ. Health Prev. Med. 2008, 13, 102–108. [Google Scholar] [CrossRef]
- Plano, D.; Ibáñez, E.; Calvo, A.; Palop, J.A.; Sanmartín, C. Novel library of selenocompounds as kinase modulators. Molecules 2011, 16, 6349–6364. [Google Scholar] [CrossRef]
- Rizvi, M.A.; Guru, S.; Naqvi, T.; Kumar, M.; Kumbhar, N.; Akhoon, S.; Banday, S.; Singh, S.K.; Bhushan, S.; Mustafa Peerzada, G.; et al. An investigation of in vitro cytotoxicity and apoptotic potential of aromatic diselenides. Bioorg. Med. Chem. Lett. 2014, 24, 3440–3446. [Google Scholar] [CrossRef]
- Li, W.; Guo, M.; Liu, Y.; Mu, W.; Deng, G.; Li, C.; Qiu, C. Selenium induces an anti-tumor effect via inhibiting intratumoral angiogenesis in a mouse model of transplanted canine mammary tumor cells. Biol. Trace Elem. Res. 2016, 171, 371–379. [Google Scholar] [CrossRef]
- de Vargas Barbosa, N.B.; Nogueira, C.W.; Guecheva, T.N.; Bellinaso Mde, L.; Rocha, J.B. Diphenyl diselenide supplementation delays the development of n-nitroso-n-methylurea-induced mammary tumors. Arch. Toxicol. 2008, 82, 655–663. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Cervi, V.F.; Sari, M.H.M.; Barbieri, A.V.; Ramos, A.P.; Copetti, P.M.; de Brum, G.F.; Nascimento, K.; Nadal, J.M.; Farago, P.V.; et al. Diphenyl diselenide loaded poly(ε-caprolactone) nanocapsules with selective antimelanoma activity: Development and cytotoxic evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hort, M.A.; Straliotto, M.R.; de Oliveira, J.; Amoêdo, N.D.; da Rocha, J.B.; Galina, A.; Ribeiro-do-Valle, R.M.; de Bem, A.F. Diphenyl diselenide protects endothelial cells against oxidized low density lipoprotein-induced injury: Involvement of mitochondrial function. Biochimie 2014, 105, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.M.; Roesler, R.; Braga, A.L.; Saffi, J.; Henriques, J.A. Pharmacology and toxicology of diphenyl diselenide in several biological models. Braz. J. Med. Biol. Res. 2007, 40, 1287–1304. [Google Scholar] [CrossRef] [PubMed]
- da Costa, N.S.; Lima, L.S.; Oliveira, F.A.M.; Galiciolli, M.E.A.; Manzano, M.I.; Garlet, Q.I.; Irioda, A.C.; Oliveira, C.S. Antiproliferative effect of inorganic and organic selenium compounds in breast cell lines. Biomedicines 2023, 11, 1346. [Google Scholar] [CrossRef]
- Laskowska, A.; Pacuła-Miszewska, A.J.; Długosz-Pokorska, A.; Janecka, A.; Wojtczak, A.; Ścianowski, J. Attachment of chiral functional groups to modify the activity of new gpx mimetics. Materials 2022, 15, 2068. [Google Scholar] [CrossRef]
- Gandhi, V.V.; Phadnis, P.P.; Kunwar, A. 2,2′-dipyridyl diselenide (py2se2) induces g1 arrest and apoptosis in human lung carcinoma (a549) cells through ros scavenging and reductive stress. Metallomics 2020, 12, 1253–1266. [Google Scholar] [CrossRef]
- Gandhi, V.V.; Bihani, S.C.; Phadnis, P.P.; Kunwar, A. Diselenide-derivative of 3-pyridinol targets redox enzymes leading to cell cycle deregulation and apoptosis in a549 cells. Biol. Chem. 2022, 403, 891–905. [Google Scholar] [CrossRef]
- Etxebeste-Mitxeltorena, M.; Plano, D.; Astrain-Redín, N.; Morán-Serradilla, C.; Aydillo, C.; Encío, I.; Moreno, E.; Espuelas, S.; Sanmartín, C. New amides and phosphoramidates containing selenium: Studies on their cytotoxicity and antioxidant activities in breast cancer. Antioxidants 2021, 10, 590. [Google Scholar] [CrossRef]
- Kunwar, A.; Patil, A.; Kumar, S.; Deshpande, R.; Gota, V.; Goda, J.S.; Jain, V.K.; Indira Priyadarsini, K. Toxicological safety evaluation of 3,3′-diselenodipropionic acid (dsepa), a pharmacologically important derivative of selenocystine. Regul. Toxicol. Pharmacol. 2018, 99, 159–167. [Google Scholar] [CrossRef]
