Polyethylene Glycol Loxenatide Accelerates Diabetic Wound Healing by Downregulating Systemic Inflammation and Improving Endothelial Progenitor Cell Functions
Abstract
1. Introduction
2. Results
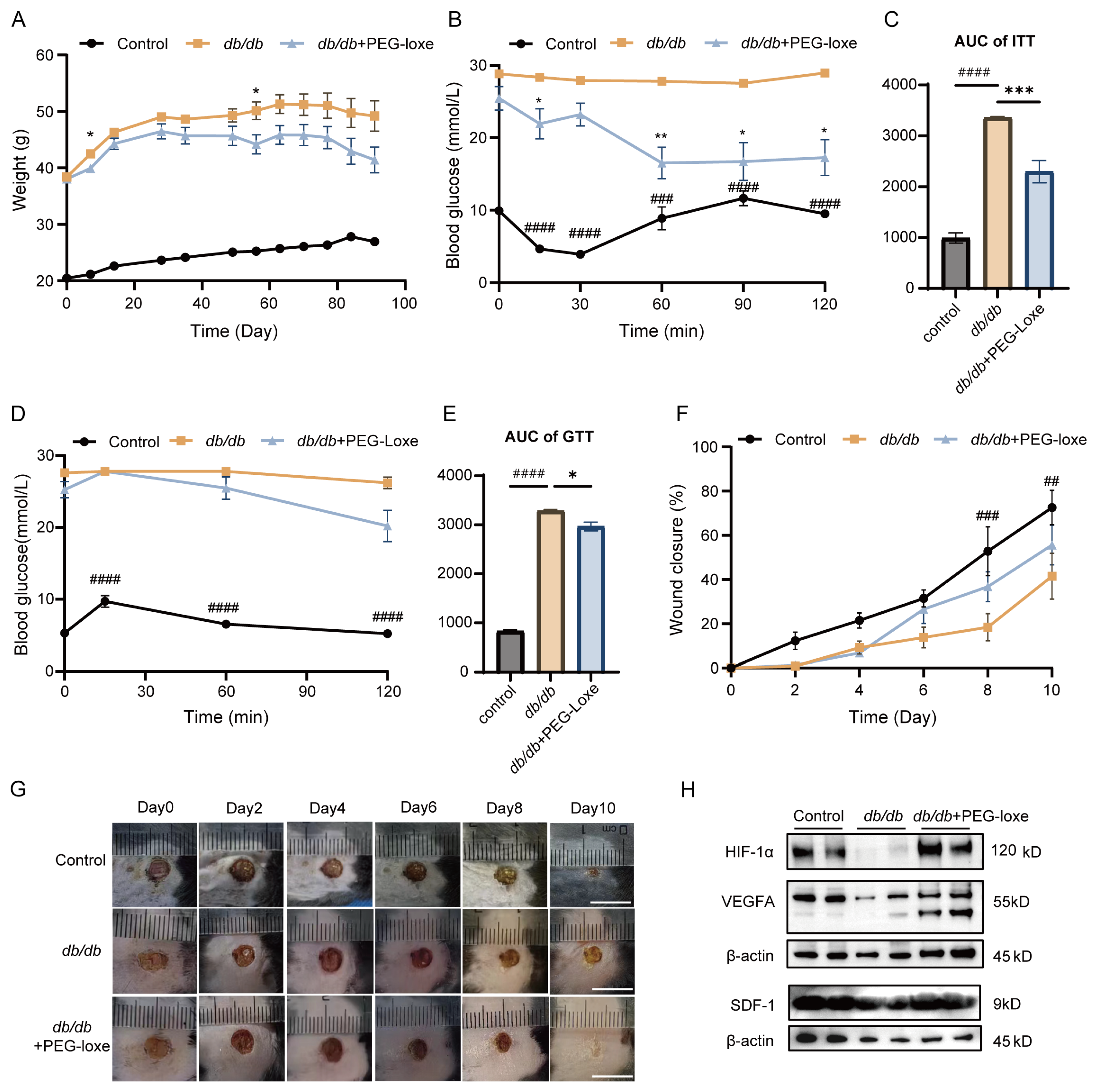
2.1. PEG-Loxe Improved Glucose Metabolism
2.2. PEG-Loxe Accelerated Diabetic Wound Healing in db/db Mice
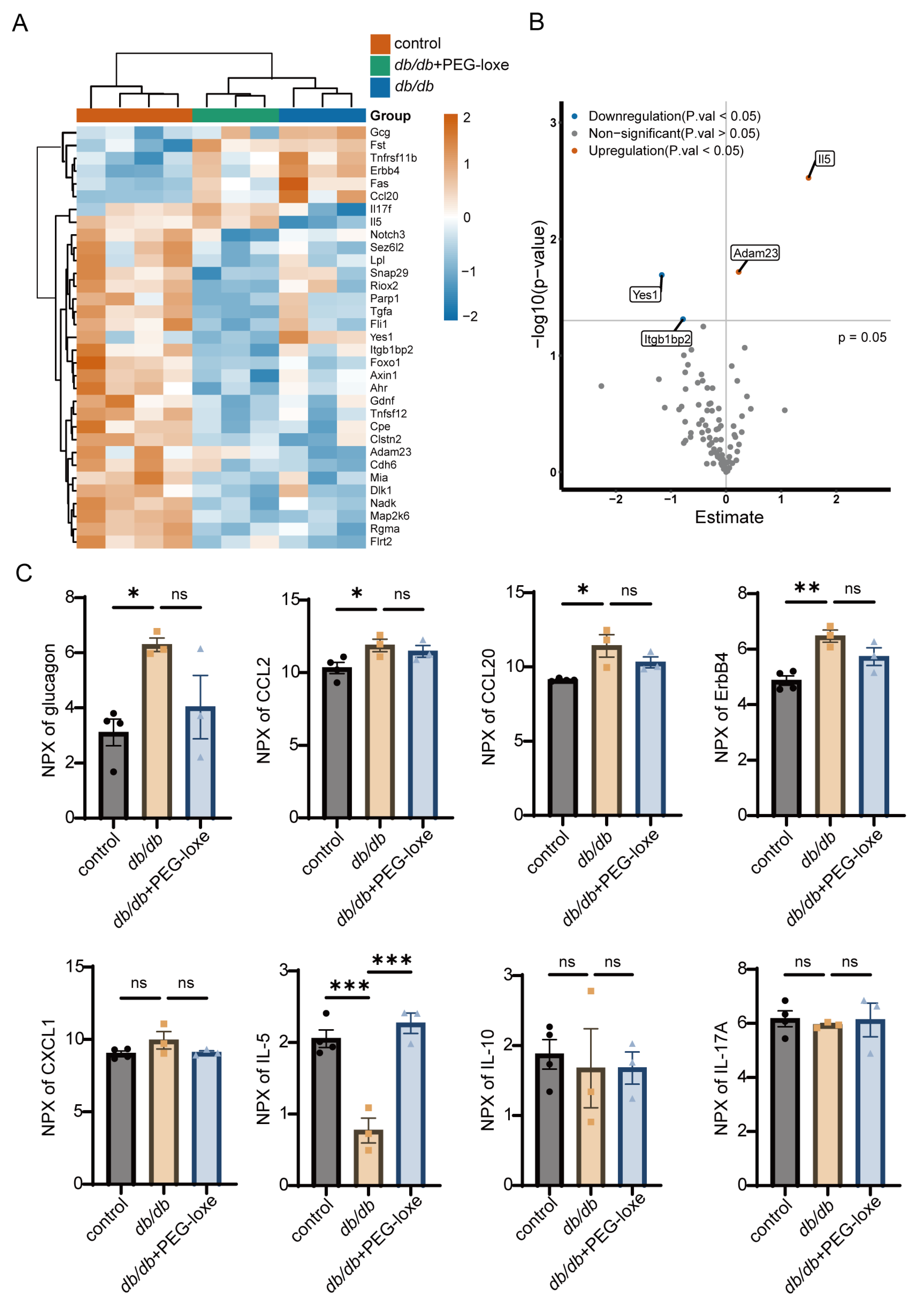
2.3. PEG-Loxe Regulated Systemic Inflammatory Response During Diabetic Wound Healing
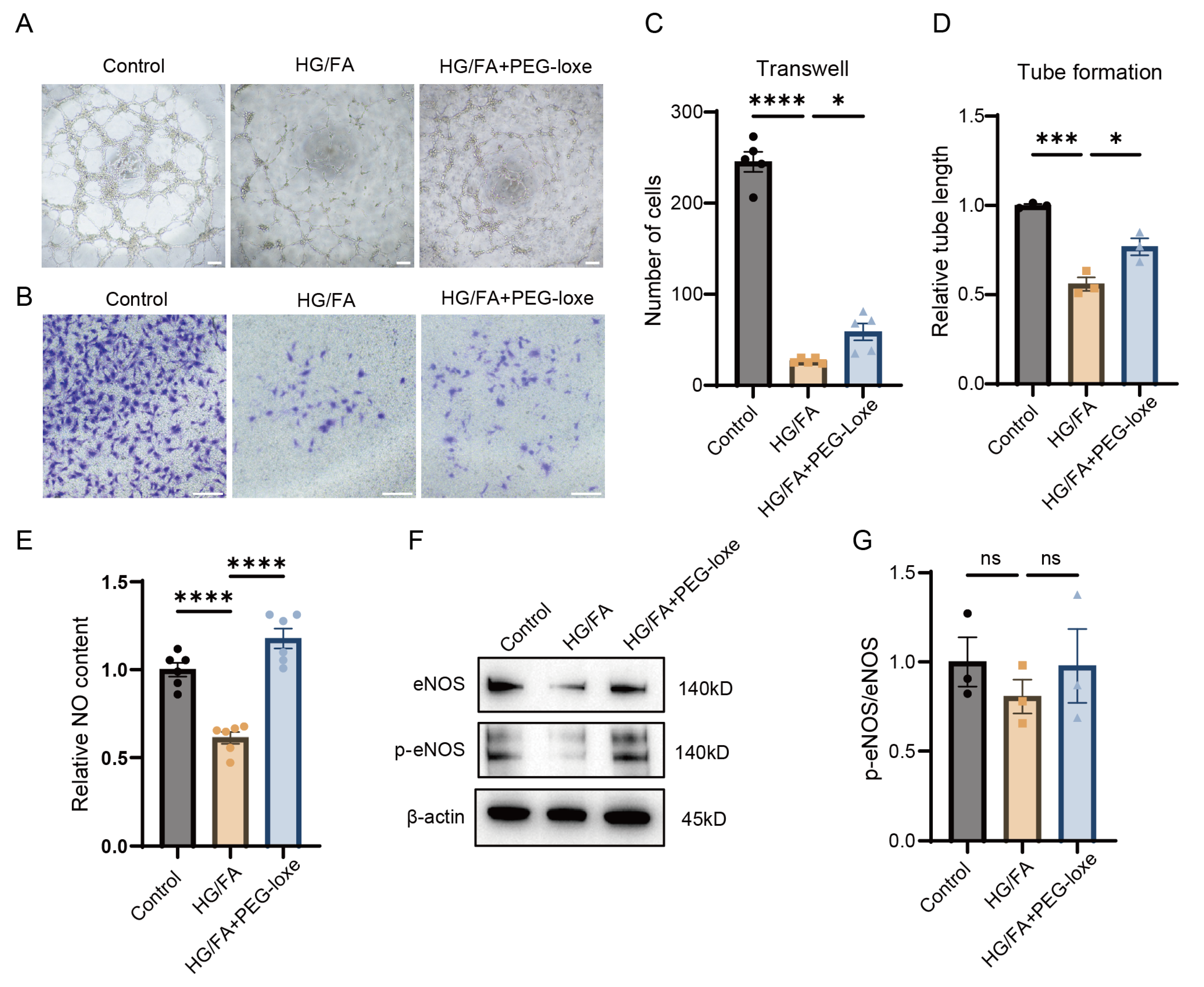
2.4. PEG-Loxe Protects Endothelial Progenitor Cell Function from Glucotoxicity and Lipotoxicity In Vitro
2.5. PEG-Loxe Increased NO Bioavailability in Endothelial Progenitor Cells Under High-Glucose and -Fatty-Acid Conditions
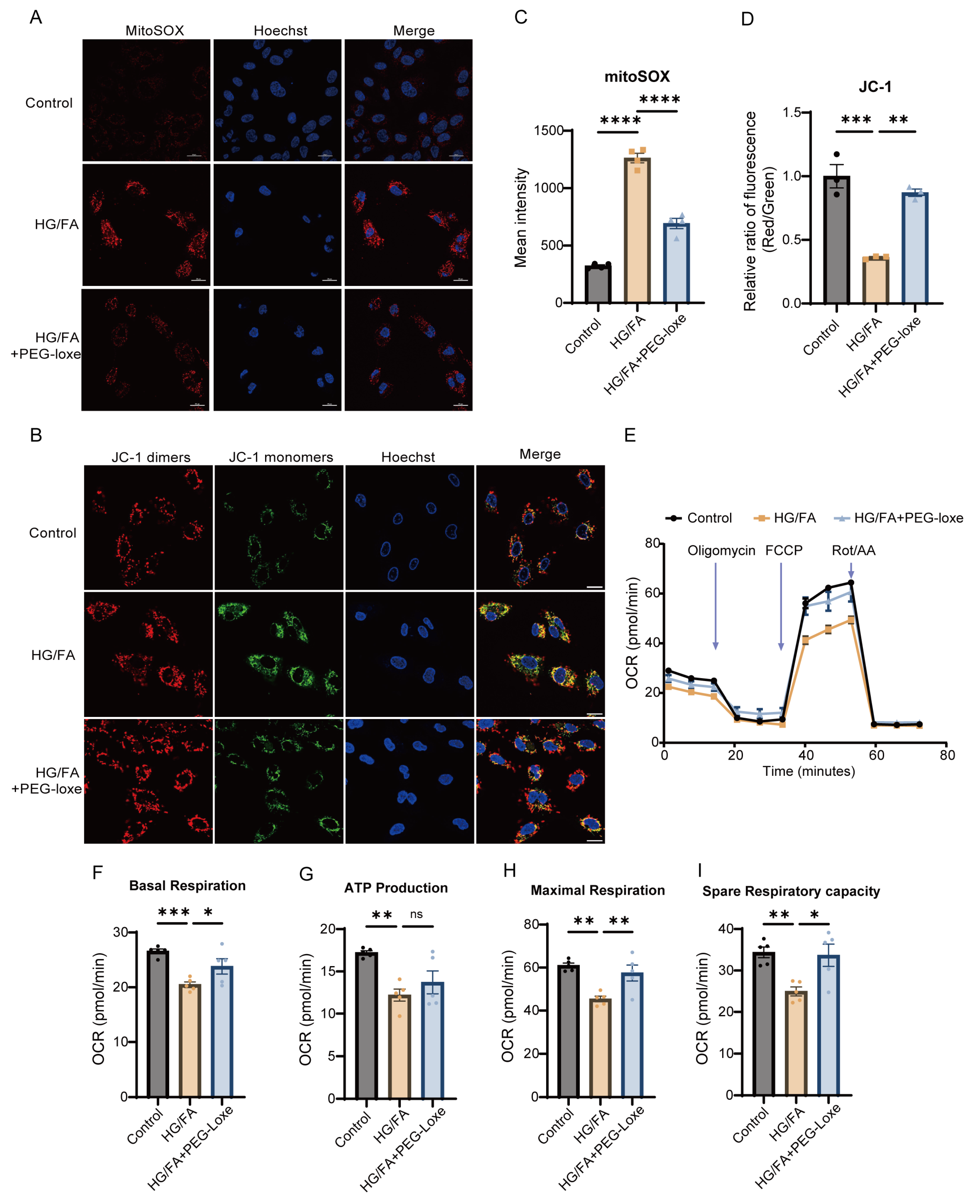
2.6. PEG-Loxe Reduces Reactive Oxygen Species and Restores Mitochondrial Membrane Potential in Endothelial Progenitor Cells
2.7. PEG-Loxe Restored Mitochondrial Respiratory Function
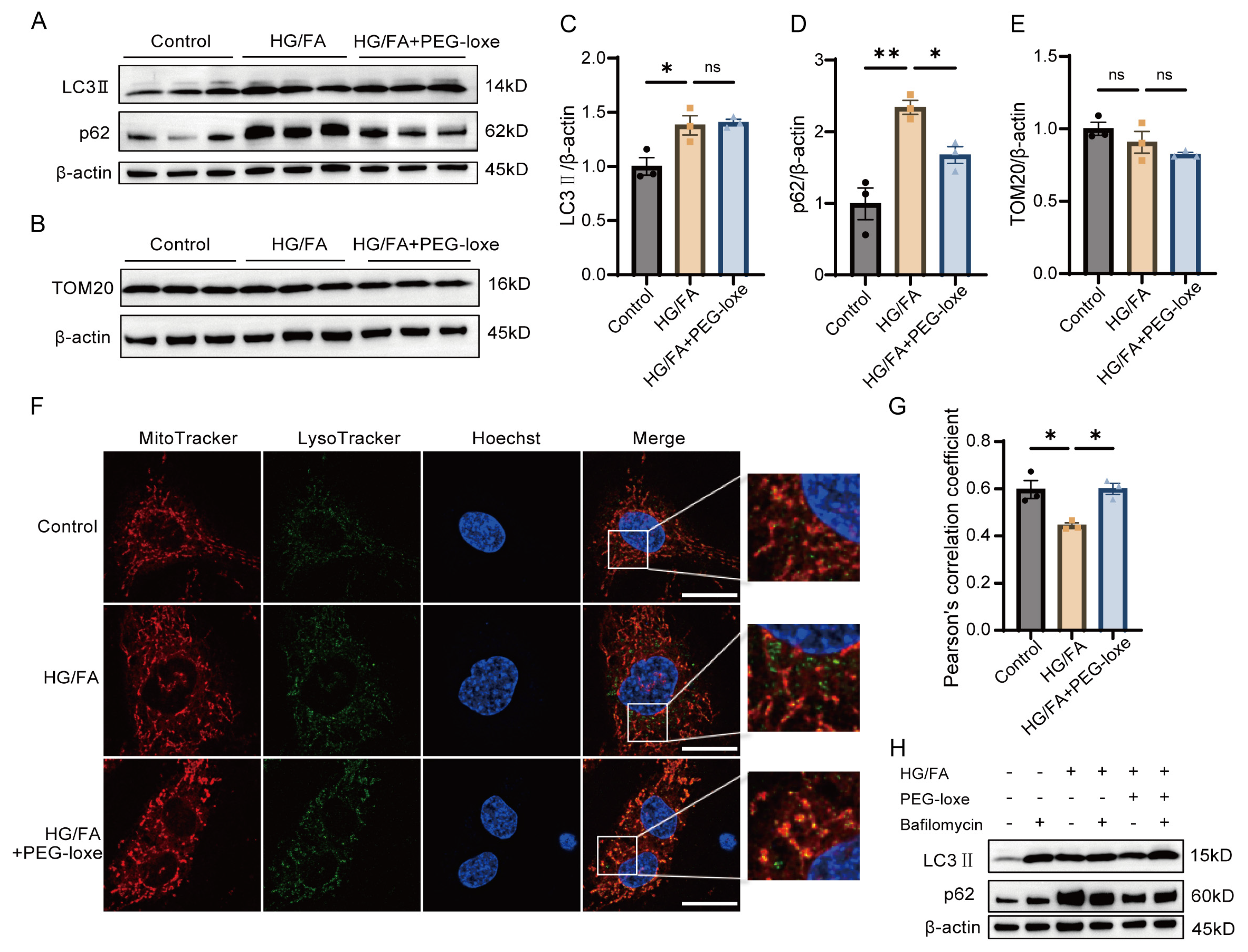
2.8. PEG-Loxe Facilitated Mitochondria Quality Control
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Insulin Tolerance Test (ITT) and Oral Glucose Tolerance Test (OGTT)
4.3. Mouse Model of Wound Healing and PEG-Loxenatide Administration
4.4. Wound-Healing Analysis
4.5. Serum Olink Analysis
4.6. Cell Culture and Treatment
4.7. NO Detection
4.8. Transwell Migration Assay
4.9. Tube Formation Assay
4.10. Detection of Reactive Oxygen Species (ROS)
4.11. Mitochondrial Membrane Potential Assay
4.12. Colocalization of Mitochondria and Lysosomes
4.13. Seahorse XF Analysis
4.14. Western Blotting
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dal Canto, E.; Ceriello, A.; Rydén, L.; Ferrini, M.; Hansen, T.B.; Schnell, O.; Standl, E.; Beulens, J.W. Diabetes as a Cardiovascular Risk Factor: An Overview of Global Trends of Macro and Micro Vascular Complications. Eur. J. Prev. Cardiol. 2019, 26, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Okonkwo, U.A.; DiPietro, L.A. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, Mortality, and Kidney Outcomes with GLP-1 Receptor Agonists in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef]
