GDF15, EGF, and Neopterin in Assessing Progression of Pediatric Chronic Kidney Disease Using Artificial Intelligence Tools—A Pilot Study
Abstract
1. Introduction
2. Results
2.1. GDF15, EGF, and Neopterin Serum Concentrations
2.2. Correlations Between GDF15, EGF, Neopterin Serum Concentrations, and Classical Markers of CKD
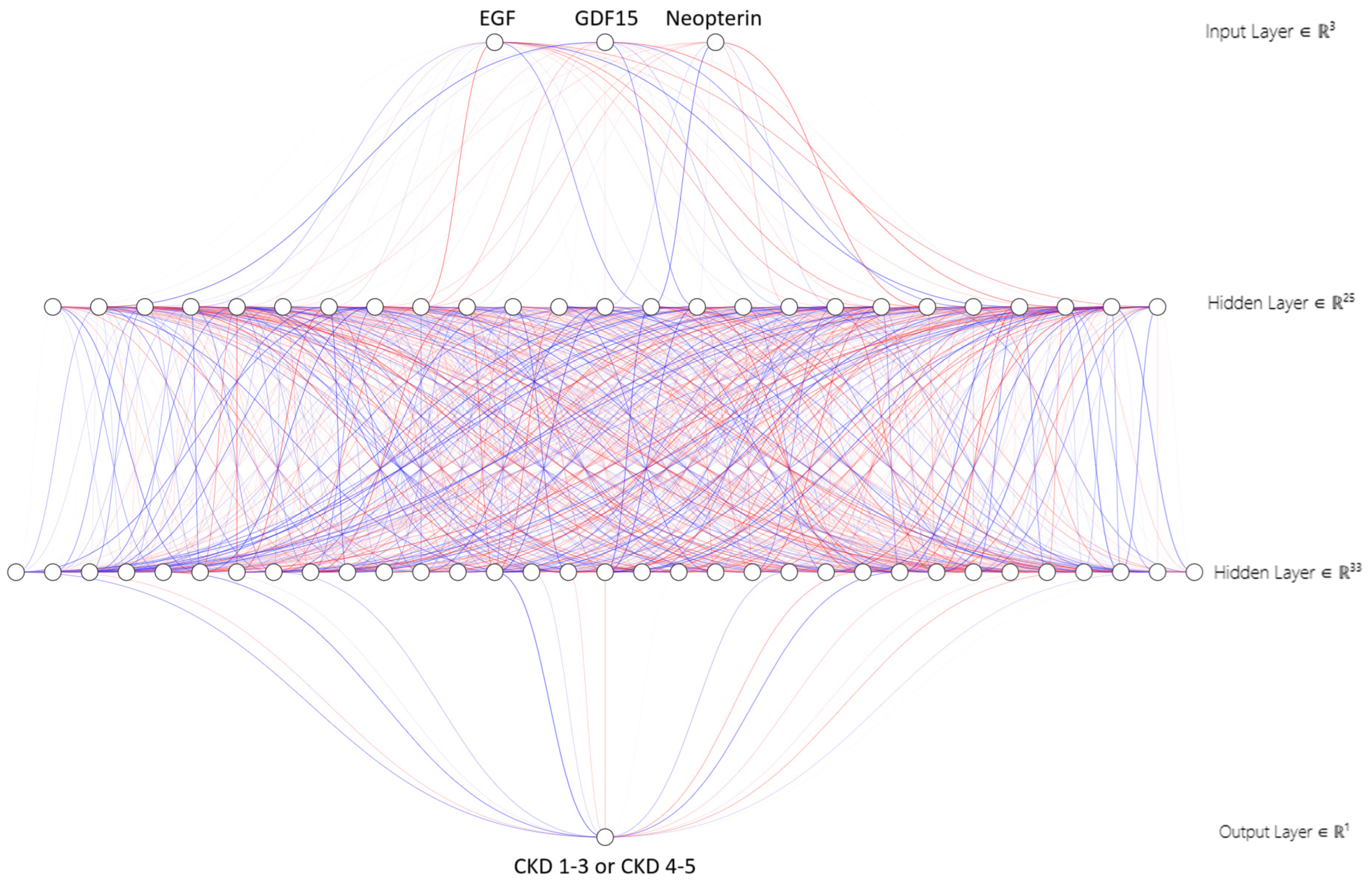
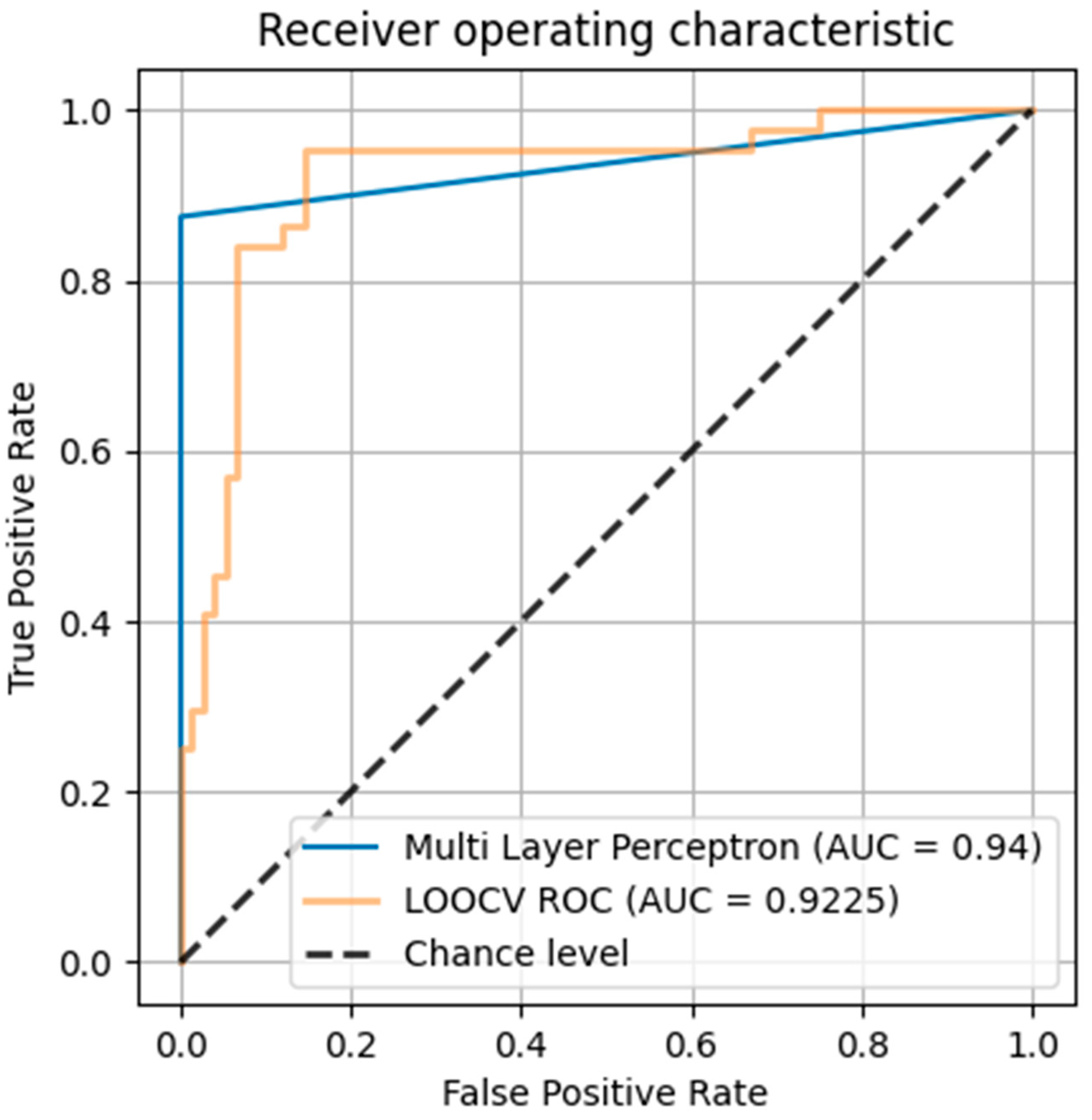
2.3. Multilayer Perceptron (MLP) Network Modeling
3. Discussion
4. Materials and Methods
4.1. Basic Characteristics
4.2. Assay Characteristics
4.3. Classical Statistical Analysis
4.4. Database Analysis by Multilayer Perceptron (MLP) Network
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bootcov, M.R.; Bauskin, A.R.; Valenzuela, S.M.; Moore, A.G.; Bansal, M.; He, X.Y.; Zhang, H.P.; Donnellan, M.; Mahler, S.; Pryor, K.; et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc. Natl. Acad. Sci. USA 1997, 94, 11514–11519. [Google Scholar] [CrossRef] [PubMed]
- Wischhusen, J.; Melero, I.; Fridman, W.H. Growth/differentiation factor-15 (GDF15): From biomarker to novel targetable immune checkpoint. Front. Immunol. 2020, 11, 951. [Google Scholar] [CrossRef]
- Li, J.; Hu, X.; Xie, Z.; Li, J.; Huang, C.; Huang, Y. Overview of growth differentiation factor 15 (GDF15) in metabolic diseases. Biomed. Pharmacother. 2024, 176, 116809. [Google Scholar] [CrossRef]
- Schwarz, A.; Kinscherf, R.; Bonaterra, G.A. Role of the stress- and inflammation-induced cytokine GDF15 in cardiovascular diseases: From basic research to clinical relevance. Rev. Cardiovasc. Med. 2023, 24, 81. [Google Scholar] [CrossRef] [PubMed]
- Adela, R.; Banerjee, S.K. GDF15 as a target and biomarker for diabetes and cardiovascular diseases: A translational prospective. J. Diabetes Res. 2015, 2015, 490842. [Google Scholar] [CrossRef]
- Tuegel, C.; Katz, R.; Alam, M.; Bhat, Z.; Bellovich, K.; de Boer, I.; Brosius, F.; Gadegbeku, C.; Gipson, D.; Hawkins, J.; et al. GDF15, galectin 3, soluble ST2, and risk of mortality and cardiovascular events in CKD. Am. J. Kidney Dis. 2018, 72, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Robinson-Cohen, C.; Smith, M.R.; Bellovich, K.A.; Bhat, Z.Y.; Bobadilla, M.; Brosius, F.; de Boer, I.H.; Essioux, L.; Formentini, I.; et al. Growth differentiation factor-15 and risk of CKD progression. J. Am. Soc. Nephrol. 2017, 28, 2233–2240. [Google Scholar] [CrossRef]