- Gota, V.; Goda, J.S.; Doshi, K.; Patil, A.; Sunderajan, S.; Kumar, K.; Varne, M.; Kunwar, A.; Jain, V.K.; Priyadarshini, I. Biodistribution and pharmacokinetic study of 3,3′ diseleno dipropionic acid (dsepa), a synthetic radioprotector, in mice. Eur. J. Drug Metab. Pharmacokinet 2016, 41, 839–844. [Google Scholar] [CrossRef]
- Gandhi, V.V.; Gandhi, K.A.; Kumbhare, L.B.; Goda, J.S.; Gota, V.; Priyadarsini, K.I.; Kunwar, A. 3,3′-diselenodipropionic acid (dsepa) induces reductive stress in a549 cells triggering p53-independent apoptosis: A novel mechanism for diselenides. Free Radic. Biol. Med. 2021, 175, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Kumar Jha, R.; Batabyal, M.; Dutta, T.; Koner, A.L.; Kumar, S. Janus -faced oxidant and antioxidant profiles of organo diselenides. Dalton Trans. 2021, 50, 14576–14594. [Google Scholar] [CrossRef] [PubMed]
- Kazberuk, A.; Chalecka, M.; Palka, J.; Surazynski, A. Nonsteroidal anti-inflammatory drugs as pparγ agonists can induce prodh/pox-dependent apoptosis in breast cancer cells: New alternative pathway in nsaid-induced apoptosis. Int. J. Mol. Sci. 2022, 23, 1510. [Google Scholar] [CrossRef] [PubMed]
- Nortcliffe, A.; Ekstrom, A.G.; Black, J.R.; Ross, J.A.; Habib, F.K.; Botting, N.P.; O’Hagan, D. Synthesis and biological evaluation of nitric oxide-donating analogues of sulindac for prostate cancer treatment. Bioorg. Med. Chem. 2014, 22, 756–761. [Google Scholar] [CrossRef]
- Hudson, L.G.; Cook, L.S.; Grimes, M.M.; Muller, C.Y.; Adams, S.F.; Wandinger-Ness, A. Dual actions of ketorolac in metastatic ovarian cancer. Cancers 2019, 11, 1049. [Google Scholar] [CrossRef]
- Ramos-Inza, S.; Aliaga, C.; Encío, I.; Raza, A.; Sharma, A.K.; Aydillo, C.; Martínez-Sáez, N.; Sanmartín, C.; Plano, D. First generation of antioxidant precursors for bioisosteric se-nsaids: Design, synthesis, and in vitro and in vivo anticancer evaluation. Antioxidants 2023, 12, 1666. [Google Scholar] [CrossRef]
- Nie, Y.; Zhong, M.; Li, S.; Li, X.; Zhang, Y.; Zhang, Y.; He, X. Synthesis and potential anticancer activity of some novel selenocyanates and diselenides. Chem. Biodivers. 2020, 17, e1900603. [Google Scholar] [CrossRef]
- Peglow, T.J.; Schumacher, R.F.; Cargnelutti, R.; Reis, A.S.; Luchese, C.; Wilhelm, E.A.; Perin, G. Preparation of bis(2-pyridyl) diselenide derivatives: Synthesis of selenazolo[5,4-b]pyridines and unsymmetrical diorganyl selenides, and evaluation of antioxidant and anticholinesterasic activities. Tetrahedron Lett. 2017, 58, 3734–3738. [Google Scholar] [CrossRef]
- Bocchini, B.; Goldani, B.; Sousa, F.S.S.; Birmann, P.T.; Brüning, C.A.; Lenardão, E.J.; Santi, C.; Savegnago, L.; Alves, D. Synthesis and antioxidant activity of new selenium-containing quinolines. Med. Chem. 2021, 17, 667–676. [Google Scholar] [CrossRef]
- Matsumura, M.; Takahashi, T.; Yamauchi, H.; Sakuma, S.; Hayashi, Y.; Hyodo, T.; Obata, T.; Yamaguchi, K.; Fujiwara, Y.; Yasuike, S. Synthesis and anticancer activity of bis(2-arylimidazo[1,2-a]pyridin-3-yl) selenides and diselenides: The copper-catalyzed tandem c-h selenation of 2-arylimidazo[1,2-a]pyridine with selenium. Beilstein J. Org. Chem. 2020, 16, 1075–1083. [Google Scholar] [CrossRef]
- Shelar, D.S.; Malankar, G.S.; Singh, P.R.; Vaidya, S.P.; Pinjari, R.V.; Manjare, S.T. Superoxide targeted “turn-on” fluorescence sensing enabled by a diselenide based quinoline probe and its in vitro anticancer activity in cancer cells. New J. Chem. 2023, 47, 6653–6660. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, E.; Wang, G.; Ding, R.; Xu, X.; Fan, P.; Zheng, Y.; Xu, D. Synthesis and in-vitro study of a novel ligustrazine diselenide as a potential chemotherapy drug for lung adenocarcinoma. Biomed. Pharmacother. 2023, 165, 114699. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Chen, Z.; Pan, C.; Ni, M.; Ruan, H.; Song, J.; Lu, S.; Bhasin, A.; Ruan, B.H. Diselenide covalent allosteric inhibitors of glutaminase with strong in vivo anticancer activity. ACS Med. Chem. Lett. 2023, 14, 920–928. [Google Scholar] [CrossRef]
