Glycosylation Regulation by TMEM230 in Aging and Autoimmunity
Abstract
1. Introduction
Background
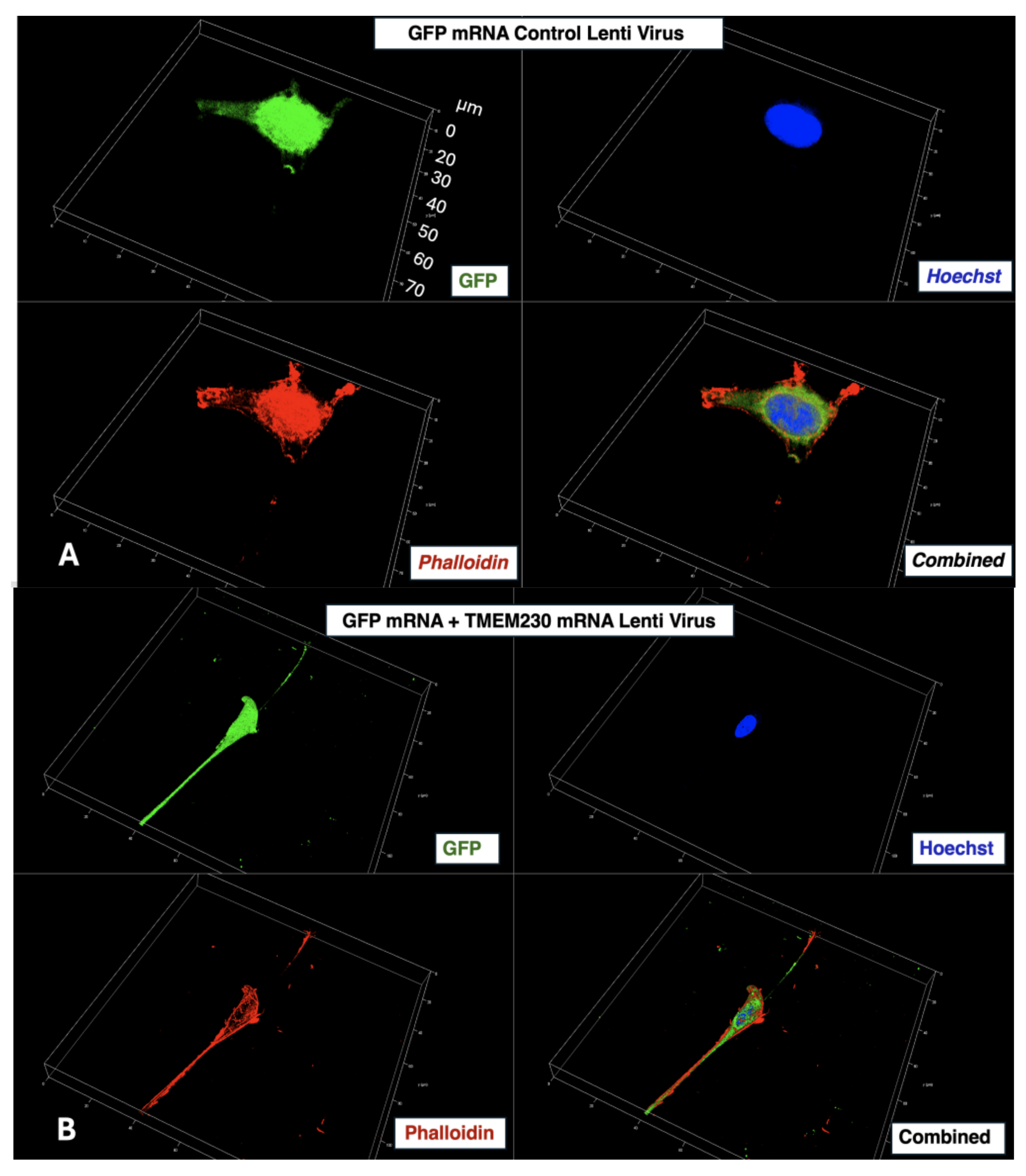
2. Results
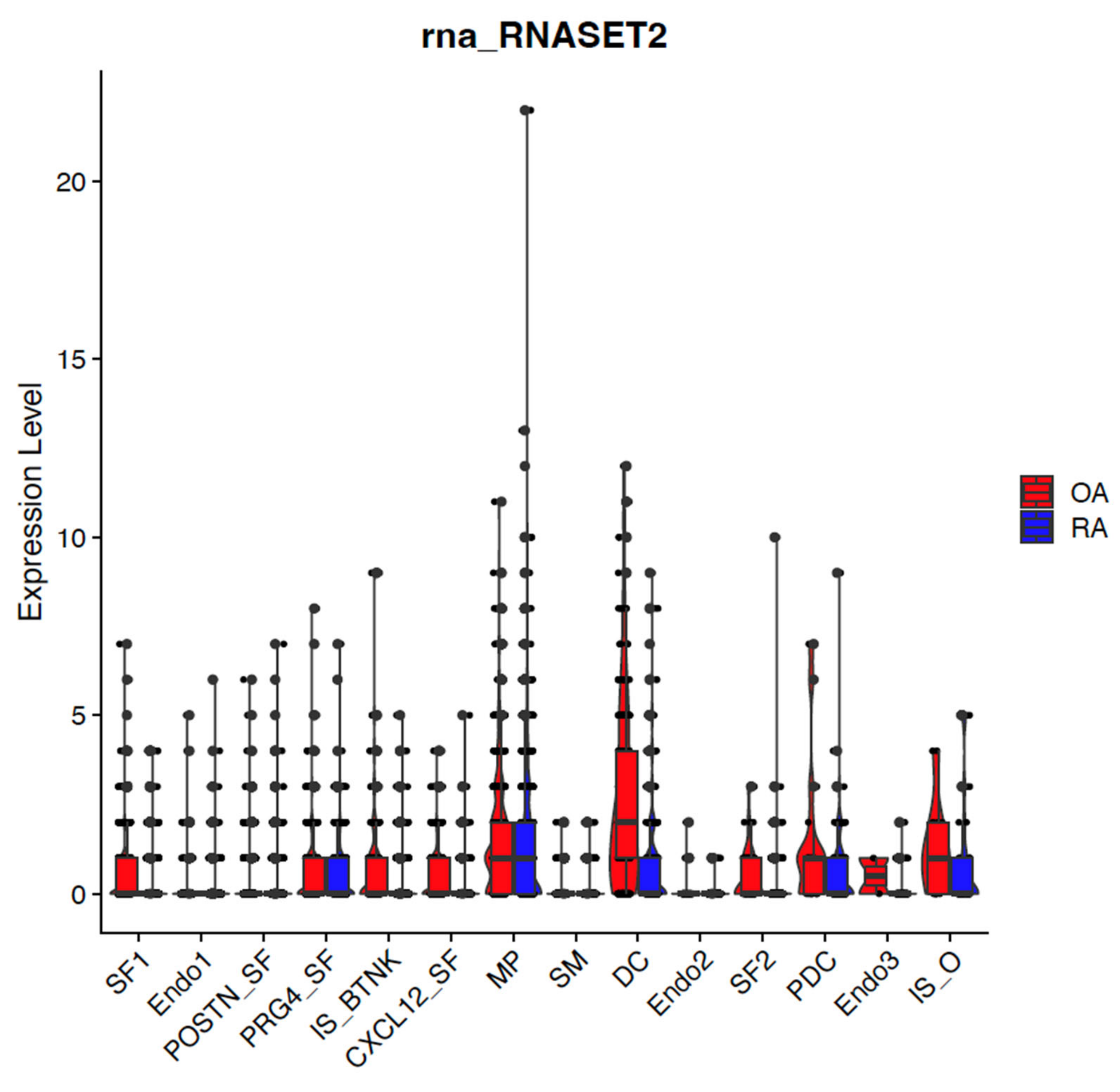
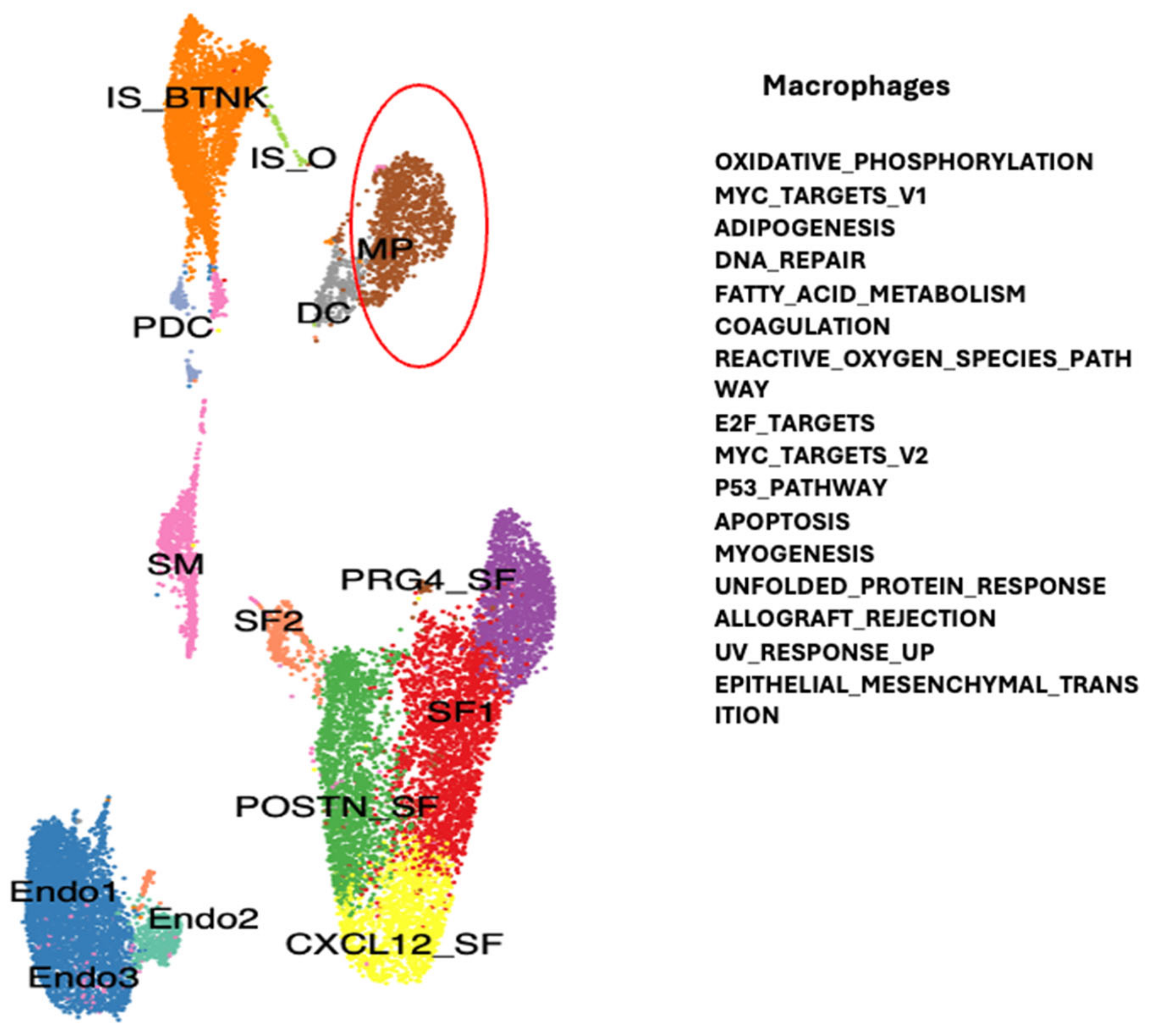
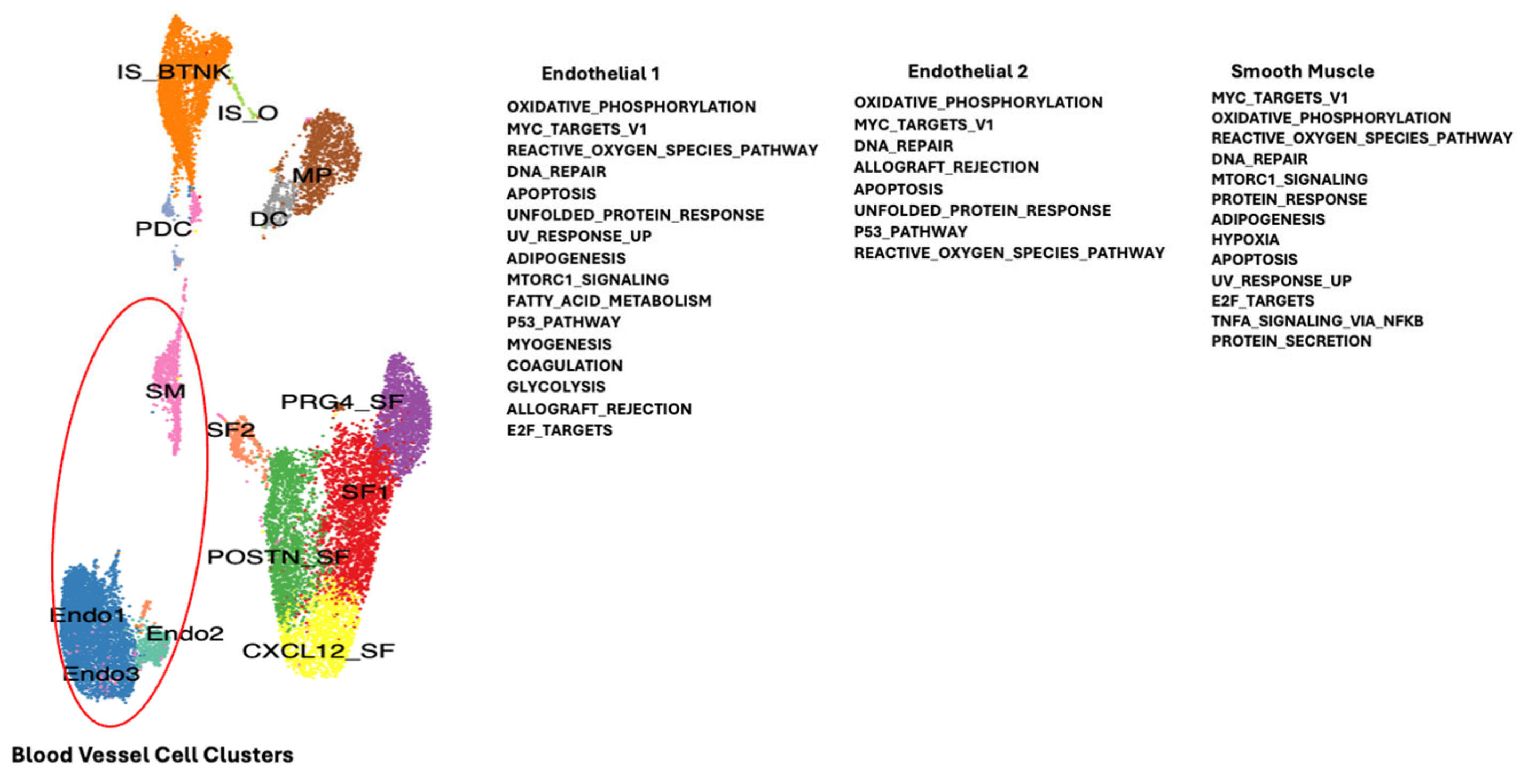
2.1. Dysregulated Genes in Autoimmunity
Single Cell RNA Sequencing Data Analysis
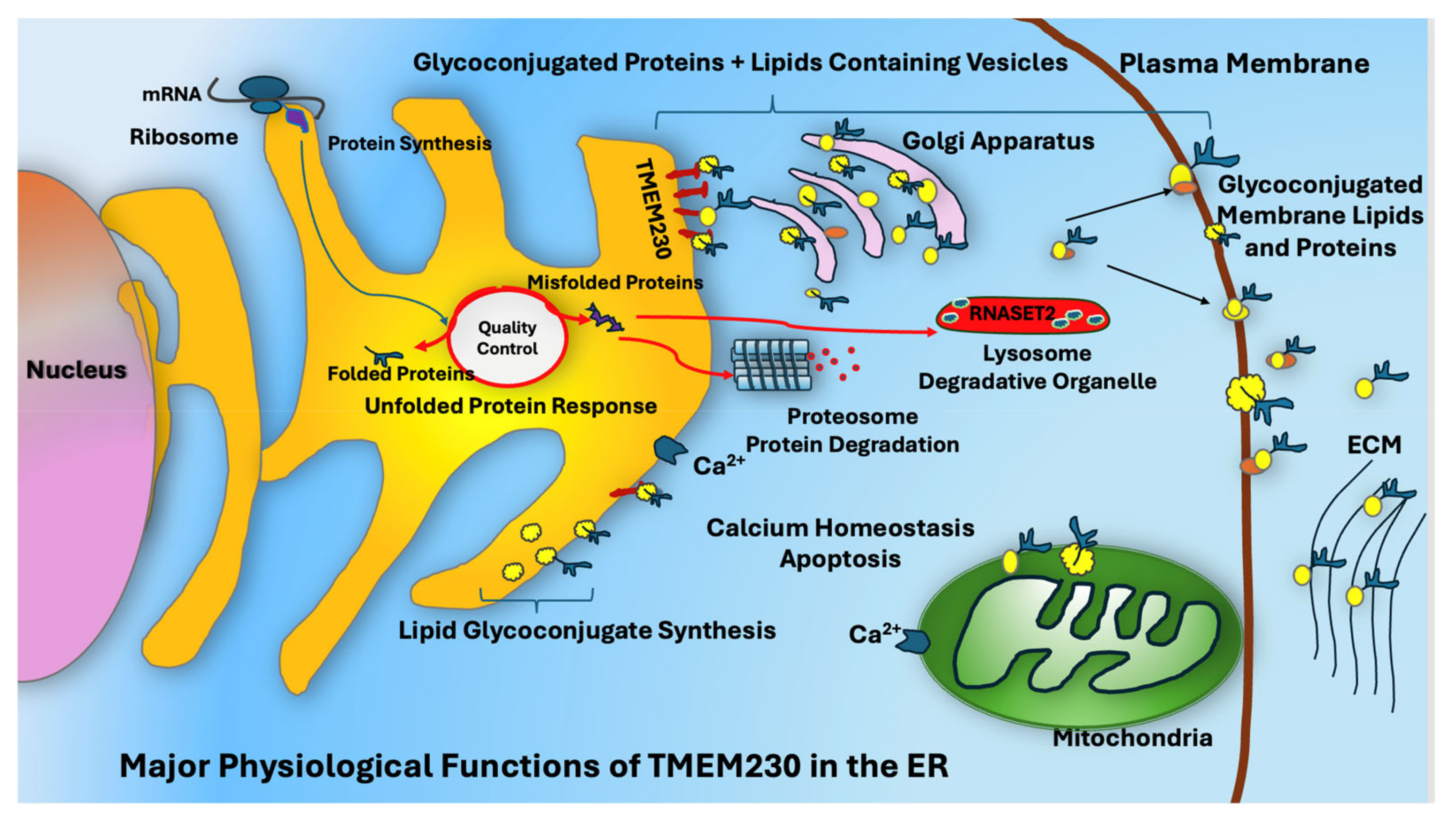
3. Discussion
4. Materials and Methods
4.1. Cloning of Lentiviral-System-Based Construct for Modulating TMEM230 Protein Expression
4.2. Adherent Cell Cultures
4.3. Immunofluorescence Analysis
4.4. Super-Resolution Microscopy
4.5. RNA-Seq Gene Expression Analysis
5. Conclusions
6. Patent
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DI Matteo, A.; Emery, P. Rheumatoid arthritis: A review of the key clinical features and ongoing challenges of the disease. Panminerva Med. 2024, 66, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Gloor, A.D.; Berry, G.J.; Goronzy, J.J.; Weyand, C.M. Age as a risk factor in vasculitis. Semin. Immunopathol. 2022, 44, 281–301. [Google Scholar] [CrossRef]
- Hetz, C.; Dillin, A. Central role of the ER proteostasis network in healthy aging. Trends Cell Biol. 2024. [Google Scholar] [CrossRef]
- Arjona, M.I.; Najafi, J.; Minc, N. Cytoplasm mechanics and cellular organization. Curr. Opin. Cell Biol. 2023, 85, 102278. [Google Scholar] [CrossRef] [PubMed]
- Ciocanel, M.V.; Chandrasekaran, A.; Mager, C.; Ni, Q.; Papoian, G.A.; Dawes, A. Simulated actin reorganization mediated by motor proteins. PLoS Comput. Biol. 2022, 18, e1010026. [Google Scholar] [CrossRef]
- Angeli, E.; Cocola, C.; Abeni, E.; Piscitelli, E.; Pelucchi, P.; Mosca, E.; Chiodi, A.; Lerma, L.; Balbino, C.; Zucchi, I.; et al. Role of TMEM230 in the Aberrant Glycosylation in Human Autoimmunity and Cancer. Proteoglycan Res. 2025, 3, e70020. [Google Scholar] [CrossRef]
- Abeni, E.; Cocola, C.; Croci, S.; Martino, V.; Piscitelli, E.; Gualtierotti, R.; Pelucchi, P.; Tria, V.; Porta, G.; Troschel, F.; et al. Single-cell transcriptomic analysis to identify endomembrane regulation of metalloproteins and motor proteins in autoimmunity. Adv. Protein Chem. Struct. Biol. 2024, 141, 299–329. [Google Scholar] [CrossRef] [PubMed]