- Wong, S.Y.; Lee, A.R.Y.B.; Sia, A.H.J.; Wo, Y.J.; Teo, Y.H.; Teo, Y.N.; Syn, N.L.; Ong, C.-C.; Teo, L.L.; Yeo, T.-C.; et al. Effects of Glucagon-Like Peptide-1 Receptor Agonist (GLP-1RA) on Cardiac Structure and Function: A Systematic Review and Meta-Analysis of Randomized-Controlled Trials. Cardiovasc. Drugs Ther. 2024, 38, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Vergès, B.; Aboyans, V.; Angoulvant, D.; Boutouyrie, P.; Cariou, B.; Hyafil, F.; Mohammedi, K.; Amarenco, P. Protection against Stroke with Glucagon-like Peptide-1 Receptor Agonists: A Comprehensive Review of Potential Mechanisms. Cardiovasc. Diabetol. 2022, 21, 242. [Google Scholar] [CrossRef]
- Ussher, J.R.; Drucker, D.J. Glucagon-like Peptide 1 Receptor Agonists: Cardiovascular Benefits and Mechanisms of Action. Nat. Rev. Cardiol. 2023, 20, 463–474. [Google Scholar] [CrossRef]
- Chen, F.; He, L.; Li, J.; Yang, S.; Zhang, B.; Zhu, D.; Wu, Z.; Zhang, S.; Hou, D.; Ouyang, C.; et al. Polyethylene Glycol Loxenatide Injection (GLP-1) Protects Vascular Endothelial Cell Function in Middle-Aged and Elderly Patients with Type 2 Diabetes by Regulating Gut Microbiota. Front. Mol. Biosci. 2022, 9, 879294. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, Y.; Wang, D.; Yan, H.; Wang, N. Loxenatide Attenuates ROS-Mediated Vascular Endothelial Progenitor Cell Damage and Mitochondrial Dysfunction via SIRT3/Foxo3 Signaling Pathway. J. Biochem. Mol. Toxicol. 2023, 37, e23452. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Y.; Wang, Y.; Wei, X.; Wu, L.; Wang, T.; Ma, A. Exendin-4 Reverses High Glucose-Induced Endothelial Progenitor Cell Dysfunction via SDF-1β/CXCR7-AMPK/P38-MAPK/IL-6 Axis. Acta Diabetol. 2020, 57, 1315–1326. [Google Scholar] [CrossRef]
- Longo, M.; Di Meo, I.; Caruso, P.; Muscio, M.F.; Scappaticcio, L.; Maio, A.; Maiorino, M.I.; Bellastella, G.; Signoriello, G.; Knop, F.K.; et al. Circulating Levels of Endothelial Progenitor Cells Are Associated with Better Cognitive Function in Older Adults with Glucagon-like Peptide 1 Receptor Agonist-Treated Type 2 Diabetes. Diabetes Res. Clin. Pract. 2023, 200, 110688. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Miorin, M.; Facco, M.; Bonamico, S.; Baesso, I.; Grego, F.; Menegolo, M.; de Kreutzenberg, S.V.; Tiengo, A.; Agostini, C.; et al. Circulating Endothelial Progenitor Cells Are Reduced in Peripheral Vascular Complications of Type 2 Diabetes Mellitus. J. Am. Coll. Cardiol. 2005, 45, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Rigato, M.; Bittante, C.; Albiero, M.; Avogaro, A.; Fadini, G.P. Circulating Progenitor Cell Count Predicts Microvascular Outcomes in Type 2 Diabetic Patients. J. Clin. Endocrinol. Metab. 2015, 100, 2666–2672. [Google Scholar] [CrossRef]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science 1997, 275, 964–967. [Google Scholar] [CrossRef]
- Hur, J.; Yoon, C.-H.; Kim, H.-S.; Choi, J.-H.; Kang, H.-J.; Hwang, K.-K.; Oh, B.-H.; Lee, M.-M.; Park, Y.-B. Characterization of Two Types of Endothelial Progenitor Cells and Their Different Contributions to Neovasculogenesis. Arter. Thromb. Vasc. Biol. 2004, 24, 288–293. [Google Scholar] [CrossRef]
- Basile, D.P.; Yoder, M.C. Circulating and Tissue Resident Endothelial Progenitor Cells. J. Cell. Physiol. 2014, 229, 10–16. [Google Scholar] [CrossRef]
- Yu, J.-W.; Deng, Y.-P.; Han, X.; Ren, G.-F.; Cai, J.; Jiang, G.-J. Metformin Improves the Angiogenic Functions of Endothelial Progenitor Cells via Activating AMPK/eNOS Pathway in Diabetic Mice. Cardiovasc. Diabetol. 2016, 15, 88. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.-Y.; Pan, Z.-W.; Li, Q.-Q.; Sun, L.-H.; Li, X.; Gong, M.-Y.; Yang, X.-W.; Wang, Y.-Y.; Li, H.-D.; et al. GDF11 Promotes Wound Healing in Diabetic Mice via Stimulating HIF-1α-VEGF/SDF-1α-Mediated Endothelial Progenitor Cell Mobilization and Neovascularization. Acta Pharmacol. Sin. 2023, 44, 999–1013. [Google Scholar] [CrossRef]
- Ceradini, D.J.; Gurtner, G.C. Homing to Hypoxia: HIF-1 as a Mediator of Progenitor Cell Recruitment to Injured Tissue. Trends Cardiovasc. Med. 2005, 15, 57–63. [Google Scholar] [CrossRef]
- Fadini, G.P.; Boscaro, E.; Albiero, M.; Menegazzo, L.; Frison, V.; de Kreutzenberg, S.; Agostini, C.; Tiengo, A.; Avogaro, A. The Oral Dipeptidyl Peptidase-4 Inhibitor Sitagliptin Increases Circulating Endothelial Progenitor Cells in Patients with Type 2 Diabetes: Possible Role of Stromal-Derived Factor-1α. Diabetes Care 2010, 33, 1607–1609. [Google Scholar] [CrossRef]