- Ramos-Inza, S.; Henriquez-Figuereo, A.; Moreno, E.; Berzosa, M.; Encío, I.; Plano, D.; Sanmartín, C. Unveiling a new selenocyanate as a multitarget candidate with anticancer, antileishmanial and antibacterial potential. Molecules 2022, 27, 7477. [Google Scholar] [CrossRef]
- Gouda, M.; Abbas, Y.J.; Abd El-Lateef, H.M.; Khalaf, M.M.; Shaaban, S. Design, synthesis, and biological evaluations of novel naphthalene-based organoselenocyanates. Biointerface Res. Appl. Chem. 2023, 13, 219. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; Zhong, M.; Xu, Q.; Li, X.; Chang, B.; Fang, J. Integration of a diselenide unit generates fluorogenic camptothecin prodrugs with improved cytotoxicity to cancer cells. J. Med. Chem. 2021, 64, 17979–17991. [Google Scholar] [CrossRef]
- Madibone, K.S.; Deshmukh, P.P.; Navalkar, A.; Maji, S.K.; Badani, P.M.; Manjare, S.T. Cyclic organoselenide bodipy-based probe: Targeting superoxide in mcf-7 cancer cells. ACS Omega 2020, 5, 14186–14193. [Google Scholar] [CrossRef]
- Rottenberg, S.; Disler, C.; Perego, P. The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer 2021, 21, 37–50. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Z.; Deng, Z.; Zhu, G. Recent advances in the synthesis, stability, and activation of platinum(iv) anticancer prodrugs. Coord. Chem. Rev. 2021, 442, 213991. [Google Scholar] [CrossRef]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef]
- El-Gammal, O.A.; Mohamed, F.S.; Rezk, G.N.; El-Bindary, A.A. Synthesis, characterization, catalytic, DNA binding and antibacterial activities of co(ii), ni(ii) and cu(ii) complexes with new schiff base ligand. J. Mol. Liq. 2021, 326, 115223. [Google Scholar] [CrossRef]
- Gladis, E.H.E.; Nagashri, K.; Suman, A.; Joseph, J. Multifunctional 1,10-phenanthroline derivative and its metal complexes as an anti-alzheimer’s agent: Structure-based drug design, synthesis, characterization and pharmacological studies. J. Coord. Chem. 2020, 73, 3267–3290. [Google Scholar] [CrossRef]
- Gomes, L.M.F.; Bataglioli, J.C.; Storr, T. Metal complexes that bind to the amyloid-β peptide of relevance to alzheimer’s disease. Coord. Chem. Rev. 2020, 412, 213255. [Google Scholar] [CrossRef]
- Ramos-Inza, S.; Plano, D.; Sanmartín, C. Metal-based compounds containing selenium: An appealing approach towards novel therapeutic drugs with anticancer and antimicrobial effects. Eur. J. Med. Chem. 2022, 244, 114834. [Google Scholar] [CrossRef]
- Gan, C.H.; Tan, K.W.; Ooi, M.L.; Liew, J.W.K.; Ng, Y.L.; Lau, Y.L.; Ng, Y.Z.; Ng, C.H.; Tan, C.H.; Wong, R.C.S. Synthesis, anticancer and antimalarial activities of organosulfur and organoselenium derivatives of cyclopentadienyliron dicarbonyl as photocorms. Inorg. Chim. Acta 2022, 536, 120872. [Google Scholar] [CrossRef]
- Mane, P.A.; Dey, S.; Pathak, A.K.; Kumar, M.; Bhuvanesh, N. Xantphos-capped pd(ii) and pt(ii) macrocycles of aryldithiolates: Structural variation and catalysis in c-c coupling reaction. Inorg. Chem. 2019, 58, 2965–2978. [Google Scholar] [CrossRef]
- Mishra, A.; Ravikumar, S.; Hong, S.H.; Kim, H.; Vajpayee, V.; Lee, H.; Ahn, B.; Wang, M.; Stang, P.J.; Chi, K.W. DNA binding and unwinding by self-assembled supramolecular hetero-bimetallacycles. Organometallics 2011, 30, 6343–6346. [Google Scholar] [CrossRef]