- Thorsteinsdottir, H.; Salvador, C.L.; Mjøen, G.; Lie, A.; Sugulle, M.; Tøndel, C.; Brun, A.; Almaas, R.; Bjerre, A. Growth differentiation factor 15 in children with chronic kidney disease and after renal transplantation. Dis. Markers 2020, 2020, 6162892. [Google Scholar] [CrossRef]
- Bargenda, A.; Musiał, K.; Zwolińska, D. Epidermal growth factor, growth differentiation factor-15, and survivin as novel biocompatibility markers in children on chronic dialysis. Biomarkers 2016, 21, 752–756. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, F.; Forrester, S.J.; Eguchi, S.; Zhang, M.-Z.; Harris, R.C. Expression and function of the epidermal growth factor receptor in physiology and disease. Physiol. Rev. 2016, 96, 1025–1069. [Google Scholar] [CrossRef]
- Harris, R.C. Potential physiologic roles for epidermal growth factor in the kidney. Am. J. Kidney Dis. 1991, 17, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Orofiamma, L.A.; Vural, D.; Antonescu, C.N. Control of cell metabolism by the epidermal growth factor receptor. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119359. [Google Scholar] [CrossRef] [PubMed]
- Abu Shady, M.M.; Fathy, H.A.; Ali, A.; Youness, E.R.; Fathy, G.A. Association of neopterin as a marker of immune system activation and juvenile rheumatoid arthritis activity. J. Pediatr. 2015, 91, 352–357. [Google Scholar] [CrossRef]
- Melichar, B.; Solichová, D.; Melicharová, K.; Malírová, E.; Cermanová, M.; Zadák, Z. Urinary neopterin in patients with advanced colorectal carcinoma. Int. J. Biol. Markers 2006, 21, 190–198. [Google Scholar] [CrossRef]
- Al-Nimer, M.S.; Dezayee, Z.M.I. Assessment of serum neopterin as an inflammatory and cardiovascular marker in type 1 and 2 diabetes complicated by diabetic foot syndrome: A comparative study. Clin. Diabetol. 2018, 7, 91–96. [Google Scholar] [CrossRef]
