NAC047/052/104 Synergistically Regulate the Dark-Induced Leaf Senescence in Non-Heading Chinese Cabbage
Abstract
1. Introduction
2. Results
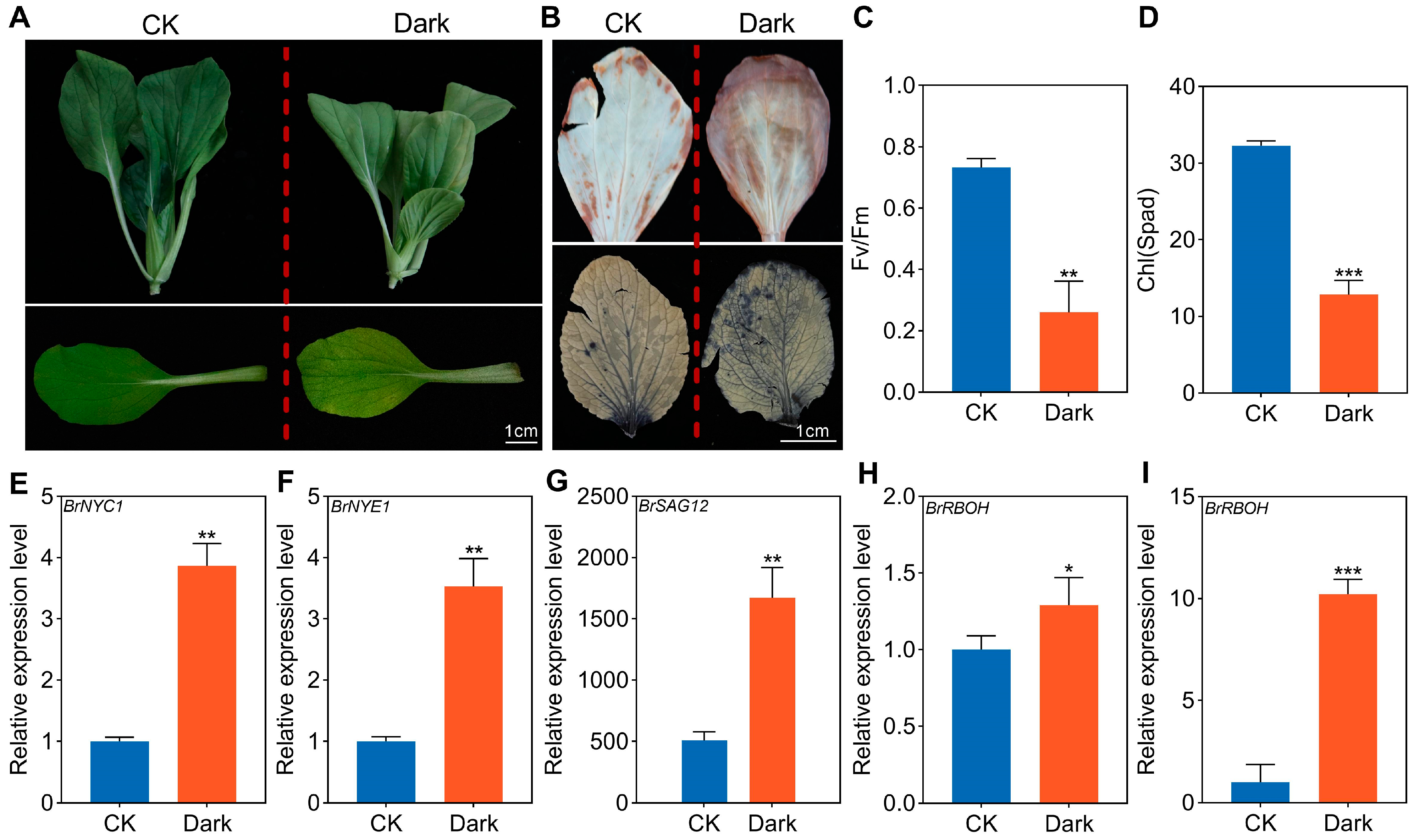
2.1. Phenotypic and Physiological Analysis of Dark-Induced Senescence in NHCC
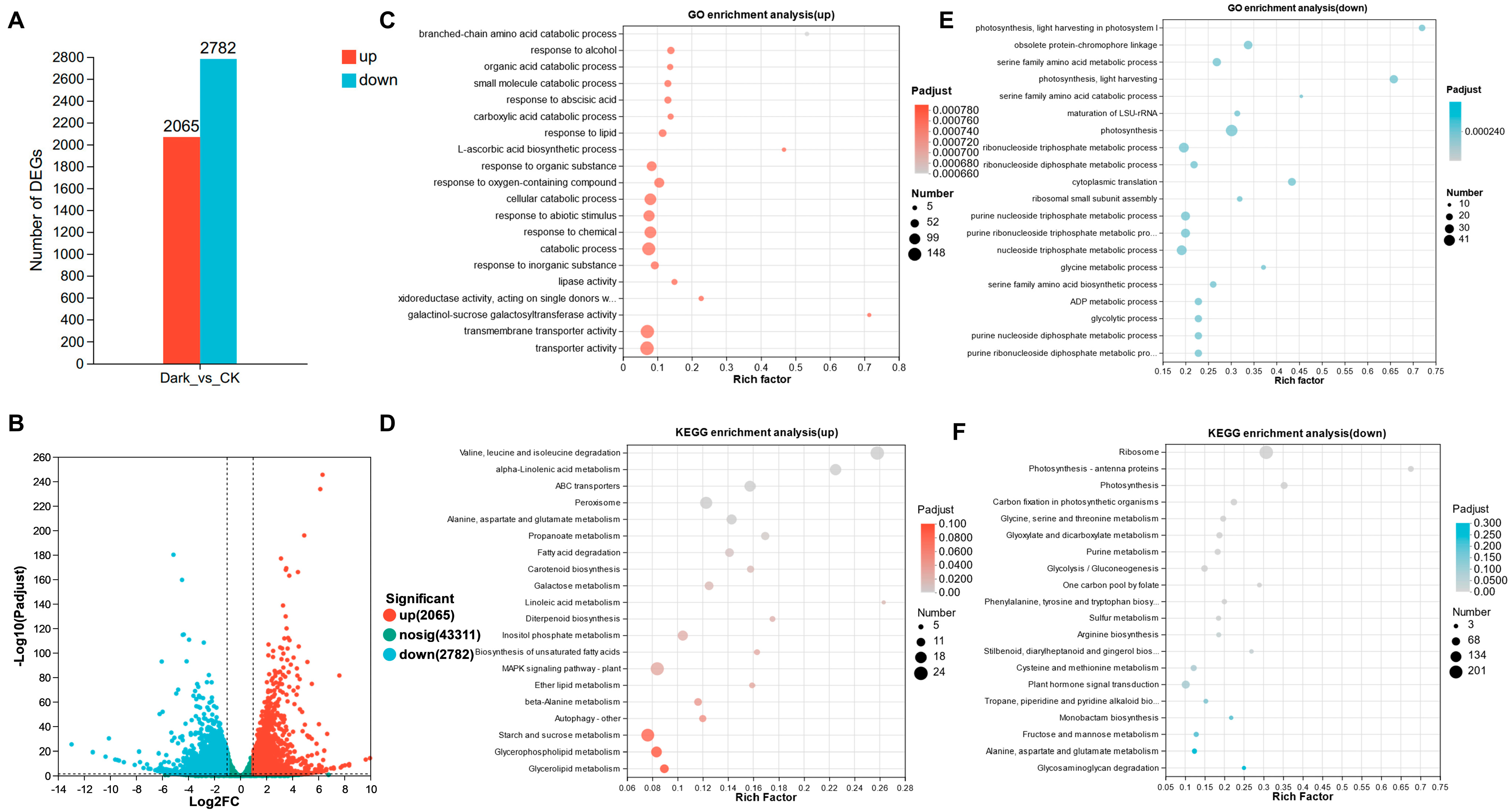
2.2. Analysis of Dark-Induced Transcriptomic Alterations in NHCC Leaves
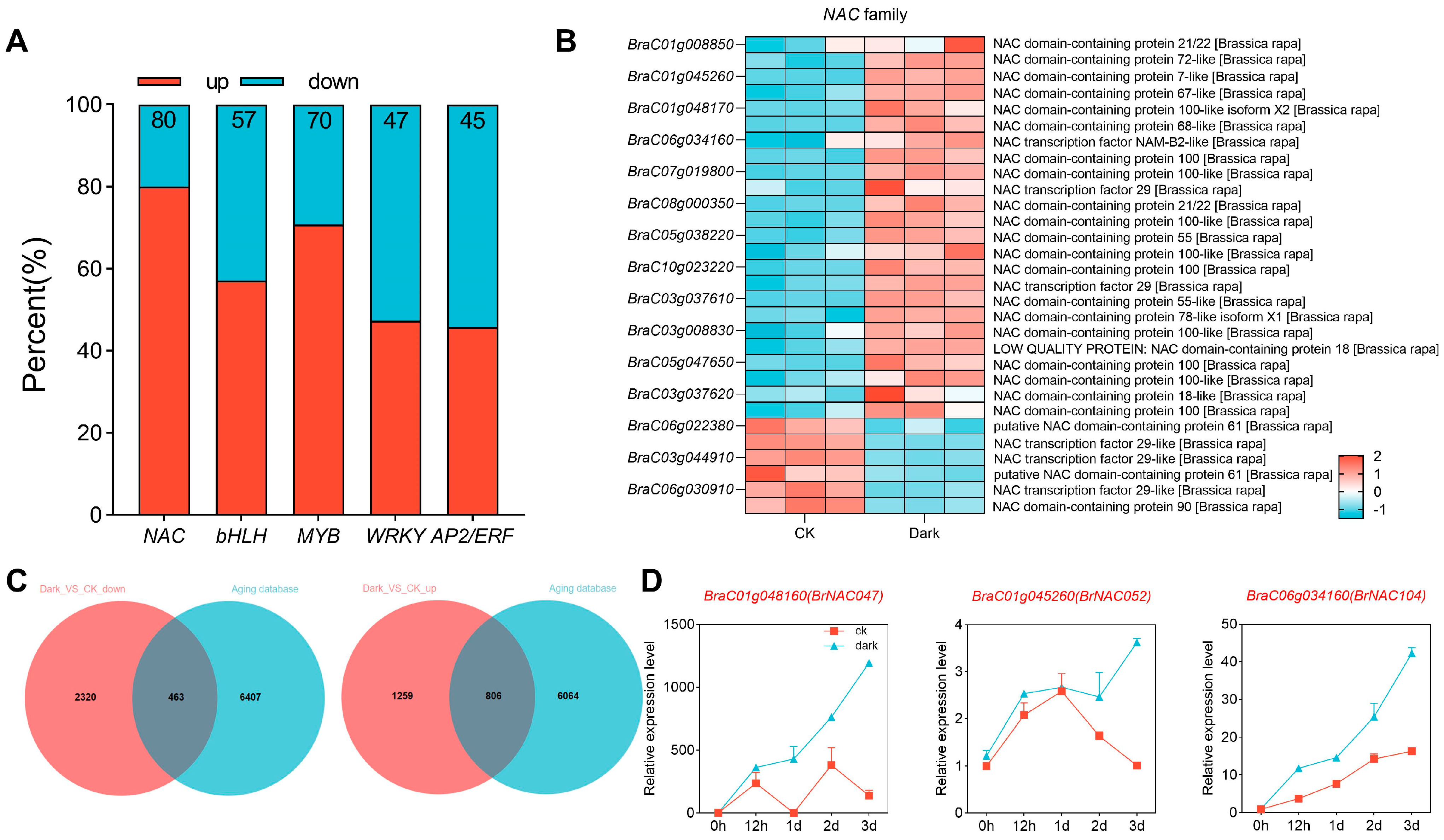
2.3. Darkness Primarily Activates NAC Transcription Factors in NHCC
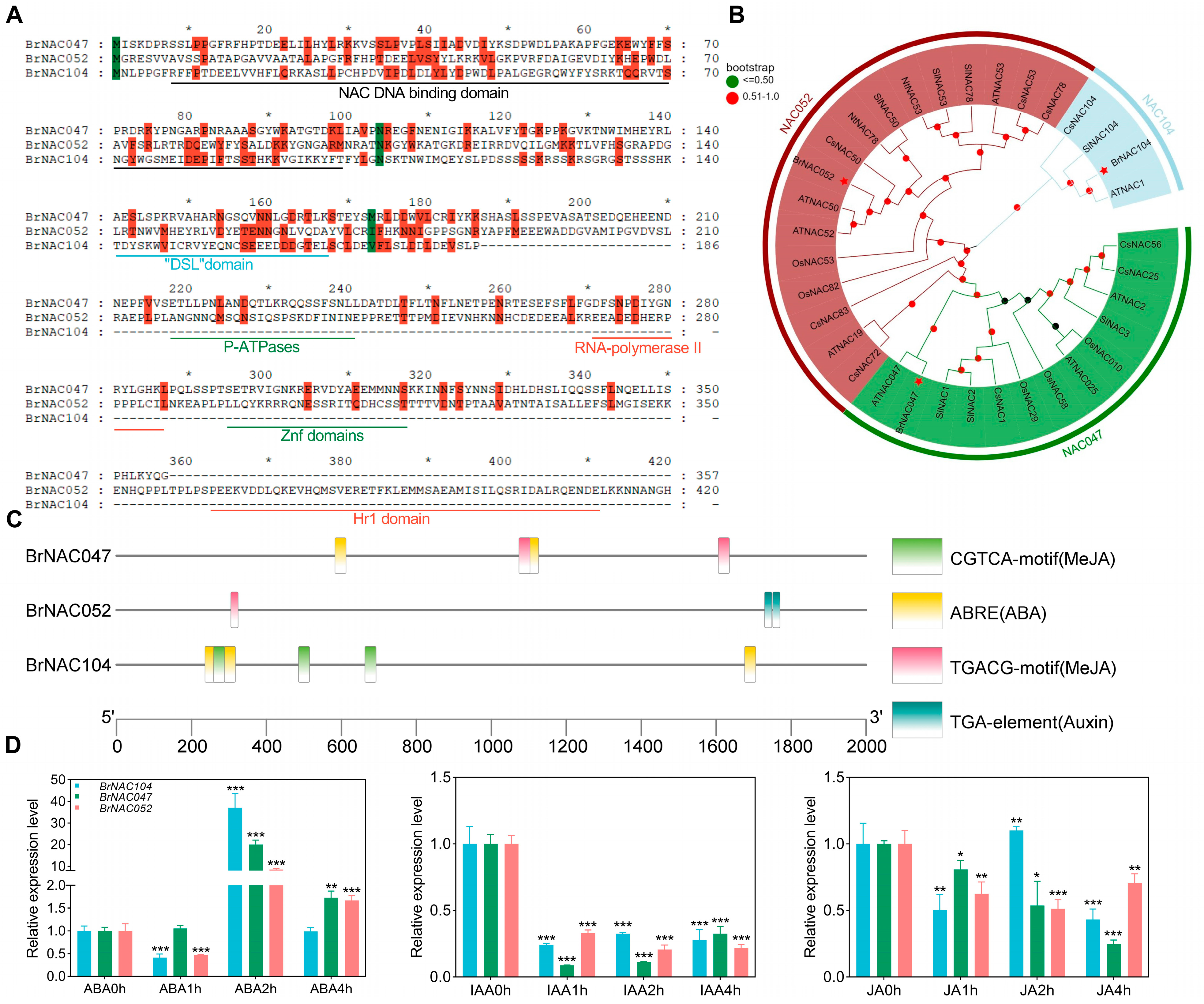
2.4. Molecular Characterization of BrNAC047, BrNAC052, and BrNAC104
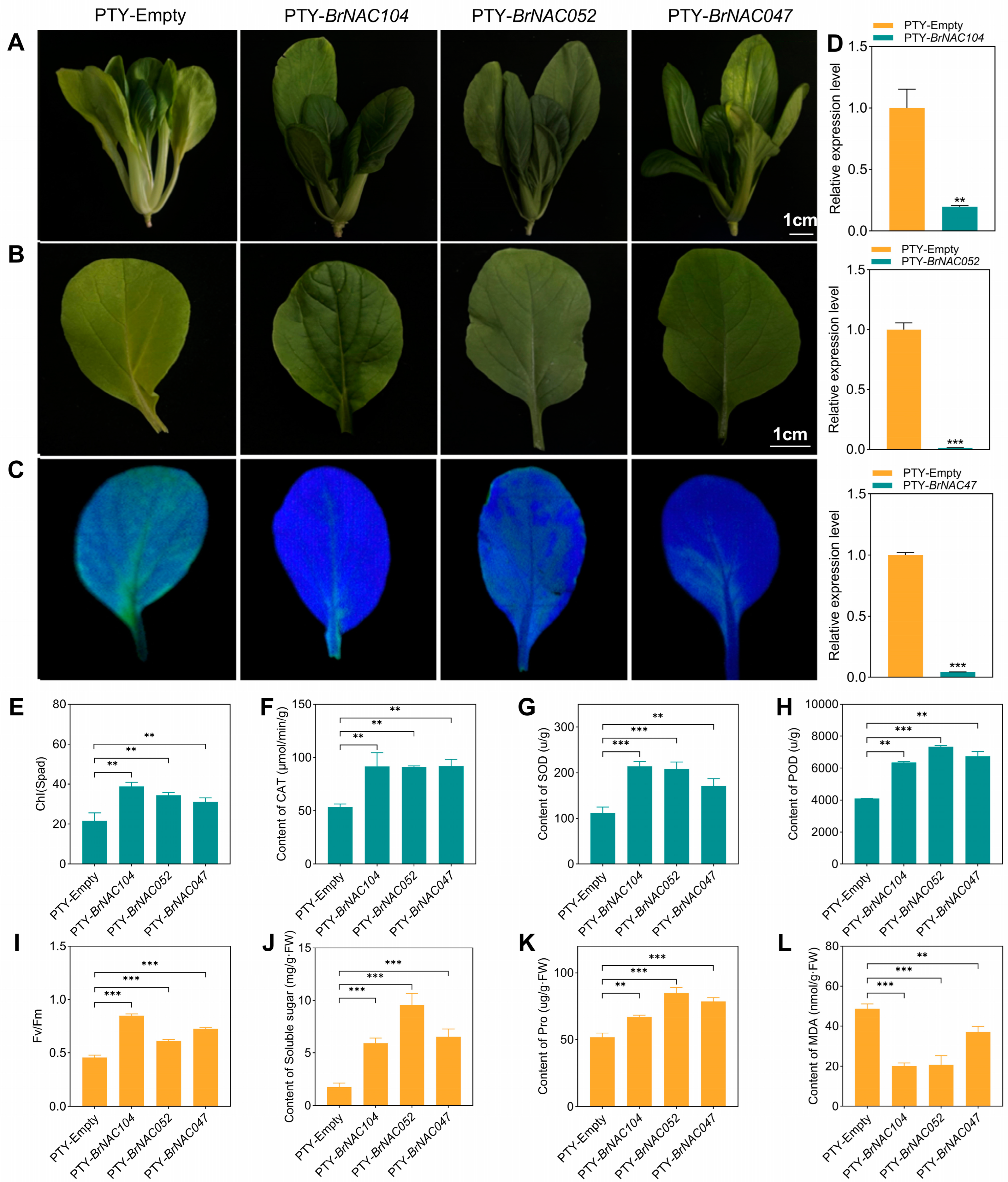
2.5. BraNAC047/BrNAC052/BrNAC104 Positively Regulate Dark-Induced Leaf Senescence in NHCC
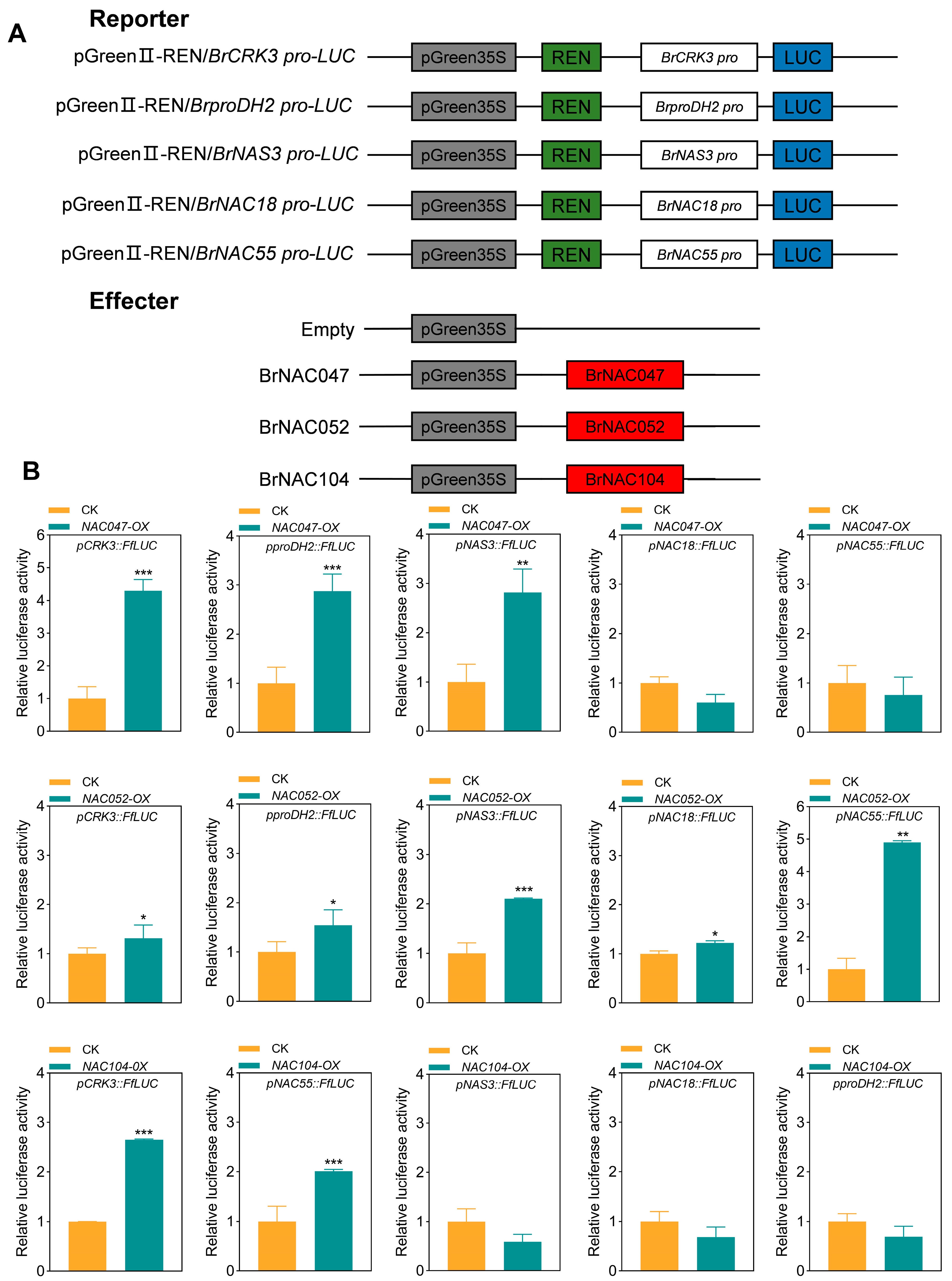
2.6. BrNAC047, BrNAC052, and BrNAC104 Activate SAG Expression in NHCC
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Dark Treatment
4.3. Total RNA Extraction, Library Construction, and Sequencing
4.4. Measurements of Chlorophyll Content and the FV/Fm Ratio
4.5. DAB and NBT Staining
4.6. Virus-Induced Gene Silencing (VIGS)
4.7. Determination of Antioxidant Activity and Soluble Sugar Content
4.8. Dual-Luciferase Assay
4.9. Yeast Two-Hybrid Assay
4.10. qRT-PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, D.; Liu, S.T.; Wei, Y.P.; Zhou, D.Y.; Hou, X.L.; Li, Y.; Hu, C.M. cDNA-AFLP analysis reveals differential gene expression in incompatible interaction between infected non-heading Chinese cabbage and Hyaloperonospora parasitica. Hortic. Res. 2016, 3, 16034. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Lim, C.J.; Kim, J.K.; Park, S.U. Comparative Metabolic Profiling of Green and Purple Pakchoi (Brassica Rapa Subsp. Chinensis). Molecules 2018, 23, 1613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tang, S.; Li, X.; Chen, Y.; Li, J.; Wang, Y.; Bian, R.; Jin, Y.; Zhu, X.; Zhang, K. Arabidopsis WRKY1 promotes monocarpic senescence by integrative regulation of flowering, leaf senescence and nitrogen remobilization. Mol. Plant 2024, 17, 1289–1306. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Peng, J.; Chen, C.; Xiong, C.; Li, S.; Ge, A.; Wang, E.; Liesack, W. Harnessing biological nitrogen fixation in plant leaves. Trends Plant Sci. 2023, 28, 1391–1405. [Google Scholar] [CrossRef] [PubMed]
- Zohner, C.M.; Mirzagholi, L.; Renner, S.S.; Mo, L.; Rebindaine, D.; Bucher, R.; Palouš, D.; Vitasse, Y.; Fu, Y.H.; Stocker, B.D.; et al. Effect of climate warming on the timing of autumn leaf senescence reverses after the summer solstice. Science 2023, 381, eadf5098. [Google Scholar] [CrossRef]
- Wingler, A.; Marès, M.; Pourtau, N. Spatial patterns and metabolic regulation of photosynthetic parameters during leaf senescence. New Phytol. 2004, 161, 781–789. [Google Scholar] [CrossRef]
- Guo, Y.; Ren, G.; Zhang, K.; Li, Z.; Miao, Y.; Guo, H. Leaf senescence: Progression, regulation, and application. Mol. Hortic. 2021, 1, 5. [Google Scholar] [CrossRef]
- Guo, Y.; Gan, S. Leaf senescence: Signals, execution, and regulation. Curr. Top. Dev. Biol. 2005, 71, 83–112. [Google Scholar] [CrossRef]
- Sun, L.; Xu, H.; Song, J.; Yang, X.; Wang, X.; Liu, H.; Pang, M.; Hu, Y.; Yang, Q.; Ning, X.; et al. OsNAC103, a NAC Transcription Factor, Positively Regulates Leaf Senescence and Plant Architecture in Rice. Rice 2024, 17, 15. [Google Scholar] [CrossRef]
- Woo, H.R.; Kim, H.J.; Nam, H.G.; Lim, P.O. Plant leaf senescence and death—Regulation by multiple layers of control and implications for aging in general. J. Cell Sci. 2013, 126, 4823–4833. [Google Scholar] [CrossRef]
- Ma, L.; Han, R.; Yang, Y.; Liu, X.; Li, H.; Zhao, X.; Li, J.; Fu, H.; Huo, Y.; Sun, L.; et al. Phytochromes enhance SOS2-mediated PIF1 and PIF3 phosphorylation and degradation to promote Arabidopsis salt tolerance. Plant Cell 2023, 35, 2997–3020. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, R.; Abe, S.; Marugami, M.; Yamagami, A.; Akema, R.; Ohashi, T.; Nishida, K.; Nosaki, S.; Miyakawa, T.; Tanokura, M.; et al. BPG4 regulates chloroplast development and homeostasis by suppressing GLK transcription factors and involving light and brassinosteroid signaling. Nat. Commun. 2024, 15, 370. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Iqbal, M.S.; Hashem, A.; Abd Allah, E.F.; Ansari, M.I. Plant Defense Responses to Biotic Stress and Its Interplay with Fluctuating Dark/Light Conditions. Front. Plant Sci. 2021, 12, 631810. [Google Scholar] [CrossRef]
- Buchanan-Wollaston, V.; Page, T.; Harrison, E.; Breeze, E.; Lim, P.O.; Nam, H.G.; Lin, J.F.; Wu, S.H.; Swidzinski, J.; Ishizaki, K.; et al. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 2005, 42, 567–585. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhao, W.; Cui, S.; Su, X.; Yu, J.; Guo, L.; Song, K. Deciphering the dual role of persistent luminescence materials: Toxicity and photoreception effects on rice development. Sci. Total Environ. 2024, 947, 174542. [Google Scholar] [CrossRef]
- Liu, X.W.; Wang, T.; Bartholomew, E.; Black, K.; Dong, M.M.; Zhang, Y.Q.; Yang, S.; Cai, Y.L.; Xue, S.D.; Weng, Y.Q.; et al. Comprehensive analysis of NAC transcription factors and their expression during fruit spine development in cucumber (Cucumis sativus L.). Hortic. Res. 2018, 5, 31. [Google Scholar] [CrossRef]
- Aida, M.; Ishida, T.; Fukaki, H.; Fujisawa, H.; Tasaka, M. Genes involved in organ separation in Arabidopsis: An analysis of the cup-shaped cotyledon mutant. Plant Cell 1997, 9, 841–857. [Google Scholar] [CrossRef]
- Hegedus, D.; Yu, M.; Baldwin, D.; Gruber, M.; Sharpe, A.; Parkin, I.; Whitwill, S.; Lydiate, D. Molecular characterization of Brassica napus NAC domain transcriptional activators induced in response to biotic and abiotic stress. Plant Mol. Biol. 2003, 53, 383–397. [Google Scholar] [CrossRef]
- Lei, L.; Wu, D.; Cui, C.; Gao, X.; Yao, Y.; Dong, J.; Xu, L.; Yang, M. Transcriptome Analysis of Early Senescence in the Post-Anthesis Flag Leaf of Wheat (Triticum aestivum L.). Plants 2022, 11, 2593. [Google Scholar] [CrossRef]