- Chaudhari, K.R.; Kunwar, A.; Bhuvanesh, N.; Dey, S. Synthesis and anti-proliferative activities of amine capped pd and pt macrocycles of 4,4′-dipyridylselenides. New J. Chem. 2020, 44, 7329–7337. [Google Scholar] [CrossRef]
- Pal, M.K.; Wadawale, A.P.; Chauhan, N.; Majumdar, A.G.; Subramanian, M.; Bhuvanesh, N.; Dey, S. Anticancer potential of pd and pt metallo-macrocycles of phosphines and 4,4′-dipyridyldiselenide. Polyhedron 2022, 211, 115547. [Google Scholar] [CrossRef]
- Sun, C.; Tan, Y.; Xu, H. From selenite to diselenide-containing drug delivery systems. ACS Mater. Lett. 2020, 2, 1173–1177. [Google Scholar] [CrossRef]
- Xu, H.; Cao, W.; Zhang, X. Selenium-containing polymers: Promising biomaterials for controlled release and enzyme mimics. Acc. Chem. Res. 2013, 46, 1647–1658. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Zhang, F.; Chen, F.; Zheng, X.; Hu, H.; Yang, C.; Tu, Z.; Wang, Z.; Chang, Z.; Lu, J.; et al. Biomimetic diselenide-bridged mesoporous organosilica nanoparticles as an x-ray-responsive biodegradable carrier for chemo-immunotherapy. Adv. Mater. 2020, 32, e2004385. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Li, Y.; Xu, H.; Wang, Z.; Zhang, X. Dual redox responsive assemblies formed from diselenide block copolymers. J. Am. Chem. Soc. 2010, 132, 442–443. [Google Scholar] [CrossRef]
- Liang, Q.; Zhou, L.; Li, Y.; Liu, J.; Liu, Y. Nano drug delivery system reconstruct tumour vasculature for the tumour vascular normalisation. J. Drug Target. 2022, 30, 119–130. [Google Scholar] [CrossRef]
- Plamper, F.A.; Richtering, W. Functional microgels and microgel systems. Acc. Chem. Res. 2017, 50, 131–140. [Google Scholar] [CrossRef]
- Jia, B.; Gao, Y.; Ouyang, Z.; Shen, S.; Shen, M.; Shi, X. Diselenide-crosslinked nanogels laden with gold nanoparticles and methotrexate for immunomodulation-enhanced chemotherapy and computed tomography imaging of tumors. J. Mater. Chem. B 2023, 11, 4808–4818. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Zhang, N.; Yang, C.; Xu, H.; Wang, Z.; Li, B.; Ding, J.; Chen, X. X-ray-responsive polypeptide nanogel for concurrent chemoradiotherapy. J. Control Release 2021, 332, 1–9. [Google Scholar] [CrossRef]
- Ding, X.; Zang, M.; Zhang, Y.; Chen, Y.; Du, J.; Yan, A.; Gu, J.; Li, Y.; Wei, S.; Xu, J.; et al. A bioresponsive diselenide-functionalized hydrogel with cascade catalytic activities for enhanced local starvation- and hypoxia-activated melanoma therapy. Acta Biomater. 2023, 167, 182–194. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Ostańska, E.; Aebisher, D.; Bartusik-Aebisher, D. The potential of photodynamic therapy in current breast cancer treatment methodologies. Biomed. Pharmacother. 2021, 137, 111302. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, M.W.; Jeong, Y.I.; Yang, H.S. Redox-sensitive and folate-receptor-mediated targeting of cervical cancer cells for photodynamic therapy using nanophotosensitizers composed of chlorin e6-conjugated β-cyclodextrin via diselenide linkage. Cells 2021, 10, 2190. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.I.; Lee, H.L.; Choi, S.H.; Kim, J.; Yu, Y.B.; Jeong, Y.I.; Kang, D.H. Reactive oxygen species-sensitive nanophotosensitizers of methoxy poly(ethylene glycol)-chlorin e6/phenyl boronic acid pinacol ester conjugates having diselenide linkages for photodynamic therapy of cervical cancer cells. Materials 2021, 15, 138. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous silica and organosilica nanoparticles: Physical chemistry, biosafety, delivery strategies, and biomedical applications. Adv. Healthc. Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef]