- Cocola, C.; Abeni, E.; Martino, V.; Piscitelli, E.; Morara, S.; Pelucchi, P.; Mosca, E.; Chiodi, A.; Mohamed, T.; Palizban, M.; et al. Transmembrane protein TMEM230, regulator of metalloproteins and motor proteins in gliomas and gliosis. Adv. Protein Chem. Struct. Biol. 2024, 141, 255–297. [Google Scholar] [CrossRef]
- Piscitelli, E.; Maya, I.C.; Cocola, C.; Martino, V.; Abeni, E.; Pelucchi, P.; Angeli, E.; Guida, P.; Consiglio, A.; Grillo, G.; et al. Long-term culture of patient-derived mammary organoids in non-biogenic electrospun scaffolds for identifying metalloprotein and motor protein activities in aging and senescence. Adv. Protein Chem. Struct. Biol. 2024, 141, 331–360. [Google Scholar] [CrossRef]
- Izdebska, M.; Zielinska, W.; Halas-Wisniewska, M.; Grzanka, A. Involvement of Actin and Actin-Binding Proteins in Carcinogenesis. Cells 2020, 9, 2245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carra, S.; Sangiorgio, L.; Pelucchi, P.; Cermenati, S.; Mezzelani, A.; Martino, V.; Palizban, M.; Albertini, A.; Gotte, M.; Kehler, J.; et al. Zebrafish Tmem230a cooperates with the Delta/Notch signaling pathway to modulate endothelial cell number in angiogenic vessels. J. Cell. Physiol. 2018, 233, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Cocola, C.; Abeni, E.; Martino, V.; Piscitelli, E.; Pelucchi, P.; Mosca, E.; Chiodi, A.; Mohamed, T.; Palizban, M.; Porta, G.; et al. Transmembrane Protein TMEM230, Regulator of Glial Cell Vascular Mimicry and Endothelial Cell Angiogenesis in High-Grade Heterogeneous Infiltrating Gliomas and Glioblastoma. Int. J. Mol. Sci. 2024, 25, 3967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cocola, C.; Magnaghi, V.; Abeni, E.; Pelucchi, P.; Martino, V.; Vilardo, L.; Piscitelli, E.; Consiglio, A.; Grillo, G.; Mosca, E.; et al. Transmembrane Protein TMEM230, a Target of Glioblastoma Therapy. Front. Cell. Neurosci. 2021, 15, 703431. [Google Scholar] [CrossRef]
- Guida, P.; Piscitelli, E.; Marrese, M.; Martino, V.; Cirillo, V.; Guarino, V.; Angeli, E.; Cocola, C.; Pelucchi, P.; Repetto, L.; et al. Integrating Microstructured Electrospun Scaffolds in an Open Microfluidic System for in Vitro Studies of Human Patient-Derived Primary Cells. ACS Biomater. Sci. Eng. 2020, 6, 3649–3663. [Google Scholar] [CrossRef]
