Temporal mRNA Expression of Purinergic P2 Receptors in the Brain Following Cerebral Ischemia and Reperfusion: Similarities and Distinct Variations Between Rats and Mice
Abstract
1. Introduction
2. Results
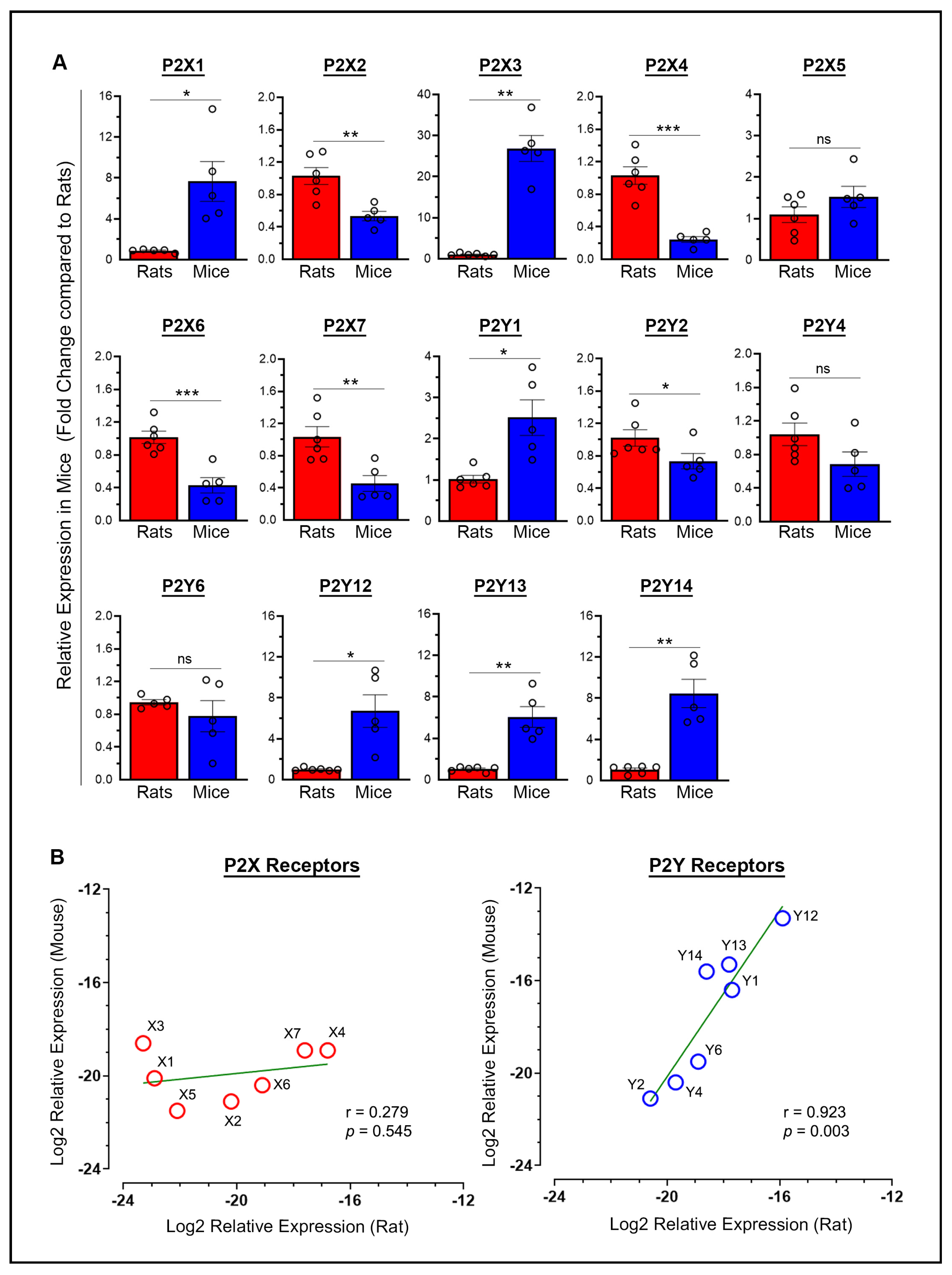
2.1. Baseline P2X and P2Y Receptor Expression: Variability and Correlation Between Species
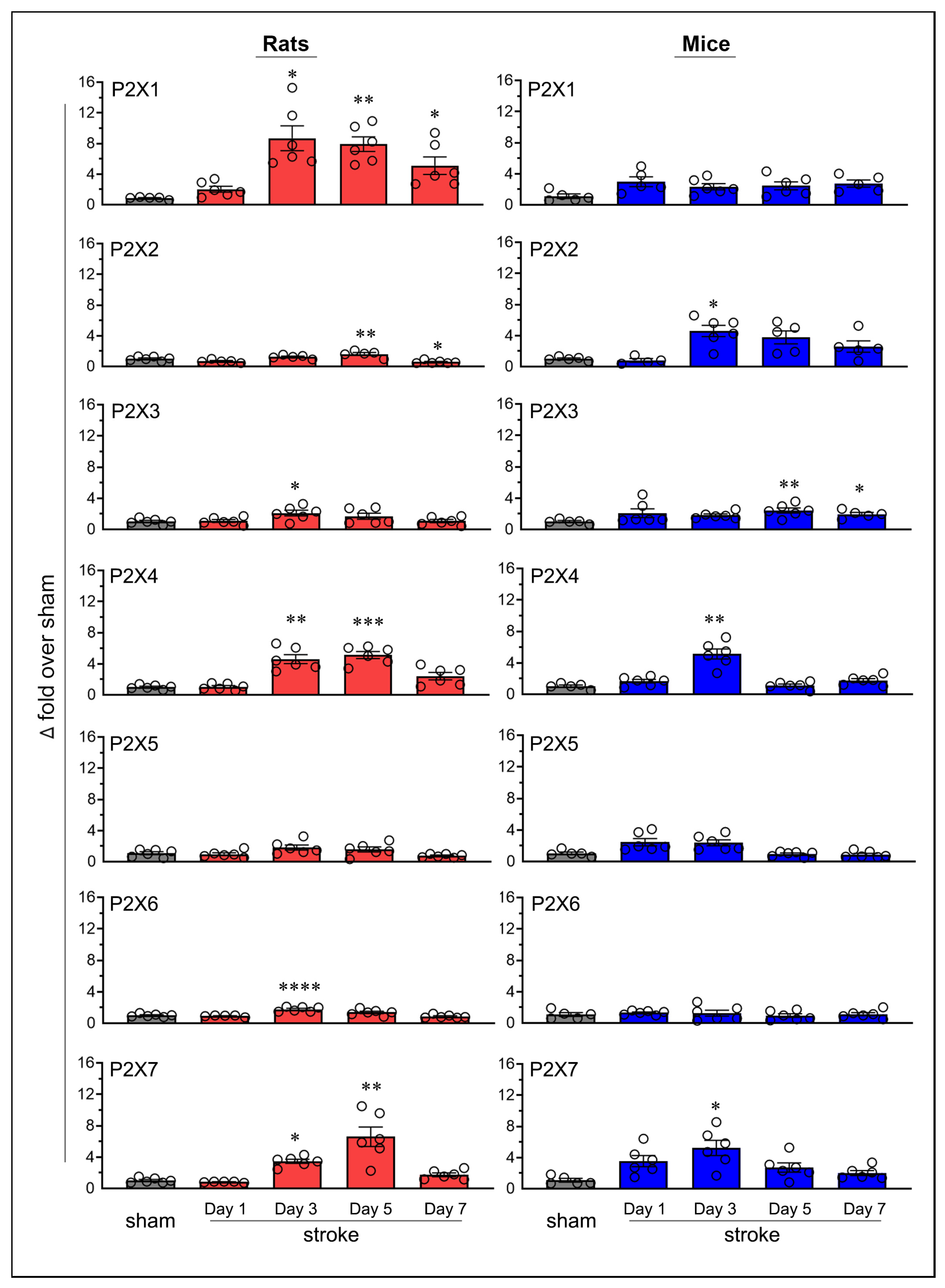
2.2. Similarities and Variations in Purinergic P2X Receptor Expression Following Stroke
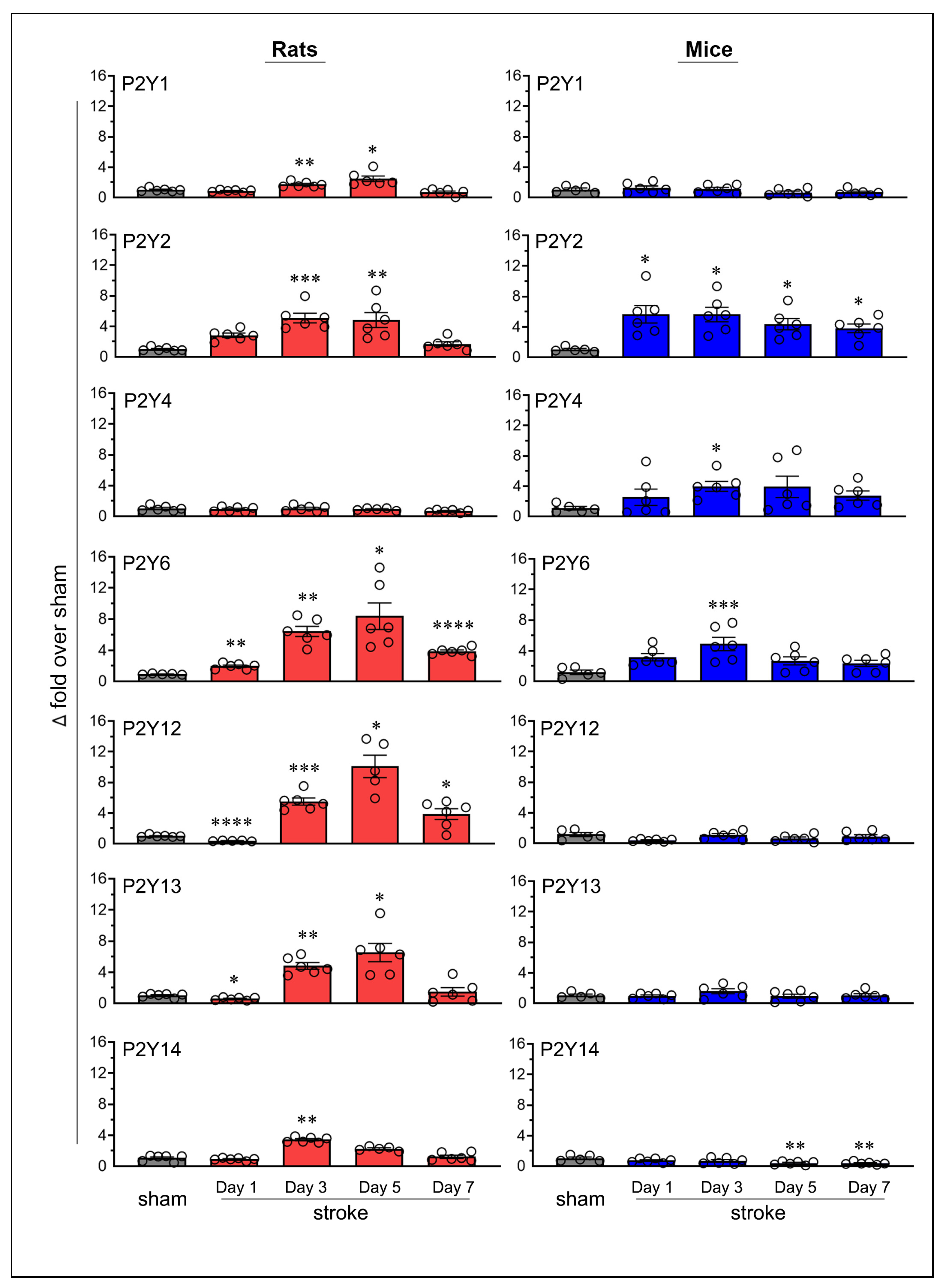
2.3. Similarities and Variations in Purinergic P2Y Receptor Expression Following Stroke
3. Discussion
4. Materials and Methods
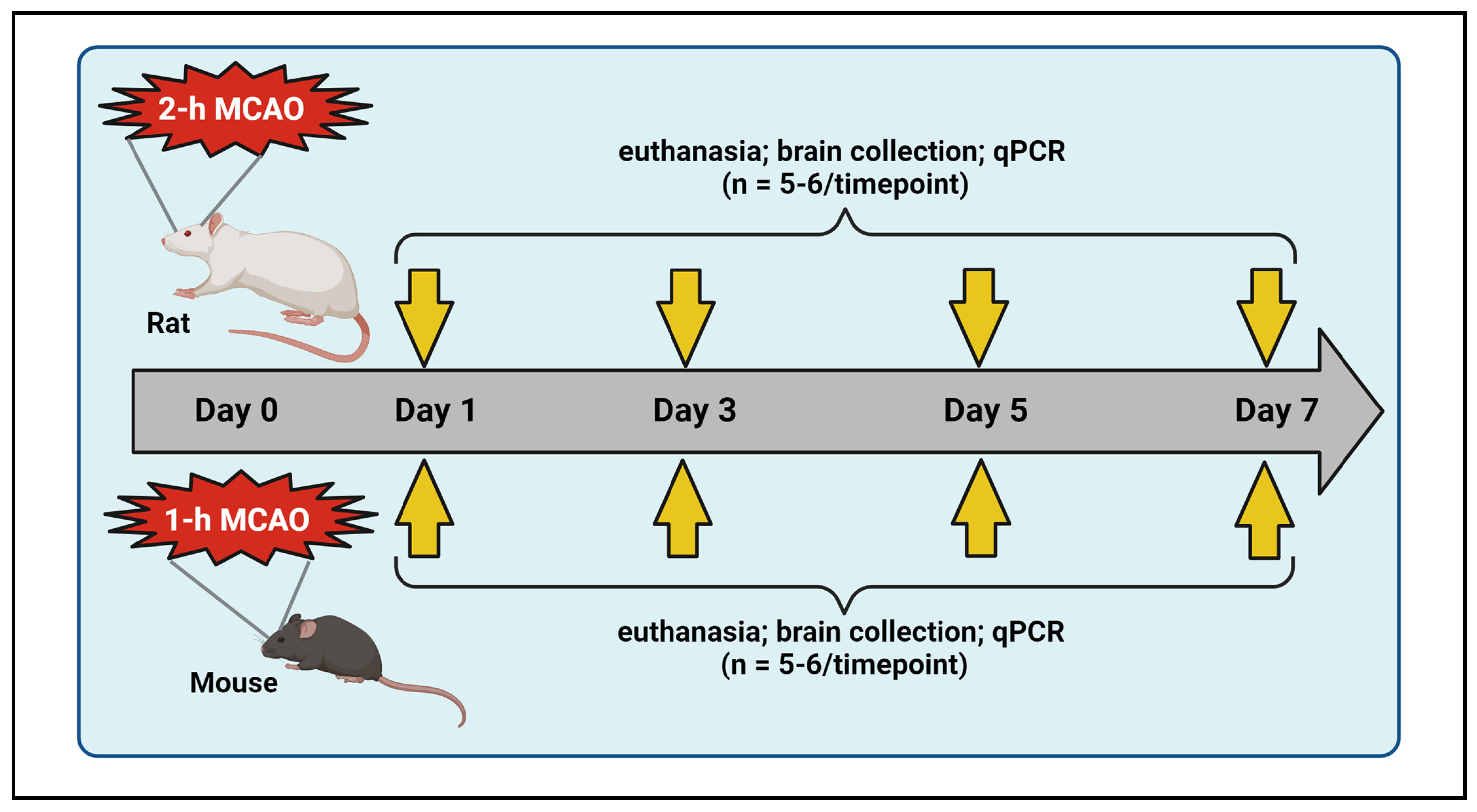
4.1. Middle Cerebral Artery Occlusion (MCAO) and Reperfusion in Rats and Mice
4.2. Modified Neurological Severity Score (mNSS) Assessment in Rats
4.3. Neurological Deficit Score (NDS) Assessment in Mice
4.4. Exclusion Criteria
4.5. RNA Isolation and cDNA Synthesis
4.6. Quantitative Real-Time PCR Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burnstock, G. Purine and Purinergic Receptors. Brain Neurosci. Adv. 2018, 2, 2398212818817494. [Google Scholar] [CrossRef]
- Burnstock, G. Introduction to Purinergic Signaling. Methods Mol. Biol. 2020, 2041, 1–15. [Google Scholar] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [PubMed]
- Cheng, R.; Ren, J.; Zhang, Y.; Ye, X. P2X4 Receptors Expressed on Microglial Cells in Post-Ischemic Inflammation of Brain Ischemic Injury. Neurochem. Int. 2014, 67, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zou, Y.; Zhong, X.Z.; Cao, Q.; Zhao, K.; Zhu, M.X.; Murrell-Lagnado, R.; Dong, X. P2X4 Forms Functional ATP-Activated Cation Channels on Lysosomal Membranes Regulated by Luminal pH. J. Biol. Chem. 2014, 289, 17658–17667. [Google Scholar] [CrossRef]
- Rivera, A.; Vanzulli, I.; Butt, A.M. A Central Role for ATP Signalling in Glial Interactions in the CNS. Curr. Drug Targets 2016, 17, 1829–1833. [Google Scholar] [CrossRef]
