Functional Characterization of PeVLN4 Involved in Regulating Pollen Tube Growth from Passion Fruit
Abstract
1. Introduction
2. Results
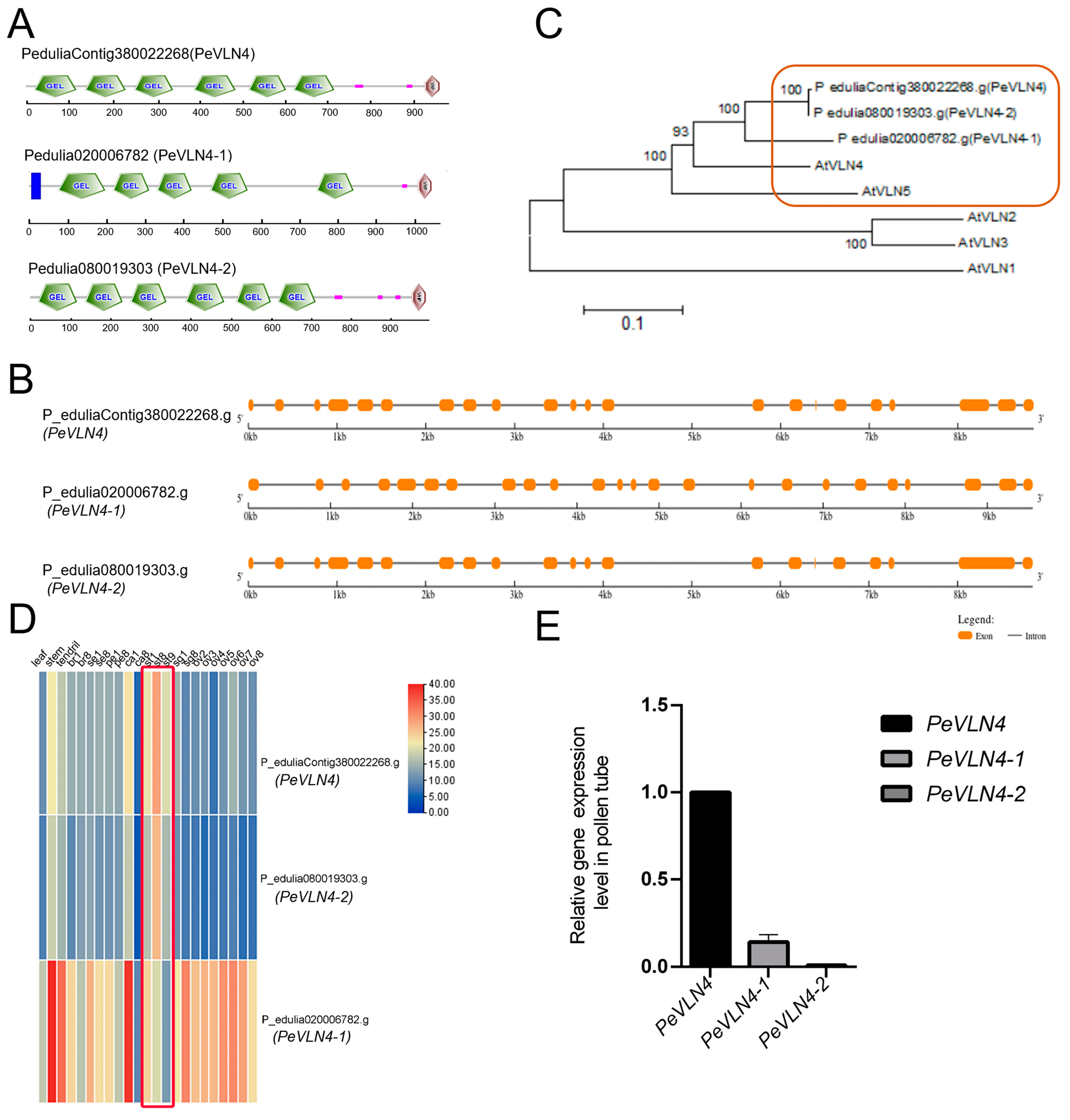
2.1. Molecular Composition and Gene Expression of VLN Genes in Passion Fruit
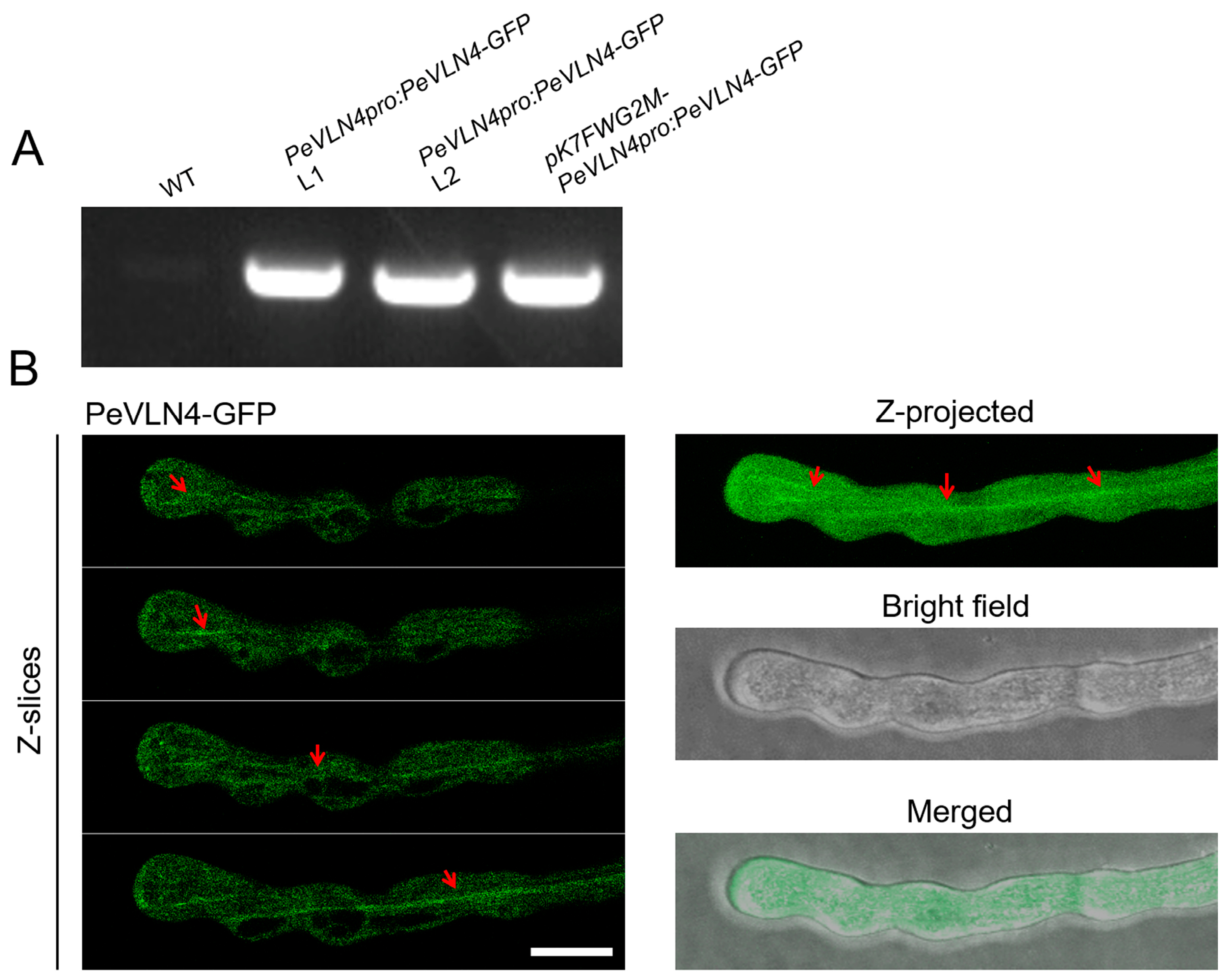
2.2. Subcellular Localization Analysis of PeVLN4 in Pollen Tubes
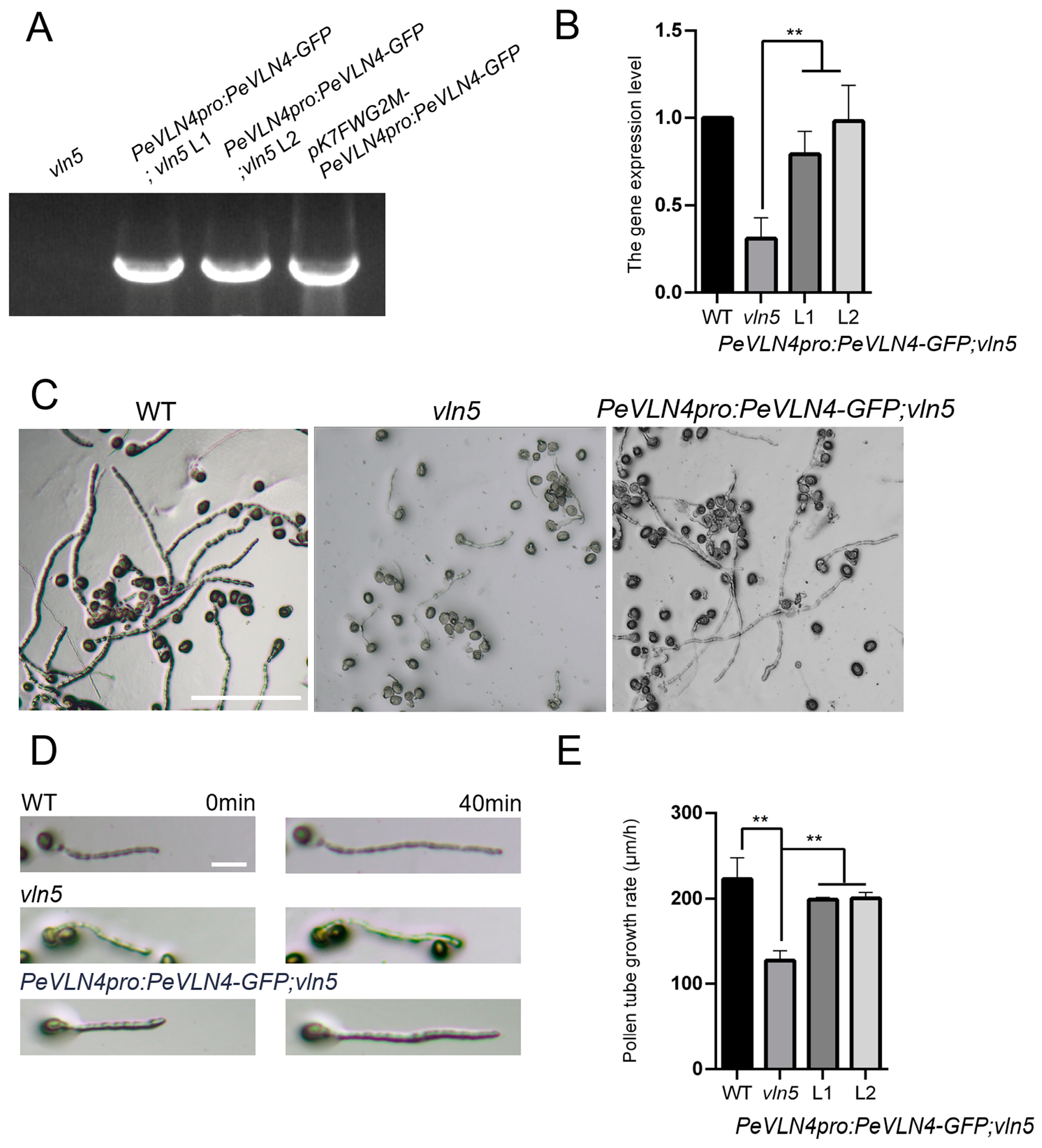
2.3. PeVLN4 Is Required for Normal Pollen Tube Growth
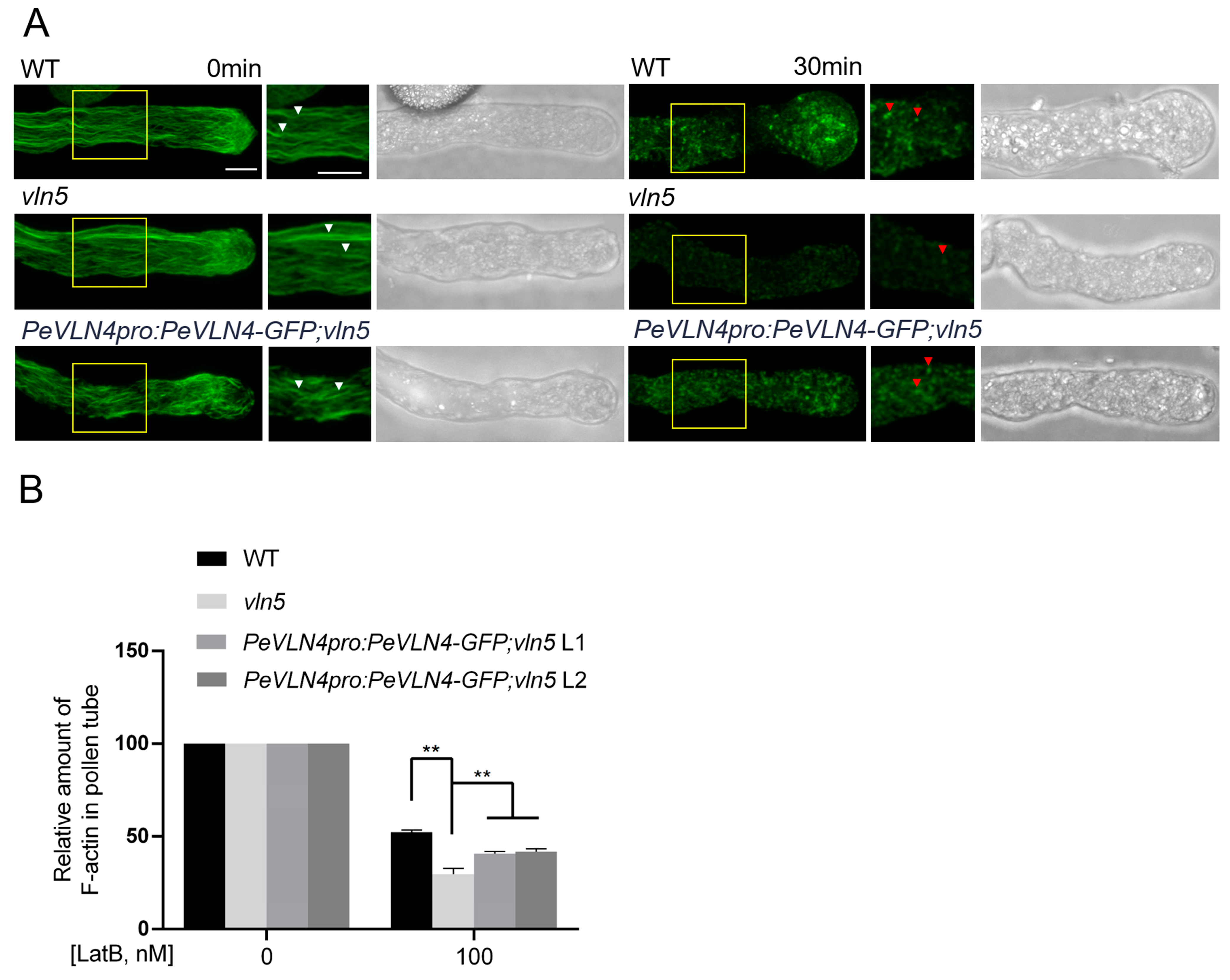
2.4. Pollen Tubes Expressing PeVLN4 Are Less Sensitive to LatB Treatment
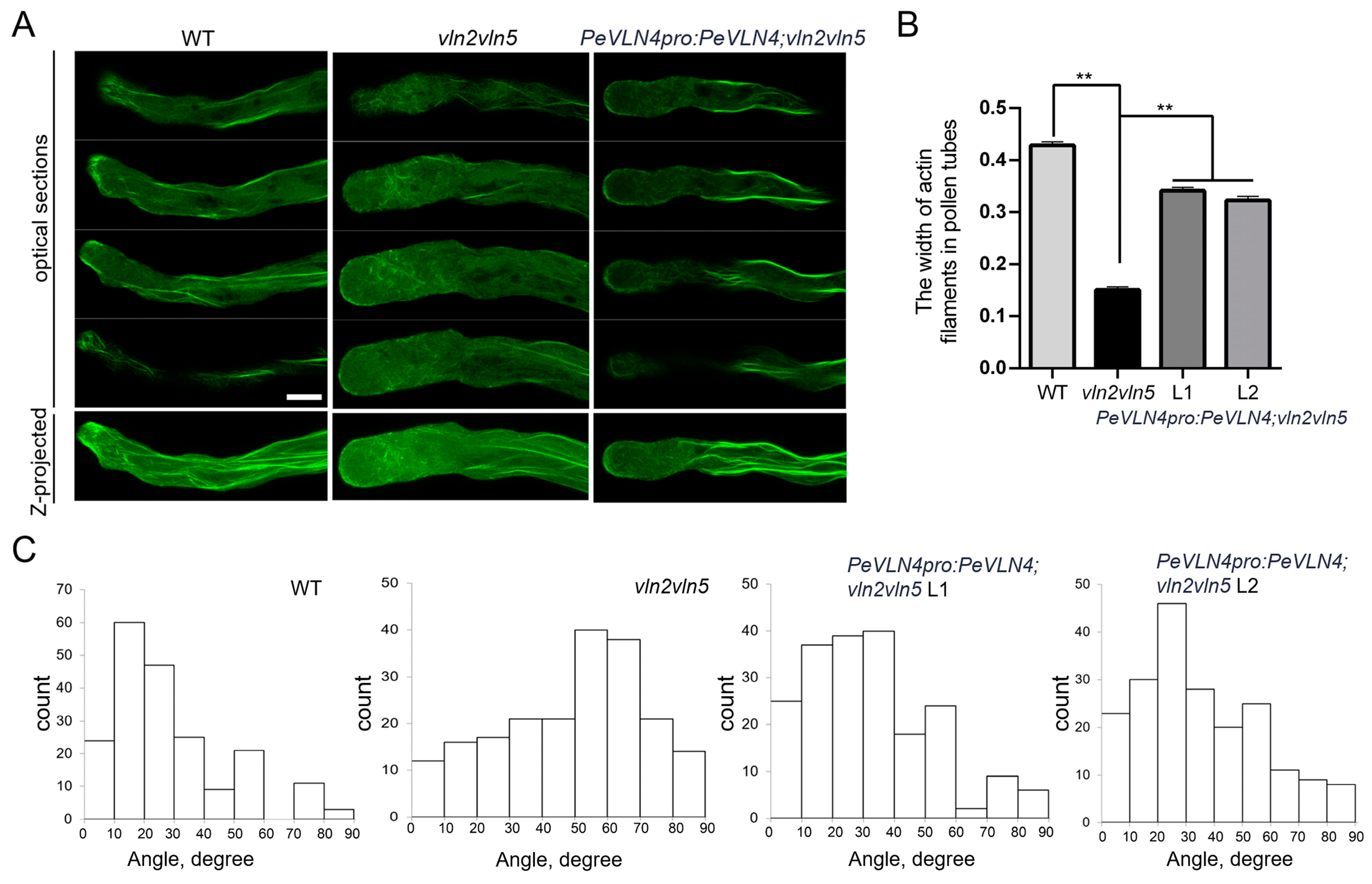
2.5. PeVLN4 Is Required for the Organization of Actin Filaments in Pollen Tubes
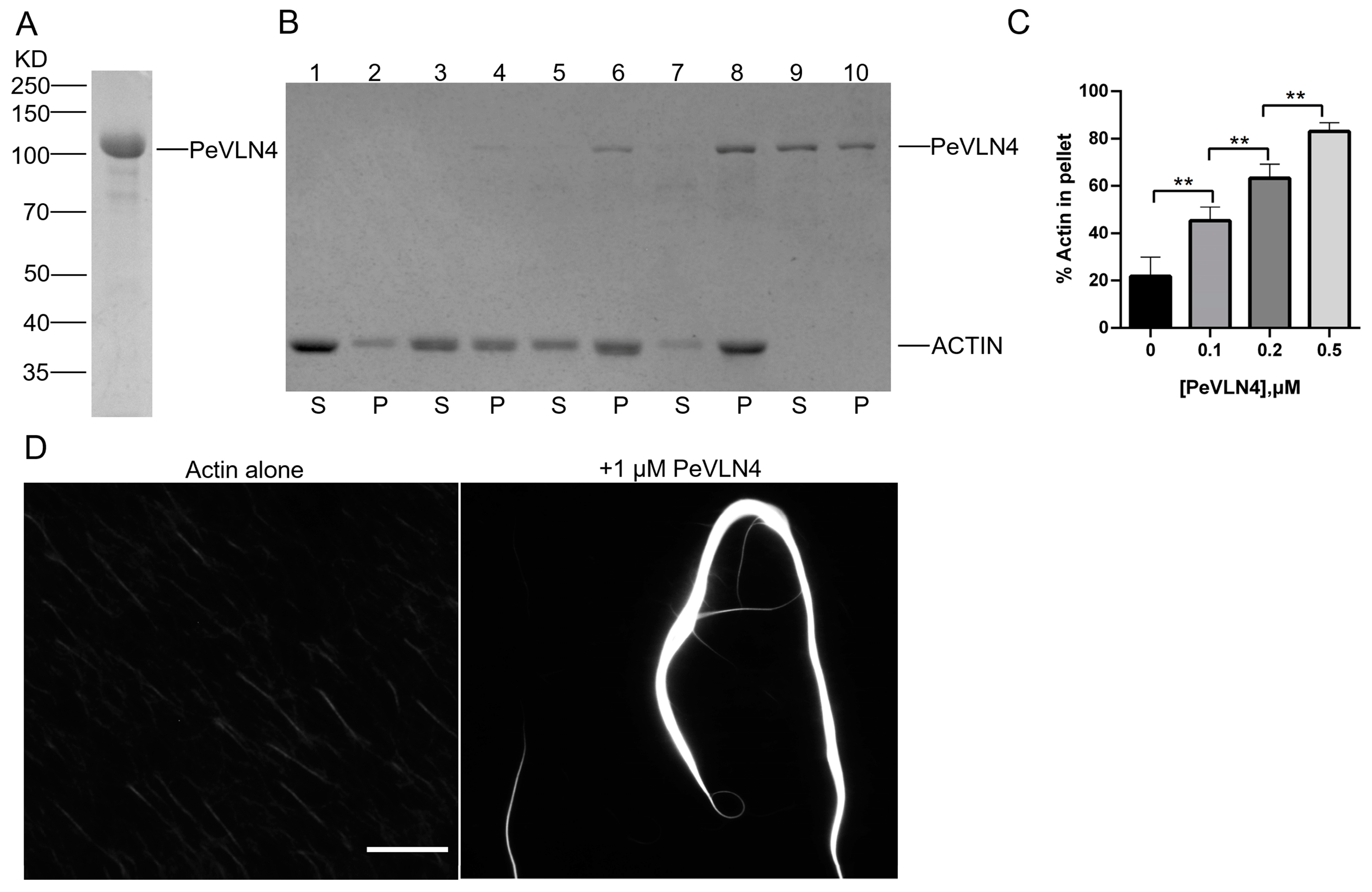
2.6. PeVLN4 Bundles Actin Filaments In Vitro
3. Discussion
4. Materials and Methods
4.1. Identification, Sequence Analysis, and Expression Analysis of VLNs from Passion Fruit
4.2. Construction and Plant Materials
4.3. Quantitative Real-Time PCR
4.4. Pollen Tube Growth and Quantification
4.5. LatB Treatment and Staining of Actin Filaments in Pollen Tubes
4.6. Visualization of Intracellular Localization of PeVLN4 in Pollen Tubes
4.7. Transient Expression Assay
4.8. Protein Production
4.9. Low-Speed F-Actin Co-Sedimentation Assay
4.10. Fluorescence Light Microscopy of Actin Filaments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, X.; Luan, F.; Yang, Y.; Wang, Z.; Li, Y. Passiflora edulis: An insight into current researches on phytochemistry and pharmacology. Front. Pharmacol. 2020, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhu, X.; Zhang, Q.; Zeng, S.; Li, Y.; Wang, Y. Genome-wide identification and expression analysis of WRKY transcription factor genes in passion fruit (Passiflora edulis). Trop. Plant Biol. 2024, 17, 214–232. [Google Scholar] [CrossRef]