- Iqbal, Z.; Toft, M. TMEM230 variants in Parkinson’s disease. Nat. Genet. 2019, 51, 366. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, G.; Zhang, J.; Aebez, N.; Liu, Z.; Liu, C.F.; Ross, C.A.; Smith, W.W. Mutant-TMEM230-induced neurodegeneration and impaired axonal mitochondrial transport. Hum. Mol. Genet. 2021, 30, 1535–1542. [Google Scholar] [CrossRef]
- Wang, X.; Whelan, E.; Liu, Z.; Liu, C.F.; Smith, W.W. Controversy of TMEM230 Associated with Parkinson’s Disease. Neuroscience 2021, 453, 280–286. [Google Scholar] [CrossRef]
- Deng, H.X.; Pericak-Vance, M.A.; Siddique, T. Reply to ‘TMEM230 variants in Parkinson’s disease’ and ‘Doubts about TMEM230 as a gene for parkinsonism’. Nat. Genet. 2019, 51, 369–371. [Google Scholar] [CrossRef]
- Farrer, M.J. Doubts about TMEM230 as a gene for parkinsonism. Nat. Genet. 2019, 51, 367–368. [Google Scholar] [CrossRef]
- Wei, Q.; Ou, R.; Zhou, Q.; Chen, Y.; Cao, B.; Gu, X.; Zhao, B.; Wu, Y.; Song, W.; Shang, H.F. TMEM230 Mutations Are Rare in Han Chinese Patients with Autosomal Dominant Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 2851–2855. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, H.; Yang, D.; Song, G.; He, J.; Zhou, Y.; Huang, C.; Huang, B. Absence of motor impairments or pathological changes in TMEM230 knockout rats. Neurosci. Lett. 2024, 837, 137921. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Hussain, S.A.; Alrubie, T.M.; Zhang, X. Neuroprotective Role of Transchalcone in Parkinson’s Disease through AMP-activated Protein Kinase-mediated Signaling Pathway. J. Physiol. Investig. 2024, 67, 312–320. [Google Scholar] [CrossRef]
- Choong, C.J.; Mochizuki, H. Involvement of Mitochondria in Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 17027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Wang, W.; Pan, S.; Cao, X.; Thomas, E.R.; Xie, M.; Zhang, C.; Wu, J. Exploring heat shock proteins as therapeutic targets for Parkinson’s disease. Biochem. Pharmacol. 2024, 230, 116633. [Google Scholar] [CrossRef]