- Parrott, D.L.; McInnerney, K.; Feller, U.; Fischer, A.M. Steam-girdling of barley (Hordeum vulgare) leaves leads to carbohydrate accumulation and accelerated leaf senescence, facilitating transcriptomic analysis of senescence-associated genes. New Phytol. 2007, 176, 56–69. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Y.; Zhou, G.; Ye, R.; Zhao, L.; Li, X.; Lin, Y. Identification of early senescence-associated genes in rice flag leaves. Plant Mol. Biol. 2008, 67, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Balazadeh, S.; Siddiqui, H.; Allu, A.D.; Matallana-Ramirez, L.P.; Caldana, C.; Mehrnia, M.; Zanor, M.I.; Köhler, B.; Mueller-Roeber, B. A gene regulatory network controlled by the NAC transcription factor ANAC092/AtNAC2/ORE1 during salt-promoted senescence. Plant J. 2010, 62, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gan, S. AtNAP, a NAC family transcription factor, has an important role in leaf senescence. Plant J. 2006, 46, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.J.; Wang, Y.; Li, B.; Chang, J.L.; Chen, M.J.; Li, K.X.; Yang, G.X.; He, G.Y. TaNAC29, a NAC transcription factor from wheat, enhances salt and drought tolerance in transgenic Arabidopsis. BMC Plant Biol. 2015, 15, 268. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Gongbuzhaxi; Wang, C.Y.; Xue, F.; Zhang, H.; Ji, W.Q. Wheat NAC transcription factor TaNAC29 is involved in response to salt stress. Plant Physiol. Biochem. 2015, 96, 356–363. [Google Scholar] [CrossRef]
- Yang, S.D.; Seo, P.J.; Yoon, H.K.; Park, C.M. The Arabidopsis NAC transcription factor VNI2 integrates abscisic acid signals into leaf senescence via the COR/RD genes. Plant Cell 2011, 23, 2155–2168. [Google Scholar] [CrossRef]
- Takasaki, H.; Maruyama, K.; Takahashi, F.; Fujita, M.; Yoshida, T.; Nakashima, K.; Myouga, F.; Toyooka, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. SNAC-As, stress-responsive NAC transcription factors, mediate ABA-inducible leaf senescence. Plant J. 2015, 84, 1114–1123. [Google Scholar] [CrossRef]
