Blood-Based DNA Methylation Biomarkers to Identify Risk and Progression of Cardiovascular Disease
Abstract
1. Background
2. Studies Profiling DNA Methylation in CVD
2.1. Global DNA Methylation Studies
2.2. Candidate Gene Studies
2.2.1. Genes Involved in Inflammation and Immunity
2.2.2. MicroRNAs
2.2.3. Genes Involved in Lipid Metabolism
2.2.4. Genes Associated with Stress and Aging
2.2.5. Genes Involved in Endothelial Function
2.3. Genome-Wide Studies
3. Future Perspectives and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5mC | 5-methylcytosine |
| 5hmC | 5-hydroxymethylcytosine |
| Alu | Arthrobacter luteus |
| ARICs | Atherosclerosis Risk in Communities Study |
| AT | Adipose tissue |
| AWHS | Aragon Workers Health Study |
| BMI | Body mass index |
| BMPR2 | Bone morphogenic protein receptor 2 |
| CaG | CARTaGENE |
| CHD | Coronary heart disease |
| CHS | Cardiovascular Health Study |
| CpGs | Cytosine-guanine dinucleotides |
| CRP | C-reactive protein |
| CVD | Cardiovascular disease |
| DILGOM | National FINRISK Study |
| ELISA | Enzyme-linked immunosorbent assay |
| EPICORs | Long-Term Follow-Up of Antithrombotic Management Patterns in Acute Coronary Syndrome Patients |
| FABP3 | Fatty Acid Binding Protein 3 |
| FHS | Framingham Heart Study |
| FOXP3 | Forkhead/winged helix transcription factor 3 |
| FOS | Framingham Offspring Study |
| FPG | Fasting plasma glucose |
| GOLDN | Genetics of Lipid Lowering Drugs and Diet Network |
| GTP | Grady Trauma Project |
| GWASs | Genome-wide association studies |
| HDL | High-density lipoprotein |
| HF | Heart failure |
| HOMA-IR | Homeostatic model assessment for insulin resistance |
| hTERT | Human telomerase reverse transcriptase |
| IFN-γ | Interferon Gamma |
| IL-6 | Interleukin-6 |
| iNOS | Inducible nitric oxide synthase |
| InCHIANTI | The Invecchiare in Chianti study |
| IUCPQ | Quebec Heart and Lung Institute |
| KORA | Kooperative Gesundheitsforschung in der Region Augsburg study |
| LBCs | Lothian Birth Cohorts |
| LDL | Low-density lipoprotein |
| LINE-1 | Long interspersed nuclear element-1 |
| LVAD | Impact of Left Ventricular Assist Devices Implantation on Micro- and Macrovascular Function |
| MCC | Methyl-capture sequencing |
| MetS | Metabolic syndrome |
| MI | Myocardial infarction |
| MeDIP | Methylated DNA immunoprecipitation |
| MiRNA | MicroRNA |
| MZ | Monozygotic |
| NAS | Normative Aging Study |
| NFBC | Northern Finland Birth Cohort |
| NPPA | Atrial natriuretic peptide |
| NR3C1 | Glucocorticoid receptor |
| NSPHS | Northern Sweden Population Health Study |
| PB | Peripheral blood |
| PBLs | Peripheral blood leukocytes |
| PBMCs | Peripheral blood mononuclear cells |
| PIVUSs | Prospective Investigation of the Vasculature in Uppsala Seniors |
| REGICOR | Girona Heart Registry/REgistre GIroní del COR |
| SOAT1 | Sterol-O-Acyltransferase |
| SNP | Single nucleotide polymorph |
| T2D | Type 2 Diabetes |
| TC | Total cholesterol |
| TGs | Triglycerides |
| TLR2 | Toll-like receptor 2 |
| TSS | Transcriptional start site |
| TXNIP | Thioredoxin-interacting protein |
| VAT | Visceral adipose tissue |
| VSIP | Vitamin Intervention for Stroke Prevention |
| VHD | Vascular heart disease |
| WB | Whole blood |
| WBCs | White blood cells |
| WHI-BAA23 | Women’s Health Initiative “Integrative genomics and risk of CHD and related phenotypes in the Women’s Health Initiative” |
| WHI-EMPC | Women’s Health Initiative “Epigenetic Mechanisms of Particulate Matter-Mediated CVD” |
| BP | Blood plasma |
| HDL-C | High-density lipoprotein cholesterol |
References
- Vineis, P.; Stringhini, S.; Porta, M. The environmental roots of non-communicable diseases (NCDs) and the epigenetic impacts of globalization. Environ. Res. 2014, 133, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Boutayeb, A.; Boutayeb, S. The burden of non communicable diseases in developing countries. Int. J. Equity Health 2005, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cardiovascular Diseases. 11 June 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 30 May 2024).
- World Health Organization. Global Status Report on Non Communicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011; Chapter 1. [Google Scholar]
- Kontis, V.; Mathers, C.D.; Rehm, J.; Stevens, G.A.; Shield, K.D.; Bonita, R.; Riley, L.M.; Poznyak, V.; Beaglehole, R.; Ezzati, M. Contribution of six risk factors to achieving the 25×25 non-communicable disease mortality reduction target: A modelling study. Lancet 2014, 384, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Marmot, M.; Friel, S.; Bell, R.; Houweling, T.A.; Taylor, S.; on behalf of the Commission on Social Determinants of Health. Closing the gap in a generation-health equity through action on the social determinants of health. Lancet 2008, 372, 661–669. [Google Scholar] [CrossRef]
- Sharp, G.C.; Relton, C.L. Epigenetics and noncommunicable diseases. Epigenomics 2017, 9, 789–791. [Google Scholar] [CrossRef]
- Eccleston, A.; DeWitt, N.; Gunter, C.; Marte, B.; Nath, D. Introduction Epigenetics. Nature 2007, 447, 395–396. [Google Scholar] [CrossRef]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef]
- Kim, M.; Long, T.I.; Arakawa, K.; Wang, R.; Yu, M.C.; Laird, P.W. DNA methylation as a biomarker for cardiovascular disease risk. PLoS ONE 2010, 5, e9692. [Google Scholar] [CrossRef]
- Al Muftah, W.A.; Al-Shafai, M.; Zaghlool, S.B.; Visconti, A.; Tsai, P.-C.; Kumar, P.; Spector, T.; Bell, J.; Falchi, M.; Suhre, K. Epigenetic associations of type 2 diabetes and BMI in an Arab population. Clin. Epigenet. 2016, 8, 13. [Google Scholar] [CrossRef]
- Kasinska, M.A.; Drzewoski, J.; Sliwinska, A. Epigenetic modifications in adipose tissue—Relation to obesity and diabetes. Arch. Med. Sci. 2016, 12, 1293–1301. [Google Scholar] [CrossRef]
- Duthie, S.J. Epigenetic modifications and human pathologies: Cancer and CVD. Proc. Nutr. Soc. 2011, 70, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Turunen, M.P.; Aavik, E.; Ylä-Herttuala, S. Epigenetics and atherosclerosis. Biochim. Biophys. Acta 2009, 1790, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lu, L.; Liu, M.; Li, X.-C.; Sun, R.-R.; Zheng, Y.; Zhang, P.-Y. Impact of epigenetics in the management of cardiovascular disease—A review. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3097–3104. [Google Scholar]
- Heyn, H.; Esteller, M. DNA methylation profiling in the clinic: Applications and challenges. Nat. Rev. Genet. 2012, 13, 679–692. [Google Scholar] [CrossRef]
- Shenker, N.S.; Polidoro, S.; van Veldhoven, K.; Sacerdote, C.; Ricceri, F.; Birrell, M.A.; Belvisi, M.G.; Brown, R.; Vineis, P.; Flanagan, J.M. Epigenome-wide association study in the European Prospective Investigation into Cancer and Nutrition (EPIC-Turin) identifies novel genetic loci associated with smoking. Hum. Mol. Genet. 2013, 22, 843–851. [Google Scholar] [CrossRef] [PubMed]
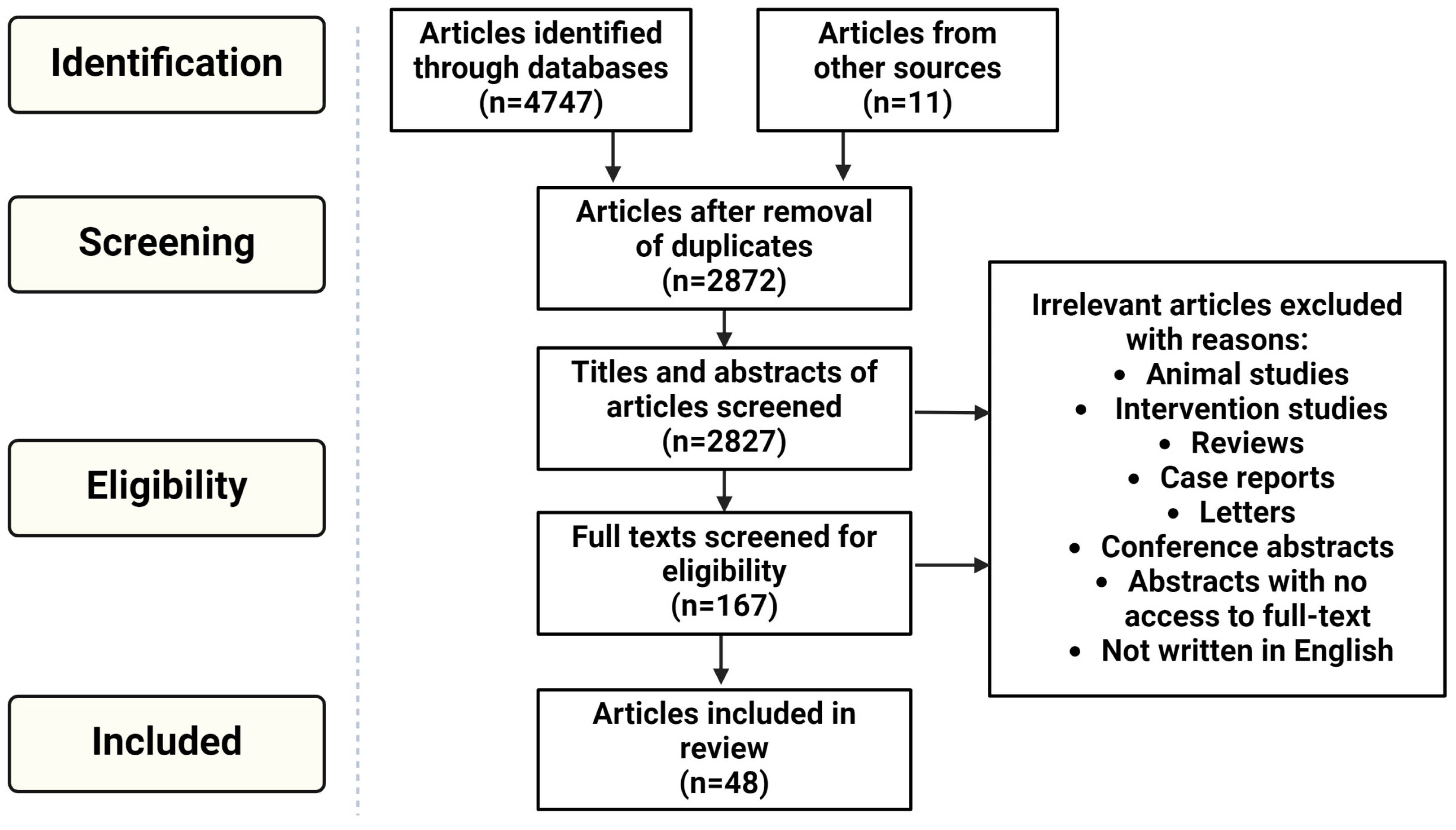
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Castro, R.; Rivera, I.; Struys, E.A.; Jansen, E.E.; Ravasco, P.; Camilo, M.E.; Blom, H.J.; Jakobs, C.; de Almeida, I.T. Increased homocysteine and S-adenosylhomocysteine concentrations and DNA hypomethylation in vascular disease. Clin. Chem. 2003, 49, 1292–1296. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, J.; Garg, G.; Kumar, A.; Patowary, A.; Karthikeyan, G.; Ramakrishnan, L.; Brahmachari, V.; Sengupta, S. Detection of Altered Global DNA Methylation in Coronary Artery Disease Patients. DNA Cell Biol. 2008, 27, 357–365. [Google Scholar] [CrossRef]
- Baccarelli, A.; Wright, R.; Bollati, V.; Litonjua, A.; Zanobetti, A.; Tarantini, L.; Sparrow, D.; Vokonas, P.; Schwartz, J. Ischemic Heart Disease and Stroke in Relation to Blood DNA Methylation. Epidemiology 2010, 21, 819–828. [Google Scholar] [CrossRef]
- Lin, R.-T.; Hsi, E.; Lin, H.-F.; Liao, Y.-C.; Wang, Y.-S.; Juo, S.-H.H. LINE-1 methylation is associated with an increased risk of ischemic stroke in men. Curr. Neurovascular Res. 2014, 11, 4–9. [Google Scholar] [CrossRef]