- Verma, R.; Cronin, C.G.; Hudobenko, J.; Venna, V.R.; McCullough, L.D.; Liang, B.T. Deletion of the P2X4 Receptor is Neuroprotective Acutely, but Induces a Depressive Phenotype during Recovery from Ischemic Stroke. Brain Behav. Immun. 2017, 66, 302–312. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Vuerich, M. Purinergic Signaling in the Immune System. Auton. Neurosci. 2015, 191, 117–123. [Google Scholar] [CrossRef]
- Cekic, C.; Linden, J. Purinergic Regulation of the Immune System. Nat. Rev. Immunol. 2016, 16, 177–192. [Google Scholar] [CrossRef]
- Zarrinmayeh, H.; Territo, P.R. Purinergic Receptors of the Central Nervous System: Biology, PET Ligands, and their Applications. Mol. Imaging 2020, 19, 1536012120927609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, D.; Lin, S.; Fang, X.; Ye, Z. Contribution of P2X Purinergic Receptor in Cerebral Ischemia Injury. Brain Res. Bull. 2022, 190, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Crain, J.M.; Nikodemova, M.; Watters, J.J. Expression of P2 Nucleotide Receptors Varies with Age and Sex in Murine Brain Microglia. J. Neuroinflamm. 2009, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Alkayed, N.J.; Harukuni, I.; Kimes, A.S.; London, E.D.; Traystman, R.J.; Hurn, P.D. Gender-Linked Brain Injury in Experimental Stroke. Stroke 1998, 29, 159–165; discussion 166. [Google Scholar] [CrossRef]
- Suzuki, S.; Gerhold, L.M.; Bottner, M.; Rau, S.W.; Dela Cruz, C.; Yang, E.; Zhu, H.; Yu, J.; Cashion, A.B.; Kindy, M.S.; et al. Estradiol Enhances Neurogenesis Following Ischemic Stroke through Estrogen Receptors Alpha and Beta. J. Comp. Neurol. 2007, 500, 1064–1075. [Google Scholar] [CrossRef]
- Toung, T.J.; Traystman, R.J.; Hurn, P.D. Estrogen-Mediated Neuroprotection After Experimental Stroke in Male Rats. Stroke 1998, 29, 1666–1670. [Google Scholar] [CrossRef]
- Toung, T.K.; Hurn, P.D.; Traystman, R.J.; Sieber, F.E. Estrogen Decreases Infarct Size After Temporary Focal Ischemia in a Genetic Model of Type 1 Diabetes Mellitus. Stroke 2000, 31, 2701–2706. [Google Scholar] [CrossRef]
- Santizo, R.A.; Xu, H.L.; Ye, S.; Baughman, V.L.; Pelligrino, D.A. Loss of Benefit from Estrogen Replacement Therapy in Diabetic Ovariectomized Female Rats Subjected to Transient Forebrain Ischemia. Brain Res. 2002, 956, 86–95. [Google Scholar] [CrossRef]