- Zhang, K.; Gan, S.S. An abscisic acid-AtNAP transcription factor-SAG113 protein phosphatase 2C regulatory chain for controlling dehydration in senescing Arabidopsis leaves. Plant Physiol. 2012, 158, 961–969. [Google Scholar] [CrossRef]
- Zareen, S.; Ali, A.; Lim, C.J.; Khan, H.A.; Park, J.; Xu, Z.Y.; Yun, D.J. The Transcriptional Corepressor HOS15 Mediates Dark-Induced Leaf Senescence in Arabidopsis. Front. Plant Sci. 2022, 13, 828264. [Google Scholar] [CrossRef]
- Chen, P.Y.; Nguyen, T.T.T.; Lee, R.H.; Hsu, T.W.; Kao, M.H.; Gojobori, T.; Chiang, T.Y.; Huang, C.L. Genome-wide expression analysis of vegetative organs during developmental and herbicide-induced whole plant senescence in Arabidopsis thaliana. BMC Genom. 2024, 25, 621. [Google Scholar] [CrossRef]
- Song, G.; Kwon, C.T.; Kim, S.H.; Shim, Y.; Lim, C.; Koh, H.J.; An, G.; Kang, K.; Paek, N.C. The Rice SPOTTED LEAF4 (SPL4) Encodes a Plant Spastin That Inhibits ROS Accumulation in Leaf Development and Functions in Leaf Senescence. Front. Plant Sci. 2018, 9, 1925. [Google Scholar] [CrossRef]
- Lee, J.; Kang, M.H.; Kim, J.Y.; Lim, P.O. The Role of Light and Circadian Clock in Regulation of Leaf Senescence. Front. Plant Sci. 2021, 12, 669170. [Google Scholar] [CrossRef] [PubMed]
- Rosado, D.; Trench, B.; Bianchetti, R.; Zuccarelli, R.; Rodrigues Alves, F.R.; Purgatto, E.; Segal Floh, E.I.; Silveira Nogueira, F.T.; Freschi, L.; Rossi, M. Downregulation of PHYTOCHROME-INTERACTING FACTOR 4 Influences Plant Development and Fruit Production. Plant Physiol. 2019, 181, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Z.; Chen, Y.; He, J.X.; Bi, Y. PHYTOCHROME-INTERACTING FACTOR 5 (PIF5) positively regulates dark-induced senescence and chlorophyll degradation in Arabidopsis. Plant Sci. 2015, 237, 57–68. [Google Scholar] [CrossRef]