- Wei, L.; Liu, S.; Su, Z.; Cheng, R.; Bai, X.; Li, X. LINE-1 hypomethylation is associated with the risk of coronary heart disease in Chinese population. Arq. Bras. Cardiol. 2014, 102, 481–488. [Google Scholar] [CrossRef]
- Guarrera, S.; Fiorito, G.; Onland-Moret, N.C.; Russo, A.; Agnoli, C.; Allione, A.; Di Gaetano, C.; Mattiello, A.; Ricceri, F.; Chiodini, P.; et al. Gene-specific DNA methylation profiles and LINE-1 hypomethylation are associated with myocardial infarction risk. Clin. Epigenet. 2015, 7, 133. [Google Scholar] [CrossRef]
- Ramos, R.B.; Fabris, V.; Lecke, S.B.; Maturana, M.A.; Spritzer, P.M. Association between global leukocyte DNA methylation and cardiovascular risk in postmenopausal women. BMC Med. Genet. 2016, 17, 71. [Google Scholar] [CrossRef]
- Istas, G.; Declerck, K.; Pudenz, M.; Szic, K.S.V.; Lendinez-Tortajada, V.; Leon-Latre, M.; Heyninck, K.; Haegeman, G.; Casasnovas, J.A.; Tellez-Plaza, M.; et al. Identification of differentially methylated BRCA1 and CRISP2 DNA regions as blood surrogate markers for cardiovascular disease. Sci. Rep. 2017, 7, 5120. [Google Scholar] [CrossRef]
- Alexeeff, S.E.; Baccarelli, A.A.; Halonen, J.; Coull, B.A.; Wright, R.O.; Tarantini, L.; Bollati, V.; Sparrow, D.; Vokonas, P.; Schwartz, J. Association between blood pressure and DNA methylation of retrotransposons and pro-inflammatory genes. Int. J. Epidemiol. 2013, 42, 270–280. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, Y.; Chang, G.; Duan, Q.; You, L.; Sun, M.; Hu, C.; Gao, L.; Wu, S.; Tao, H.; et al. DNA hydroxymethylation combined with carotid plaques as a novel biomarker for coronary atherosclerosis. Aging 2019, 11, 3170–3181. [Google Scholar] [CrossRef]
- Jiang, D.; Sun, M.; You, L.; Lu, K.; Gao, L.; Hu, C.; Wu, S.; Chang, G.; Tao, H.; Zhang, D. DNA methylation and hydroxymethylation are associated with the degree of coronary atherosclerosis in elderly patients with coronary heart disease. Life Sci. 2019, 224, 241–248. [Google Scholar] [CrossRef]
- Li, N.; Zhu, L.; Zhu, C.; Zhou, H.; Zheng, D.; Xu, G.; Shi, H.; Gao, J.; Li, A.J.; Wang, Z.; et al. BMPR2 promoter methylation and its expression in valvular heart disease complicated with pulmonary artery hypertension. Aging 2021, 13, 24580–24604. [Google Scholar] [CrossRef]
- Zhu, L.; Jia, L.; Liu, Z.; Zhang, Y.; Wang, J.; Yuan, Z.; Hui, R. Elevated Methylation of FOXP3 (Forkhead Box P3)-TSDR (Regulatory T-Cell-Specific Demethylated Region) Is Associated with Increased Risk for Adverse Outcomes in Patients with Acute Coronary Syndrome. Hypertension 2019, 74, 581–589. [Google Scholar] [CrossRef]
- Jia, L.; Zhu, L.; Wang, J.Z.; Wang, X.J.; Chen, J.Z.; Song, L.; Wu, Y.J.; Sun, K.; Yuan, Z.Y.; Hui, R. Methylation of FOXP3 in regulatory T cells is related to the severity of coronary artery disease. Atherosclerosis 2013, 228, 346–352. [Google Scholar] [CrossRef]
- Krishnan, R.; Mani, P.; Sivakumar, P.; Gopinath, V.; Sekar, D. Expression and methylation of circulating microRNA-510 in essential hypertension. Hypertens. Res. 2017, 40, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kent, J.W.; Lee, A.; Cerjak, D.; Ali, O.; Diasio, R.; Olivier, M.; Blangero, J.; A Carless, M.; Kissebah, A.H. Fatty acid binding protein 3 (fabp3) is associated with insulin, lipids and cardiovascular phenotypes of the metabolic syndrome through epigenetic modifications in a northern european family population. BMC Med. Genom. 2013, 6, 9. [Google Scholar] [CrossRef]
- Zhang, D.; Wen, X.; Wu, W.; Xu, E.; Zhang, Y.; Cui, W. Homocysteine-related hTERT DNA demethylation contributes to shortened leukocyte telomere length in atherosclerosis. Atherosclerosis 2013, 231, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.-P.; Guo, Y.-Y.; Che, L.; Wu, X.-Z. Hypomethylation of Interleukin-6 Promoter is Associated with the Risk of Coronary Heart Disease. Arq. Bras. Cardiol. 2016, 107, 131–136. [Google Scholar] [CrossRef]
- Li, Z.; Yu, F.; Zhou, X.; Zeng, S.; Zhan, Q.; Yuan, M.; Yang, Q.; Liu, Y.; Xia, J. Promoter hypomethylation of microRNA223 gene is associated with atherosclerotic cerebral infarction. Atherosclerosis 2017, 263, 237–243. [Google Scholar] [CrossRef]
- Zhao, J.; An, Q.; Goldberg, J.; Quyyumi, A.A.; Vaccarino, V. Promoter methylation of glucocorticoid receptor gene is associated with subclinical atherosclerosis: A monozygotic twin study. Atherosclerosis 2015, 242, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpanah, M.; Heidari, M.M.; Khatami, M.; Hadadzadeh, M. Relationship of hypomethylation CpG islands in interleukin-6 gene promoter with IL-6 mRNA levels in patients with coronary atherosclerosis. J. Cardiovasc. Thorac. Res. 2020, 12, 221–228. [Google Scholar] [CrossRef]
- Li, J.; Zhu, J.; Zhang, Q.; Chen, L.; Ma, S.; Lu, Y.; Shen, B.; Zhang, R.; Zhang, M.; He, Y.; et al. NPPA Promoter Hypomethylation Predicts Central Obesity Development: A Prospective Longitudinal Study in Chinese Adults. Obes. Facts 2022, 15, 257–270. [Google Scholar] [CrossRef]
- Abuzhalihan, J.; Wang, Y.-T.; Ma, Y.-T.; Fu, Z.-Y.; Yang, Y.-N.; Ma, X.; Li, X.-M.; Liu, F.; Chen, B.-D. SOAT1 methylation is associated with coronary heart disease. Lipids Health Dis. 2019, 18, 192. [Google Scholar] [CrossRef]
- Rong, J.; Xu, X.; Xiang, Y.; Yang, G.; Ming, X.; He, S.; Liang, B.; Zhang, X.; Zheng, F. Thioredoxin-interacting protein promotes activation and inflammation of monocytes with DNA demethylation in coronary artery disease. J. Cell. Mol. Med. 2020, 24, 3560–3571. [Google Scholar] [CrossRef]
- Sharma, P.; Garg, G.; Kumar, A.; Mohammad, F.; Kumar, S.R.; Tanwar, V.S.; Sati, S.; Sharma, A.; Karthikeyan, G.; Brahmachari, V.; et al. Genome wide DNA methylation profiling for epigenetic alteration in coronary artery disease patients. Gene 2014, 541, 31–40. [Google Scholar] [CrossRef]
- Rask-Andersen, M.; Martinsson, D.; Ahsan, M.; Enroth, S.; Ek, W.E.; Gyllensten, U.; Johansson, Å. Epigenome-wide association study reveals differential DNA methylation in individuals with a history of myocardial infarction. Hum. Mol. Genet. 2016, 25, 4739–4748. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, N.M.D.; Chen, W.-M.; Brewer, M.S.; Williams, S.R.; Sale, M.M.; Worrall, B.B.; Keene, K.L. Epigenome-Wide Analyses Identify Two Novel Associations with Recurrent Stroke in the Vitamin Intervention for Stroke Prevention Clinical Trial. Front. Genet. 2018, 9, 358. [Google Scholar] [CrossRef]
- Fernández-Sanlés, A.; Sayols-Baixeras, S.; Curcio, S.; Subirana, I.; Marrugat, J.; Elosua, R. DNA methylation and age-Independent cardiovascular risk, an epigenome-Wide approach the REGICOR study (REgistre GIroní del COR). Arterioscler. Thromb. Vasc. Biol. 2018, 38, 645–652. [Google Scholar] [CrossRef]
- Bain, C.R.; Ziemann, M.; Kaspi, A.; Khan, A.W.; Taylor, R.; Trahair, H.; Khurana, I.; Kaipananickal, H.; Wallace, S.; El-Osta, A.; et al. DNA methylation patterns from peripheral blood separate coronary artery disease patients with and without heart failure. ESC Heart Fail. 2020, 7, 2468–2478. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Pang, H.; Chen, B.; Wu, C.; Wang, Y.; Hou, L.; Wang, S.; Sun, D.; Zheng, X. Genome-wide analysis of DNA methylation and risk of cardiovascular disease in a Chinese population. BMC Cardiovasc. Disord. 2021, 21, 240. [Google Scholar] [CrossRef]
- Ammous, F.; Zhao, W.; Lin, L.; Ratliff, S.M.; Mosley, T.H.; Bielak, L.F.; Zhou, X.; Peyser, P.A.; Kardia, S.L.R.; Smith, J.A. Epigenetics of single-site and multi-site atherosclerosis in African Americans from the Genetic Epidemiology Network of Arteriopathy (GENOA). Clin. Epigenetics 2022, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sanlés, A.; Sayols-Baixeras, S.; Subirana, I.; Sentí, M.; Pérez-Fernández, S.; Moura, M.d.C.; Esteller, M.; Marrugat, J.; Elosua, R. DNA methylation biomarkers of myocardial infarction and cardiovascular disease. Clin. Epigenet. 2021, 13, 86. [Google Scholar] [CrossRef]
- Hedman, Å.K.; Mendelson, M.M.; Marioni, R.E.; Gustafsson, S.; Joehanes, R.; Irvin, M.R.; Zhi, D.; Sandling, J.K.; Yao, C.; Liu, C.; et al. Epigenetic Patterns in Blood Associated with Lipid Traits Predict Incident Coronary Heart Disease Events and Are Enriched for Results from Genome-Wide Association Studies. Circ. Cardiovasc. Genet. 2017, 10, e001487. [Google Scholar] [CrossRef]