- Schadlich, I.S.; Winzer, R.; Stabernack, J.; Tolosa, E.; Magnus, T.; Rissiek, B. The Role of the ATP-Adenosine Axis in Ischemic Stroke. Semin. Immunopathol. 2023, 45, 347–365. [Google Scholar] [CrossRef]
- Nalamolu, K.R.; Chelluboina, B.; Fornal, C.A.; Challa, S.R.; Pinson, D.M.; Wang, D.Z.; Klopfenstein, J.D.; Veeravalli, K.K. Stem Cell Treatment Improves Post Stroke Neurological Outcomes: A Comparative Study in Male and Female Rats. Stroke Vasc. Neurol. 2021, 6, 519–527. [Google Scholar] [CrossRef]
- Arruri, V.; Chokkalla, A.K.; Jeong, S.; Chelluboina, B.; Mehta, S.L.; Veeravalli, K.K.; Vemuganti, R. MMP-12 Knockdown Prevents Secondary Brain Damage After Ischemic Stroke in Mice. Neurochem. Int. 2022, 161, 105432. [Google Scholar] [CrossRef] [PubMed]
- King, B.F. Rehabilitation of the P2X5 Receptor: A Re-Evaluation of Structure and Function. Purinergic Signal. 2023, 19, 421–439. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, F.; Dinkel, K.; Reymann, K. Microglia Response and P2 Receptor Participation in Oxygen/Glucose Deprivation-Induced Cortical Damage. Neuroscience 2005, 136, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Erdling, A.; Johansson, S.E.; Radziwon-Balicka, A.; Ansar, S.; Edvinsson, L. Changes in P2Y(6) Receptor-Mediated Vasoreactivity Following Focal and Global Ischemia. Physiol. Rep. 2022, 10, e15283. [Google Scholar] [CrossRef]
- Peterson, T.S.; Camden, J.M.; Wang, Y.; Seye, C.I.; Wood, W.G.; Sun, G.Y.; Erb, L.; Petris, M.J.; Weisman, G.A. P2Y2 Nucleotide Receptor-Mediated Responses in Brain Cells. Mol. Neurobiol. 2010, 41, 356–366. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the Mechanism of IL-1beta Secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Hou, M.; Moller, S.; Edvinsson, L.; Erlinge, D. Cytokines Induce Upregulation of Vascular P2Y(2) Receptors and Increased Mitogenic Responses to UTP and ATP. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2064–2069. [Google Scholar] [CrossRef]
- Stegner, D.; Klaus, V.; Nieswandt, B. Platelets as Modulators of Cerebral Ischemia/Reperfusion Injury. Front. Immunol. 2019, 10, 2505. [Google Scholar] [CrossRef]