- Song, Y.; Yang, C.; Gao, S.; Zhang, W.; Li, L.; Kuai, B. Age-triggered and dark-induced leaf senescence require the bHLH transcription factors PIF3, 4, and 5. Mol. Plant 2014, 7, 1776–1787. [Google Scholar] [CrossRef] [PubMed]
- Thiele, A.; Herold, M.; Lenk, I.; Quail, P.H.; Gatz, C. Heterologous expression of Arabidopsis phytochrome B in transgenic potato influences photosynthetic performance and tuber development. Plant Physiol. 1999, 120, 73–82. [Google Scholar] [CrossRef]
- Chun, I.; Kim, H.J.; Hong, S.; Kim, Y.G.; Kim, M.S. Structural basis of DNA binding by the NAC transcription factor ORE1, a master regulator of plant senescence. Plant Commun. 2023, 4, 100510. [Google Scholar] [CrossRef]
- Garapati, P.; Xue, G.P.; Munné-Bosch, S.; Balazadeh, S. Transcription Factor ATAF1 in Arabidopsis Promotes Senescence by Direct Regulation of Key Chloroplast Maintenance and Senescence Transcriptional Cascades. Plant Physiol. 2015, 168, 1122–1139. [Google Scholar] [CrossRef]
- Kim, H.J.; Nam, H.G.; Lim, P.O. Regulatory network of NAC transcription factors in leaf senescence. Curr. Opin. Plant Biol. 2016, 33, 48–56. [Google Scholar] [CrossRef]
- Oda-Yamamizo, C.; Mitsuda, N.; Sakamoto, S.; Ogawa, D.; Ohme-Takagi, M.; Ohmiya, A. The NAC transcription factor ANAC046 is a positive regulator of chlorophyll degradation and senescence in Arabidopsis leaves. Sci. Rep. 2016, 6, 23609. [Google Scholar] [CrossRef]
- Liang, C.; Wang, Y.; Zhu, Y.; Tang, J.; Hu, B.; Liu, L.; Ou, S.; Wu, H.; Sun, X.; Chu, J.; et al. OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice. Proc. Natl. Acad. Sci. USA 2014, 111, 10013–10018. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Lu, S.; Lv, B.; Zhang, B.; Shen, J.; He, J.; Luo, L.; Xi, D.; Chen, X.; Ming, F. A Rice NAC Transcription Factor Promotes Leaf Senescence via ABA Biosynthesis. Plant Physiol. 2017, 174, 1747–1763. [Google Scholar] [CrossRef]