- Rasmi, Y.; Heidari, N.; Kirboğa, K.K.; Hatamkhani, S.; Tekin, B.; Alipour, S.; Naderi, R.; Farnamian, Y.; Akca, I. The importance of neopterin in COVID-19: The prognostic value and relation with the disease severity. Clin. Biochem. 2022, 104, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Sharma, V.; Jha, V. Association between serum neopterin and inflammatory activation in chronic kidney disease. Mediat. Inflamm. 2012, 2012, 476979. [Google Scholar] [CrossRef]
- Ünüvar, S.; Aslanhan, H. Clinical significance of increased serum neopterin in chronic kidney failure as a biomarker of cell-mediated immunity. J. Med. Biochem. 2019, 38, 1–5. [Google Scholar] [CrossRef]
- Musiał, K.; Zwolińska, D. New markers of cell migration and inflammation in children with chronic kidney disease. Biomarkers 2019, 24, 295–302. [Google Scholar] [CrossRef]
- Lajer, M.; Jorsal, A.; Tarnow, L.; Parving, H.-H.; Rossing, P. Plasma growth differentiation factor-15 independently predicts all-cause mortality and cardiovascular mortality as well as deterioration of kidney function in type 1 diabetic patients with nephropathy. Diabetes Care 2010, 33, 1567–1572. [Google Scholar] [CrossRef]
- Bao, X.; Xu, B.; Borné, Y.; Orho-Melaner, M.; Melander, O.; Nilsson, J.; Christensson, A.; Engström, G. Growth differentiation factor-15 and incident chronic kidney disease: A population-based cohort study. BMC Nephrol 2021, 22, 351. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.; Zhang, J.; Ding, F.; Ma, L. Role of growth differentiation factor 15 in cancer cachexia. Oncol. Lett. 2023, 26, 462. [Google Scholar] [CrossRef]
- Ono, K.; Maeshima, A.; Nagayama, I.; Kubo, T.; Yagisawa, T.; Nagata, D. Urinary epidermal growth factor level as a noinvasive indicator of tubular repair in patients with acute kidney injury. Diagnostics 2024, 14, 947. [Google Scholar] [CrossRef]
- Amatruda, J.G.; Katz, R.; Rebholz, C.M.; Sarnak, M.J.; Gutierrez, O.M.; Schrauben, S.J.; Greenberg, J.H.; Coresh, J.; Cushman, M.; Waikar, S.; et al. Urine biomarkers of kidney tubule health and risk of incident CKD in persons without diabetes: The ARIC, MESA, and REGARDS studies. Kidney Med. 2024, 6, 100834. [Google Scholar] [CrossRef]