- Hou, Z.; Liang, J.; Cai, X.; Lin, J.; Wang, X.; Liu, R.; Lu, L.; Chai, G.; An, C.; Chen, S.; et al. PeHVA22 gene family in passion fruit (Passiflora edulis): Initial characterization and expression profiling diversity. Front. Plant Sci. 2024, 14, 1279001. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Hou, Z.; Liao, J.; Qin, Y.; Wang, L.; Wang, X.; Su, W.; Cai, Z.; Fang, Y.; Aslam, M.; et al. Genome-wide identification and expression analysis of LBD transcription factor genes in passion fruit (Passiflora edulis). Int. J. Mol. Sci. 2022, 23, 4700. [Google Scholar] [CrossRef]
- Pereira, Z.C.; Cruz, J.M.D.A.; Corrêa, R.F.; Sanches, E.A.; Campelo, P.H.; Bezerra, J.A. Passion fruit (Passiflora spp.) Pulp: A review on bioactive properties, health benefits and technological potential. Food Res. Int. 2023, 166, 112626. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, Y.; Yi, R.; Shen, J.; Huang, S. Actin cytoskeleton in the control of vesicle transport, cytoplasmic organization, and pollen tube tip growth. Plant Physiol. 2023, 193, 9–25. [Google Scholar] [CrossRef]
- Qu, X.; Jiang, Y.; Chang, M.; Liu, X.; Zhang, R.; Huang, S. Organization and regulation of the actin cytoskeleton in the pollen tube. Front. Plant Sci. 2015, 5, 786. [Google Scholar] [CrossRef]
- Janda, M.; Matoušková, J.; Burketová, L.; Valentová, O. Interconnection between actin cytoskeleton and plant defense signaling. Plant Signal Behav. 2014, 9, e976486. [Google Scholar] [CrossRef]
- Yuan, H.; Yang, T. Exploring the role of the plant actin cytoskeleton: From signaling to cellular functions. Int. J. Mol. Sci. 2023, 24, 15480. [Google Scholar] [CrossRef]
- Dominguez, R.; Holmes, K.C. Actin structure and function. Annu. Rev. Biophys. 2011, 40, 169–186. [Google Scholar] [CrossRef]
- Qu, X.; Zhang, R.; Zhang, M.; Diao, M.; Xue, Y.; Huang, S. Organizational innovation of apical actin filaments drives rapid pollen tube growth and turning. Mol. Plant. 2017, 10, 930–947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qu, X.; Bao, C.; Khurana, P.; Wang, Q.; Xie, Y.; Zheng, Y.; Chen, N.; Blanchoin, L.; Staiger, C.J. Arabidopsis VILLIN5, an actin filament bundling and severing protein, is necessary for normal pollen tube growth. Plant Cell. 2010, 22, 2749–2767. [Google Scholar] [CrossRef]