- Kong, J.; Sun, Y.; Ge, X.; Mao, M.; Yu, H.; Wang, Y. A chlorotoxin-directed diselenide-bridged tumor-homing persistent luminescence nanoprobes mediating inhibition of oxidative phosphorylation for long-term near-infrared imaging and therapy of glioblastoma. Adv. Funct. Mater. 2023, 33, 2209579. [Google Scholar] [CrossRef]
- Yan, H.; Dong, J.; Huang, X.; Du, X. Protein-gated upconversion nanoparticle-embedded mesoporous silica nanovehicles via diselenide linkages for drug release tracking in real time and tumor chemotherapy. ACS Appl. Mater. Interfaces 2021, 13, 29070–29082. [Google Scholar] [CrossRef]
- Zhang, R.X.; Wong, H.L.; Xue, H.Y.; Eoh, J.Y.; Wu, X.Y. Nanomedicine of synergistic drug combinations for cancer therapy—Strategies and perspectives. J. Control Release 2016, 240, 489–503. [Google Scholar] [CrossRef]
- Wu, S.Y.; Chou, H.Y.; Yuh, C.H.; Mekuria, S.L.; Kao, Y.C.; Tsai, H.C. Radiation-sensitive dendrimer-based drug delivery system. Adv. Sci. 2018, 5, 1700339. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Xu, J.; Li, Y.; He, J.; Yang, Y.; Huynh, T.; Ni, P.; Duan, G.; Yang, Z.; et al. Low-dose x-ray-responsive diselenide nanocarriers for effective delivery of anticancer agents. ACS Appl. Mater. Interfaces 2020, 12, 43398–43407. [Google Scholar] [CrossRef]
- Li, T.; Pan, S.; Gao, S.; Xiang, W.; Sun, C.; Cao, W.; Xu, H. Diselenide-pemetrexed assemblies for combined cancer immuno-, radio-, and chemotherapies. Angew. Chem. Int. Ed. Engl. 2020, 59, 2700–2704. [Google Scholar] [CrossRef]
- Gao, S.; Li, T.; Guo, Y.; Sun, C.; Xianyu, B.; Xu, H. Selenium-containing nanoparticles combine the nk cells mediated immunotherapy with radiotherapy and chemotherapy. Adv. Mater. 2020, 32, e1907568. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Pan, S.; Zhuang, H.; Gao, S.; Xu, H. Selenium-containing carrier-free assemblies with aggregation-induced emission property combine cancer radiotherapy with chemotherapy. ACS Appl. Bio Mater. 2020, 3, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Luan, T.; Liu, D.; Cheng, J.; Li, H.; Wei, H.; Zhang, L.; Lan, J.; Liu, Y.; Zhao, G. Diblock copolymer glyco-nanomicelles constructed by a maltoheptaose-based amphiphile for reduction- and ph-mediated intracellular drug delivery. Polym. Chem. 2018, 9, 1337–1347. [Google Scholar] [CrossRef]
- Yang, J.; Pan, S.; Gao, S.; Li, T.; Xu, H. Co/chemosensitization/antiangiogenesis synergistic therapy with H2O2-responsive diselenide-containing polymer. Biomaterials 2021, 271, 120721. [Google Scholar] [CrossRef]
- Tawfik, S.M.; Azizov, S.; Elmasry, M.R.; Sharipov, M.; Lee, Y.I. Recent advances in nanomicelles delivery systems. Nanomaterials 2020, 11, 70. [Google Scholar] [CrossRef]
- Ramesh, K.; Yadav, S.; Mishra, A.K.; Jo, S.-H.; Park, S.-H.; Oh, C.-W.; Lim, K.T. Interface-cross-linked micelles of poly(d,l-lactide)-b-poly(furfuryl methacrylate)-b-poly(n-acryloylmorpholine) for near-infrared-triggered drug delivery application. Polym. Adv. Technol. 2022, 33, 2137–2148. [Google Scholar] [CrossRef]
- Miao, Z.; Huang, D.; Wang, Y.; Li, W.-J.; Fan, L.; Wang, J.; Ma, Y.; Zhao, Q.; Zha, Z. Safe-by-design exfoliation of niobium diselenide atomic crystals as a theory-oriented 2d nanoagent from anti-inflammation to antitumor. Adv. Funct. Mater. 2020, 30, 2001593. [Google Scholar] [CrossRef]
- Venkatesan, J.; Jeong Lee, S.; Hur, W.; Gupta, P.K.; Eun Son, S.; Been Lee, H.; Yeon Park, J.; Nyeon Kim, S.; Hun Seong, G. Preparation and photothermal effect of chitosan-alginate-molybdenum diselenide nanocomposite scaffolds for cancer therapy. J. Photochem. Photobiol. A Chem. 2023, 442, 114759. [Google Scholar] [CrossRef]