- Postalcioglu, M.; Scherzer, R.; Ix, J.H.; Jacobs, D.R., Jr.; Lewis, C.E.; Vaigankar, S.; Estrella, M.M.; Gutierrez, O.M.; Shlipak, M.G. Urine epidermal growth factor and kidney function decline in middle-aged adults. Kidney Med. 2024, 6, 100846. [Google Scholar] [CrossRef]
- Bienaimé, F.; Muorah, M.; Metzger, M.; Broeuilh, M.; Houiller, P.; Flamant, M.; Haymann, J.-P.; Vonderscher, J.; Mizrahi, J.; Friedlander, G.; et al. Combining robust urine biomarkers to assess chronic kidney disease progression. EBioMedicine 2023, 93, 104635. [Google Scholar] [CrossRef]
- Azukatis, K.; Ju, W.; Kirchner, M.; Nair, V.; Smith, M.; Fang, Z.; Thurn-Valsassina, D.; Bayazit, A.; Niemirska, A.; Canpolat, N.; et al. Low levels of urinary epidermal growth factor predict chronic kidney disease progression in children. Kidney Int. 2019, 96, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.H.; Abraham, A.G.; Xu, Y.; Schelling, J.R.; Feldman, H.I.; Sabbisetti, V.S.; Ix, J.H.; Jogalekar, M.P.; Coca, S.; Waikar, S.S.; et al. Urine biomarkers of kidney tubule health, injury, and inflammation are associated with progression of CKD in children. J. Am. Soc. Nephrol. 2021, 32, 2664–2677. [Google Scholar] [CrossRef] [PubMed]
- Lanser, L.; Pölzl, G.; Fuchs, D.; Weiss, G.; Kurz, K. Neopterin is associated with disease severity and outcome in patients with non-ischaemic heart failure. J. Clin. Med. 2019, 8, 2230. [Google Scholar] [CrossRef]
- Pihlstrøm, H.; Mjøen, G.; März, W.; Dahle, D.O.; Abedini, S.; Holme, I.; Fellström, B.; Jardine, A.; Pilz, S.; Holdaas, H. Neopterin is associated with cardiovascular events and all-cause mortality in renal transplant patients. Clin. Transplant. 2014, 28, 111–119. [Google Scholar] [CrossRef]
- Schwartz, G.J.; Munoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 2009, 20, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, T.J.; Huemann, Z.; Hu, J.; Rahmim, A. A Guide to Cross-Validation for Artificial Intelligence in Medical Imaging. Radiol. Artif. Intell. 2023, 5, e220232. [Google Scholar] [CrossRef]
