Neuroprotective Activity of Oligomeric Stilbenes from Alpha Grape Stems in In Vitro Models of Parkinson’s Disease
Abstract
1. Introduction
2. Results
2.1. Isolation and Structure Determination of Compounds
2.2. Antioxidant Activity of Stilbenes 1–8
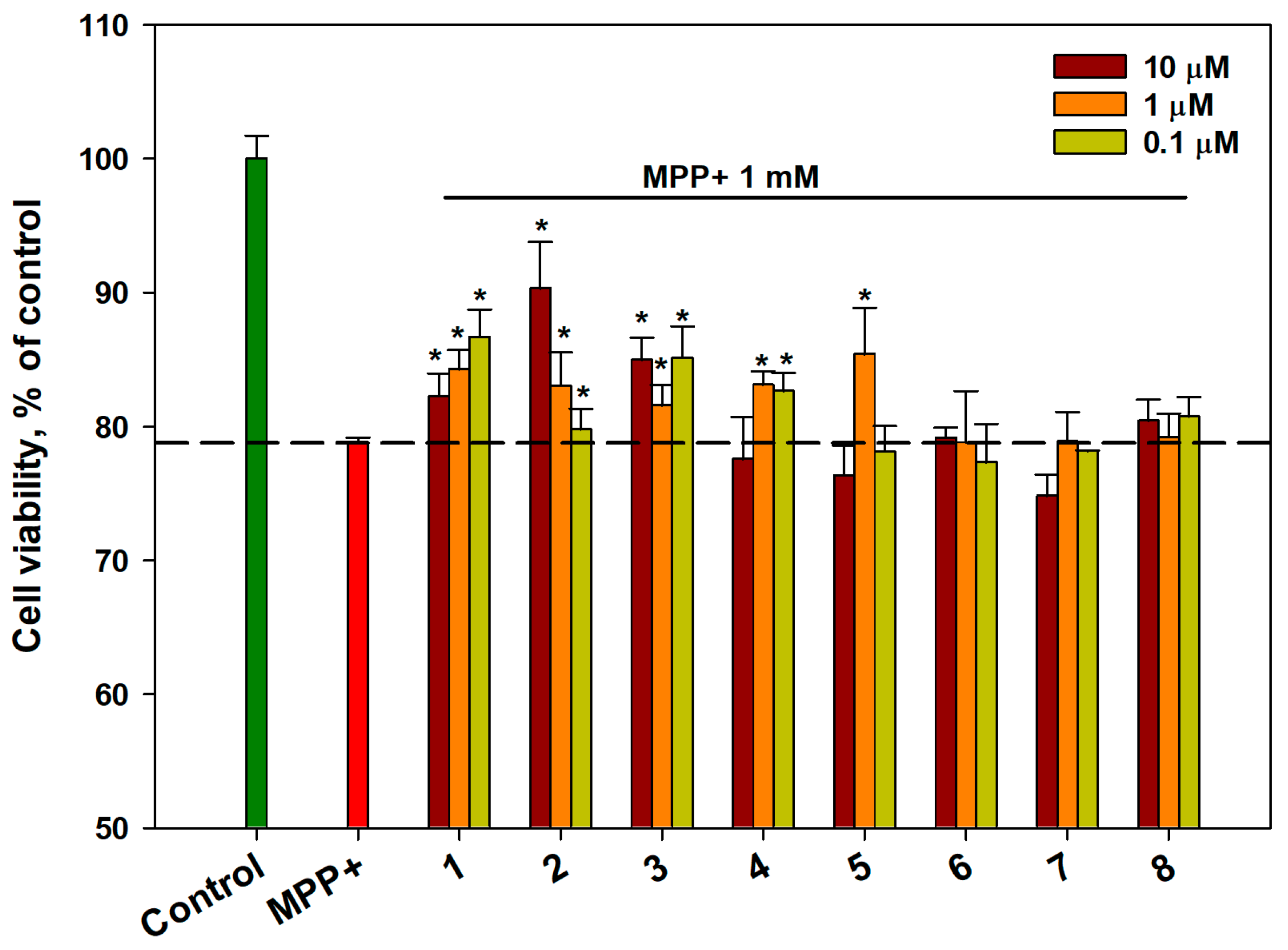
2.3. Effect of OSs on the Viability of MPP+-Treated Neuro-2a Cells
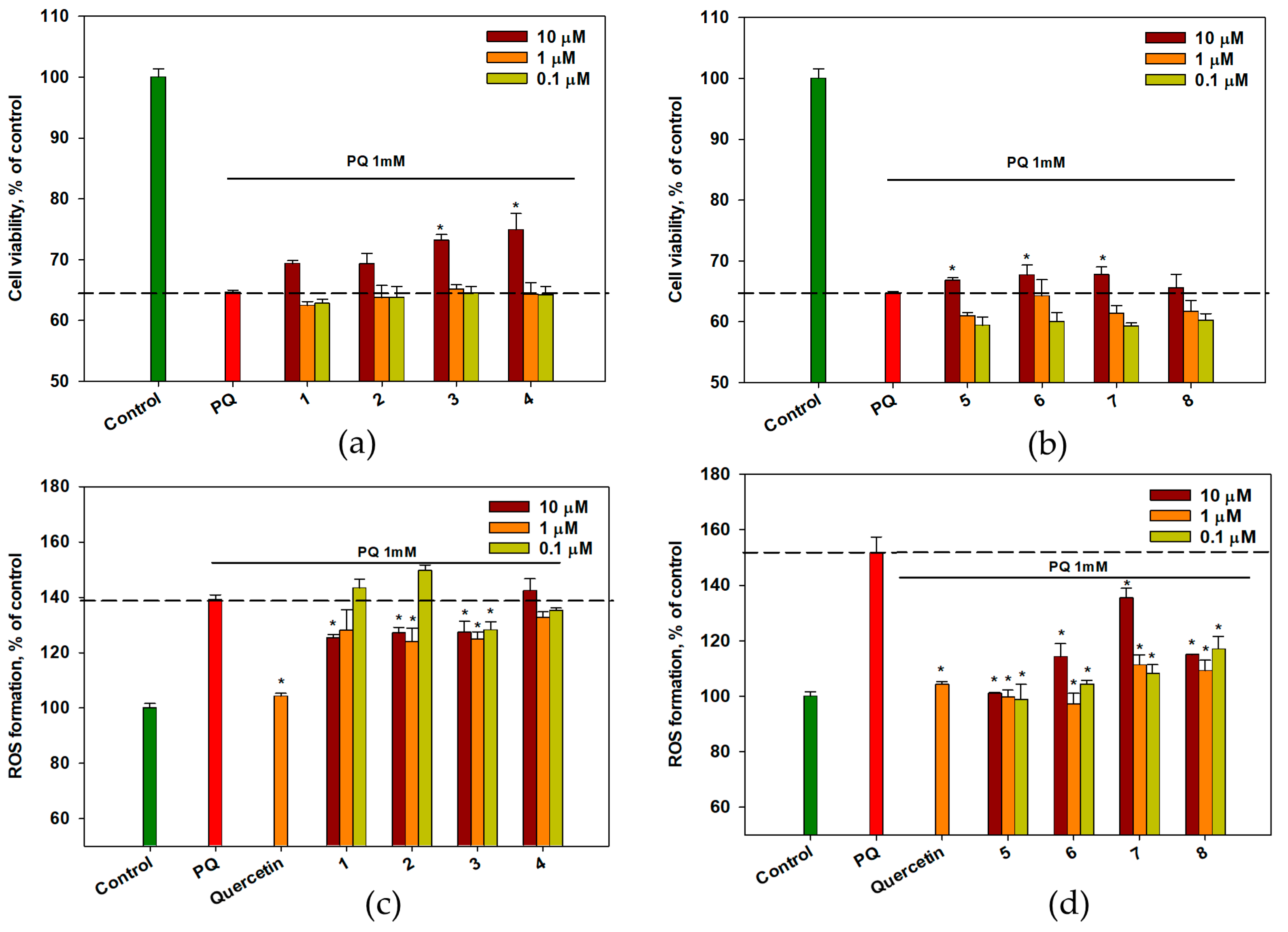