- Lim, D.; Tapella, L.; Dematteis, G.; Genazzani, A.A.; Corazzari, M.; Verkhratsky, A. The endoplasmic reticulum stress and unfolded protein response in Alzheimer’s disease: A calcium dyshomeostasis perspective. Ageing Res. Rev. 2023, 87, 101914. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.K.; Nakamura, T.; Zhang, X.; Lipton, S.A. Redox regulation, protein S-nitrosylation, and synapse loss in Alzheimer’s and related dementias. Neuron 2024, 112, 3823–3850. [Google Scholar] [CrossRef]
- Pal, C. Mitochondria-targeting by small molecules against Alzheimer’s disease: A mechanistic perspective. Biochim. Biophys. Acta Mol. Basis. Dis. 2024, 1871, 167617. [Google Scholar] [CrossRef] [PubMed]
- Palma, F.R.; Gantner, B.N.; Sakiyama, M.J.; Kayzuka, C.; Shukla, S.; Lacchini, R.; Cunniff, B.; Bonini, M.G. ROS production by mitochondria: Function or dysfunction? Oncogene 2024, 43, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Tapella, L.; Dematteis, G.; Moro, M.; Pistolato, B.; Tonelli, E.; Vanella, V.V.; Giustina, D.; La Forgia, A.; Restelli, E.; Barberis, E.; et al. Protein synthesis inhibition and loss of homeostatic functions in astrocytes from an Alzheimer’s disease mouse model: A role for ER-mitochondria interaction. Cell Death Dis. 2022, 13, 878. [Google Scholar] [CrossRef]
- Acosta-Alvear, D.; Harnoss, J.M.; Walter, P.; Ashkenazi, A. Homeostasis control in health and disease by the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2025, 26, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Barrera, M.J.; Aguilera, S.; Castro, I.; Gonzalez, S.; Carvajal, P.; Molina, C.; Hermoso, M.A.; Gonzalez, M.J. Endoplasmic reticulum stress in autoimmune diseases: Can altered protein quality control and/or unfolded protein response contribute to autoimmunity? A critical review on Sjogren’s syndrome. Autoimmun. Rev. 2018, 17, 796–808. [Google Scholar] [CrossRef]
- Bieberich, E. Synthesis, Processing, and Function of N-Glycans in N-Glycoproteins. Adv. Neurobiol. 2023, 29, 65–93. [Google Scholar] [CrossRef]
- Chen, J.; Wen, P.; Tang, Y.H.; Li, H.; Wang, Z.; Wang, X.; Zhou, X.; Gao, X.D.; Fujita, M.; Yang, G. Proteome and Glycoproteome Analyses Reveal Regulation of Protein Glycosylation Site-Specific Occupancy and Lysosomal Hydrolase Maturation by N-Glycan-Dependent ER-Quality Control. J. Proteome Res. 2024, 23, 4409–4421. [Google Scholar] [CrossRef] [PubMed]