- Ceradini, D.J.; Yao, D.; Grogan, R.H.; Callaghan, M.J.; Edelstein, D.; Brownlee, M.; Gurtner, G.C. Decreasing Intracellular Superoxide Corrects Defective Ischemia-Induced New Vessel Formation in Diabetic Mice. J. Biol. Chem. 2008, 283, 10930–10938. [Google Scholar] [CrossRef]
- Cunnion, K.M.; Krishna, N.K.; Pallera, H.K.; Pineros-Fernandez, A.; Rivera, M.G.; Hair, P.S.; Lassiter, B.P.; Huyck, R.; Clements, M.A.; Hood, A.F.; et al. Complement Activation and STAT4 Expression Are Associated with Early Inflammation in Diabetic Wounds. PLoS ONE 2017, 12, e0170500. [Google Scholar] [CrossRef]
- Lörchner, H.; Cañes Esteve, L.; Góes, M.E.; Harzenetter, R.; Brachmann, N.; Gajawada, P.; Günther, S.; Doll, N.; Pöling, J.; Braun, T. Neutrophils for Revascularization Require Activation of CCR6 and CCL20 by TNFα. Circ. Res. 2023, 133, 592–610. [Google Scholar] [CrossRef]
- Thum, T.; Fraccarollo, D.; Schultheiss, M.; Froese, S.; Galuppo, P.; Widder, J.D.; Tsikas, D.; Ertl, G.; Bauersachs, J. Endothelial Nitric Oxide Synthase Uncoupling Impairs Endothelial Progenitor Cell Mobilization and Function in Diabetes. Diabetes 2007, 56, 666–674. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2012, 33, 829–837, 837a–837d. [Google Scholar] [CrossRef] [PubMed]
- Onishi, M.; Yamano, K.; Sato, M.; Matsuda, N.; Okamoto, K. Molecular Mechanisms and Physiological Functions of Mitophagy. EMBO J. 2021, 40, e104705. [Google Scholar] [CrossRef]
- Kelly, A.G.; Panigrahy, D. Targeting Angiogenesis via Resolution of Inflammation. Cold Spring Harb. Perspect. Med. 2023, 13, a041172. [Google Scholar] [CrossRef]
- Lin, C.-P.; Lin, F.-Y.; Huang, P.-H.; Chen, Y.-L.; Chen, W.-C.; Chen, H.-Y.; Huang, Y.-C.; Liao, W.-L.; Huang, H.-C.; Liu, P.-L.; et al. Endothelial Progenitor Cell Dysfunction in Cardiovascular Diseases: Role of Reactive Oxygen Species and Inflammation. BioMed Res. Int. 2013, 2013, 845037. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Ghanim, H.; Vora, M.; Sia, C.L.; Korzeniewski, K.; Dhindsa, S.; Makdissi, A.; Dandona, P. Exenatide Exerts a Potent Antiinflammatory Effect. J. Clin. Endocrinol. Metab. 2012, 97, 198–207. [Google Scholar] [CrossRef]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef]