- Tu, J.; Wang, L. Therapeutic Potential of Extracellular ATP and P2 Receptors in Nervous System Diseases. Neurosci. Bull. 2009, 25, 27–32. [Google Scholar] [CrossRef]
- Alberto, A.V.P.; Ferreira, N.C.d.S.; Bonavita, A.G.C.; Nihei, O.K.; Farias, F.P.d.; Bisaggio, R.d.C.; Albuquerque, C.d.; Savino, W.; Coutinho-Silva, R.; Persechini, P.M.; et al. Physiologic Roles of P2 Receptors in Leukocytes. J. Leukoc. Biol. 2022, 112, 983–1012. [Google Scholar] [CrossRef]
- Ozaki, T.; Muramatsu, R.; Sasai, M.; Yamamoto, M.; Kubota, Y.; Fujinaka, T.; Yoshimine, T.; Yamashita, T. The P2X4 Receptor is Required for Neuroprotection Via Ischemic Preconditioning. Sci. Rep. 2016, 6, 25893. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Chen, S.; Xue, S.; Zhang, X.; Lian, Y. P2RY2 Alleviates Cerebral Ischemia-Reperfusion Injury by Inhibiting YAP Phosphorylation and Reducing Mitochondrial Fission. Neuroscience 2022, 480, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic Signaling in the Cardiovascular System. Circ. Res. 2017, 120, 207–228. [Google Scholar] [CrossRef]
- Srivastava, P.; Cronin, C.G.; Scranton, V.L.; Jacobson, K.A.; Liang, B.T.; Verma, R. Neuroprotective and Neuro-Rehabilitative Effects of Acute Purinergic Receptor P2X4 (P2X4R) Blockade After Ischemic Stroke. Exp. Neurol. 2020, 329, 113308. [Google Scholar] [CrossRef]
- Chu, K.; Yin, B.; Wang, J.; Peng, G.; Liang, H.; Xu, Z.; Du, Y.; Fang, M.; Xia, Q.; Luo, B. Inhibition of P2X7 Receptor Ameliorates Transient Global Cerebral Ischemia/Reperfusion Injury Via Modulating Inflammatory Responses in the Rat Hippocampus. J. Neuroinflamm. 2012, 9, 69. [Google Scholar] [CrossRef]
- Wilmes, M.; Pinto Espinoza, C.; Ludewig, P.; Stabernack, J.; Liesz, A.; Nicke, A.; Gelderblom, M.; Gerloff, C.; Falzoni, S.; Tolosa, E.; et al. Blocking P2X7 by Intracerebroventricular Injection of P2X7-Specific Nanobodies Reduces Stroke Lesions. J. Neuroinflamm. 2022, 19, 256. [Google Scholar] [CrossRef]