2.4. Effect of OSs on PQ-Treated Neuro-2a Cells
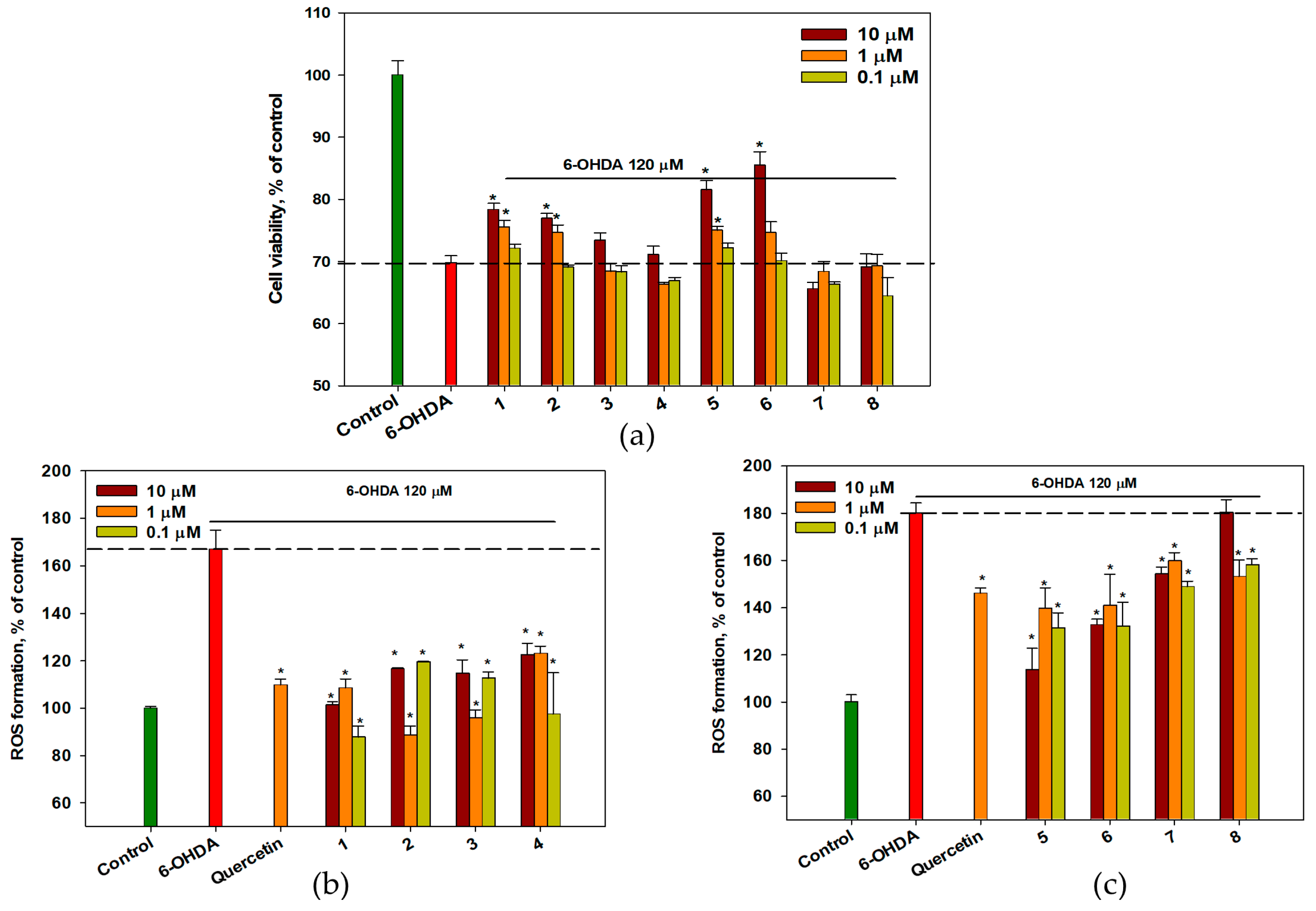
2.5. Effect of OSs on 6-OHDA-Treated Neuro-2a Cells
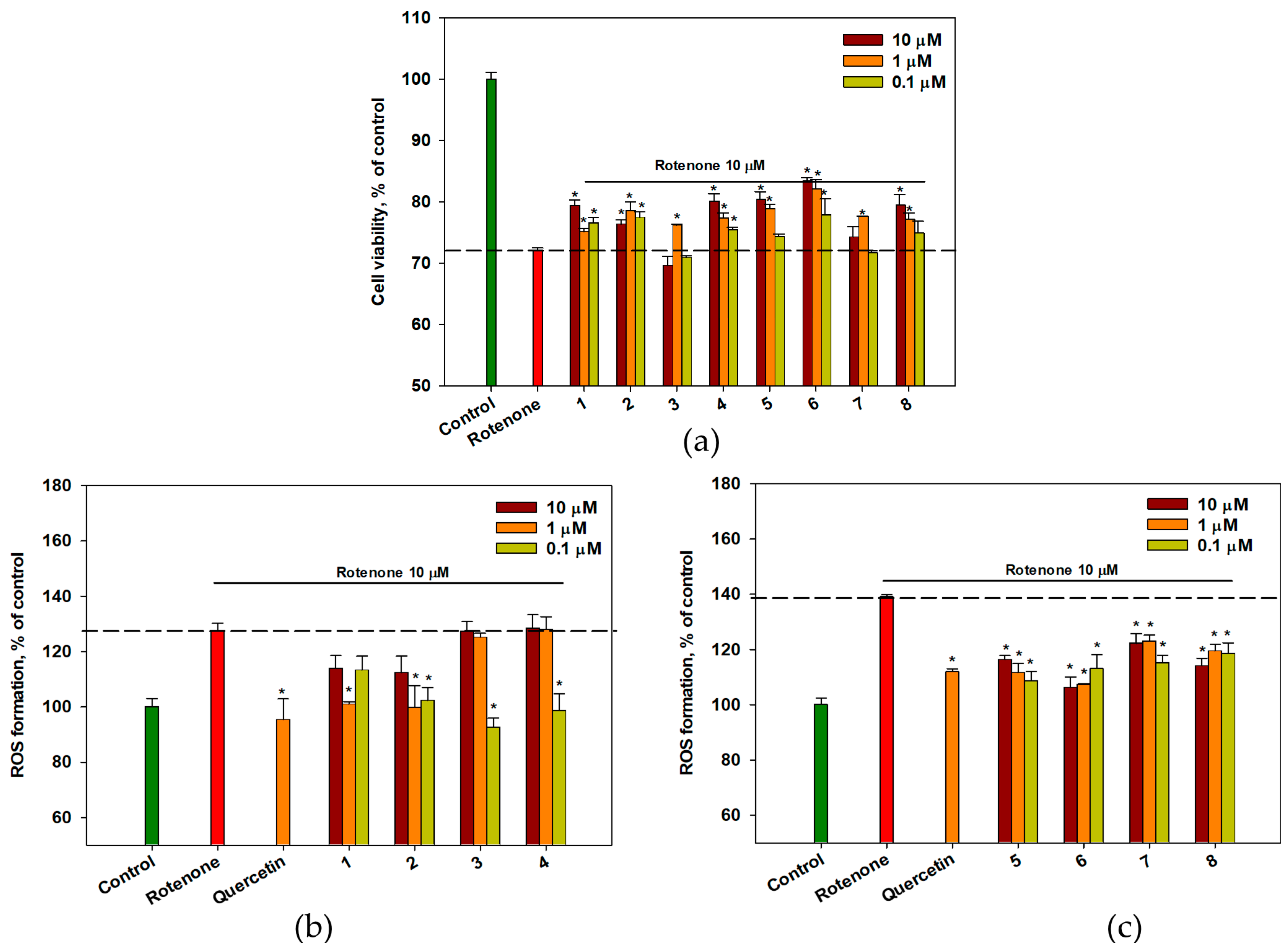
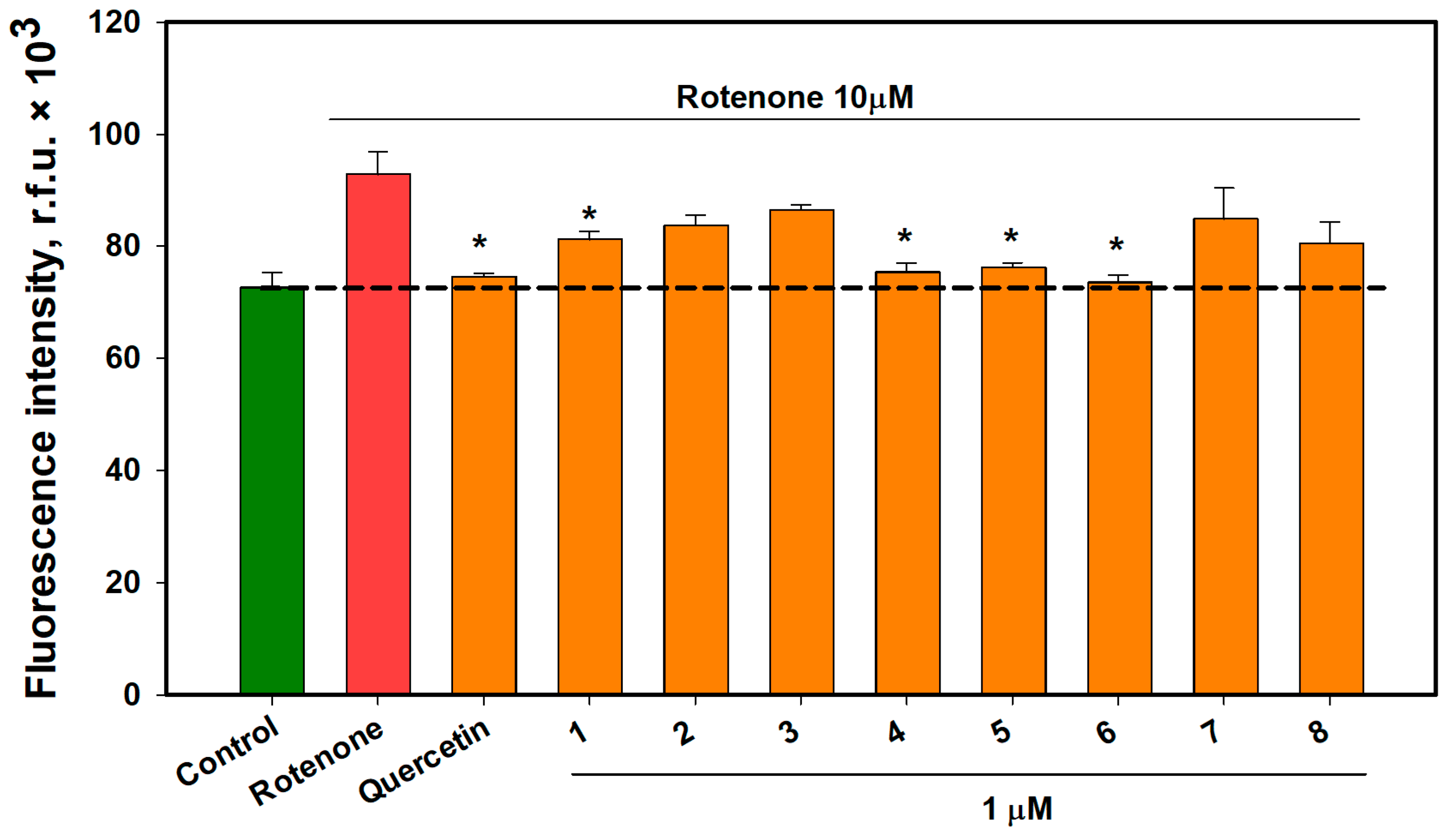
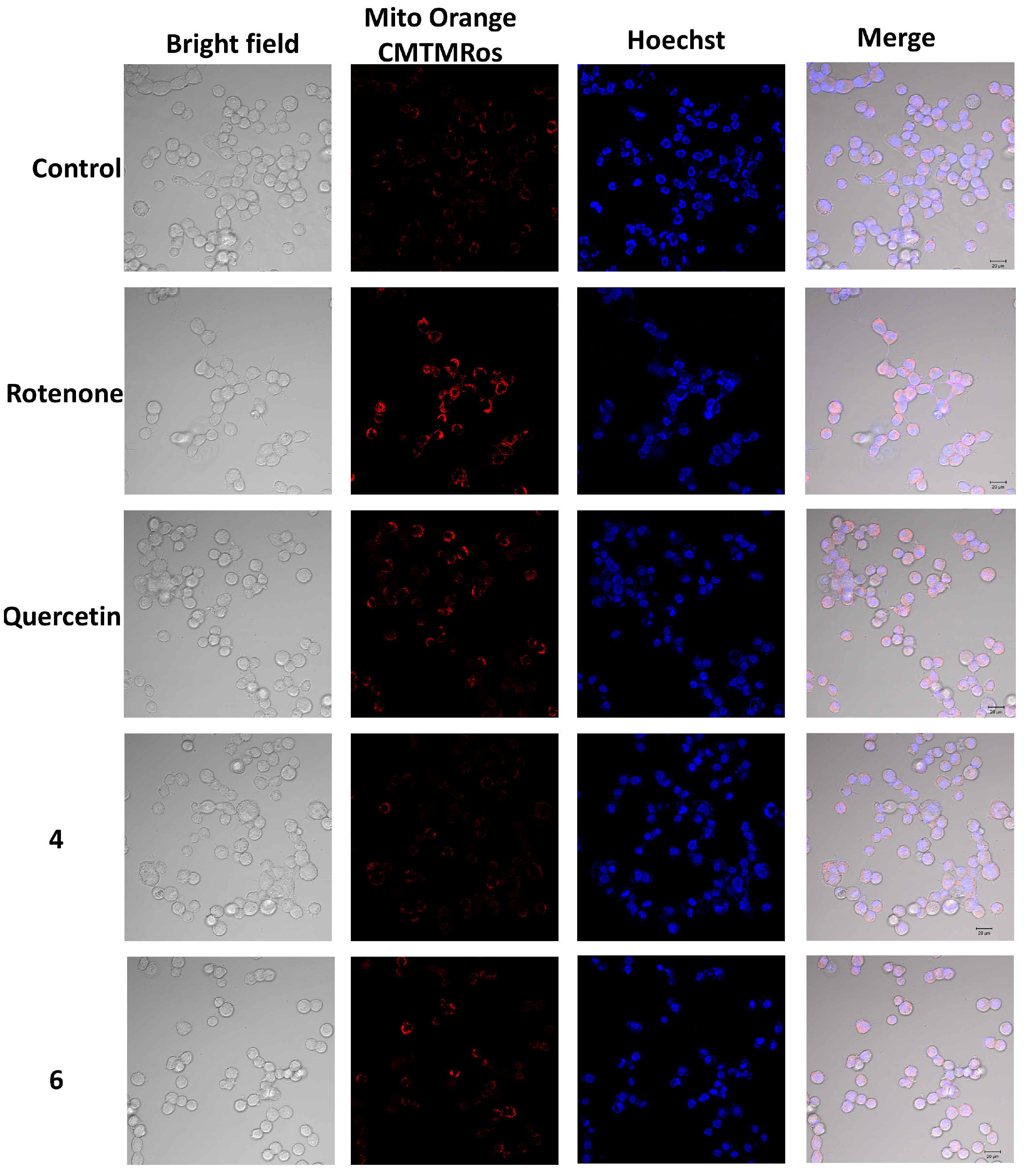
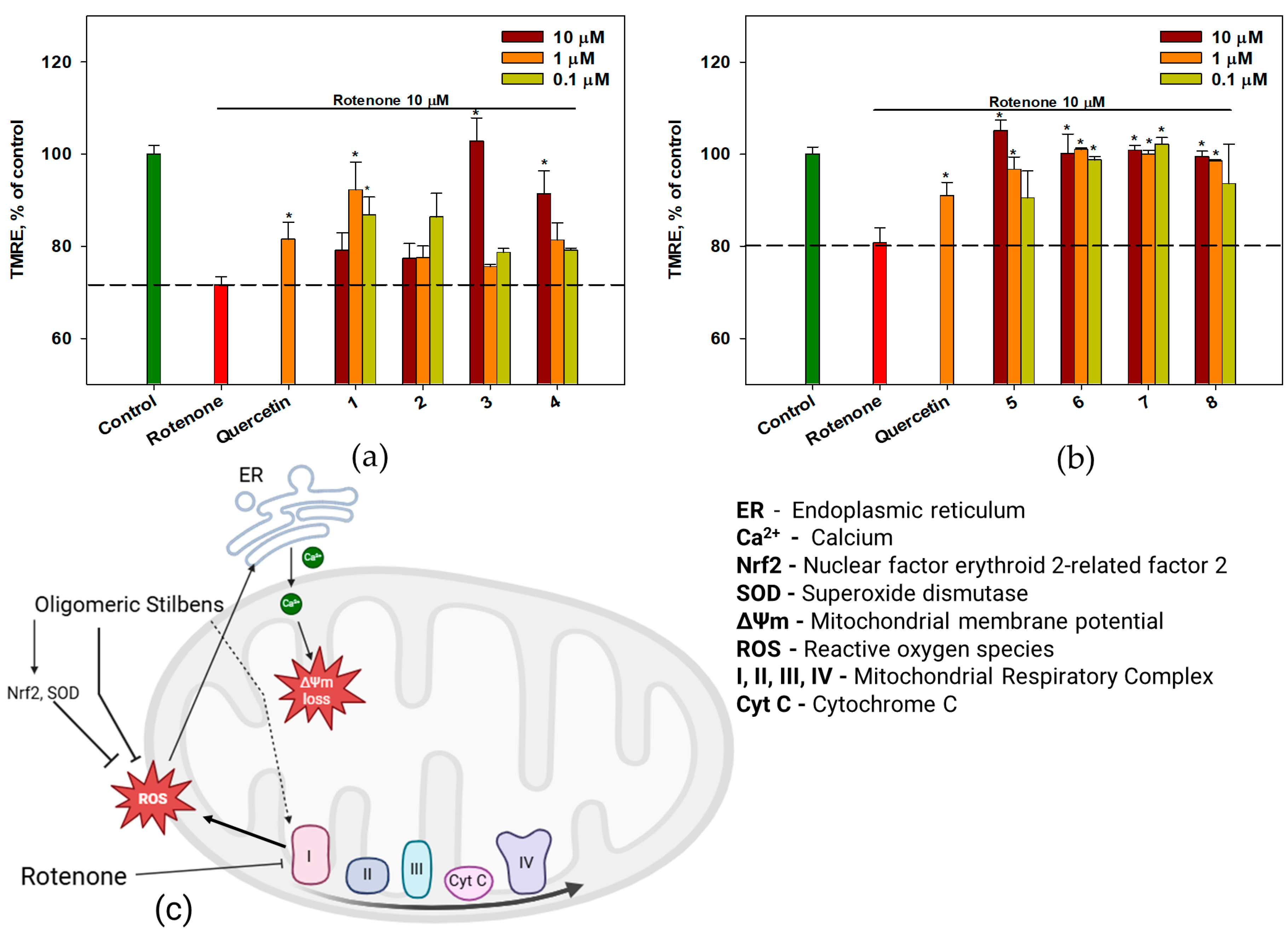
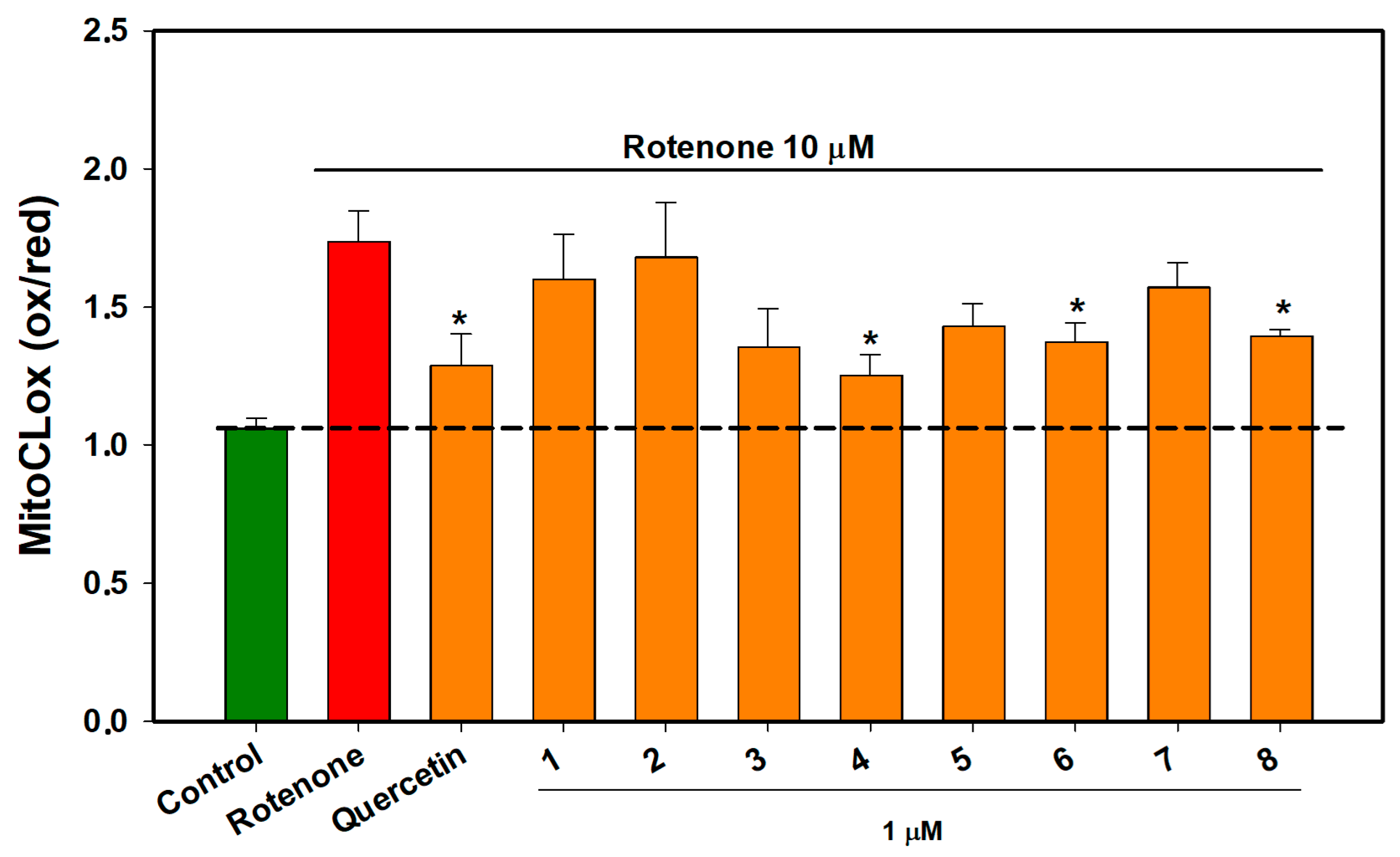
2.6. The Effect of OSs on Neuro-2a Cells Treated with Rotenone