- Dabsan, S.; Twito, G.; Biadsy, S.; Igbaria, A. Less is better: Various means to reduce protein load in the endoplasmic reticulum. FEBS J. 2024. [Google Scholar] [CrossRef] [PubMed]
- Fasana, E.; Fregno, I.; Galli, C.; Solda, T.; Molinari, M. ER-to-lysosome-associated degradation acts as failsafe mechanism upon ERAD dysfunction. EMBO Rep. 2024, 25, 2773–2785. [Google Scholar] [CrossRef] [PubMed]
- Fregno, I.; Fasana, E.; Solda, T.; Galli, C.; Molinari, M. N-glycan processing selects ERAD-resistant misfolded proteins for ER-to-lysosome-associated degradation. EMBO J. 2021, 40, e107240. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, L.; Sorrentino, C.; Roti, G. Targeting Notch Trafficking and Processing in Cancers. Cells 2020, 9, 2212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rudinskiy, M.; Molinari, M. ER-to-lysosome-associated degradation in a nutshell: Mammalian, yeast, and plant ER-phagy as induced by misfolded proteins. FEBS Lett. 2023, 597, 1928–1945. [Google Scholar] [CrossRef]
- Banerjee, D.K. Birth of a Glycotherapy for Breast Cancer. Trends Carbohydr. Res. 2023, 15, 25–37. [Google Scholar]
- Deng, X.; Liu, X.; Zhang, Y.; Ke, D.; Yan, R.; Wang, Q.; Tian, X.; Li, M.; Zeng, X.; Hu, C. Changes of serum IgG glycosylation patterns in rheumatoid arthritis. Clin. Proteom. 2023, 20, 7. [Google Scholar] [CrossRef]
- Esmail, S.; Manolson, M.F. Advances in understanding N-glycosylation structure, function, and regulation in health and disease. Eur. J. Cell Biol. 2021, 100, 151186. [Google Scholar] [CrossRef] [PubMed]
- Kissel, T.; Toes, R.E.M.; Huizinga, T.W.J.; Wuhrer, M. Glycobiology of rheumatic diseases. Nat. Rev. Rheumatol. 2023, 19, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Zabczynska, M.; Link-Lenczowski, P.; Pochec, E. Glycosylation in Autoimmune Diseases. Adv. Exp. Med. Biol. 2021, 1325, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Abbasi, R.; Rezaie, J. Tumor immune escape: Extracellular vesicles roles and therapeutics application. Cell Commun. Signal. 2024, 22, 9. [Google Scholar] [CrossRef]