- Nguyen, V.Q.; You, D.G.; Oh, B.H.; Bui, V.D.; An, J.Y.; Um, W.; Park, J.H. Diselenide-bearing liposomes for intracellular delivery of a vitamin c derivative in cancer cells. Macromol. Res. 2021, 29, 327–330. [Google Scholar] [CrossRef]
- Wan, S.C.; Ye, M.J.; Yang, Q.C.; Zhang, T.; Zhang, M.J.; Ma, X.B.; Xu, J.M.; Wang, S.; Wu, Z.Z.; Yang, L.L.; et al. Diselenide-based dual-responsive prodrug as pyroptosis inducer potentiates cancer immunotherapy. Adv. Healthc. Mater. 2023, 12, e2202135. [Google Scholar] [CrossRef]
- Kim, S.G.; Robby, A.I.; Lee, B.C.; Lee, G.; Park, S.Y. Mitochondria-targeted ros- and gsh-responsive diselenide-crosslinked polymer dots for programmable paclitaxel release. J. Ind. Eng. Chem. 2021, 99, 98–106. [Google Scholar] [CrossRef]
- Wei, C.; Liang, B.; Li, Y.; Yan, B.; Zhou, Y.; Liu, Y.; Lang, M. A drug-free therapeutic system for cancer therapy by diselenide-based polymers themselves. Adv. Healthc. Mater. 2021, 10, e2001471. [Google Scholar] [CrossRef] [PubMed]
- Vetrik, M.; Kucka, J.; Kobera, L.; Konefal, R.; Lobaz, V.; Pavlova, E.; Bajecny, M.; Heizer, T.; Brus, J.; Sefc, L.; et al. Fluorinated diselenide nanoparticles for radiosensitizing therapy of cancer. Free Radic. Biol. Med. 2022, 187, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.M.; Sari, M.H.M.; Azambuja, J.H.; da Silveira, E.F.; Cervi, V.F.; Marchiori, M.C.L.; Maria-Engler, S.S.; Wink, M.R.; Azevedo, J.G.; Nogueira, C.W.; et al. Xanthan gum-based hydrogel containing nanocapsules for cutaneous diphenyl diselenide delivery in melanoma therapy. Investig. New Drugs 2020, 38, 662–674. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Sari, M.H.M.; Cervi, V.F.; Prado, V.C.; Nadal, J.M.; Azambuja, J.H.; da Silveira, E.F.; Nogueira, C.W.; Farago, P.V.; Braganhol, E.; et al. Design of pegylated-nanocapsules to diphenyl diselenide administration: In vitro evidence of hemocompatible and selective antiglioma formulation. AAPS PharmSciTech 2020, 21, 307. [Google Scholar] [CrossRef]
- Mosolygó, T.; Kincses, A.; Csonka, A.; Tönki, Á.S.; Witek, K.; Sanmartín, C.; Marć, M.A.; Handzlik, J.; Kieć-Kononowicz, K.; Domínguez-Álvarez, E.; et al. Selenocompounds as novel antibacterial agents and bacterial efflux pump inhibitors. Molecules 2019, 24, 1487. [Google Scholar] [CrossRef]
- Stefanello, S.T.; Mizdal, C.R.; Gonçalves, D.F.; Hartmann, D.D.; Dobrachinski, F.; de Carvalho, N.R.; Salman, S.M.; Sauer, A.C.; Dornelles, L.; de Campos, M.M.A.; et al. The insertion of functional groups in organic selenium compounds promote changes in mitochondrial parameters and raise the antibacterial activity. Bioorg. Chem. 2020, 98, 103727. [Google Scholar] [CrossRef]
- He, R.; Ding, C.; Luo, Y.; Guo, G.; Tang, J.; Shen, H.; Wang, Q.; Zhang, X. Congener-induced sulfur-related metabolism interference therapy promoted by photothermal sensitization for combating bacteria. Adv. Mater. 2021, 33, e2104410. [Google Scholar] [CrossRef]
- Bonilla, M.; Krull, E.; Irigoín, F.; Salinas, G.; Comini, M.A. Selenoproteins of african trypanosomes are dispensable for parasite survival in a mammalian host. Mol. Biochem. Parasitol. 2016, 206, 13–19. [Google Scholar] [CrossRef]
- Garnica, P.; Etxebeste-Mitxeltorena, M.; Plano, D.; Moreno, E.; Espuelas, S.; Antonio Palop, J.; Jiménez-Ruiz, A.; Sanmartín, C. Pre-clinical evidences of the antileishmanial effects of diselenides and selenocyanates. Bioorg. Med. Chem. Lett. 2020, 30, 127371. [Google Scholar] [CrossRef]