- El Mannai, Y.; Akabane, K.; Hiratsu, K.; Satoh-Nagasawa, N.; Wabiko, H. The NAC Transcription Factor Gene OsY37 (ONAC011) Promotes Leaf Senescence and Accelerates Heading Time in Rice. Int. J. Mol. Sci. 2017, 18, 2165. [Google Scholar] [CrossRef] [PubMed]
- Kamranfar, I.; Xue, G.P.; Tohge, T.; Sedaghatmehr, M.; Fernie, A.R.; Balazadeh, S.; Mueller-Roeber, B. Transcription factor RD26 is a key regulator of metabolic reprogramming during dark-induced senescence. New Phytol. 2018, 218, 1543–1557. [Google Scholar] [CrossRef]
- Wang, H.L.; Zhang, Y.; Wang, T.; Yang, Q.; Yang, Y.; Li, Z.; Li, B.; Wen, X.; Li, W.; Yin, W.; et al. An alternative splicing variant of PtRD26 delays leaf senescence by regulating multiple NAC transcription factors in Populus. Plant Cell 2021, 33, 1594–1614. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Z. PIF4 and PIF4-Interacting Proteins: At the Nexus of Plant Light, Temperature and Hormone Signal Integrations. Int. J. Mol. Sci. 2021, 22, 10304. [Google Scholar] [CrossRef]
- Cackett, L.; Luginbuehl, L.H.; Schreier, T.B.; Lopez-Juez, E.; Hibberd, J.M. Chloroplast development in green plant tissues: The interplay between light, hormone, and transcriptional regulation. New Phytol. 2021, 233, 2000–2016. [Google Scholar] [CrossRef]
- Mahapatra, K.; Dwivedi, S.; Mukherjee, A.; Pradhan, A.A.; Rao, K.V.; Singh, D.; Bhagavatula, L.; Datta, S. Interplay of Light and ABA signaling to modulate plant development. J. Exp. Bot. 2024, 76, 730–745. [Google Scholar] [CrossRef] [PubMed]
- Liebsch, D.; Keech, O. Dark-induced leaf senescence: New insights into a complex light-dependent regulatory pathway. New Phytol. 2016, 212, 563–570. [Google Scholar] [CrossRef]
- Deepika; Ankit; Sagar, S.; Singh, A. Dark-Induced Hormonal Regulation of Plant Growth and Development. Front. Plant Sci. 2020, 11, 581666. [Google Scholar] [CrossRef]