- Tougui, I.; Jilbab, A.; Mhamdi, J.E. Impact of the Choice of Cross-Validation Techniques on the Results of Machine Learning-Based Diagnostic Applications. Healthc. Inf. Res. 2021, 27, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Boughorbel, S.; Jarray, F.; El-Anbari, M. Optimal classifier for imbalanced data using Matthews Correlation Coefficient metric. PLoS ONE 2017, 12, e0177678. [Google Scholar] [CrossRef]
- Chicco, D.; Jurman, G. The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genom. 2020, 21, 6. [Google Scholar] [CrossRef] [PubMed]

| Parameters | eGFR [mL/min/1.73 m2] | Uric Acid [mg/dL] | Albumin [g/dL] | Hemoglobin [g/dL] | Parathormone [pg/mL] | CKD Stage |
|---|---|---|---|---|---|---|
| EGF | R = 0.37 p = 0.00001 | R = −0.19 p = 0.024 | R = 0.49 p = 0.00001 | R = 0.29 p = 0.0004 | R = −0.39 p = 0.000004 | R = −0.42 p = 0.000001 |
| GDF15 | R = −0.36 p = 0.00001 | R = 0.18 p = 0.029 | R = −0.47 p = 0.00001 | R = −0.26 p = 0.001 | R = 0.39 p = 0.000004 | R = 0.43 p = 0.000001 |
| Neopterin | R = −0.51 p = 0.0000001 | R = 0.26 p = 0.003 | R = −0.09 p = 0.34 | R = −0.38 p = 0.000007 | R = 0.24 p = 0.008 | R = 0.58 p = 0.0000001 |
| Dependent Variable | Independent Variable | Regression Coefficient β | Constant Term | Coefficient of Determination R2 | p |
|---|---|---|---|---|---|
| Serum EGF | Serum GDF15 | −0.97 | 397.57 | 0.91 | 0.000001 |
| CKD stage | −0.7 | 339.11 | 0.12 | 0.0009 | |
| Serum GDF15 | CKD stage | 0.53 | 145.68 | 0.69 | 0.007 |
| Serum neopterin | CKD stage | 0.74 | 5.48 | 0.24 | 0.02 |
| CKD stage | Serum neopterin | 0.54 | 0.07 | 0.39 | 0.000001 |
| Examined Groups | Number of Patients | Gender F M | Age [Years] Median Values (Lower–Upper Quartile) | BMI [kg/m2] Median Values (Lower–Upper Quartile) |
|---|---|---|---|---|
| CKD 1 | 26 | 9 | 12.7 | 17.7 |
| 17 | (8.4–14.1) | (16.9–20.3) | ||
| CKD 2 | 25 | 9 | 9.5 | 16.5 |
| 16 | (5.1–13.4) | (15.5–18.4) | ||
| CKD 3 | 51 | 19 | 11.1 | 16.5 |
| 21 | (7.3–14.9) | (14.7–19.7) | ||
| CKD 4 | 28 | 14 | 10.9 | 15.8 |
| 14 | (9.9–14.5) | (15.0–19.3) | ||