2.7. Impact of OSs on Superoxide Dismutase (SOD) Activity in Neurotoxin-Treated Cells
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction and Isolation
4.3. General Experimental Procedures
4.4. Analytical HPLC-UV-MS
4.5. DPPH Radical Scavenging Assay
4.6. Ferric-Reducing Antioxidant Power Assay (FRAP)
4.7. Cell Line and Culture Conditions
4.8. In Vitro Model of Neurotoxicity Induced by PQ, Rotenone, MPP+, and 6-OHDA
4.9. Cell Viability MTT Assay
4.10. Assessment of Intracellular Reactive Oxygen Species (ROS) Levels
4.11. Mitochondrial ROS Level Assay
4.12. Determination of Mitochondrial Membrane Potential (MMP)
4.13. Cardiolipin Peroxidation Assay
4.14. Superoxide Dismutase Activity Detection
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.; Cui, Y.; Zhang, J.; Yan, R.; Su, D.; Zhao, D.; Wang, A.; Feng, T. Temporal trends in the prevalence of Parkinson’s disease from 1980 to 2023: A systematic review and meta-analysis. Lancet Healthy Longev. 2024, 5, e464–e479. [Google Scholar] [CrossRef] [PubMed]
- IHME. Parkinson’s Disease—Level 3 Cause. Available online: https://www.healthdata.org/research-analysis/diseases-injuries-risks/factsheets?title=Parkinson%27s%20disease%20-%20Level%203%20cause (accessed on 10 January 2025).