- Xu, F.; Othman, B.; Lim, J.; Batres, A.; Ponugoti, B.; Zhang, C.; Yi, L.; Liu, J.; Tian, C.; Hameedaldeen, A.; et al. Foxo1 Inhibits Diabetic Mucosal Wound Healing but Enhances Healing of Normoglycemic Wounds. Diabetes 2015, 64, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage Plasticity, Polarization, and Function in Health and Disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Al Sadoun, H. Macrophage Phenotypes in Normal and Diabetic Wound Healing and Therapeutic Interventions. Cells 2022, 11, 2430. [Google Scholar] [CrossRef]
- Asosingh, K.; Swaidani, S.; Aronica, M.; Erzurum, S.C. Th1- and Th2-Dependent Endothelial Progenitor Cell Recruitment and Angiogenic Switch in Asthma. J. Immunol. 2007, 178, 6482–6494. [Google Scholar] [CrossRef]
- Bucher, F.; Lee, J.; Shin, S.; Kim, M.S.; Oh, Y.-S.; Ha, S.; Zhang, H.; Yea, K. Interleukin-5 Suppresses Vascular Endothelial Growth Factor-Induced Angiogenesis through STAT5 Signaling. Cytokine 2018, 110, 397–403. [Google Scholar] [CrossRef]
- Pan, X.; Yang, L.; Wang, S.; Liu, Y.; Yue, L.; Chen, S. Semaglutide Alleviates Inflammation-Induced Endothelial Progenitor Cells Injury by Inhibiting MiR-155 Expression in Macrophage Exosomes. Int. Immunopharmacol. 2023, 119, 110196. [Google Scholar] [CrossRef]
- Tecilazich, F.; Dinh, T.; Pradhan-Nabzdyk, L.; Leal, E.; Tellechea, A.; Kafanas, A.; Gnardellis, C.; Magargee, M.L.; Dejam, A.; Toxavidis, V.; et al. Role of Endothelial Progenitor Cells and Inflammatory Cytokines in Healing of Diabetic Foot Ulcers. PLoS ONE 2013, 8, e83314. [Google Scholar] [CrossRef]
- Hoenig, M.R.; Bianchi, C.; Rosenzweig, A.; Sellke, F.W. Decreased Vascular Repair and Neovascularization with Ageing: Mechanisms and Clinical Relevance with an Emphasis on Hypoxia-Inducible Factor-1. Curr. Mol. Med. 2008, 8, 754–767. [Google Scholar] [CrossRef]
- Ceradini, D.J.; Kulkarni, A.R.; Callaghan, M.J.; Tepper, O.M.; Bastidas, N.; Kleinman, M.E.; Capla, J.M.; Galiano, R.D.; Levine, J.P.; Gurtner, G.C. Progenitor Cell Trafficking Is Regulated by Hypoxic Gradients through HIF-1 Induction of SDF-1. Nat. Med. 2004, 10, 858–864. [Google Scholar] [CrossRef]