- Kuboyama, K.; Harada, H.; Tozaki-Saitoh, H.; Tsuda, M.; Ushijima, K.; Inoue, K. Astrocytic P2Y(1) Receptor is Involved in the Regulation of Cytokine/Chemokine Transcription and Cerebral Damage in a Rat Model of Cerebral Ischemia. J. Cereb. Blood Flow Metab. 2011, 31, 1930–1941. [Google Scholar] [CrossRef]
- Chin, Y.; Kishi, M.; Sekino, M.; Nakajo, F.; Abe, Y.; Terazono, Y.; Hiroyuki, O.; Kato, F.; Koizumi, S.; Gachet, C.; et al. Involvement of Glial P2Y(1) Receptors in Cognitive Deficit After Focal Cerebral Stroke in a Rodent Model. J. Neuroinflamm. 2013, 10, 95. [Google Scholar] [CrossRef]
- Milde, S.; Brown, G.C. Knockout of the P2Y(6) Receptor Prevents Peri-Infarct Neuronal Loss After Transient, Focal Ischemia in Mouse Brain. Int. J. Mol. Sci. 2022, 23, 2304. [Google Scholar] [CrossRef]
- Yamauchi, K.; Imai, T.; Shimazawa, M.; Iwama, T.; Hara, H. Effects of Ticagrelor in a Mouse Model of Ischemic Stroke. Sci. Rep. 2017, 7, 12088. [Google Scholar] [CrossRef]
- Webster, C.M.; Hokari, M.; McManus, A.; Tang, X.N.; Ma, H.; Kacimi, R.; Yenari, M.A. Microglial P2Y12 Deficiency/Inhibition Protects Against Brain Ischemia. PLoS ONE 2013, 8, e70927. [Google Scholar] [CrossRef]
- Sasaki, Y.; Hoshi, M.; Akazawa, C.; Nakamura, Y.; Tsuzuki, H.; Inoue, K.; Kohsaka, S. Selective Expression of Gi/O-Coupled ATP Receptor P2Y12 in Microglia in Rat Brain. Glia 2003, 44, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yu, C.; Pi, S.; Mao, L.; Hu, B. The Role of P2Y(12) Receptor in Ischemic Stroke of Atherosclerotic Origin. Cell. Mol. Life Sci. 2019, 76, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Tantry, U.S.; Gurbel, P.A. P2Y12 Receptor Inhibitors for Secondary Prevention of Ischemic Stroke. Expert Opin. Pharmacother. 2015, 16, 1149–1165. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, X.; Zhao, Y.; Guo, X.; Jiang, H.; Li, H.; Gu, Z. Evolution of Gene Regulation during Transcription and Translation. Genome Biol. Evol. 2015, 7, 1155–1167. [Google Scholar] [CrossRef]
- Klaver, D.; Thurnher, M. Control of Macrophage Inflammation by P2Y Purinergic Receptors. Cells 2021, 10, 1098. [Google Scholar] [CrossRef]