- Mendelson, M.M.; Marioni, R.E.; Joehanes, R.; Liu, C.; Hedman, Å.K.; Aslibekyan, S.; Demerath, E.W.; Guan, W.; Zhi, D.; Yao, C.; et al. Association of Body Mass Index with DNA Methylation and Gene Expression in Blood Cells and Relations to Cardiometabolic Disease: A Mendelian Randomization Approach. PLoS Med. 2017, 14, e1002215. [Google Scholar] [CrossRef]
- Allum, F.; Hedman, Å.K.; Shao, X.; Cheung, W.A.; Vijay, J.; Guénard, F.; Kwan, T.; Simon, M.-M.; Ge, B.; Moura, C.; et al. Dissecting features of epigenetic variants underlying cardiometabolic risk using full-resolution epigenome profiling in regulatory elements. Nat. Commun. 2019, 10, 1209. [Google Scholar] [CrossRef] [PubMed]
- Chilunga, F.P.; Henneman, P.; Venema, A.; Meeks, K.A.C.; Requena-Méndez, A.; Beune, E.; Mockenhaupt, F.P.; Smeeth, L.; Bahendeka, S.; Danquah, I.; et al. Genome-wide DNA methylation analysis on C-reactive protein among Ghanaians suggests molecular links to the emerging risk of cardiovascular diseases. npj Genom. Med. 2021, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Antoun, E.; Issarapu, P.; di Gravio, C.; Shrestha, S.; Betts, M.; Saffari, A.; Sahariah, S.A.; Sankareswaran, A.; Arumalla, M.; Prentice, A.M.; et al. DNA methylation signatures associated with cardiometabolic risk factors in children from India and The Gambia: Results from the EMPHASIS study. Clin. Epigenet. 2022, 14, 6. [Google Scholar] [CrossRef]
- Agha, G.; Mendelson, M.M.; Ward-Caviness, C.K.; Joehanes, R.; Huan, T.; Gondalia, R.; Salfati, E.; Brody, J.A.; Fiorito, G.; Bressler, J.; et al. Blood Leukocyte DNA Methylation Predicts Risk of Future Myocardial Infarction and Coronary Heart Disease. Circulation 2019, 140, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Feng, Z.-H.; Sun, H.; Zhao, Z.-H.; Yang, S.-B.; Yang, P. The blood genome-wide DNA methylation analysis reveals novel epigenetic changes in human heart failure. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1828–1836. [Google Scholar] [CrossRef]
- Huan, T.; Joehanes, R.; Song, C.; Peng, F.; Guo, Y.; Mendelson, M.; Yao, C.; Liu, C.; Ma, J.; Richard, M.; et al. Genome-wide identification of DNA methylation QTLs in whole blood highlights pathways for cardiovascular disease. Nat. Commun. 2019, 10, 4267. [Google Scholar] [CrossRef]
- Qin, X.; Karlsson, I.K.; Wang, Y.; Li, X.; Pedersen, N.; Reynolds, C.A.; Hägg, S. The epigenetic etiology of cardiovascular disease in a longitudinal Swedish twin study. Clin. Epigenet. 2021, 13, 129. [Google Scholar] [CrossRef]
- Koseler, A.; Ma, F.; Kilic, I.D.; Morselli, M.; Kilic, O.; Pellegrini, M. Genome-wide DNA methylation profiling of blood from monozygotic twins discordant for myocardial infarction. In Vivo 2020, 34, 361–367. [Google Scholar] [CrossRef]
- Nakatochi, M.; Ichihara, S.; Yamamoto, K.; Naruse, K.; Yokota, S.; Asano, H.; Matsubara, T.; Yokota, M. Epigenome-wide association of myocardial infarction with DNA methylation sites at loci related to cardiovascular disease. Clin. Epigenet. 2017, 9, 54. [Google Scholar] [CrossRef]
- Ward-Caviness, C.K.; Agha, G.; Chen, B.H.; Pfeiffer, L.; Wilson, R.; Wolf, P.; Gieger, C.; Schwartz, J.; Vokonas, P.S.; Hou, L.; et al. Analysis of repeated leukocyte DNA methylation assessments reveals persistent epigenetic alterations after an incident myocardial infarction. Clin. Epigenet. 2018, 10, 161. [Google Scholar] [CrossRef]
- Suzuki, M.M.; Bird, A. DNA methylation landscapes: Provocative insights from epigenomics. Nat. Rev. Genet. 2008, 9, 465–476. [Google Scholar] [CrossRef]
- Willmer, T.; Johnson, R.; Louw, J.; Pheiffer, C. Blood-Based DNA Methylation Biomarkers for Type 2 Diabetes: Potential for Clinical Applications. Front. Endocrinol. 2018, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef]
- Cordaux, R.; Batzer, M.A. The impact of retrotransposons on human genome evolution. Nat. Rev. Genet. 2009, 10, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Cash, H.L.; McGarvey, S.T.; Houseman, E.A.; Marsit, C.J.; Hawley, N.L.; Lambert-Messerlian, G.M.; Viali, S.; Tuitele, J.; Kelsey, K.T. Cardiovascular disease risk factors and DNA methylation at the LINE-1 repeat region in peripheral blood from Samoan Islanders. Epigenetics 2011, 6, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Thongsroy, J.; Mutirangura, A. The association between Alu hypomethylation and the severity of hypertension. PLoS ONE 2022, 17, e0270004. [Google Scholar] [CrossRef]
- Chen, R.; Meng, X.; Zhao, A.; Wang, C.; Yang, C.; Li, H.; Cai, J.; Zhao, Z.; Kan, H. DNA hypomethylation and its mediation in the effects of fine particulate air pollution on cardiovascular biomarkers: A randomized crossover trial. Environ. Int. 2016, 94, 614–619. [Google Scholar] [CrossRef]
- Bellavia, A.; Urch, B.; Speck, M.; Brook, R.D.; Scott, J.A.; Albetti, B.; Behbod, B.; North, M.; Valeri, L.; Bertazzi, P.A.; et al. DNA hypomethylation, ambient particulate matter, and increased blood pressure: Findings from controlled human exposure experiments. J. Am. Heart Assoc. 2013, 2, e000212. [Google Scholar] [CrossRef]
- Tsaprouni, L.G.; Yang, T.-P.; Bell, J.; Dick, K.J.; Kanoni, S.; Nisbet, J.; Viñuela, A.; Grundberg, E.; Nelson, C.P.; Meduri, E.; et al. Cigarette smoking reduces DNA methylation levels at multiple genomic loci but the effect is partially reversible upon cessation. Epigenetics 2014, 9, 1382–1396. [Google Scholar] [CrossRef]
- Zakhari, S. Alcohol Metabolism and Epigenetics Changes. Alcohol Res. Curr. Rev. 2013, 35, 6–16. [Google Scholar]
- Bock, C.; Halbritter, F.; Carmona, F.J.; Tierling, S.; Datlinger, P.; Assenov, Y.; Berdasco, M.; Bergmann, A.K.; Booher, K.; Busato, F.; et al. Quantitative comparison of DNA methylation assays for biomarker development and clinical applications. Nat. Biotechnol. 2016, 34, 726–737. [Google Scholar]
- Hartman, J.; Frishman, W.H. Inflammation and atherosclerosis: A review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol. Rev. 2014, 22, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Hashmat, S.; Rudemiller, N.; Lund, H.; Abais-Battad, J.M.; Van Why, S.; Mattson, D.L. Interleukin-6 inhibition attenuates hypertension and associated renal damage in Dahl salt-sensitive rats. Am. J. Physiol. Renal. Physiol. 2016, 311, F555–F561. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ye, D.; Wang, Z.; Pan, H.; Lu, X.; Wang, M.; Xu, Y.; Yu, J.; Zhang, J.; Zhao, M.; et al. The Role of Interleukin-6 Family Members in Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 9, 818890. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.; Zanobetti, A.; Silverman, F.; Schwartz, J.; Coull, B.; Urch, B.; Speck, M.; Brook, J.R.; Manno, M.; Gold, D.R. Baseline repeated measures from controlled human exposure studies: Associations between ambient air pollution exposure and the systemic inflammatory biomarkers IL-6 and fibrinogen. Environ. Health Perspect. 2010, 118, 120–124. [Google Scholar] [CrossRef]
- Frantz, S.; Ertl, G.; Bauersachs, J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, 444–454. [Google Scholar] [CrossRef]
- Liu, V.W.; Huang, P.L. Cardiovascular roles of nitric oxide: A review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc. Res. 2008, 77, 19–29. [Google Scholar] [CrossRef]
- McLaren, J.E.; Ramji, D.P. Interferon gamma: A master regulator of atherosclerosis. Cytokine Growth Factor Rev. 2009, 20, 125–135. [Google Scholar] [CrossRef]
- Kleemann, R.; Zadelaar, S.; Kooistra, T. Cytokines and atherosclerosis: A comprehensive review of studies in mice. Cardiovasc. Res. 2008, 79, 360–376. [Google Scholar] [CrossRef]
- Roshan, M.H.K.; Tambo, A.; Pace, N.P. The Role of TLR2, TLR4, and TLR9 in the Pathogenesis of Atherosclerosis. Int. J. Inflamm. 2016, 2016, 1532832. [Google Scholar] [CrossRef]