- Wils, J.; Favre, J.; Bellien, J. Modulating Putative Endothelial Progenitor Cells for the Treatment of Endothelial Dysfunction and Cardiovascular Complications in Diabetes. Pharmacol. Ther. 2017, 170, 98–115. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Shvets, E.; Fass, E.; Shorer, H.; Gil, L.; Elazar, Z. Reactive Oxygen Species Are Essential for Autophagy and Specifically Regulate the Activity of Atg4. EMBO J. 2019, 38, e101812. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes-Novais, S.; Blecha, J.; Naraine, R.; Mikesova, J.; Abaffy, P.; Pecinova, A.; Milosevic, M.; Bohuslavova, R.; Prochazka, J.; Khan, S.; et al. Mitochondrial Respiration Supports Autophagy to Provide Stress Resistance during Quiescence. Autophagy 2022, 18, 2409–2426. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Ardehali, H.; Balaban, R.S.; DiLisa, F.; Dorn, G.W.; Kitsis, R.N.; Otsu, K.; Ping, P.; Rizzuto, R.; Sack, M.N.; et al. Mitochondrial Function, Biology, and Role in Disease: A Scientific Statement from the American Heart Association. Circ. Res. 2016, 118, 1960–1991. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Janaszak-Jasiecka, A.; Płoska, A.; Wierońska, J.M.; Dobrucki, L.W.; Kalinowski, L. Endothelial Dysfunction Due to eNOS Uncoupling: Molecular Mechanisms as Potential Therapeutic Targets. Cell. Mol. Biol. Lett. 2023, 28, 21. [Google Scholar] [CrossRef]
- Lee, J.; Giordano, S.; Zhang, J. Autophagy, Mitochondria and Oxidative Stress: Cross-Talk and Redox Signalling. Biochem. J. 2012, 441, 523–540. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Lin, S.-J.; Lin, F.-Y.; Wu, T.-C.; Tsao, C.-R.; Huang, P.-H.; Liu, P.-L.; Chen, Y.-L.; Chen, J.-W. High Glucose Impairs Early and Late Endothelial Progenitor Cells by Modifying Nitric Oxide-Related but Not Oxidative Stress-Mediated Mechanisms. Diabetes 2007, 56, 1559–1568. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takabatake, Y.; Takahashi, A.; Kimura, T.; Namba, T.; Matsuda, J.; Minami, S.; Kaimori, J.-Y.; Matsui, I.; Matsusaka, T.; et al. High-Fat Diet-Induced Lysosomal Dysfunction and Impaired Autophagic Flux Contribute to Lipotoxicity in the Kidney. J. Am. Soc. Nephrol. 2017, 28, 1534–1551. [Google Scholar] [CrossRef]