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Gutiérrez, M.T.; Serrano-García, N.; Orozco-Ibarra, M. Rotenone-Induced Model of Parkinson’s Disease: Beyond Mitochondrial Complex I Inhibition. Mol. Neurobiol. 2023, 60, 1929–1948. [Google Scholar] [CrossRef]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef]
- Pajares, M.; Rojo, A.I.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef]
- Pyatha, S.; Kim, H.; Lee, D.; Kim, K. Association between Heavy Metal Exposure and Parkinson’s Disease: A Review of the Mechanisms Related to Oxidative Stress. Antioxidants 2022, 11, 2467. [Google Scholar] [CrossRef]
- Morris, H.R.; Spillantini, M.G.; Sue, C.M.; Williams-Gray, C.H. The pathogenesis of Parkinson’s disease. Lancet 2024, 403, 293–304. [Google Scholar] [CrossRef]
- Prasad, E.M.; Hung, S.-Y. Current Therapies in Clinical Trials of Parkinson’s Disease: A 2021 Update. Pharmaceuticals 2021, 14, 717. [Google Scholar] [CrossRef]
- Deus, C.M.; Teixeira, J.; Raimundo, N.; Tucci, P.; Borges, F.; Saso, L.; Oliveira, P.J. Modulation of cellular redox environment as a novel therapeutic strategy for Parkinson’s disease. Eur. J. Clin. Investig. 2022, 52, e13820. [Google Scholar] [CrossRef] [PubMed]
- Talebi, S.; Ghoreishy, S.M.; Jayedi, A.; Travica, N.; Mohammadi, H. Dietary Antioxidants and Risk of Parkinson’s Disease: A Systematic Review and Dose–Response Meta-analysis of Observational Studies. Adv. Nutr. 2022, 13, 1493–1504. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Allelopathy of Knotweeds as Invasive Plants. Plants 2021, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.K.; Lee, W.B.; Ko, H.H. Trans-resveratrol modulates the catalytic activity and mRNA expression of the procarcinogen-activating human cytochrome P450 1B1. Can. J. Physiol. Pharmacol. 2000, 78, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Thu, Z.M.; Myo, K.K.; Aung, H.T.; Armijos, C.; Vidari, G. Flavonoids and Stilbenoids of the Genera Dracaena and Sansevieria: Structures and Bioactivities. Molecules 2020, 25, 2608. [Google Scholar] [CrossRef]
- Wen, H.; Fu, Z.; Wei, Y.; Zhang, X.; Ma, L.; Gu, L.; Li, J. Antioxidant Activity and Neuroprotective Activity of Stilbenoids in Rat Primary Cortex Neurons via the PI3K/Akt Signalling Pathway. Molecules 2018, 23, 2328. [Google Scholar] [CrossRef]
- Fan, Y.; He, X.; Chen, M.; Guo, S.; Dong, Z. Pterostilbene alleviates MPTP-induced neurotoxicity by targeting neuroinflammation and oxidative stress. Biochem. Biophys. Res. Commun. 2024, 729, 150358. [Google Scholar] [CrossRef]
- Lange, K.W.; Li, S. Resveratrol, pterostilbene, and dementia. Biofactors 2018, 44, 83–90. [Google Scholar] [CrossRef]
- Rivière, C.; Pawlus, A.D.; Mérillon, J.M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef]
- Pawlus, A.; Waffo-Téguo, P.; Shaver, J.; Mérillon, J.-M. Stilbenoid chemistry from wine and the genus Vitis, a review. J. Int. Sci. Vigne Vin 2012, 46, 57–111. [Google Scholar] [CrossRef]
- Fedoreyev, S.A.; Mishchenko, N.P.; Tarbeeva, D.V.; Vasileva, E.A.; Pokhilo, N.D.; Grigorchuk, V.P.; Emelyanov, A.N. Stilbenes from Stems of Primorsky Grape Variety Alpha and their Antioxidant Activity. Chem. Nat. Compd. 2024, 60, 697–700. [Google Scholar] [CrossRef]
- Guo, X.L.; Gao, Y.Y.; Yang, Y.X.; Zhu, Q.F.; Guan, H.Y.; He, X.; Zhang, C.L.; Wang, Y.; Xu, G.B.; Zou, S.H.; et al. Amelioration effects of α-viniferin on hyperuricemia and hyperuricemia-induced kidney injury in mice. Phytomedicine 2023, 116, 154868. [Google Scholar] [CrossRef]
- Oshima, Y.; Kamijou, A.; Ohizumi, Y.; Niwa, M.; Ito, J.; Hisamichi, K.; Takeshita, M. Novel oligostilbenes from Vitis coignetiae1. Tetrahedron 1995, 51, 11979–11986. [Google Scholar] [CrossRef]
- Matsuda, H.; Asao, Y.; Nakamura, S.; Hamao, M.; Sugimoto, S.; Hongo, M.; Pongpiriyadacha, Y.; Yoshikawa, M. Antidiabetogenic constituents from the Thai traditional medicine Cotylelobium melanoxylon. Chem. Pharm. Bull. 2009, 57, 487–494. [Google Scholar] [CrossRef]
- Samuel Kumi, O.; Chaorong, Z.; Dong, Y.; Yanchun, H. Neurotoxic Mechanism and Shortcomings of MPTP, 6-OHDA, Rotenone and Paraquat-induced Parkinson’s Disease Animal Models. Venoms Toxins 2021, 1, 27–40. [Google Scholar] [CrossRef]
- Powers, R.; Lei, S.; Anandhan, A.; Marshall, D.; Worley, B.; Cerny, R.; Dodds, E.; Huang, Y.; Panayiotidis, M.; Pappa, A.; et al. Metabolic Investigations of the Molecular Mechanisms Associated with Parkinson’s Disease. Metabolites 2017, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Jagmag, S.A.; Tripathi, N.; Shukla, S.D.; Maiti, S.; Khurana, S. Evaluation of Models of Parkinson’s Disease. Front. Neurosci. 2016, 9, 503. [Google Scholar] [CrossRef]
- Lim, K.G.; Gray, A.I.; Pyne, S.; Pyne, N.J. Resveratrol dimers are novel sphingosine kinase 1 inhibitors and affect sphingosine kinase 1 expression and cancer cell growth and survival. Br. J. Pharmacol. 2012, 166, 1605–1616. [Google Scholar] [CrossRef]
- Huang, C.; Huang, Y.L.; Wang, C.C.; Pan, Y.L.; Lai, Y.H.; Huang, H.C. Ampelopsins A and C Induce Apoptosis and Metastasis through Downregulating AxL, TYRO3, and FYN Expressions in MDA-MB-231 Breast Cancer Cells. J. Agric. Food Chem. 2019, 67, 2818–2830. [Google Scholar] [CrossRef]