- Voloshyna, I.; Littlefield, M.J.; Reiss, A.B. Atherosclerosis and interferon-γ: New insights and therapeutic targets. Trends Cardiovasc. Med. 2014, 24, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Matsui-Hirai, H.; Fukatsu, A.; Sumi, D.; Kano-Hayashi, H.; P, J.A.R.; Iguchi, A. Selective iNOS inhibitor, ONO1714 successfully retards the development of high-cholesterol diet induced atherosclerosis by novel mechanism. Atherosclerosis 2006, 187, 316–324. [Google Scholar] [CrossRef]
- Chong, C.-R.; Chan, W.P.A.; Nguyen, T.H.; Liu, S.; Procter, N.E.K.; Ngo, D.T.; Sverdlov, A.L.; Chirkov, Y.Y.; Horowitz, J.D. Thioredoxin-interacting protein: Pathophysiology and emerging pharmacotherapeutics in cardiovascular disease and diabetes. Cardiovasc. Drugs Ther. 2014, 28, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Tárraga, C.; Jiménez-Conde, J.; Giralt-Steinhauer, E.; Mola-Caminal, M.; Vivanco-Hidalgo, R.M.; Ois, A.; Rodríguez-Campello, A.; Cuadrado-Godia, E.; Sayols-Baixeras, S.; Elosua, R.; et al. Epigenome-wide association study identifies TXNIP gene associated with type 2 diabetes mellitus and sustained hyperglycemia. Hum. Mol. Genet. 2015, 25, 609–619. [Google Scholar] [CrossRef]
- Kulkarni, H.; Kos, M.Z.; Neary, J.; Dyer, T.D.; Kent, J.W.; Göring, H.H.; Cole, S.A.; Comuzzie, A.G.; Almasy, L.; Mahaney, M.C.; et al. Novel epigenetic determinants of type 2 diabetes in Mexican-American families. Hum. Mol. Genet. 2015, 24, 5330–5344. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.C.; Loh, M.; Lehne, B.; Drong, A.; Kriebel, J.; Motta, V.; Wahl, S.; Elliott, H.R.; Rota, F.; Scott, W.R.; et al. Epigenome-wide association of DNA methylation markers in peripheral blood from Indian Asians and Europeans with incident type 2 diabetes: A nested case-control study. Lancet Diabetes Endocrinol. 2015, 3, 526–534. [Google Scholar] [CrossRef]
- Florath, I.; Butterbach, K.; Heiss, J.; Bewerunge-Hudler, M.; Zhang, Y.; Schöttker, B.; Brenner, H. Type 2 diabetes and leucocyte DNA methylation: An epigenome-wide association study in over 1500 older adults. Diabetologia 2016, 59, 130–138. [Google Scholar] [CrossRef]
- Iung, B.; Vahanian, A. Epidemiology of valvular heart disease in the adult. Nat. Rev. Cardiol. 2011, 8, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.W.; Girerd, B.; Montani, D.; Wang, X.-J.; Galiè, N.; Austin, E.D.; Elliott, G.; Asano, K.; Grünig, E.; Yan, Y.; et al. BMPR2 mutations and survival in pulmonary arterial hypertension: An individual participant data meta-analysis. Lancet Respir. Med. 2016, 4, 129–137. [Google Scholar] [CrossRef]
- Guignabert, C.; Bailly, S.; Humbert, M. Restoring BMPRII functions in pulmonary arterial hypertension: Opportunities, challenges and limitations. Expert Opin. Ther. Targets 2017, 21, 181–190. [Google Scholar] [CrossRef]
- Hautefort, A.; Mendes-Ferreira, P.; Sabourin, J.; Manaud, G.; Bertero, T.; Rucker-Martin, C.; Riou, M.; Adão, R.; Manoury, B.; Lambert, M.; et al. Bmpr2 mutant rats develop pulmonary and cardiac characteristics of pulmonary arterial hypertension. Circulation 2019, 139, 932–948. [Google Scholar] [CrossRef]
- Ali, A.J.; Makings, J.; Ley, K. Regulatory T cell stability and plasticity in atherosclerosis. Cells 2020, 9, 2665. [Google Scholar] [CrossRef] [PubMed]
- Joly, A.-L.; Seitz, C.; Liu, S.; Kuznetsov, N.V.; Gertow, K.; Westerberg, L.S.; Paulsson-Berne, G.; Hansson, G.K.; Andersson, J. Alternative splicing of FOXP3 controls regulatory T cell effector functions and is associated with human atherosclerotic plaque stability. Circ. Res. 2018, 122, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.M.; Sanad, E.F.; Hamdy, N.M. MicroRNAs Role in Non-Communicable Diseases and Link to Multidrug Resistance, Regulation or Alteration. Preprints 2021. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Yu, Z.; Li, Y.; Su, Z.; NI, L.; Yang, S.; Gui, Y.; Lai, Y. Downregulated microRNA-510-5p acts as a tumor suppressor in renal cell carcinoma. Mol. Med. Rep. 2015, 12, 3061–3066. [Google Scholar] [CrossRef]
- Gaj, P.; Zagozdzon, R. In silicoanalysis of microRNA-510 as a potential oncomir in human breast cancer. Breast Cancer Res. 2014, 16, 403. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.J.; Mills, J.N.; Bandurraga, S.G.; Nogueira, L.M.; Mason, N.J.; Camp, E.R.; Larue, A.C.; Turner, D.P.; Findlay, V.J. MicroRNA-510 promotes cell and tumor growth by targeting peroxiredoxin1 in breast cancer. Breast Cancer Res. 2013, 15, R70. [Google Scholar] [CrossRef]
- Zhang, M.-W.; Shen, Y.-J.; Shi, J.; Yu, J.-G. MiR-223-3p in cardiovascular diseases: A biomarker and potential therapeutic target. Front. Cardiovasc. Med. 2021, 7, 610561. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Metabolic syndrome: From the genetics to the pathophysiology. Curr. Hypertens. Rep. 2011, 13, 149–157. [Google Scholar] [CrossRef]
- Blüher, S.; Käpplinger, J.; Herget, S.; Reichardt, S.; Böttcher, Y.; Grimm, A.; Kratzsch, J.; Petroff, D. Cardiometabolic risk markers, adipocyte fatty acid binding protein (aFABP) and the impact of high-intensity interval training (HIIT) in obese adolescents. Metab. Clin. Exp. 2017, 68, 77–87. [Google Scholar] [CrossRef]
- Zhuang, L.; Mao, Y.; Liu, Z.; Li, C.; Jin, Q.; Lu, L.; Tao, R.; Yan, X.; Chen, K. FABP3 deficiency exacerbates metabolic derangement in cardiac hypertrophy and heart failure via PPARα pathway. Front. Cardiovasc. Med. 2021, 8, 722908. [Google Scholar] [CrossRef]
- Qian, Q.; Kuo, L.; Yu, Y.-T.; Rottman, J.N. A concise promoter region of the heart fatty acid–binding protein gene dictates tissue-appropriate expression. Circ. Res. 1999, 84, 276–289. [Google Scholar] [CrossRef]
- Hai, Q.; Smith, J.D. Acyl-Coenzyme A: Cholesterol Acyltransferase (ACAT) in Cholesterol Metabolism: From Its Discovery to Clinical Trials and the Genomics Era. Metabolites 2021, 11, 543. [Google Scholar] [CrossRef]
- Xu, N.; Meng, H.; Liu, T.-Y.; Feng, Y.-L.; Qi, Y.; Zhang, D.-H.; Wang, H.-L. Sterol O-acyltransferase 1 deficiency improves defective insulin signaling in the brains of mice fed a high-fat diet. Biochem. Biophys. Res. Commun. 2018, 499, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.-K.; Wang, C.-Y. Telomeres and telomerase in cardiovascular diseases. Genes 2016, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Kyo, S.; Takakura, M.; Fujiwara, T.; Inoue, M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 2008, 99, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Li-Tempel, T.; Larra, M.F.; Sandt, E.; Mériaux, S.B.; Schote, A.B.; Schächinger, H.; Muller, C.P.; Turner, J.D. The cardiovascular and hypothalamus-pituitary-adrenal axis response to stress is controlled by glucocorticoid receptor sequence variants and promoter methylation. Clin. Epigenetics 2016, 8, 12. [Google Scholar] [CrossRef]
- Willmer, T.; Goedecke, J.H.; Dias, S.; Louw, J.; Pheiffer, C. DNA methylation of FKBP5 in South African women: Associations with obesity and insulin resistance. Clin. Epigenet. 2020, 12, 141. [Google Scholar] [CrossRef]
- Volpe, M.; Rubattu, S.; Burnett, J.J. Natriuretic peptides in cardiovascular diseases: Current use and perspectives. Eur. Heart J. 2014, 35, 419–425. [Google Scholar] [CrossRef]
- Zois, N.E.; Bartels, E.D.; Hunter, I.; Kousholt, B.S.; Olsen, L.H.; Goetze, J.P. Natriuretic peptides in cardiometabolic regulation and disease. Nat. Rev. Cardiol. 2014, 11, 403–412. [Google Scholar] [CrossRef]
- Gouil, Q.; Keniry, A. Latest techniques to study DNA methylation. Essays Biochem. 2019, 63, 639–648. [Google Scholar] [PubMed]
- Lister, R.; Pelizzola, M.; Dowen, R.H.; Hawkins, R.D.; Hon, G.; Tonti-Filippini, J.; Nery, J.R.; Lee, L.; Ye, Z.; Ngo, Q.-M.; et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 2009, 462, 315–322. [Google Scholar] [CrossRef]
- Jin, Z.; Liu, Y. DNA methylation in human diseases. Genes Dis. 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Brott, B.K.; Sokol, S.Y. A Vertebrate Homolog of the Cell Cycle Regulator Dbf4 Is an Inhibitor of Wnt Signaling Required for Heart Development. Dev. Cell 2005, 8, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Kempf, T.; Eden, M.; Strelau, J.; Naguib, M.; Willenbockel, C.; Tongers, J.; Heineke, J.; Kotlarz, D.; Xu, J.; Molkentin, J.D.; et al. The transforming growth factor-β superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ. Res. 2006, 98, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.Q.; Ng, K.; Dhillon, O.; Kelly, D.; Quinn, P.; Squire, I.B.; Davies, J.E.; Ng, L.L. Growth differentiation factor-15 as a prognostic marker in patients with acute myocardial infarction. Eur. Heart J. 2009, 30, 1057–1065. [Google Scholar] [CrossRef]