- Cai, G.; Parrotta, L.; Cresti, M. Organelle trafficking, the cytoskeleton, and pollen tube growth. J. Integr. Plant Biol. 2015, 57, 63–78. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Duanhong, Q.; Costa, S.S.; de Graaf, B.H.J.; Di Stilio, V.S.; Feijo, J.; Wu, H. The dynamic pollen tube cytoskeleton: Live cell studies using actin-binding and microtubule-binding reporter proteins. Mol. Plant. 2008, 1, 686–702. [Google Scholar] [CrossRef]
- Chen, N.; Qu, X.; Wu, Y.; Huang, S. Regulation of actin dynamics in pollen tubes: Control of actin polymer level. J. Integr. Plant Biol. 2009, 51, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Skruber, K.; Read, T.A.; Vitriol, E.A. Reconsidering an active role for G-actin in cytoskeletal regulation. J. Cell Sci. 2018, 131, 1. [Google Scholar] [CrossRef]
- Xue, B.; Robinson, R.C. Guardians of the actin monomer. Eur. J. Cell Biol. 2013, 92, 316–332. [Google Scholar] [CrossRef]
- Chang, M.; Huang, S. Arabidopsis ACT 11 modifies actin turnover to promote pollen germination and maintain the normal rate of tube growth. Plant J. 2015, 83, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Li, X.; Huang, S. Arabidopsis AIP1-1 regulates the organization of apical actin filaments by promoting their turnover in pollen tubes. Sci. China Life Sci. 2020, 63, 239–250. [Google Scholar] [CrossRef]
- McCurdy, D.W.; Kovar, D.R.; Staiger, C.J. Actin and actin-binding proteins in higher plants. Protoplasma. 2001, 215, 89–104. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, X.; Wang, T.; Zhang, Y.; Liu, Q.; Hussey, P.J.; Ren, H. Actin binding protein29 from lilium pollen plays an important role in dynamic actin remodeling. Plant Cell. 2007, 19, 1930–1946. [Google Scholar] [CrossRef] [PubMed]
- Beckley, S.J.; Hunter, M.C.; Kituyi, S.N.; Wingate, I.; Chakraborty, A.; Schwarz, K.; Makhubu, M.P.; Rousseau, R.P.; Ruck, D.K.; de la Mare, J.-A.; et al. STIP1/HOP regulates the actin cytoskeleton through interactions with actin and changes in actin-binding proteins cofilin and profilin. Int. J. Mol. Sci. 2020, 21, 3152. [Google Scholar] [CrossRef] [PubMed]