- Henriquez-Figuereo, A.; Alcon, M.; Moreno, E.; Sanmartín, C.; Espuelas, S.; Lucio, H.; Jiménez-Ruiz, A.; Plano, D. Next generation of selenocyanate and diselenides with upgraded leishmanicidal activity. Bioorg. Chem. 2023, 138, 106624. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Lalani, R.; Bardoliwala, D.; Ghosh, S.; Misra, A. Lipid-based oral formulation strategies for lipophilic drugs. AAPS PharmSciTech 2018, 19, 3609–3630. [Google Scholar] [CrossRef] [PubMed]
- Etxebeste-Mitxeltorena, M.; Moreno, E.; Carvalheiro, M.; Calvo, A.; Navarro-Blasco, I.; González-Peñas, E.; Álvarez-Galindo, J.I.; Plano, D.; Irache, J.M.; Almeida, A.J.; et al. Oral efficacy of a diselenide compound loaded in nanostructured lipid carriers in a murine model of visceral leishmaniasis. ACS Infect. Dis. 2021, 7, 3197–3209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Saad, R.; Taylor, E.W.; Rayman, M.P. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020, 37, 101715. [Google Scholar] [CrossRef]
- Nogara, P.A.; Omage, F.B.; Bolzan, G.R.; Delgado, C.P.; Aschner, M.; Orian, L.; Teixeira Rocha, J.B. In silico studies on the interaction between mpro and plpro from SARS-CoV-2 and ebselen, its metabolites and derivatives. Mol. Inform. 2021, 40, e2100028. [Google Scholar] [CrossRef]
- Zmudzinski, M.; Rut, W.; Olech, K.; Granda, J.; Giurg, M.; Burda-Grabowska, M.; Kaleta, R.; Zgarbova, M.; Kasprzyk, R.; Zhang, L.; et al. Ebselen derivatives inhibit SARS-CoV-2 replication by inhibition of its essential proteins: Pl(pro) and m(pro) proteases, and nsp14 guanine n7-methyltransferase. Sci. Rep. 2023, 13, 9161. [Google Scholar] [CrossRef]
- Omage, F.B.; Madabeni, A.; Tucci, A.R.; Nogara, P.A.; Bortoli, M.; Rosa, A.D.S.; Neuza Dos Santos Ferreira, V.; Teixeira Rocha, J.B.; Miranda, M.D.; Orian, L. Diphenyl diselenide and SARS-CoV-2: In silico exploration of the mechanisms of inhibition of main protease (m(pro)) and papain-like protease (pl(pro)). J. Chem. Inf. Model. 2023, 63, 2226–2239. [Google Scholar] [CrossRef]
- Amaral, B.P.; Cargnelutti, J.F.; Mortari, A.P.G.; Merchioratto, I.; Feio, L.M.; Nogueira, C.W.; Weiblen, R.; Flores, E. Diphenyl diselenide and cidofovir present anti-viral activity against bovine alphaherpesvirus 2 in vitro and in a sheep model. Res. Vet. Sci. 2021, 134, 78–85. [Google Scholar] [CrossRef]
- Weller, J.; Budson, A. Current understanding of alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7, 1161. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Song, G.L. Roles of selenoproteins in brain function and the potential mechanism of selenium in alzheimer’s disease. Front. Neurosci. 2021, 15, 646518. [Google Scholar] [CrossRef]
- Barbosa, F.A.R.; Canto, R.F.S.; Teixeira, K.F.; de Souza, A.S.; de Oliveira, A.S.; Braga, A.L. Selenium-derivative compounds: A review of new perspectives in the treatment of alzheimer’s disease. Curr. Med. Chem. 2023, 30, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, A.; Raheem, S.; Ali, F.; Dar, T.A.; Chakrabarty, S.; Rizvi, M.A. Organoselenium compounds as acetylcholinesterase inhibitors: Evidence and mechanism of mixed inhibition. J. Phys. Chem. B 2021, 125, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Refaay, D.A.; Ahmed, D.M.; Mowafy, A.M.; Shaaban, S. Evaluation of novel multifunctional organoselenium compounds as potential cholinesterase inhibitors against alzheimer’s disease. Med. Chem. Res. 2022, 31, 894–904. [Google Scholar] [CrossRef]
- Yuan, X.; Jia, Z.; Li, J.; Liu, Y.; Huang, Y.; Gong, Y.; Guo, X.; Chen, X.; Cen, J.; Liu, J. A diselenide bond-containing ros-responsive ruthenium nanoplatform delivers nerve growth factor for alzheimer’s disease management by repairing and promoting neuron regeneration. J. Mater. Chem. B 2021, 9, 7835–7847. [Google Scholar] [CrossRef]