- Liu, G.; He, X.; Zhao, G.; Lu, Z. Complement regulation in tumor immune evasion. Semin. Immunol. 2024, 76, 101912. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Werner, A. Sweet Rules: Linking Glycosylation to Antibody Function; Experientia Supplementum; Springer: Berlin/Heidelberg, Germany, 2021; Volume 112, pp. 365–393. [Google Scholar] [CrossRef]
- Radtke, F.; MacDonald, H.R.; Tacchini-Cottier, F. Regulation of innate and adaptive immunity by Notch. Nat. Rev. Immunol. 2013, 13, 427–437. [Google Scholar] [CrossRef]
- Xu, X.; Peng, Q.; Jiang, X.; Tan, S.; Yang, W.; Han, Y.; Oyang, L.; Lin, J.; Shen, M.; Wang, J.; et al. Altered glycosylation in cancer: Molecular functions and therapeutic potential. Cancer Commun. 2024, 44, 1316–1336. [Google Scholar] [CrossRef]
- Guay, K.P.; Ke, H.; Canniff, N.P.; George, G.T.; Eyles, S.J.; Mariappan, M.; Contessa, J.N.; Gershenson, A.; Gierasch, L.M.; Hebert, D.N. ER chaperones use a protein folding and quality control glyco-code. Mol. Cell 2023, 83, 4524–4537.e4525. [Google Scholar] [CrossRef]
- Zhang, Y.; Srivastava, V.; Zhang, B. Mammalian cargo receptors for endoplasmic reticulum-to-Golgi transport: Mechanisms and interactions. Biochem. Soc. Trans. 2023, 51, 971–981. [Google Scholar] [CrossRef]
- Micheroli, R.; Elhai, M.; Edalat, S.; Frank-Bertoncelj, M.; Burki, K.; Ciurea, A.; MacDonald, L.; Kurowska-Stolarska, M.; Lewis, M.J.; Goldmann, K.; et al. Role of synovial fibroblast subsets across synovial pathotypes in rheumatoid arthritis: A deconvolution analysis. RMD Open 2022, 8, e001949. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corazzari, M.; Gagliardi, M.; Fimia, G.M.; Piacentini, M. Endoplasmic Reticulum Stress, Unfolded Protein Response, and Cancer Cell Fate. Front. Oncol. 2017, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, Z.; Zheng, Y.; Chen, Z.; Yue, X.; Bian, E.; Zhao, B. Identification of an endoplasmic reticulum stress-related signature associated with clinical prognosis and immune therapy in glioma. BMC Neurol. 2022, 22, 192. [Google Scholar] [CrossRef] [PubMed]