- Sakai, S.; Yamamoto, T.; Takabatake, Y.; Takahashi, A.; Namba-Hamano, T.; Minami, S.; Fujimura, R.; Yonishi, H.; Matsuda, J.; Hesaka, A.; et al. Proximal Tubule Autophagy Differs in Type 1 and 2 Diabetes. J. Am. Soc. Nephrol. 2019, 30, 929–945. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Chen, X.; Wang, Z.; Wang, X.; Zhou, Q.; Fang, W.; Zheng, C. Liraglutide Prevents High Glucose Induced HUVECs Dysfunction via Inhibition of PINK1/Parkin-Dependent Mitophagy. Mol. Cell. Endocrinol. 2022, 545, 111560. [Google Scholar] [CrossRef]
- Peña, O.A.; Martin, P. Cellular and Molecular Mechanisms of Skin Wound Healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Rak, G.D.; Osborne, L.C.; Siracusa, M.C.; Kim, B.S.; Wang, K.; Bayat, A.; Artis, D.; Volk, S.W. IL-33-Dependent Group 2 Innate Lymphoid Cells Promote Cutaneous Wound Healing. J. Investig. Dermatol. 2016, 136, 487–496. [Google Scholar] [CrossRef]
- Kingo, K.; Kõks, S.; Nikopensius, T.; Silm, H.; Vasar, E. Polymorphisms in the Interleukin-20 Gene: Relationships to Plaque-Type Psoriasis. Genes Immun. 2004, 5, 117–121. [Google Scholar] [CrossRef]
- Keermann, M.; Kõks, S.; Reimann, E.; Prans, E.; Abram, K.; Kingo, K. Transcriptional Landscape of Psoriasis Identifies the Involvement of IL36 and IL36RN. BMC Genom. 2015, 16, 322. [Google Scholar] [CrossRef]
- Bacci, S.; Laurino, A.; Manni, M.E.; Landucci, E.; Musilli, C.; De Siena, G.; Mocali, A.; Raimondi, L. The Pro-Healing Effect of Exendin-4 on Wounds Produced by Abrasion in Normoglycemic Mice. Eur. J. Pharmacol. 2015, 764, 346–352. [Google Scholar] [CrossRef]
- Nagae, K.; Uchi, H.; Morino-Koga, S.; Tanaka, Y.; Oda, M.; Furue, M. Glucagon-like Peptide-1 Analogue Liraglutide Facilitates Wound Healing by Activating PI3K/Akt Pathway in Keratinocytes. Diabetes Res. Clin. Pract. 2018, 146, 155–161. [Google Scholar] [CrossRef]
- Yue, H.; Zhang, X.; Zhao, Z.; Gong, S.; Shao, S. Pro-Healing Impact of Liraglutide on Skin Wounds in Normoglycemic Mice. Int. Immunopharmacol. 2025, 147, 114050. [Google Scholar] [CrossRef]