- Alberto, E.E.; do Nascimento, V.; Braga, A.L. Catalytic application of selenium and tellurium compounds as glutathione peroxidase enzyme mimetics. J. Braz. Chem. Soc. 2010, 21, 2032–2041. [Google Scholar] [CrossRef]
- Hara, T.; Okazaki, T.; Hashiya, T.; Nozawa, K.; Yasuike, S.; Kurita, J.; Yamamoto, C.; Hamada, N.; Kaji, T. Effects of substitution on cytotoxicity of diphenyl ditelluride in cultured vascular endothelial cells. Int. J. Mol. Sci. 2021, 22, 10520. [Google Scholar] [CrossRef]
- Juchem, A.L.M.; Trindade, C.; da Silva, J.B.; Machado, M.D.S.; Guecheva, T.N.; Rocha, J.C.; Saffi, J.; de Oliveira, I.M.; Henriques, J.A.P.; Escargueil, A. Diphenyl ditelluride anticancer activity and DNA topoisomerase i poisoning in human colon cancer hct116 cells. Oncotarget 2023, 14, 637–649. [Google Scholar] [CrossRef]
- Pinheiro, F.C.; Bortolotto, V.C.; Araujo, S.M.; Couto, S.F.; Dahleh, M.M.M.; Cancela, M.; Neto, J.; Zeni, G.; Zaha, A.; Prigol, M. Oxidative stress response system in escherichia coli arising from diphenyl ditelluride (phte)(2) exposure. Toxicol. Vitr. 2022, 83, 105404. [Google Scholar] [CrossRef]
- Yang, F.; Wong, K.H.; Yang, Y.; Li, X.; Jiang, J.; Zheng, W.; Wu, H.; Chen, T. Purification and in vitro antioxidant activities of tellurium-containing phycobiliproteins from tellurium-enriched spirulina platensis. Drug Des. Dev. Ther. 2014, 8, 1789–1800. [Google Scholar] [CrossRef]
- Liu, X.; Silks, L.A.; Liu, C.; Ollivault-Shiflett, M.; Huang, X.; Li, J.; Luo, G.; Hou, Y.M.; Liu, J.; Shen, J. Incorporation of tellurocysteine into glutathione transferase generates high glutathione peroxidase efficiency. Angew. Chem. Int. Ed. Engl. 2009, 48, 2020–2023. [Google Scholar] [CrossRef]
- Tripathi, A.; Khan, A.; Srivastava, R. Synthesis and screening for anticancer activity of two novel telluro-amino acids: 1,3-tellurazolidine-4-carboxylic acid and tellurohomocystine. Amino Acids 2023, 55, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Zhou, J.; Sun, C. Ditelluride-bridged peg-pcl copolymer as folic acid-targeted and redox-responsive nanoparticles for enhanced cancer therapy. Front. Chem. 2020, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xue, R.; Lyu, J.; Gao, A.; Sun, C. Tumor acidity/redox hierarchical-activable nanoparticles for precise combination of x-ray-induced photodynamic therapy and hypoxia-activated chemotherapy. J. Mater. Chem. B 2022, 10, 3849–3860. [Google Scholar] [CrossRef]


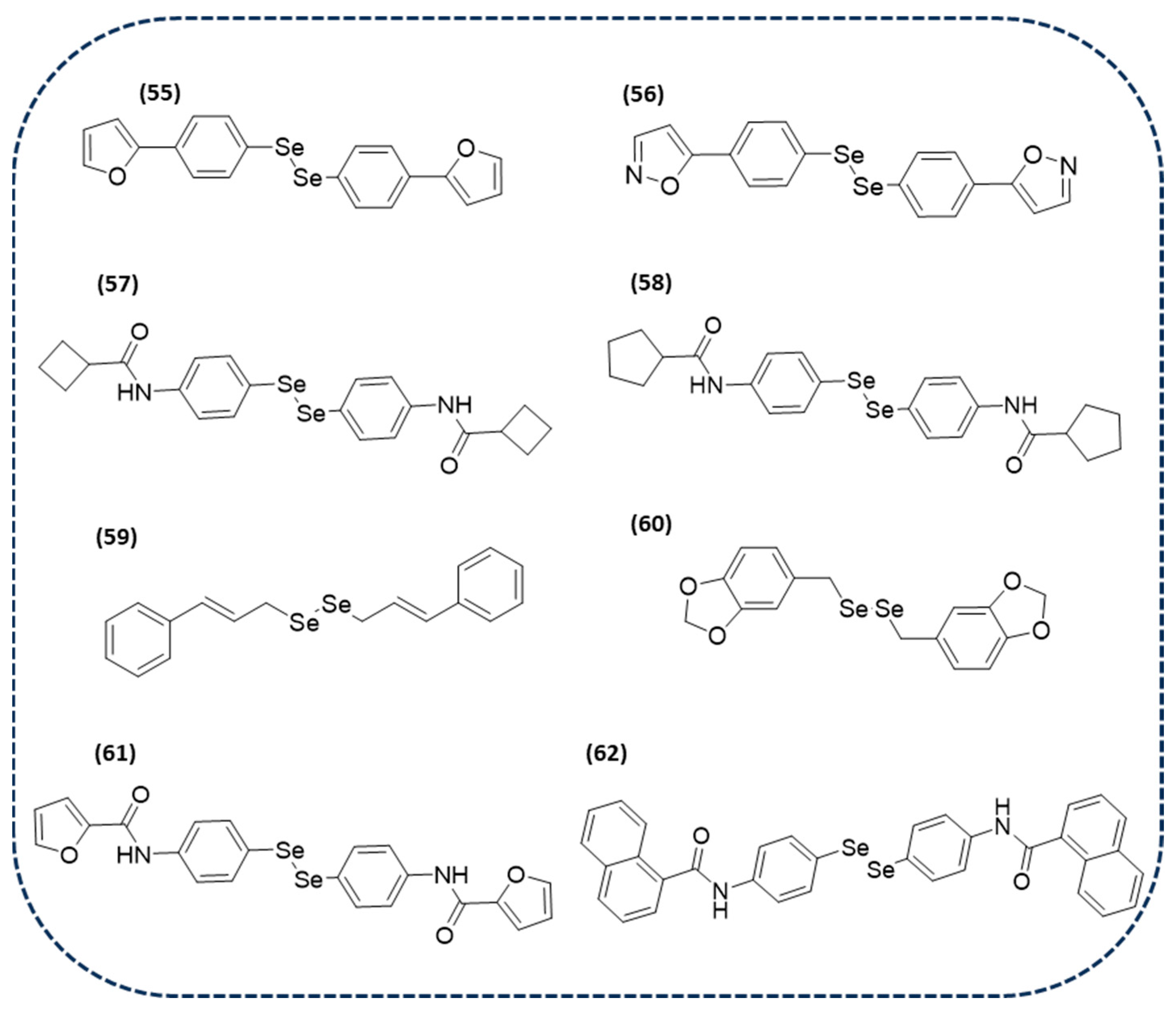
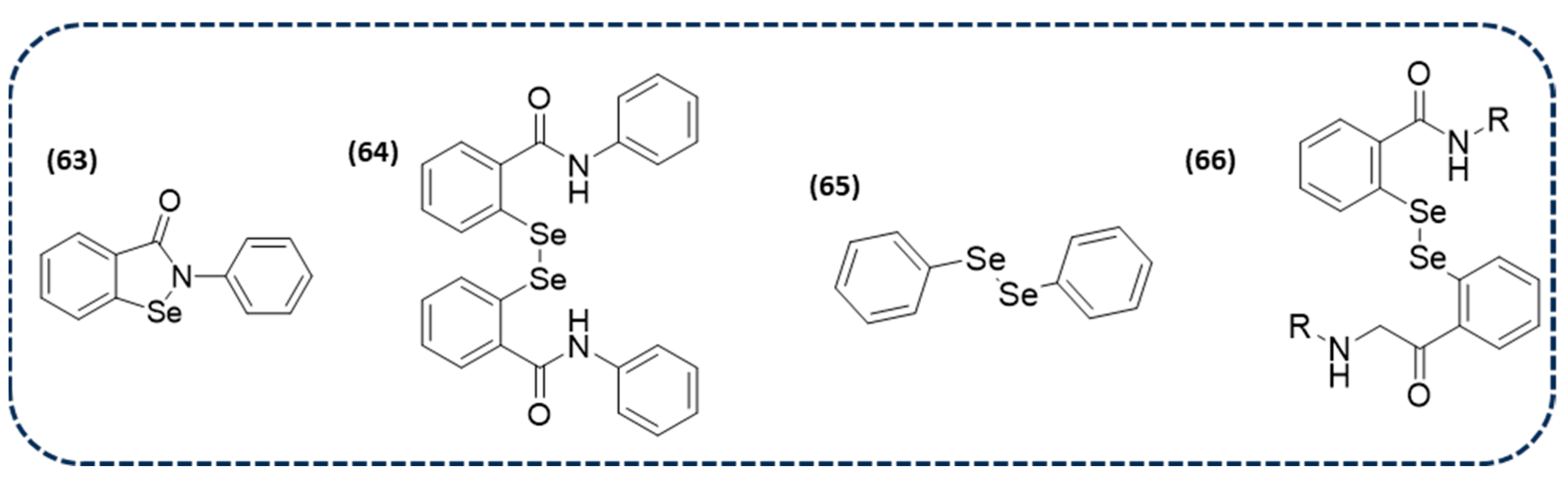
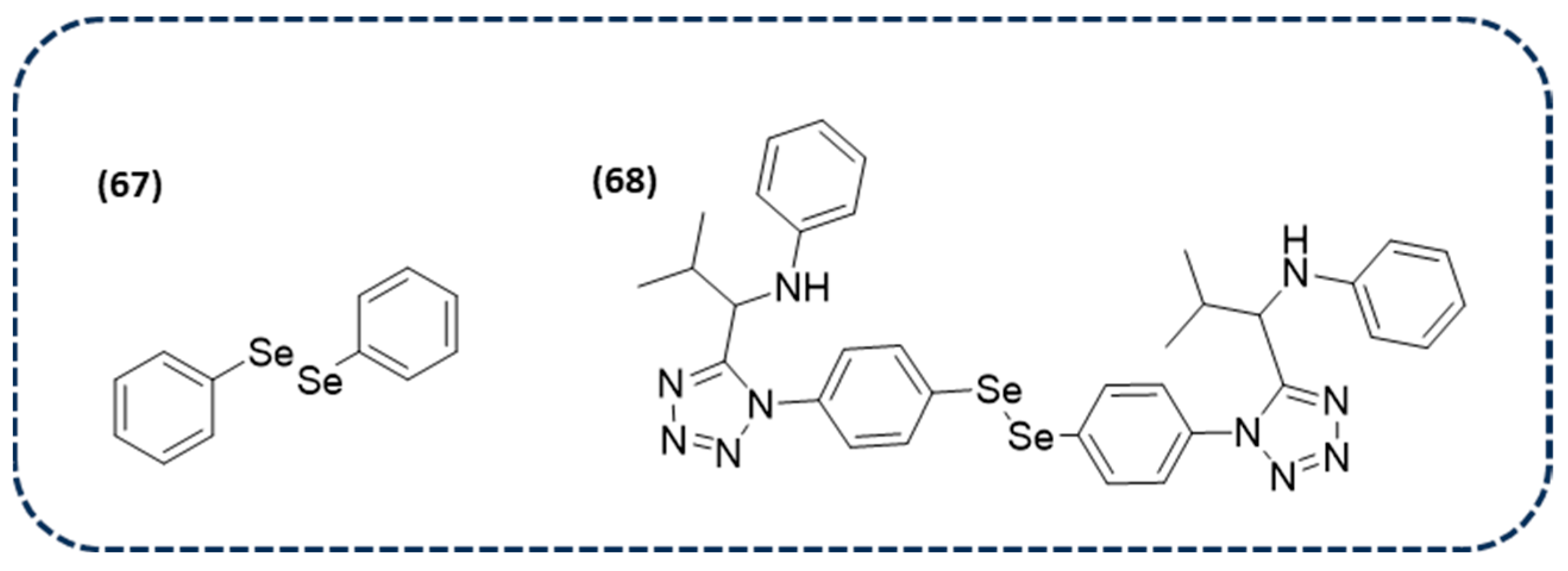
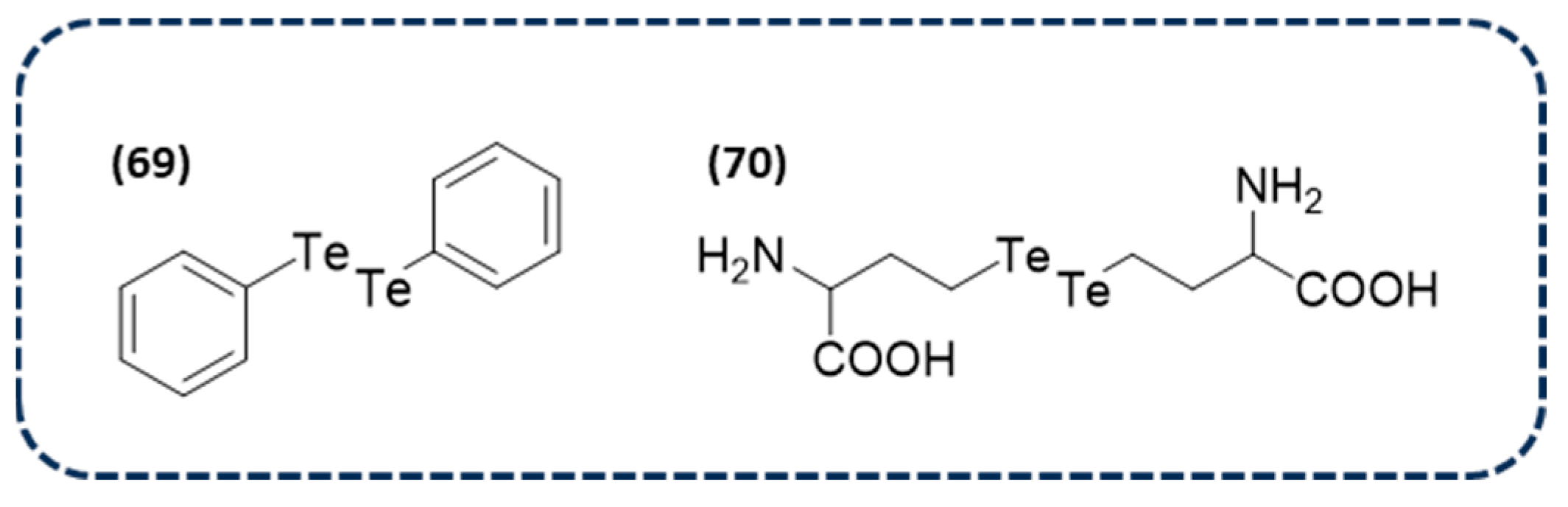
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaviria-Soteras, L.; Sharma, A.K.; Sanmartín, C.; Plano, D. Recent Insights into Bioactive Dichalcogen Derivatives: From Small Molecules to Complex Materials. Int. J. Mol. Sci. 2025, 26, 2436. https://doi.org/10.3390/ijms26062436
Gaviria-Soteras L, Sharma AK, Sanmartín C, Plano D. Recent Insights into Bioactive Dichalcogen Derivatives: From Small Molecules to Complex Materials. International Journal of Molecular Sciences. 2025; 26(6):2436. https://doi.org/10.3390/ijms26062436
Chicago/Turabian StyleGaviria-Soteras, Leire, Arun K. Sharma, Carmen Sanmartín, and Daniel Plano. 2025. "Recent Insights into Bioactive Dichalcogen Derivatives: From Small Molecules to Complex Materials" International Journal of Molecular Sciences 26, no. 6: 2436. https://doi.org/10.3390/ijms26062436
APA StyleGaviria-Soteras, L., Sharma, A. K., Sanmartín, C., & Plano, D. (2025). Recent Insights into Bioactive Dichalcogen Derivatives: From Small Molecules to Complex Materials. International Journal of Molecular Sciences, 26(6), 2436. https://doi.org/10.3390/ijms26062436