- Inada, N. Plant actin depolymerizing factor: Actin microfilament disassembly and more. J. Plant Res. 2017, 130, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Blanchoin, L.; Amann, K.J.; Higgs, H.N.; Marchand, J.B.; Kaiser, D.A.; Pollard, T.D. Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature. 2000, 404, 1007–1011. [Google Scholar] [CrossRef]
- Zhu, J.; Nan, Q.; Qin, T.; Qian, D.; Mao, T.; Yuan, S.; Wu, X.; Niu, Y.; Bai, Q.; An, L.; et al. Higher-ordered actin structures remodeled by Arabidopsis actin-depolymerizing factor5 are important for pollen germination and pollen tube growth. Mol. Plant. 2017, 10, 1065–1081. [Google Scholar] [CrossRef]
- Andrianantoandro, E.; Pollard, T.D. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/Cofilin. Mol. Cell. 2006, 24, 13–23. [Google Scholar] [CrossRef]
- Fu, Y.; Duan, X.; Tang, C.; Li, X.; Voegele, R.T.; Wang, X.; Wei, G.; Kang, Z. TaADF7, an actin-depolymerizing factor, contributes to wheat resistance against Puccinia striiformis f. sp. tritici. Plant J. 2014, 78, 16–30. [Google Scholar] [CrossRef]
- Bi, S.; Li, M.; Liu, C.; Liu, X.; Cheng, J.; Wang, L.; Wang, J.; Lv, Y.; He, M.; Cheng, X.; et al. Actin depolymerizing factor ADF7 inhibits actin bundling protein VILLIN1 to regulate root hair formation in response to osmotic stress in Arabidopsis. PLoS Genet. 2022, 18, e1010338. [Google Scholar] [CrossRef]
- Tanaka, K. Formin family proteins in cytoskeletal control. Biochem. Biophys. Res. Commun. 2000, 267, 479–481. [Google Scholar] [CrossRef]
- Diao, M.; Ren, S.; Wang, Q.; Qian, L.; Shen, J.; Liu, Y.; Huang, S. Arabidopsis Formin 2 regulates cell-to-cell trafficking by capping and stabilizing actin filaments at plasmodesmata. Elife. 2018, 7, e36316. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Ren, H. Profilin promotes formin-mediated actin filament assembly and vesicle transport during polarity formation in pollen. Plant Cell. 2021, 33, 1252–1267. [Google Scholar] [CrossRef] [PubMed]