- Chelluboina, B.; Nalamolu, K.R.; Mendez, G.G.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Veeravalli, K.K. Mesenchymal Stem Cell Treatment Prevents Post-Stroke Dysregulation of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases. Cell. Physiol. Biochem. 2017, 44, 1360–1369. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Alfieri, A.; Allan, S.M.; Carswell, H.V.; Deuchar, G.A.; Farr, T.D.; Flecknell, P.; Gallagher, L.; Gibson, C.L.; Haley, M.J.; et al. The IMPROVE Guidelines (Ischaemia Models: Procedural Refinements of in Vivo Experiments). J. Cereb. Blood Flow Metab. 2017, 37, 3488–3517. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. Br. J. Pharmacol. 2020, 177, 3617–3624. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, L.; Zhang, Z.; Lu, D.; Lu, M.; Chopp, M. Therapeutic Benefit of Intravenous Administration of Bone Marrow Stromal Cells After Cerebral Ischemia in Rats. Stroke 2001, 32, 1005–1011. [Google Scholar] [CrossRef]


| Group Name | Group Description | Number of Animals | ||
|---|---|---|---|---|
| For Real Time PCR | Excluded | Total | ||
| Mice | ||||
| Sham: Mice subjected to surgery without intraluminal monofilament suture insertion to simulate stroke conditions | ||||
| - Sham mice euthanized on day 1 post-surgery | 6♂ | 0 | 6♂ | |
| - Sham mice euthanized on day 3 post-surgery | 6♂ | 0 | 6♂ | |
| - Sham mice euthanized on day 5 post-surgery | 5♂ | 0 | 5♂ | |
| - Sham mice euthanized on day 7 post-surgery | 5♂ | 0 | 5♂ | |
| Stroke: Mice subjected to 1-h MCAO | ||||
| - Stroke mice euthanized on day 1 post-MCAO | 6♂ | 2♂ | 8♂ | |
| - Stroke mice euthanized on day 3 post-MCAO | 6♂ | 3♂ | 9♂ | |
| - Stroke mice euthanized on day 5 post-MCAO | 6♂ | 4♂ | 10♂ | |
| - Stroke mice euthanized on day 7 post-MCAO | 6♂ | 3♂ | 9♂ | |
| Rats | ||||
| Sham: Rats subjected to surgery without intraluminal monofilament suture insertion to simulate stroke conditions | ||||
| - Sham rats euthanized on day 7 post-surgery | 6♂ | 0 | 6♂ | |
| Stroke: Rats subjected to 2-h MCAO | ||||
| - Stroke rats euthanized on day 1 post-MCAO | 6♂ | 2♂ | 8♂ | |
| - Stroke rats euthanized on day 3 post-MCAO | 6♂ | 2♂ | 8♂ | |