- Urra, H.; Aravena, R.; Gonzalez-Johnson, L.; Hetz, C. The UPRising connection between endoplasmic reticulum stress and the tumor microenvironment. Trends. Cancer 2024, 10, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.S.; Pery, N.; Butler, L.; Shafiq, M.I.; Batool, N.; Rehman, M.F.U.; Grahame-Dunn, L.G.; Yetisen, A.K. Challenges for biosimilars: Focus on rheumatoid arthritis. Crit. Rev. Biotechnol. 2021, 41, 121–153. [Google Scholar] [CrossRef]
- Kemberi, M.; Minns, A.F.; Santamaria, S. Soluble Proteoglycans and Proteoglycan Fragments as Biomarkers of Pathological Extracellular Matrix Remodeling. Proteoglycan Res. 2024, 2, e70011. [Google Scholar] [CrossRef]
- Koers, J.; Derksen, N.; Falkenburg, W.; Ooijevaar-de Heer, P.; Nurmohamed, M.T.; Wolbink, G.J.; Rispens, T. Elevated Fab glycosylation of anti-hinge antibodies. Scand. J. Rheumatol. 2023, 52, 25–32. [Google Scholar] [CrossRef]
- Mayboroda, O.A.; Lageveen-Kammeijer, G.S.M.; Wuhrer, M.; Dolhain, R. An Integrated Glycosylation Signature of Rheumatoid Arthritis. Biomolecules 2023, 13, 1106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mueller, A.L.; Payandeh, Z.; Mohammadkhani, N.; Mubarak, S.M.H.; Zakeri, A.; Alagheband Bahrami, A.; Brockmueller, A.; Shakibaei, M. Recent Advances in Understanding the Pathogenesis of Rheumatoid Arthritis: New Treatment Strategies. Cells 2021, 10, 3017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weyand, C.M.; Goronzy, J.J. Metabolic checkpoints in rheumatoid arthritis. Semin. Arthritis Rheum. 2025, 70S, 152586. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zack, S.R.; Alzoubi, O.; Satoeya, N.; Singh, K.P.; Deen, S.; Nijim, W.; Lewis, M.J.; Pitzalis, C.; Sweiss, N.; Ivashkiv, L.B.; et al. Another Notch in the Belt of Rheumatoid Arthritis. Arthritis Rheumatol. 2024, 76, 1475–1487. [Google Scholar] [CrossRef]
- Zheng, Y.; Wei, K.; Jiang, P.; Zhao, J.; Shan, Y.; Shi, Y.; Zhao, F.; Chang, C.; Li, Y.; Zhou, M.; et al. Macrophage polarization in rheumatoid arthritis: Signaling pathways, metabolic reprogramming, and crosstalk with synovial fibroblasts. Front. Immunol. 2024, 15, 1394108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Motta, F.; Selmi, C.; Ridgway, W.M.; Gershwin, M.E.; Zhang, W. Antibody glycosylation in autoimmune diseases. Autoimmun. Rev. 2021, 20, 102804. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Lu, W.; Chen, W.; Wu, Y.; Wang, Q.; Liu, Y. A narrative review of the role of the Notch signaling pathway in rheumatoid arthritis. Ann. Transl. Med. 2022, 10, 371. [Google Scholar] [CrossRef] [PubMed]