- Schnee, S.; Queiroz, E.F.; Voinesco, F.; Marcourt, L.; Dubuis, P.H.; Wolfender, J.L.; Gindro, K. Vitis vinifera canes, a new source of antifungal compounds against Plasmopara viticola, Erysiphe necator, and Botrytis cinerea. J. Agric. Food Chem. 2013, 61, 5459–5467. [Google Scholar] [CrossRef]
- Hong, Y.; Choi, Y.H.; Han, Y.E.; Oh, S.J.; Lee, A.; Lee, B.; Magnan, R.; Ryu, S.Y.; Choi, C.W.; Kim, M.S. Central Administration of Ampelopsin A Isolated from Vitis vinifera Ameliorates Cognitive and Memory Function in a Scopolamine-Induced Dementia Model. Antioxidants 2021, 10, 835. [Google Scholar] [CrossRef] [PubMed]
- Temsamani, H.; Krisa, S.; Decossas-Mendoza, M.; Lambert, O.; Mérillon, J.M.; Richard, T. Piceatannol and Other Wine Stilbenes: A Pool of Inhibitors against α-Synuclein Aggregation and Cytotoxicity. Nutrients 2016, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.T.; Chen, L.G.; Tseng, S.H.; Huang, J.S.; Hsieh, M.S.; Wang, C.C. Anti-inflammatory effects of resveratrol and oligostilbenes from Vitis thunbergii var. taiwaniana against lipopolysaccharide-induced arthritis. J. Agric. Food Chem. 2011, 59, 3649–3656. [Google Scholar] [CrossRef]
- Liao, H.R.; Lin, C.H.; Chen, J.J.; Liu, F.C.; Tseng, C.P. The anti-inflammatory effect of ε-viniferin by specifically targeting formyl peptide receptor 1 on human neutrophils. Chem. Biol. Interact. 2021, 345, 109490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ma, Y.; Feng, J. Neuroprotective mechanisms of ε-viniferin in a rotenone-induced cell model of Parkinson’s disease: Significance of SIRT3-mediated FOXO3 deacetylation. Neural Regen. Res. 2020, 15, 2143–2153. [Google Scholar] [CrossRef]
- Lv, H.; Zhou, W.; Wang, X.; Wang, Z.; Suo, Y.; Wang, H. Extraction and Separation of Vitisin D, Ampelopsin B and cis-Vitisin A from Iris lactea Pall. var. chinensis (Fisch.) Koidz by Alkaline Extraction-Acid Precipitation and High-Speed Counter-Current Chromatography. J. Chromatogr. Sci. 2016, 54, 744–751. [Google Scholar] [CrossRef]
- Tie, F.F.; Luan, G.X.; Zhou, W.N.; Wang, Z.H.; Shi, X.B.; Li, G.; Wang, H.L. Effects of the oligostilbenes from Iris lactea Pall. var. chinensis (Fisch.) Koidz on the adipocytes differentiation of 3T3-L1 cells. Pharmazie 2018, 73, 98–103. [Google Scholar] [CrossRef]
- Chen, L.G.; Wang, C.C.; Lee, Y.S.; Sie, Y.Y.; Chang, C.I.; Hou, W.C. Vitisin A, a Resveratrol Tetramer, Improves Scopolamine-Induced Impaired Learning and Memory Functions in Amnesiac ICR Mice. Biomedicines 2022, 10, 273. [Google Scholar] [CrossRef]
- Huang, Y.L.; Tsai, W.J.; Shen, C.C.; Chen, C.C. Resveratrol derivatives from the roots of Vitis thunbergii. J. Nat. Prod. 2005, 68, 217–220. [Google Scholar] [CrossRef]
- Mi Jeong, S.; Davaatseren, M.; Kim, W.; Sung Kwang, P.; Kim, S.H.; Haeng Jeon, H.; Myung Sunny, K.; Kim, Y.S.; Dae Young, K. Vitisin A suppresses LPS-induced NO production by inhibiting ERK, p38, and NF-kappaB activation in RAW 264.7 cells. Int. Immunopharmacol. 2009, 9, 319–323. [Google Scholar] [CrossRef]
- Jang, M.H.; Piao, X.L.; Kim, H.Y.; Cho, E.J.; Baek, S.H.; Kwon, S.W.; Park, J.H. Resveratrol oligomers from Vitis amurensis attenuate beta-amyloid-induced oxidative stress in PC12 cells. Biol. Pharm. Bull. 2007, 30, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Cho, E.J.; Piao, X.L. Protective effects of resveratrol oligomers from Vitis amurensis against sodium nitroprusside-induced neurotoxicity in human neuroblastoma SH-SY5Y cells. Arch. Pharm. Res. 2015, 38, 1263–1269. [Google Scholar] [CrossRef]
- Fan, Y.; Zhao, L.; Huang, X.; Jia, Q.; Wang, W.; Gao, M.; Jia, X.; Chang, Y.; Ouyang, H.; He, J. Pharmacokinetic and bioavailability studies of α-viniferin after intravenous and oral administration to rats. J. Pharm. Biomed. Anal. 2020, 188, 113376. [Google Scholar] [CrossRef]
- Dilshara, M.G.; Lee, K.T.; Kim, H.J.; Lee, H.J.; Choi, Y.H.; Lee, C.M.; Kim, L.K.; Kim, G.Y. Anti-inflammatory mechanism of α-viniferin regulates lipopolysaccharide-induced release of proinflammatory mediators in BV2 microglial cells. Cell. Immunol. 2014, 290, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, C.; Barman, R.; Darshani, P.; Pillai, M.; Ahuja, S.; Mondal, R.; Pragadheesh, V.S.; Chakraborty, J.; Kumar, D. α-Viniferin, a dietary phytochemical, inhibits Monoamine oxidase and alleviates Parkinson’s disease associated behavioral deficits in a mice model. Neurochem. Int. 2024, 174, 105698. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Ewald, P.; Yasui, Y.; Yokokawa, H.; Wagner, A.E.; Matsugo, S.; Winterhalter, P.; Rimbach, G. Chemical Characterization, Free Radical Scavenging, and Cellular Antioxidant and Anti-Inflammatory Properties of a Stilbenoid-Rich Root Extract of Vitis vinifera. Oxidative Med. Cell. Longev. 2016, 2016, 8591286. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, K.D.; Lee, M.; Cho, Y.; Choi, G.; Jang, H.; Kim, B.; Jung, D.-H.; Oh, J.-G.; Kim, G.-W.; et al. Identification of a resveratrol tetramer as a potent inhibitor of hepatitis C virus helicase. Br. J. Pharmacol. 2016, 173, 191–211. [Google Scholar] [CrossRef]