- Kovar, D.R.; Gibbon, B.C.; McCurdy, D.W.; Staiger, C.J. Fluorescently-labeled fimbrin decorates a dynamic actin filament network in live plant cells. Planta. 2001, 213, 390–395. [Google Scholar] [CrossRef]
- Zhang, R.; Qu, X.; Zhang, M.; Jiang, Y.; Dai, A.; Zhao, W.; Cao, D.; Lan, Y.; Yu, R.; Wang, H. The balance between actin-bundling factors controls actin architecture in pollen tubes. iScience 2019, 16, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Qu, X.; Zhang, R. Plant villins: Versatile actin regulatory proteins. J. Integr. Plant Biol. 2015, 57, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, S.; Kameyama, K.; Kanzawa, N.; Tamiya, T.; Mabucbi, I.; Tsuchiya, T. The GELSOLIN/PRAGMIN family protein identified in the higher plant Mimosa pudica. J. Biochem. 2001, 130, 243–249. [Google Scholar] [CrossRef]
- Huang, S.; Robinson, R.C.; Gao, L.Y.; Matsumoto, T.; Brunet, A.; Blanchoin, L.; Staiger, C.J. Arabidopsis VILLIN1 generates actin filament cables that are resistant to depolymerization. Plant Cell. 2005, 17, 486–501. [Google Scholar] [CrossRef]
- Khurana, P.; Henty, J.L.; Huang, S.; Staiger, A.M.; Blanchoin, L.; Staiger, C.J. Arabidopsis VILLIN1 and VILLIN3 have overlapping and distinct activities in actin bundle formation and turnover. Plant Cell. 2010, 22, 2727–2748. [Google Scholar] [CrossRef]
- Pandey, D.K. Genome-wide analysis of VILLIN Gene family associated with stress responses in cotton (Gossypium spp.). Curr. Issues Mol. Biol. 2024, 46, 2278–2300. [Google Scholar] [CrossRef]
- Shimmen, Y.T. The 135-kDa actin-bundling protein from lily pollen tubes arranges F-actin into bundles with uniform polarity. Planta 1999, 209, 264–266. [Google Scholar] [CrossRef]