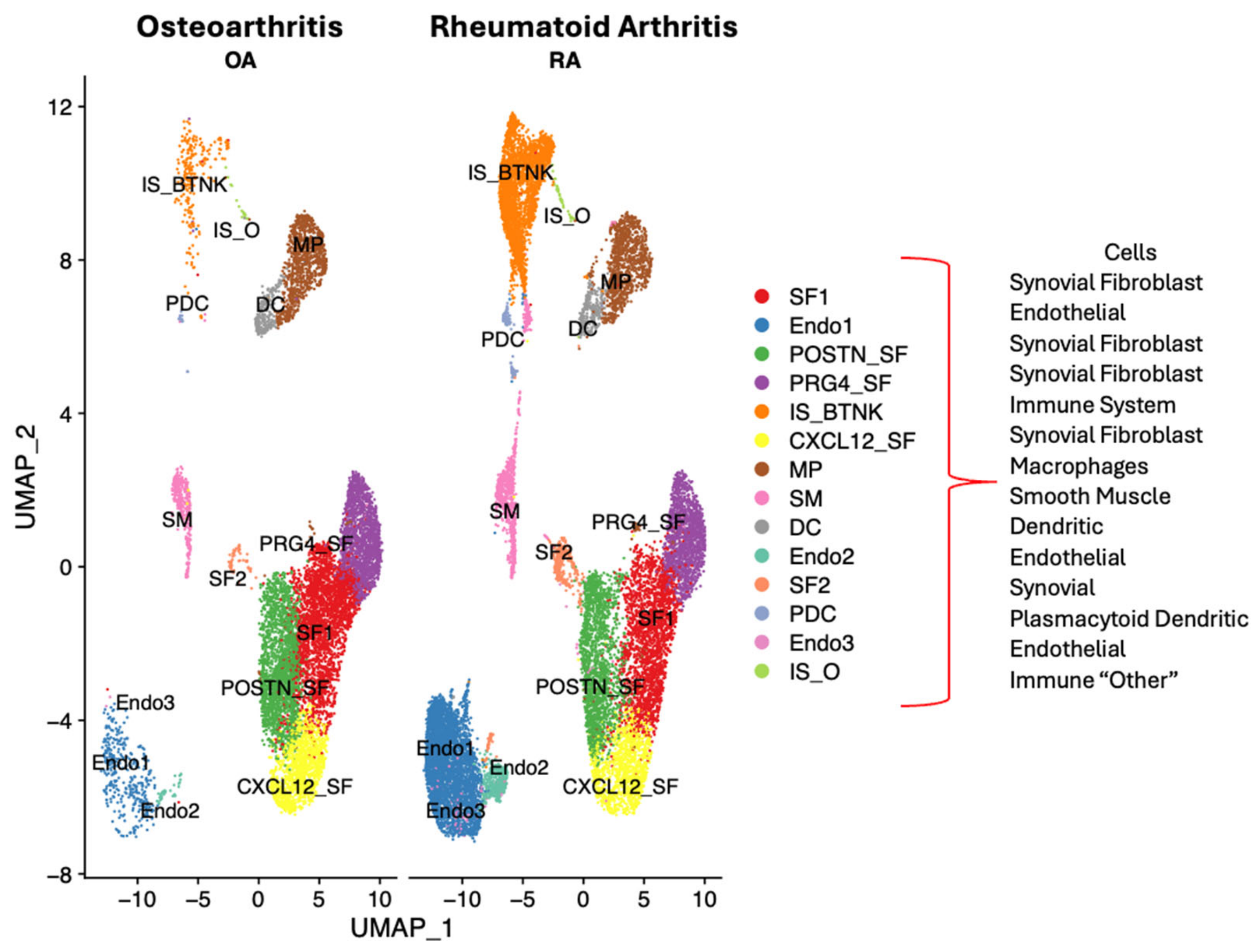




| SF-1 | ENDO1 | POSTN_SF | PRG4_SF | IS_BTNK | CXCL12_SF | MP | SM | DC | ENDO2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycosyl Transferases | GT2 | 0.02 | 0.05 | ||||||||
| GT8 | 0.03 | ||||||||||
| GT13 | 0.036 | 0.034 | |||||||||
| GT21 | 0.029 | 0.011 | 0.043 | 0.024 | 0.037 | 0.019 | |||||
| GT29 | 0.024 | ||||||||||
| GT31 | 0.05 | ||||||||||
| GT39 | 0.042 | ||||||||||
| GT41 | 0.022 | 0.046 | 0.046 | ||||||||
| Glycosyl Hydrolases | GH13 | 0.036 | 0.03 | ||||||||
| GH20 | 0.024 | 0.01 | |||||||||
| GH22 | 0.0058 | 0.043 | |||||||||
| SULFO-TRANSFGERASES | 0.0095 | 0.036 | 0.034 | 0.022 | |||||||
| OTHER | 0.0037 | 0.0095 | 0.0091 | 0.025 | 0.043 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piscitelli, E.; Abeni, E.; Balbino, C.; Angeli, E.; Cocola, C.; Pelucchi, P.; Palizban, M.; Diaspro, A.; Götte, M.; Zucchi, I.; et al. Glycosylation Regulation by TMEM230 in Aging and Autoimmunity. Int. J. Mol. Sci. 2025, 26, 2412. https://doi.org/10.3390/ijms26062412
Piscitelli E, Abeni E, Balbino C, Angeli E, Cocola C, Pelucchi P, Palizban M, Diaspro A, Götte M, Zucchi I, et al. Glycosylation Regulation by TMEM230 in Aging and Autoimmunity. International Journal of Molecular Sciences. 2025; 26(6):2412. https://doi.org/10.3390/ijms26062412
Chicago/Turabian StylePiscitelli, Eleonora, Edoardo Abeni, Cristiana Balbino, Elena Angeli, Cinzia Cocola, Paride Pelucchi, Mira Palizban, Alberto Diaspro, Martin Götte, Ileana Zucchi, and et al. 2025. "Glycosylation Regulation by TMEM230 in Aging and Autoimmunity" International Journal of Molecular Sciences 26, no. 6: 2412. https://doi.org/10.3390/ijms26062412
APA StylePiscitelli, E., Abeni, E., Balbino, C., Angeli, E., Cocola, C., Pelucchi, P., Palizban, M., Diaspro, A., Götte, M., Zucchi, I., & Reinbold, R. A. (2025). Glycosylation Regulation by TMEM230 in Aging and Autoimmunity. International Journal of Molecular Sciences, 26(6), 2412. https://doi.org/10.3390/ijms26062412