| - Stroke rats euthanized on day 5 post-MCAO | 6♂ | 3♂ | 9♂ | |
| - Stroke rats euthanized on day 7 post-MCAO | 6♂ | 3♂ | 9♂ | |
| Gene | NCBI Reference Sequence | Primer Sequence | Amplicon Length (bp) | |
|---|---|---|---|---|
| Forward (5′-3′) | Reverse (5′-3′) | |||
| Rat | ||||
| P2X1 | NM_012997 | ttgtgcagagaacccagaag | acagttgcctgtgcgaata | 100 |
| P2X2 | NM_053656 | gtgacctggacttgtctgaat | tggtggtagtgccgtttatc | 135 |
| P2X3 | NM_031075 | gtcatggacgtgtcggattat | cagcggtacttctcttcattct | 128 |
| P2X4 | NM_031594 | caatgtgtctcctggctacaa | ttcccagcctttccaaaca | 129 |
| P2X5 | NM_080780 | gaatgggactgtgaccttgat | gtaatacctggcgaacctgaa | 126 |
| P2X6 | NM_012721 | acctgggacaacacctatttc | agcaatgcaaggtcctcaa | 125 |
| P2X7 | NM_019256 | tttggccaccgtgtgtatt | gatgggctcacacttctttct | 139 |
| P2Y1 | NM_012800 | tttgtatgtgctcaccctacc | agatgctgccatagaggtttac | 125 |
| P2Y2 | NM_017255 | tgccgctgctggtttatta | gatgctgcagtagaggttagtg | 107 |
| P2Y4 | NM_031680 | tcgatttgcaagccttctct | catggcacaggatggtagtt | 113 |
| P2Y6 | NM_057124 | cctcttctatgccaacctacac | agccaaacgactccacatac | 147 |
| P2Y12 | NM_022800 | ctgaagaccaccagaccattta | cctcctgttggtgagaatcat | 129 |
| P2Y13 | NM_001002853 | ggcatcaaccgtgaagaaatg | tcttggcaatcaccgtgtaa | 140 |
| P2Y14 | NM_133577 | catggccataacgaggaagat | gcacgaaacagacgacaaatac | 125 |
| 18S rRNA | NR_046237 | acgtctgccctatcaactttc | ttggatgtggtagccgtttc | 117 |
| Mouse | ||||
| P2X1 | NM_008771 | ttgtgcagagaacccagaag | acagttgcctgtgcgaata | 100 |
| P2X2 | NM_001164834 | gggtcatcatcaactggaac | aagaggcagggtcatactt | 102 |
| P2X3 | NM_145526 | gaagggaaacctccttcctaac | aatcctgcccagcaaactta | 125 |
| P2X4 | NM_001310720 | ctcatcctggcttacgtcatt | agctgagaagtgttggtcac | 125 |
| P2X5 | NM_033321 | cacttcagctccaccaatct | atcaaggtcacagtcccattc | 138 |
| P2X6 | NM_001159561 | tgtcatcaccaaactcaaggg | ggaagttggttaccaggaagaa | 128 |
| P2X7 | NM_001038887 | aagtgggtcttgcacatgat | cacctctgctatgcctttga | 126 |
| P2Y1 | NM_001282016 | ggcaggctcaagaagaagaa | tcattggacgtggtgtcatag | 146 |
| P2Y2 | NM_001302347 | ccgagagctctttagccattt | ggccataagcacgtaacaga | 103 |