- Wang, X.; Bi, S.; Wang, L.; Li, H.; Gao, B.; Huang, S.; Qu, X.; Cheng, J.; Wang, S.; Liu, C.; et al. GLABRA2 regulates actin bundling protein VILLIN1 in root hair growth in response to osmotic stress. Plant Physiol. 2020, 184, 176–193. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Y.; Du, F.; Cao, L.; Dong, H.; Ren, H. Arabidopsis VILLIN4 is involved in root hair growth through regulating actin organization in a Ca2+-dependent manner. New Phytol. 2011, 190, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Wang, J.; Zhang, R.; Zhang, B.; Zhang, H.; Zhou, Y.; Huang, S. Arabidopsis VILLIN2 and VILLIN3 act redundantly in sclerenchyma development via bundling of actin filaments. Plant J. 2012, 71, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Van Der Honing, H.S.; Kieft, H.; Emons, A.M.C.; Ketelaar, T. Arabidopsis VILLIN2 and VILLIN3 are required for the generation of thick actin filament bundles and for directional organ growth. Plant Physiol. 2012, 158, 1426–1438. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhuang, Y.; Zhao, W.; Qu, X.; Wang, J.; Chang, M.; Shen, J.; Chen, N.; Huang, S. Molecular and functional adaption of Arabidopsis VILLINs. New Phytol. 2025, 245, 1158–1179. [Google Scholar] [CrossRef]
- Wu, S.; Xie, Y.; Zhang, J.; Ren, Y.; Zhang, X.; Wang, J.; Guo, X.; Wu, F.; Sheng, P.; Wang, J.; et al. VLN2 regulates plant architecture by affecting microfilament dynamics and polar auxin transport in rice. Plant Cell. 2015, 27, 2829–2845. [Google Scholar] [CrossRef]
- Li, W.B.; Song, S.W.; Zhong, M.M.; Liu, L.G.; Su, L.; Han, L.B.; Xia, G.X.; Sun, Y.D.; Wang, H.Y. VILLIN2 regulates cotton defense against Verticillium dahlia by modulating actin cytoskeleton remodeling. Plant Physiol. 2023, 192, 666–679. [Google Scholar] [CrossRef]
- Xia, Z.; Huang, D.; Zhang, S.; Wang, W.; Ma, F.; Wu, B.; Xu, Y.; Xu, B.; Chen, D.; Zou, M.; et al. Chromosome-scale genome assembly provides insights into the evolution and flavor synthesis of passion fruit (Passiflora edulis Sims). Hortic. Res. 2021, 8, 14. [Google Scholar] [CrossRef]
- Qu, X.; Zhang, H.; Xie, Y.; Wang, J.; Chen, N.; Huang, S. Arabidopsis VILLINs promote actin turnover at pollen tube tips and facilitate the construction of actin collars. Plant Cell. 2013, 25, 1803–1817. [Google Scholar] [CrossRef]