- Kwon, E.-B.; Li, W.; Kim, Y.S.; Kim, B.; Chung, H.-S.; Go, Y.; Ko, H.-J.; Song, J.-H.; Kim, Y.H.; Choi, C.W.; et al. Vitisin B inhibits influenza A virus replication by multi-targeting neuraminidase and virus-induced oxidative stress. Acta Pharm. Sin. B 2023, 13, 174–191. [Google Scholar] [CrossRef]
- Ihara, Y.; Chuda, M.; Kuroda, S.; Hayabara, T. Hydroxyl radical and superoxide dismutase in blood of patients with Parkinson’s disease: Relationship to clinical data. J. Neurol. Sci. 1999, 170, 90–95. [Google Scholar] [CrossRef]
- Liu, C.; Fang, J.; Liu, W. Superoxide dismutase coding of gene polymorphisms associated with susceptibility to Parkinson’s disease. J. Integr. Neurosci. 2019, 18, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, M.; Newman, J.R.; Shan, J.; Bentley, S.; Wood, S.A.; Silburn, P.A.; Mellick, G.D. Comprehensive assessment of genetic sequence variants in the antioxidant ’master regulator’ NRF2 in idiopathic Parkinson’s disease. PLoS ONE 2015, 10, e0128030. [Google Scholar] [CrossRef] [PubMed]
- Gueguen, N.; Desquiret-Dumas, V.; Leman, G.; Chupin, S.; Baron, S.; Nivet-Antoine, V.; Vessières, E.; Ayer, A.; Henrion, D.; Lenaers, G.; et al. Resveratrol Directly Binds to Mitochondrial Complex I and Increases Oxidative Stress in Brain Mitochondria of Aged Mice. PLoS ONE 2015, 10, e0144290. [Google Scholar] [CrossRef]
- Utkina, N.K.; Kulesh, N.I. Antioxidant activity of polyphenols and polyphenol complex from the far-eastern tree Maackia amurensis. Pharm. Chem. J. 2012, 46, 488–491. [Google Scholar] [CrossRef]
- Dehghan, G.; Khoshkam, Z. Tin(II)–quercetin complex: Synthesis, spectral characterisation and antioxidant activity. Food Chem. 2012, 131, 422–426. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Lyamzaev, K.G.; Panteleeva, A.A.; Karpukhina, A.A.; Galkin, I.I.; Popova, E.N.; Pletjushkina, O.Y.; Rieger, B.; Busch, K.B.; Mulkidjanian, A.Y.; Chernyak, B.V. Novel Fluorescent Mitochondria-Targeted Probe MitoCLox Reports Lipid Peroxidation in Response to Oxidative Stress In Vivo. Oxidative Med. Cell Longev. 2020, 2020, 3631272. [Google Scholar] [CrossRef]
- Lyamzaev, K.G.; Sumbatyan, N.V.; Nesterenko, A.M.; Kholina, E.G.; Voskoboynikova, N.; Steinhoff, H.J.; Mulkidjanian, A.Y.; Chernyak, B.V. MitoCLox: A Novel Mitochondria-Targeted Fluorescent Probe for Tracing Lipid Peroxidation. Oxidative Med. Cell Longev. 2019, 2019, 9710208. [Google Scholar] [CrossRef]
| Comp | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Quercetin |
|---|---|---|---|---|---|---|---|---|---|
| DPPH, IC50 µM | 717.5 ± 26.6 * | 63.0 ± 1.1 * | 62.7 ± 0.63 * | 59.1 ± 2.5 * | 409.0 ± 41.4 * | 314.0 ± 33.2 * | 333.8± 36.6 * | 528.9 ± 56.5 * | 9.5 ± 0.5 |
| FRAP, µM | 0.56 ± 0.06 * | 1.48 ± 0.13 * | 0.76 ± 0.08 * | 0.71 ± 0.07 * | 0.66 ± 0.07 * | 0.34 ± 0.03 * | 0.99 ± 0.12 * | 1.10 ± 0.14 * | 3.30 ± 0.23 |
| Compound | 6-OHDA | Rotenone | ||
|---|---|---|---|---|
| 1 h | 3 h | 1 h | 3 h | |
| control | 9.43 ± 0.78 | 7.29 ± 1.15 | 8.75 ± 2.27 | 7.74 ± 2.38 |
| neurotoxin | 8.69 ± 0.48 | 0.92 ± 0.28 # | 8.69 ± 1.93 | 2.10 ± 0.34 # |
| 1 | 10.47 ± 2.40 | 7.24 ± 2.44 * | 6.73 ± 4.25 | 8.68 ± 2.33 * |
| 2 | 8.88 ± 0.46 | 4.93 ± 1.13 * | 6.06 ± 3.73 | 5.92 ± 2.35 |
| 3 | 7.24 ± 5.58 | 6.80 ± 4.24 | 4.63 ± 1.15 | 8.16 ± 1.09 * |
| 4 | 12.84 ± 2.72 * | 5.51 ± 1.37 * | 4.10 ± 1.34 | 6.6 ± 2.65 * |
| 5 | 3.51 ± 0.80 | 4.59 ± 0.23 * | 8.64 ± 0.87 | 5.50 ± 1.28 |
| 6 | 14.21 ± 5.03 * | 2.57 ± 1.49 | 19.94 ± 1.33 * | 3.09 ± 0.79 |
| 7 | 5.42 ± 0.45 | 7.24 ± 4.44 * | 6.73 ± 4.25 | 8.68 ± 2.33 * |
| 8 | 5.27 ± 5.51 | 2.06 ± 2.16 | 7.66 ± 1.41 | 6.47 ± 1.59 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pislyagin, E.A.; Tarbeeva, D.V.; Yurchenko, E.A.; Menchinskaya, E.S.; Gorpenchenko, T.Y.; Pokhilo, N.D.; Kalinovskiy, A.I.; Aminin, D.L.; Fedoreyev, S.A. Neuroprotective Activity of Oligomeric Stilbenes from Alpha Grape Stems in In Vitro Models of Parkinson’s Disease. Int. J. Mol. Sci. 2025, 26, 2411. https://doi.org/10.3390/ijms26062411
Pislyagin EA, Tarbeeva DV, Yurchenko EA, Menchinskaya ES, Gorpenchenko TY, Pokhilo ND, Kalinovskiy AI, Aminin DL, Fedoreyev SA. Neuroprotective Activity of Oligomeric Stilbenes from Alpha Grape Stems in In Vitro Models of Parkinson’s Disease. International Journal of Molecular Sciences. 2025; 26(6):2411. https://doi.org/10.3390/ijms26062411
Chicago/Turabian StylePislyagin, Evgeny A., Darya V. Tarbeeva, Ekaterina A. Yurchenko, Ekaterina S. Menchinskaya, Tatiana Y. Gorpenchenko, Natalya D. Pokhilo, Anatoly I. Kalinovskiy, Dmitry L. Aminin, and Sergey A. Fedoreyev. 2025. "Neuroprotective Activity of Oligomeric Stilbenes from Alpha Grape Stems in In Vitro Models of Parkinson’s Disease" International Journal of Molecular Sciences 26, no. 6: 2411. https://doi.org/10.3390/ijms26062411
APA StylePislyagin, E. A., Tarbeeva, D. V., Yurchenko, E. A., Menchinskaya, E. S., Gorpenchenko, T. Y., Pokhilo, N. D., Kalinovskiy, A. I., Aminin, D. L., & Fedoreyev, S. A. (2025). Neuroprotective Activity of Oligomeric Stilbenes from Alpha Grape Stems in In Vitro Models of Parkinson’s Disease. International Journal of Molecular Sciences, 26(6), 2411. https://doi.org/10.3390/ijms26062411