- Gorgogietas, V.; Rajaei, B.; Heeyoung, C.; Santacreu, B.J.; Marín-Cañas, S.; Salpea, P.; Sawatani, T.; Musuaya, A.; Arroyo, M.N.; Moreno-Castro, C.; et al. GLP-1R Agonists Demonstrate Potential to Treat Wolfram Syndrome in Human Preclinical Models. Diabetologia 2023, 66, 1306–1321. [Google Scholar] [CrossRef]
- Kõks, S. Genomics of Wolfram Syndrome 1 (WFS1). Biomolecules 2023, 13, 1346. [Google Scholar] [CrossRef]
- Lebbar, M.; Timsit, J.; Luyton, C.; Marchand, L. Glucagon-like Peptide-1 Receptor Agonists (GLP1-RA) in the Treatment of Mitochondrial Diabetes. Acta Diabetol. 2021, 58, 1281–1282. [Google Scholar] [CrossRef]
- Liu, B.; Li, A.; Qin, Y.; Chen, L.; Gao, M.; Gong, G. Visualizing Mitophagy with Fluorescent Dyes for Mitochondria and Lysosome. J. Vis. Exp. 2022, 189, e64647. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Z.; Yang, C.; Zhai, X.; Xia, Y.; Liu, J.; Yu, M. Polyethylene Glycol Loxenatide Accelerates Diabetic Wound Healing by Downregulating Systemic Inflammation and Improving Endothelial Progenitor Cell Functions. Int. J. Mol. Sci. 2025, 26, 2367. https://doi.org/10.3390/ijms26052367
Ding Z, Yang C, Zhai X, Xia Y, Liu J, Yu M. Polyethylene Glycol Loxenatide Accelerates Diabetic Wound Healing by Downregulating Systemic Inflammation and Improving Endothelial Progenitor Cell Functions. International Journal of Molecular Sciences. 2025; 26(5):2367. https://doi.org/10.3390/ijms26052367
Chicago/Turabian StyleDing, Zerui, Chunru Yang, Xiaojun Zhai, Yuqi Xia, Jieying Liu, and Miao Yu. 2025. "Polyethylene Glycol Loxenatide Accelerates Diabetic Wound Healing by Downregulating Systemic Inflammation and Improving Endothelial Progenitor Cell Functions" International Journal of Molecular Sciences 26, no. 5: 2367. https://doi.org/10.3390/ijms26052367
APA StyleDing, Z., Yang, C., Zhai, X., Xia, Y., Liu, J., & Yu, M. (2025). Polyethylene Glycol Loxenatide Accelerates Diabetic Wound Healing by Downregulating Systemic Inflammation and Improving Endothelial Progenitor Cell Functions. International Journal of Molecular Sciences, 26(5), 2367. https://doi.org/10.3390/ijms26052367