| P2Y4 | NM_020621 | tctgcctgaggagtttga | atgagtccatagcagacca | 102 |
| P2Y6 | NM_183168 | ggcttgttattgtcgcatgg | aggaagctgatggcaaaga | 132 |
| P2Y12 | AJ312130 | gaagaccaccaggccattta | cctcctgttggtgagaatcat | 127 |
| P2Y13 | NM_028808 | tcacacctgccagttcattt | cctgctgtccttactcctaaac | 115 |
| P2Y14 | NM_133200 | cgtgttgtacggtatggtctt | gtcagccaccactatgttctt | 124 |
| 18S rRNA | NR_003278 | tgagaaacggctaccacatc | gcctcgaaagagtcctgtatt | 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Challa, S.R.; Levingston, H.; Fornal, C.A.; Baker, I.M.; Boston, J.; Shanthappa, N.; Unnam, P.; Klopfenstein, J.D.; Veeravalli, K.K. Temporal mRNA Expression of Purinergic P2 Receptors in the Brain Following Cerebral Ischemia and Reperfusion: Similarities and Distinct Variations Between Rats and Mice. Int. J. Mol. Sci. 2025, 26, 2379. https://doi.org/10.3390/ijms26062379
Challa SR, Levingston H, Fornal CA, Baker IM, Boston J, Shanthappa N, Unnam P, Klopfenstein JD, Veeravalli KK. Temporal mRNA Expression of Purinergic P2 Receptors in the Brain Following Cerebral Ischemia and Reperfusion: Similarities and Distinct Variations Between Rats and Mice. International Journal of Molecular Sciences. 2025; 26(6):2379. https://doi.org/10.3390/ijms26062379
Chicago/Turabian StyleChalla, Siva Reddy, Hunter Levingston, Casimir A. Fornal, Isidra M. Baker, Joseph Boston, Nidhi Shanthappa, Pavani Unnam, Jeffrey D. Klopfenstein, and Krishna Kumar Veeravalli. 2025. "Temporal mRNA Expression of Purinergic P2 Receptors in the Brain Following Cerebral Ischemia and Reperfusion: Similarities and Distinct Variations Between Rats and Mice" International Journal of Molecular Sciences 26, no. 6: 2379. https://doi.org/10.3390/ijms26062379
APA StyleChalla, S. R., Levingston, H., Fornal, C. A., Baker, I. M., Boston, J., Shanthappa, N., Unnam, P., Klopfenstein, J. D., & Veeravalli, K. K. (2025). Temporal mRNA Expression of Purinergic P2 Receptors in the Brain Following Cerebral Ischemia and Reperfusion: Similarities and Distinct Variations Between Rats and Mice. International Journal of Molecular Sciences, 26(6), 2379. https://doi.org/10.3390/ijms26062379