- Zheng, Q.; Teng, Z.; Zhang, J.; Ye, N. ABA Inhibits Rice Seed Aging by Reducing H2O2 Accumulation in the Radicle of Seeds. Plants 2024, 13, 809. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Wu, S.D.; Li, N.; Gao, J.; Liu, S.H.; Zhu, S.; Li, Z.L.; Ren, G.D.; Kuai, B.K. Chemical induction of leaf senescence and powdery mildew resistance involves ethylene-mediated chlorophyll degradation and ROS metabolism in cucumber. Hortic. Res. 2022, 9, uhac101. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.Y.; Liu, T.K.; Tang, J.; Duan, W.K.; Hou, X.L. BcMAF2 activates BcTEM1 and represses flowering in Pak-choi (Brassica rapa ssp. chinensis). Plant Mol. Biol. 2019, 100, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhu, Z.; Gao, J.; Zhou, X.; Zhu, S.; Wang, X.; Wang, X.; Ren, G.; Kuai, B. The NPR1-WRKY46-WRKY6 signaling cascade mediates probenazole/salicylic acid-elicited leaf senescence in Arabidopsis thaliana. J. Integr. Plant Biol. 2021, 63, 924–936. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Zhang, D.; Meng, Z.; Yin, Y.; Yang, X.; Cao, M.; Li, R.; Song, Y.; Zhu, H. NAC047/052/104 Synergistically Regulate the Dark-Induced Leaf Senescence in Non-Heading Chinese Cabbage. Int. J. Mol. Sci. 2025, 26, 2340. https://doi.org/10.3390/ijms26052340
Yang B, Zhang D, Meng Z, Yin Y, Yang X, Cao M, Li R, Song Y, Zhu H. NAC047/052/104 Synergistically Regulate the Dark-Induced Leaf Senescence in Non-Heading Chinese Cabbage. International Journal of Molecular Sciences. 2025; 26(5):2340. https://doi.org/10.3390/ijms26052340
Chicago/Turabian StyleYang, Bing, Dingyu Zhang, Zitong Meng, Yijiang Yin, Xiao Yang, Mengqin Cao, Ruixin Li, Yishan Song, and Hongfang Zhu. 2025. "NAC047/052/104 Synergistically Regulate the Dark-Induced Leaf Senescence in Non-Heading Chinese Cabbage" International Journal of Molecular Sciences 26, no. 5: 2340. https://doi.org/10.3390/ijms26052340
APA StyleYang, B., Zhang, D., Meng, Z., Yin, Y., Yang, X., Cao, M., Li, R., Song, Y., & Zhu, H. (2025). NAC047/052/104 Synergistically Regulate the Dark-Induced Leaf Senescence in Non-Heading Chinese Cabbage. International Journal of Molecular Sciences, 26(5), 2340. https://doi.org/10.3390/ijms26052340