- Wollert, K.C.; Kempf, T.; Peter, T.; Olofsson, S.; James, S.; Johnston, N.; Lindahl, B.; Horn-Wichmann, R.; Brabant, G.; Simoons, M.L.; et al. Prognostic value of growth-differentiation factor-15 in patients with non–ST-elevation acute coronary syndrome. Circulation 2007, 115, 962–971. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, L.; Hong, X.; Si, J.; Cao, W.; Yu, C.; Huang, T.; Sun, D.; Liao, C.; Pang, Y.; et al. Temporal associations between leukocytes DNA methylation and blood lipids: A longitudinal study. Clin. Epigenet. 2022, 14, 132. [Google Scholar] [CrossRef]
- Jitrapakdee, S.; Wallace, J.C. Structure, function and regulation of pyruvate carboxylase. Biochem. J. 1999, 340 Pt 1, 1–16. [Google Scholar] [CrossRef]
- Singh, K.K.; Shukla, P.C.; Quan, A.; Al-Omran, M.; Lovren, F.; Pan, Y.; Brezden-Masley, C.; Ingram, A.J.; Stanford, W.L.; Teoh, H.; et al. BRCA1 is a novel target to improve endothelial dysfunction and retard atherosclerosis. J. Thorac. Cardiovasc. Surg. 2013, 146, 949–960.e4. [Google Scholar] [CrossRef]
- Liu, X.S.; Yang, J.W.; Zeng, J.; Chen, X.Q.; Gao, Y.; Kui, X.Y.; Liu, X.Y.; Zhang, Y.; Zhang, Y.H.; Pei, Z.J. SLC2A1 is a Diagnostic Biomarker Involved in Immune Infiltration of Colorectal Cancer and Associated With m6A Modification and ceRNA. Front. Cell Dev. Biol. 2022, 10, 853596. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Wang, Q.; Brainard, R.E.; Watson, L.J.; Jones, S.P.; Epstein, P.N. Reduced cardiac fructose 2,6 bisphosphate increases hypertrophy and decreases glycolysis following aortic constriction. PLoS ONE 2013, 8, e53951. [Google Scholar] [CrossRef]
- Iida, R.; Ueki, M.; Yasuda, T. Deficiency of M-LP/Mpv17L leads to development of β-cell hyperplasia and improved glucose tolerance via activation of the Wnt and TGF-β pathways. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166318. [Google Scholar] [CrossRef]
- Szabadosova, V.; Boronova, I.; Ferenc, P.; Tothova, I.; Bernasovska, J.; Zigova, M.; Kmec, J.; Bernasovsky, I. Analysis of selected genes associated with cardiomyopathy by next-generation sequencing. J. Clin. Lab. Anal. 2018, 32, e22254. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, T.; Feng, R.; Xia, T.; Huang, H.; Liu, C.; Sun, C. Hoxa5 alleviates obesity-induced chronic inflammation by reducing ER stress and promoting M2 macrophage polarization in mouse adipose tissue. J. Cell. Mol. Med. 2019, 23, 7029–7042. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.J.H.; Ghafoor, A.A.; Liu, Y.; Sandford, A.; Jen, R.; Daniele, P.; Taylor, C.; Peres, B.U.; Ayas, N.T. The rs579459 ABO gene polymorphism and risk of incident cardiovascular events in obstructive sleep apnea: A pilot study. Sleep Breath 2023, 27, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, P.; Lee, D.; Hoffmann, T.J.; Ehret, G.B.; Arking, D.; Ranatunga, D.; Li, M.; Grove, M.L.; Boerwinkle, E.; Schaefer, C.; et al. Analysis of putative cis-regulatory elements regulating blood pressure variation. Hum. Mol. Genet. 2020, 29, 1922–1932. [Google Scholar] [CrossRef]
- Marston, N.A.; Giugliano, R.P.; Melloni, G.E.; Park, J.G.; Morrill, V.; Blazing, M.A.; Ference, B.; Stein, E.; Stroes, E.S.; Braunwald, E.; et al. Association of Apolipoprotein B-Containing Lipoproteins and Risk of Myocardial Infarction in Individuals with and Without Atherosclerosis: Distinguishing Between Particle Concentration, Type, and Content. JAMA Cardiol. 2022, 7, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Talmud, P.J.; Drenos, F.; Shah, S.; Shah, T.; Palmen, J.; Verzilli, C.; Gaunt, T.R.; Pallas, J.; Lovering, R.; Li, K.; et al. Gene-centric association signals for lipids and apolipoproteins identified via the HumanCVD BeadChip. Am. J. Hum. Genet. 2009, 85, 628–642. [Google Scholar] [CrossRef]
- Turcot, V.; Lu, Y.; Highland, H.M.; Schurmann, C.; Justice, A.E.; Fine, R.S.; Bradfield, J.P.; Esko, T.; Giri, A.; Graff, M.; et al. Protein-altering variants associated with body mass index implicate pathways that control energy intake and expenditure in obesity. Nat. Genet. 2018, 50, 26–41. [Google Scholar] [CrossRef]
- Liang, X.; Ding, Y.; Zhang, Y.; Chai, Y.-H.; He, J.; Chiu, S.-M.; Gao, F.; Tse, H.-F.; Lian, Q. Activation of NRG1-ERBB4 signaling potentiates mesenchymal stem cell-mediated myocardial repairs following myocardial infarction. Cell Death Dis. 2015, 6, e1765. [Google Scholar] [CrossRef]
- Zhang, X.; He, D.; Xiang, Y.; Wang, C.; Liang, B.; Li, B.; Qi, D.; Deng, Q.; Yu, H.; Lu, Z.; et al. DYSF promotes monocyte activation in atherosclerotic cardiovascular disease as a DNA methylation-driven gene. Transl. Res. 2022, 247, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.-Q.; Li, L.; Girelli, D.; Seidelmann, S.B.; Rao, S.; Fan, C.; Park, J.E.; Xi, Q.; Li, J.; Hu, Y.; et al. An LRP8 variant is associated with familial and premature coronary artery disease and myocardial infarction. Am. J. Hum. Genet. 2007, 81, 780–791. [Google Scholar] [CrossRef]
- Rahm, A.-K.; Gramlich, D.; Wieder, T.; Müller, M.E.; Schoeffel, A.; A El Tahry, F.; Most, P.; Heimberger, T.; Sandke, S.; Weis, T.; et al. Trigger-Specific Remodeling of KCa2 Potassium Channels in Models of Atrial Fibrillation. Pharmacogenom. Pers. Med. 2021, 14, 579–590. [Google Scholar] [CrossRef]
- Zhong, K.; Kant, S.; Sellke, F.; Feng, J. SK channels and heart disease. In Ion Transporters—From Basic Properties to Medical Treatment; IntechOpen: London, UK, 2022. [Google Scholar]
- Ma, B.; Wilker, E.H.; Willis-Owen, S.A.G.; Byun, H.-M.; Wong, K.C.C.; Motta, V.; Baccarelli, A.A.; Schwartz, J.; Cookson, W.O.C.M.; Khabbaz, K.; et al. Predicting DNA methylation level across human tissues. Nucleic Acids Res. 2014, 42, 3515–3528. [Google Scholar] [CrossRef] [PubMed]
- Houseman, E.A.; Accomando, W.P.; Koestler, D.C.; Christensen, B.C.; Marsit, C.J.; Nelson, H.H.; Wiencke, J.K.; Kelsey, K.T. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinform. 2012, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sanlés, A.; Sayols-Baixeras, S.; Subirana, I.; Degano, I.R.; Elosua, R. Association between DNA methylation and coronary heart disease or other atherosclerotic events: A systematic review. Atherosclerosis 2017, 263, 325–333. [Google Scholar] [CrossRef]
- Muka, T.; Koromani, F.; Portilla, E.; O’Connor, A.; Bramer, W.M.; Troup, J.; Chowdhury, R.; Dehghan, A.; Franco, O.H. The role of epigenetic modifications in cardiovascular disease: A systematic review. Int. J. Cardiol. 2016, 212, 174–183. [Google Scholar] [CrossRef]
- Braun, K.V.; Voortman, T.; Dhana, K.; Troup, J.; Bramer, W.M.; Troup, J.; Chowdhury, R.; Dehghan, A.; Muka, T.; Franco, O.H. The role of DNA methylation in dyslipidaemia: A systematic review. Prog. Lipid Res. 2016, 64, 178–191. [Google Scholar] [CrossRef]
- Anderson, D.R.; Poterucha, J.T.; Mikuls, T.R.; Duryee, M.J.; Garvin, R.P.; Klassen, L.W.; Shurmur, S.W.; Thiele, G.M. IL-6 and its receptors in coronary artery disease and acute myocardial infarction. Cytokine 2013, 62, 395–400. [Google Scholar] [CrossRef]
- Klingenberg, R.; Gerdes, N.; Badeau, R.M.; Gisterå, A.; Strodthoff, D.; Ketelhuth, D.F.; Lundberg, A.M.; Rudling, M.; Nilsson, S.K.; Olivecrona, G.; et al. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J. Clin. Investig. 2013, 123, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Michels, K.B.; Binder, A.M.; Dedeurwaerder, S.; Epstein, C.B.; Greally, J.M.; Gut, I.; Houseman, E.A.; Izzi, B.; Kelsey, K.T.; Meissner, A.; et al. Recommendations for the design and analysis of epigenome-wide association studies. Nat. Methods 2013, 10, 949–955. [Google Scholar] [CrossRef]