- Carmel, L.; Chorev, M. The function of introns. Front. Genet. 2012, 3, 55. [Google Scholar] [CrossRef]
- Jo, B.S.; Choi, S.S. Introns: The functional benefits of introns in genomes. Genom. Inform. 2015, 13, 112–118. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wang, Y.; Jiao, C.; Shang, Z.; Huang, S. Arabidopsis VILLIN5 bundles actin filaments using a novel mechanism. Plant J. 2024, 119, 2854–2866. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, C.H.; Casali, V.W.D.; de Moraes, C.F. Self-incompatibility in passion fruit (Passiflora edulis Sims). Acta Hortic. 1995, 370, 45–58. [Google Scholar] [CrossRef]
- Thomas, S.G.; Huang, S.; Li, S.; Staiger, C.J.; Franklin-Tong, V.E. Actin depolymerization is sufficient to induce programmed cell death in self-incompatible pollen. J. Cell Biol. 2006, 174, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Qu, X.; Zhuang, Y.; Wang, L.; Bosch, M.; Franklin-Tong, V.E.; Xue, Y.; Huang, S. Villin controls the formation and enlargement of punctate actin foci in pollen tubes. J. Cell Sci. 2020, 133, jcs237404. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, J.; Lai, M.; Zhang, Y.; Qiu, W.; Li, Y.; Tu, H.; Ling, Q.; Fu, X. Differential gene expression analysis and physiological response characteristics of passion fruit (Passiflora edulis) buds under high-temperature stress. Peer J. 2023, 11, e14839. [Google Scholar] [CrossRef]
- Xu, Y.; Li, P.; Ma, F.; Huang, D.; Xing, W.; Wu, B.; Sun, P.; Xu, B.; Song, S. Characterization of the NAC Transcription Factor in Passion Fruit (Passiflora edulis) and Functional Identification of PeNAC-19 in Cold Stress. Plants 2023, 12, 1393. [Google Scholar] [CrossRef]