| CKD 5 | 21 | 10 | 11.6 | 17.2 |
| 11 | (8.1–14.4) | (15.2–19.0) | ||
| Control group | 25 | 15 | 10.3 | 18.2 |
| 10 | (5.9–15.2) | (16.1–21.0) |
| CKD Stage | eGFR [mL/min/1.73 m2] | CRP [ng/L] | Albumin [g/dL] | Hemoglobin [g/dL] | Parathormone [pg/mL] |
|---|---|---|---|---|---|
| 1 | 114 | 0.29 | 4.4 | 13.5 | 29.5 |
| (110–135) | (0.13–0.96) | (4.2–4.7) | (12.9–14.7) | (23.2–42.4) | |
| 2 | 74 | 0.33 | 4.5 | 12.9 | 63.0 |
| (70–81) | (0.22–1.22) | (4.1–4.6) | (11.8–13.7) | (30.2–88.4) | |
| 3 | 45 | 0.35 | 4.4 | 12.6 | 84.1 |
| (36–51) | (0.20–0.62) | (4.2–4.6) | (11.3–13.5) | (59.0–120.0) | |
| 4 | 23 | 0.21 | 4.4 | 11.8 | 190.8 |
| (19–27) | (0.14–0.73) | (3.8–4.7) | (10.4–12.3) | (126.9–344.3) | |
| 5 | 10 | 0.36 | 4.3 | 10.5 | 296.9 |
| (8–12) | (0.16–0.82) | (3.7–4.5) | (9.0–12.0) | (190.6–456.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musiał, K.; Stojanowski, J.; Bargenda-Lange, A.; Gołębiowski, T. GDF15, EGF, and Neopterin in Assessing Progression of Pediatric Chronic Kidney Disease Using Artificial Intelligence Tools—A Pilot Study. Int. J. Mol. Sci. 2025, 26, 2344. https://doi.org/10.3390/ijms26052344
Musiał K, Stojanowski J, Bargenda-Lange A, Gołębiowski T. GDF15, EGF, and Neopterin in Assessing Progression of Pediatric Chronic Kidney Disease Using Artificial Intelligence Tools—A Pilot Study. International Journal of Molecular Sciences. 2025; 26(5):2344. https://doi.org/10.3390/ijms26052344
Chicago/Turabian StyleMusiał, Kinga, Jakub Stojanowski, Agnieszka Bargenda-Lange, and Tomasz Gołębiowski. 2025. "GDF15, EGF, and Neopterin in Assessing Progression of Pediatric Chronic Kidney Disease Using Artificial Intelligence Tools—A Pilot Study" International Journal of Molecular Sciences 26, no. 5: 2344. https://doi.org/10.3390/ijms26052344
APA StyleMusiał, K., Stojanowski, J., Bargenda-Lange, A., & Gołębiowski, T. (2025). GDF15, EGF, and Neopterin in Assessing Progression of Pediatric Chronic Kidney Disease Using Artificial Intelligence Tools—A Pilot Study. International Journal of Molecular Sciences, 26(5), 2344. https://doi.org/10.3390/ijms26052344