- McGarry, J.D.; Brown, N.F. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur. J. Biochem. 1997, 244, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Keung, W.; Ussher, J.R.; Jaswal, J.S.; Raubenheimer, M.; Lam, V.H.; Wagg, C.S.; Lopaschuk, G.D. Inhibition of carnitine palmitoyltransferase-1 activity alleviates insulin resistance in diet-induced obese mice. Diabetes 2013, 62, 711–720. [Google Scholar] [CrossRef]
- Rohlenova, K.; Veys, K.; Miranda-Santos, I.; De Bock, K.; Carmeliet, P. Endothelial cell metabolism in health and disease. Trends Cell Biol. 2018, 28, 224–236. [Google Scholar] [CrossRef]
- Xia, Y.; Brewer, A.; Bell, J.T. DNA methylation signatures of incident coronary heart disease: Findings from epigenome-wide association studies. Clin. Epigenet. 2021, 13, 186. [Google Scholar] [CrossRef]
- Grieshober, L.; Graw, S.; Barnett, M.J.; Thornquist, M.D.; Goodman, G.E.; Chen, C.; Koestler, D.C.; Marsit, C.J.; Doherty, J.A. AHRR methylation in heavy smokers: Associations with smoking, lung cancer risk, and lung cancer mortality. BMC Cancer 2020, 20, 905. [Google Scholar] [CrossRef] [PubMed]
- Philibert, R.A.; Dogan, M.V.; Mills, J.A.; Long, J.D. AHRR Methylation is a Significant Predictor of Mortality Risk in Framingham Heart Study. J. Insur. Med. 2019, 48, 79–89. [Google Scholar] [CrossRef]
- Bacalini, M.G.; Boattini, A.; Gentilini, D.; Giampieri, E.; Pirazzini, C.; Giuliani, C.; Fontanesi, E.; Remondini, D.; Capri, M.; Del Rio, A.; et al. A meta-analysis on age-associated changes in blood DNA methylation: Results from an original analysis pipeline for Infinium 450k data. Aging 2015, 7, 97–109. [Google Scholar] [CrossRef]
- Chamberlain, J.D.; Nusslé, S.; Chapatte, L.; Kinnaer, C.; Petrovic, D.; Pradervand, S.; Bochud, M.; Harris, S.E.; Corley, J.; Cox, S.R.; et al. Blood DNA methylation signatures of lifestyle exposures: Tobacco and alcohol consumption. Clin. Epigenet. 2022, 14, 155. [Google Scholar] [CrossRef]
- Wang, Y.; Hannon, E.; Grant, O.A.; Gorrie-Stone, T.J.; Kumari, M.; Mill, J.; Zhai, X.; McDonald-Maier, K.D.; Schalkwyk, L.C. DNA methylation-based sex classifier to predict sex and identify sex chromosome aneuploidy. BMC Genom. 2021, 22, 484. [Google Scholar] [CrossRef] [PubMed]

| Author | Population | Tissue Type | Method | Study Outcome |
|---|---|---|---|---|
| [19] | European; 7 male atherosclerotic vascular patients, 7 healthy male controls | WBCs | 5mC | Atherosclerosis patients had significantly higher plasma homocysteine concentrations and significantly lower genomic DNA methylation. |
| [20] | Indian; 137 coronary artery disease patients, 150 controls | PBLs | HpaII/MspI ratio | Global hypermethylation was associated with coronary artery disease. |
| [21] | American; 712 elderly men | WB | LINE-1 | Blood LINE-1 hypomethylation was associated with ischemic heart disease. |
| [10] | Singapore Chinese; 129 men, 157 women | PBLs | ALU and Satellite 2 repetitive element methylation | Positive correlation in global DNA methylation in men with a history of CVD or its predisposing conditions but not in women. |
| [22] | Chinese; 280 ischemic stroke patients and 280 healthy controls; 40 and 80 years | WB | LINE-1 | Global hypomethylation was associated with higher risk for ischemic stroke in men but not in women. |
| [23] | Chinese; 334 CHD patients and 788 healthy controls | PBLs | LINE-1 | LINE-1 hypomethylation was significantly associated with age, elevated total cholesterol, diagnosis of diabetes, and risk of CHD. |
| [24] | Italian European; 292 myocardial infarction patients and 292 matched controls | WBCs | LINE-1 | Global hypomethylation was associated with myocardial infarction risk in men. |
| [25] | Brazilian; 90 postmenopausal women between 45 and 65 years | PBLs | 5mC | Hypomethylation was associated with higher CVD risk. |
| [26] | German; 8 atherosclerosis patients and 8 healthy controls; 35–60 years | WB | 5mC/5hmC | No statistically significant changes in global DNA methylation in atherosclerosis patients compared to healthy individuals. |
| [27] | American; longitudinal study of 789 elderly subjects followed over 10 years | WB | LINE-1 and Alu | Positive association identified between Alu hypermethylation and diastolic blood pressure. |
| [28] | Chinese; 91 atherosclerosis patients and 22 controls | PBMCs | 5mC/5hmC | 5mC and 5hmC levels were significantly correlated with Gensini (severity of atherosclerosis). |
| [29] | Chinese; 44 patients with cardiovascular disease and 42 controls | PBMCs | 5mC/5hmC | 5mC and 5hmC levels were higher in CVD patients than in control subjects. |
| Author (Year) | Genes Investigated | Country | Sample Size | Sex | Tissue Type | Method | Study Outcome |
|---|---|---|---|---|---|---|---|
| [27] | TLR2, IFN-γ, and iNOS | United States | Longitudinal study of 789 subjects | M | WB | Bisulphite pyrosequencing | Methylation of TLR2 and iNOS positively correlated with blood pressure, whilst IFN-γ promoter methylation negatively associated with blood pressure. |
| [30] | BMPR2 | China | VHD = 82 Controls = 57 | M and F | PB | Bisulphite pyrosequencing | The BMPR2 promoter was hypermethylated in the VHD patients compared to healthy controls and correlated with VHD severity. |
| [31] | FOXP3 | China | 171 patients with CHD | M | PB | Methylation specific PCR | Increased DNA methylation of FOXP3 associated with severity of atherosclerosis. |
| [32] | FOXP3 | China | CHD = 188 Controls = 68 | M | WB | Bisulfite sequencing | Increased DNA methylation of FOXP3 associated with severity of CHD. |
| [33] | microRNA-510 | India | Hypertension = 20 Controls = 20 | M and F | WB | Methylation specific PCR | The miR-510 promoter was hypermethylated in the hypertensive patients versus healthy controls. |
| [34] | FABP3 (17 CpGs) | Europe | 517 individuals with varying parameters of metabolic syndrome | M and F | PBMCs | EpiTYPER MassArray | Average methylation of 17 CpG sites within the FABP3 positively associated with total cholesterol, whilst individual CpG methylation associated with diastolic blood pressure, insulin resistance, and LDL-cholesterol. |
| [35] | hTERT (5 CpGs) | China | Atherosclerosis = 197 Controls = 165 | M and F | PBLs | Methylation-specific PCR | hTERT was hypomethylated in atherosclerosis patients compared to healthy individuals and associated with a concomitant decrease in hTERT mRNA levels. |
| [36] | IL-6 (2 CpGs) | China | CHD = 212 Controls = 218 | M and F | PBLs | Bisulfite pyrosequencing | Hypomethylation at two promoter CpGs in CHD patients compared to controls. Combined methylation scores of both CpGs correlated with CHD risk. |
| [37] | miRNA-233 (9 CpGs) | China | Atherosclerosis = 23 Controls = 32 | M and F | PBLs | Bisulfite sequencing | Hypomethylation of seven CpG sites were detected in the miRNA-233 promoter in participants with atherosclerotic cerebral infarction compared to healthy controls. DNA methylation of these CpG sites correlated with total plasma cholesterol levels. |
| [38] | NR3C1 (22 CpGs) | United States | 84 MZ twin pairs | M | PBLs | Bisulfite pyrosequencing | Altered DNA methylation in exon 1F of the NR3C1 promoter positively associated with markers of early subclinical atherosclerosis. |
| [39] | IL-6 (6 CpGs) | Iran | Atherosclerosis = 35 Controls = 30 | M and F | PB | Bisulfite sequencing | Six evolutionary conserved CpG sites in the distal IL-6 promoter were hypomethylated in the atherosclerosis patients compared to the controls. DNA methylation of these CpG sites inversely correlated with IL-6 mRNA levels. |
| [40] | NPPA (9 CpGs) | China | Longitudinal study of 2498 subjects | M and F | PB | Bisulfite sequencing | Hypermethylation of a single CpG site situated −459 bp from the NPPA TSS associated with reduced risk of developing CVDs. |
| [41] | SOAT1 (26 CpGs) | China | CHD = 99 Controls = 89 | M and F | WB | Bisulfite sequencing | Hypomethylation of seven CpG sites associated with incidence of CHD. |
| [42] | TXNIP (1 CpG) | China | CAD = 54 Controls = 54 | M and F | PBLs | Pyrosequencing | DNA methylation of TXNIP at a single CpG site, located at cg19693031, was significantly decreased in cases compared to controls. DNA methylation inversely correlated with TXNIP mRNA expression, fasting plasma glucose, and total cholesterol. |