- Cai, X.; Li, D.; Liu, C.; Chen, J.; Wei, X.; Hu, S.; Lu, L.; Chen, S.; Yao, Q.; Xie, S.; et al. Identification and characterization of GRAS genes in passion fruit (Passiflora edulis Sims) revealed their roles in development regulation and stress response. Plant Cell Rep. 2025, 44, 46. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, J.; Zhang, R.; Qu, X.; Ren, S.; Chen, N.; Huang, S. Arabidopsis FIMBRIN5, an actin bundling factor, is required for pollen germination and pollen tube growth. Plant Cell 2010, 22, 3745–3763. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Y.; Zhao, S.; Jiang, Y.; Yi, R.; Guo, Y.; Huang, S. Activation of actin-depolymerizing factor by CDPK16-mediated phosphorylation promotes actin turnover in Arabidopsis pollen tubes. PLoS Biol. 2023, 21, e3002073. [Google Scholar] [CrossRef]
- Kovar, D.R.; Staiger, C.J.; Weaver, E.A.; McCurdy, D.W. AtFim1 is an actin filament crosslinking protein from Arabidopsis thaliana. Plant J. 2000, 24, 625–636. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Wei, X.; Wang, L.; Zheng, P.; Li, J.; Zou, Y.; Wang, L.; Feng, X.; Xu, J.; Qin, Y.; et al. Functional Characterization of PeVLN4 Involved in Regulating Pollen Tube Growth from Passion Fruit. Int. J. Mol. Sci. 2025, 26, 2348. https://doi.org/10.3390/ijms26052348
Yang H, Wei X, Wang L, Zheng P, Li J, Zou Y, Wang L, Feng X, Xu J, Qin Y, et al. Functional Characterization of PeVLN4 Involved in Regulating Pollen Tube Growth from Passion Fruit. International Journal of Molecular Sciences. 2025; 26(5):2348. https://doi.org/10.3390/ijms26052348
Chicago/Turabian StyleYang, Hanbing, Xiuqing Wei, Lifeng Wang, Ping Zheng, Junzhang Li, Yutong Zou, Lulu Wang, Xinyuan Feng, Jiahui Xu, Yuan Qin, and et al. 2025. "Functional Characterization of PeVLN4 Involved in Regulating Pollen Tube Growth from Passion Fruit" International Journal of Molecular Sciences 26, no. 5: 2348. https://doi.org/10.3390/ijms26052348
APA StyleYang, H., Wei, X., Wang, L., Zheng, P., Li, J., Zou, Y., Wang, L., Feng, X., Xu, J., Qin, Y., & Zhuang, Y. (2025). Functional Characterization of PeVLN4 Involved in Regulating Pollen Tube Growth from Passion Fruit. International Journal of Molecular Sciences, 26(5), 2348. https://doi.org/10.3390/ijms26052348