| Author (Year) | Country | Sample Size | Sex | Tissue Type | Method | Study Outcome |
|---|---|---|---|---|---|---|
| [43] | India | Discovery cohort CHD = 18 Controls = 18 Validation cohort CHD = 48 Controls = 48 | M | PBMCs | Bisulphite sequencing via 454 platform | Hypermethylation was identified within 19 regions in CHD patients versus controls. Twelve differentially methylated regions were assessed in the validation cohort, which identified the hypermethylation of 6 CpG sites in the CHD group compared to controls. Three of these sites mapped to the intronic region of STE20 related adaptor alpha (STRADA), one was situated in the intronic region of heat shock protein 90 beta family member 3 pseudogene (HSP90B3P), and two were situated within exon 1 of Complement C1q like 4 (C1QL4). |
| [24] | Italian and Dutch | Discovery cohort MI = 292 Controls = 292 Validation cohort MI = 317 Controls = 262 | M and F | WBCs | Discovery: Illumina Infinium HumanMethylation450 BeadChip Validation: MALDI-TOF mass spectrometry | Results from the discovery cohort revealed the hypomethylation of 15 CpG sites within exon 1 of the zinc finger and BTB domain-containing protein 12 (ZBTB12) gene, which correlated with MI, and was replicated in the validation cohort. |
| [44] | Swedish | CVD = 238 Controls = 491 | M | PB | Illumina Infinium HumanMethylation450 Beadchip | Altered DNA methylation was observed at 211 CpG sites, mapping to 196 genes, which collectively associated with a history of MI. Forty-two of the differentially methylated regions mapped to genes previously associated with MI in the literature. Some of these genes identified included Dysferlin (DYSF) and Neuregulin 1 (NRG1). The link between MI and methylation was independent of BMI. |
| [45] | European and African | Stroke patients = 180 | M and F | WB | Illumina Infinium HumanMethylation450 BeadChip | Hypermethylation at two CpG sites located in Ankyrin Repeat And SOCS Box Containing 10 (cg22812874, ASB10) and Tetratricopeptide repeat domain 37 (cg00340919, TTC37) associated with plasma homocysteine levels in individuals with recurrent stroke. This association was found in Europeans but not in Africans. |
| [46] | American and European | Discovery REGICOR cohort n = 645 Validation FOS cohort n = 2542 | M and F | WB | Illumina Infinium HumanMethylation450 BeadChip | Eight CpG sites annotating to four genes [Aryl hydrocarbon receptor repressor (AHRR), Carnitine Palmitoyltransferase 1A (CPT1A), Peptidylprolyl Isomerase F (PPIF), and Strawberry Notch Homolog 2 (SBNO2)], and three intergenic regions were differentially methylated and associated with cardiovascular risk, independent of age. |
| [47] | European | T2D + HF = 10 Control = 10 | M | WB | Discovery: Methyl-binding domain-capture sequencing Validation: Bisulfite sequencing | Differential methylation was identified in both gene body and enhancer elements, with reduced methylation signatures observed in coding regions of [Histone deacetylase 9 (HDAC9), MicroRNA 3675 (MIR3675), Jumonji- and AT-rich interaction domain (ARID)-domain-containing protein (JARID2), and Gremlin 1 (GREM1)], and with increased methylation in Decaprenyl Diphosphate Synthase Subunit 2 (PDSS2), linked to the extreme phenotypes of heart failure. |
| [48] | Chinese | CVD = 83 Controls = 83 | M and F | WB | Illumina Infinium HumanMethylation850 EPIC BeadChip | Eight CVD-related CpGs sites were identified, with the combined use of all eight showing an excellent predictive power for CVD development. The 8 CpGs included: 1 hypermethylated CpG of cytochrome P450, family 8, subfamily B, and polypeptide 1 (cg07655795, CYP8B1), being associated with increased CVD risk, whilst the remaining seven hypomethylated CpGs of Spondin 1 (cg06901278, cg11651314, and SPON1), Phosphofurin acidic cluster sorting protein 1 (cg03914662, PACS1), Uveal Autoantigen With Coiled-Coil Domains And Ankyrin Repeats (cg09306458, UACA), Coiled-coil domain containing 50 (cg05359217, cg05946546, CCDC50), and Haemagglutinin stalk domain 17811 (cg02518222, HASD17811) were associated with an increased CVD risk. |
| [49] | African American and European | Atherosclerosis = 93 | M and F | WB | Illumina Infinium HumanMethylation850 EPIC BeadChip | Four CpGs were associated with atherosclerosis after adjusting for CVD risk. The CpGs included Aryl hydrocarbon receptor repressor (cg05575921, cg21161138, AHRR), Growth differentiation factor 11 (cg09936388, GF11), and Leucine Rich Repeat Containing 52 (cg181168448, LRRC52). |
| [50] | American and European | Discovery cohort (REGICOR-1) MI = 191 Controls = 195 Validation cohort (REGICOR-2) MI = 101 Controls = 103 Follow-up association studies WHI = 1863 FOS = 2540 | M and F | WB | Illumina Infinium HumanMethylation850 EPIC BeadChip | A total of 34 of the identified CpG sites associated with MI in both REGICOR-1 and REGICOR-2 samples. Of these, 25 were located within gene-coding regions, whereas 9 mapped to intergenic regions. Importantly, 3 of the identified CpG sites associated with CVD in follow up association studies using the WHI and FOS cohorts. The 3 identified CpGs were Aryl hydrocarbon receptor repressor (cg05575921, AHRR), F2R like thrombin or trypsin receptor 3 (cg21566642, F2RL3), and myeloperoxidase (cg04988978, MPO). More importantly, DNA methylation changes in cg21566642 showed a genetic influence in childhood (rs139595493) and adolescence (rs72617176). |
| [51] | American and European | Discovery cohorts FHS =1494 PIVUSs = 812 Validation cohorts LBCs1921 ≤ 380 LBCs1936 ≤ 654 GOLDN = 991 | M and F | WB | Illumina Infinium HumanMethylation450 BeadChip | Differential methylation was identified at 33 CpG sites in 5 different study cohorts. Of these, 25 CpG sites showed novel associations with circulating lipid traits (HDL, LDL, and TGs), including a CpG site annotated to the ATP Binding Cassette Subfamily G Member 1 locus (cg27243685, ABCG1). Methylation of this site correlated with a 38% increased risk of CHD. |
| [52] | USA, UK, Africa, Europe, Sweden | Discovery cohorts FHS = 2377 LBCs = 1366 Validation cohorts ARICs = 2096 GOLDN = 992 PIVUSs = 967 | M and F | WB and CD4+ cells | Illumina Infinium HumanMethylation450 BeadChip | Differential methylation in 135 CpGs were identified in the discovery cohorts, of which 83 could be replicated in the three validation cohorts. The study reported a three-way association between 11 differentially methylated CpGs, expression of 7 genes, and BMI. These associations included the DNA methylation and expression status of ATP Binding Cassette Subfamily G Member 1 (cg01881899, ABCG1), Carnitine palmitoyltransferase 1A (cg00574958, cg17058475, CPT1A), and Sterol regulatory element-binding protein 1 (cg11024682, SREBF1). |
| [53] | Canadian | Discovery cohort IUCPQ = 206 Validation cohort CaG = 137 | M and F | VAT WB | Methyl-binding domain-capture sequencing | This study identified 565 differentially methylated regulatory regions in adipose tissue, which were associated with plasma lipid levels. Of these, 340 were reflected in the whole blood, of which 68 (mapping to 13 putative enhancers and 55 promoters) shared similar associations with plasma lipid traits, including serine/threonine kinase 1 (AKT1), histone deacetylase 4 (HDAC4), Bone morphogenetic protein 4 (BMP4), Growth differentiation factor 7 (GDF7), Ceramide Kinase (CERK), Vestigial-like family member 3 (VGLL3), and ATP binding cassette subfamily C member 5 (ABCC5). Of the 68 CpG sites identified in the IUCPQ cohort, 22 could be validated in the CARTaGENE cohort. |
| [54] | Ghanaian | T2D = 589 | M and F | WB | Illumina Infinium HumanMethylation450 BeadChip | The study identified 14 novel differentially methylated loci associated with CRP levels up to 40 mg/L. Three of the differentially methylated CpGs, annotating to Pyruvate Carboxylase (cg14653250, PC), BTG Anti-Proliferation Factor 4 (cg13767940, BTG4), and Peptidyl Arginine Deiminase 1 (cg21010178, PADI1), showed an association with CVD risk. |
| [26] | German | Discovery cohort: Atherosclerosis = 8 Controls = 8 Validation cohort: AWHS = 24 | M and F | WB | Discovery: Illumina Infinium HumanMethylation450 BeadChip Validation: Epityper MassARRAY | Differential methylation was identified in CpGs (287 hypo- and 229 hypermethylated) in cases versus controls which related to genes involved in cell cycle, adhesion, and death, among others. DNA methylation of breast cancer 1 (BRCA1) and Cysteine Rich Secretory Protein 2 (CRISP2) were validated using an Epityper MassARRAY and showed, in the additional AWHS cohort, that three CpG sites in CRISP2 (cg12440062, cg25390787, cg01076129) and one CpG site in BRCA1 (cg16630982) associated with subclinical atherosclerosis measures. |
| [55] | Gambian and Indian | n = 293 Gambian n = 698 Indian | M and F | WB | Illumina Infinium HumanMethylation850 BeadChip | Several differentially methylated regions were associated with diastolic blood pressure, insulin sensitivity, triglycerides, and LDL-cholesterol in the Gambian population, and insulin sensitivity and HDL-cholesterol in the Indian population. Importantly, there was no overlap of differentially methylated CpGs identified between the cohorts. The study identified significant quantitative trait loci (cis-methQTLs) at three LDL-cholesterol-associated differentially methylated CpGs in Gambians; however, methylation did not mediate genotype effects on the CVD outcomes in the Indian population. |
| [56] | American and European | ARICs = 2567 CHS = 197 EPICORs = 584 FHS = 2375 InCHIANTI = 457 KORA = 1377 NAS = 484 WHI-EMPC = 1610 WHI-BAA23 = 1622 | M and F | PBLs | Illumina Infinium HumanMethylation450 BeadChip | An association was found between methylation at 52 CpG sites and the risk of developing CHD or myocardial infarction in nine population-based cohorts. These CpG sites mapped to key regulatory regions of genes associated with calcium flux [ATPase Plasma Membrane Ca2+ Transporting 2 (ATP2B2), calcium sensing receptor (CASR), guanylate cyclase activator 1B (GUCAIB), and hippocalcin like 1 (HPCAL1)], arterial plaque calcification [Protein Tyrosine Phosphatase Receptor Type N2 (PTPRN2)], and kidney function [Cadherin Related 23 (CDH23) and Hippocalcin-like 1 (HPCAL1)]. Identified loci remained significant across all nine cohorts and significance was not influenced by race. |
| [57] | China | HF = 27 Controls = 20 | M and F | PBLs | Reduced Representation Bisulfite Sequencing | Assessment of DNA methylation in African American males younger or equal to 30 years of age revealed an increase in methylation of two CpGs in the Sulfatase 1 (SULF1) gene in HF patients, as compared to age-matched normotensive controls. |
| [58] | European and African American | Discovery cohorts FHS offspring cohort = 2648 FHS third generation cohort = 1522 Validation cohorts ARICs = 963 GTP = 384 | M and F | WB (buffy coat) | Illumina Infinium HumanMethylation450 BeadChip | Differential methylation was observed at 92 CpGs and replicated in the validation cohorts. These CpG sites were associated with cardiovascular disease traits. Some of these CpGs were annotated to genes, such as Lipase A (LIPA), the ABO blood group gene (ABO), and Serologically defined colon cancer antigen 8 (SDCCAG8) that showed putative causality for CHD and MI |
| [59] | Sweden | Monozygotic twin pairs: 83 Dizygotic twin pairs 155 Single twins: 59 | M and F | WB | Illumina Infinium HumanMethylation450 BeadChip | Differential methylation at 20 top-ranked CpGs associated with non-stroke CVD, overall stroke, and ischemic stroke. The methylation of these CpGs was shown to determine the levels of cardiometabolic trait (eg., BMI and blood pressure), with the latter mediating CVD risk. |
| [60] | Turkey | CAD = 1 Control = 1 | M | WB | Reduced Representation Bisulfite Sequencing | Eleven genes [Lipid Droplet Associated Hydrolase (LDAH), Apolipoprotein B (APOB), Acyl-CoA synthetase members 2A, 5 and 3 (ACSM2A, ACSM5, ACSM3), Carboxylesterase 1 (CES1), Carboxylesterase 1 Pseudogene 1 (CES1P1), AFG3 Like Matrix AAA Peptidase Subunit 2 (AFG3L2), Iron-Sulfur Cluster Assembly Enzyme (ISCU), SEC14 Like Lipid Binding 2 (SEC14L2), and Microsomal triglyceride transfer protein (MTTP)] involved in fatty acid and cholesterol metabolism were hypomethylated in the healthy twin as compared with twin that presenting with MI. |
| [61] | Japan | MI = 192 Control = 192 | M | WB | Illumina Infinium HumanMethylation450 BeadChip | An association was identified between DNA methylation at 2 CpG sites [cg07786668 in zinc finer homeobox 3 (ZFHX3) and cg17218495 in SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a, member 4 (SMARCA4)] and MI, which were independent of CVD risk factors, such as BMI, blood lipid levels, and T2D. |
| [62] | Germany | Discovery Cohorts: KORA = 1103 NAS = 344 Validation Cohort: InCHIANTI = 443 | M and F | PBLs | Illumina Infinium HumanMethylation450 BeadChip | Methylation at 11 CpGs was related to incidents of MI, although after adjusting for medication usage, 9 CpGs remained and composed an epigenetic fingerprint for MI. This included genes such as LDL Receptor Related Protein 8 (LRP8) and KCNN1 potassium calcium-activated channel subfamily N member 1 (KCCN1). |
| Disease Phenotype | Studies | Main Genes Identified | Summary of Conclusions |
|---|---|---|---|
| MI and CHD | [24,30,32,36,39,41,42,44,50,56,61,62] | ZBTB12, BMPR2, IL-6, DYSF, NRG1, AHRR, F2RL3, MPO, ZFHX3, FOXP3, SOAT1, SMARCA4 and TXNIP, CASR, GUCAIB, HPCAL1, PTPRN2, and CDH23 | Identified genes involved in pathways related to inflammation, vascular remodeling, and oxidative stress. Identified gene associations independent from traditional risk factors, suggesting an intrinsic epigenetic contribution to disease risk. |
| Stroke | [45,59] | ASB10 and TTC37 | Identified methylation changes may contribute to stroke pathophysiology through mechanisms involving homocysteine metabolism, blood pressure, and BMI regulation. Some stroke-related epigenetic associations were population-specific (e.g., observed only in Europeans). |
| HF, general CVD, and atherosclerosis | [26,31,37,38,40,43,46,47,48,49,54] | HF severity: HDAC9, JARID2, GREM1, and SULF1 CVD risk: CYP8B1, SPON1, PACS1, UACA, NPPA, AHRR, and CPT1A Atherosclerosis: IL-6, AHRR, hTERT, GF11, LRRC52, NR3C1, miRNA-233, CRISP2, and BRCA1 | Hypo- and hypermethylation of distinct CpGs linked to cardiac stress responses, atherosclerosis, and inflammation. Associations with CRP levels and kidney function genes suggest inflammation and metabolic dysfunction as key contributors to CVD risk. |
| Multiple CVD traits | [27,34,51,52,53,55,58,60] | ABCG1, CPT1A, FABP3, SREBF1, LIPA, SDCCAG8, ABO, TLR2, IFN-γ, and iNOS | Identified differentially methylated genes associated with CVD risk factors: lipid metabolism, insulin sensitivity, diastolic blood pressure, and BMI. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willmer, T.; Mabasa, L.; Sharma, J.; Muller, C.J.F.; Johnson, R. Blood-Based DNA Methylation Biomarkers to Identify Risk and Progression of Cardiovascular Disease. Int. J. Mol. Sci. 2025, 26, 2355. https://doi.org/10.3390/ijms26052355
Willmer T, Mabasa L, Sharma J, Muller CJF, Johnson R. Blood-Based DNA Methylation Biomarkers to Identify Risk and Progression of Cardiovascular Disease. International Journal of Molecular Sciences. 2025; 26(5):2355. https://doi.org/10.3390/ijms26052355
Chicago/Turabian StyleWillmer, Tarryn, Lawrence Mabasa, Jyoti Sharma, Christo J. F. Muller, and Rabia Johnson. 2025. "Blood-Based DNA Methylation Biomarkers to Identify Risk and Progression of Cardiovascular Disease" International Journal of Molecular Sciences 26, no. 5: 2355. https://doi.org/10.3390/ijms26052355
APA StyleWillmer, T., Mabasa, L., Sharma, J., Muller, C. J. F., & Johnson, R. (2025). Blood-Based DNA Methylation Biomarkers to Identify Risk and Progression of Cardiovascular Disease. International Journal of Molecular Sciences, 26(5), 2355. https://doi.org/10.3390/ijms26052355









