Bacterial Amino Acid Auxotrophies Enable Energetically Costlier Proteomes
Abstract
1. Introduction
2. Results
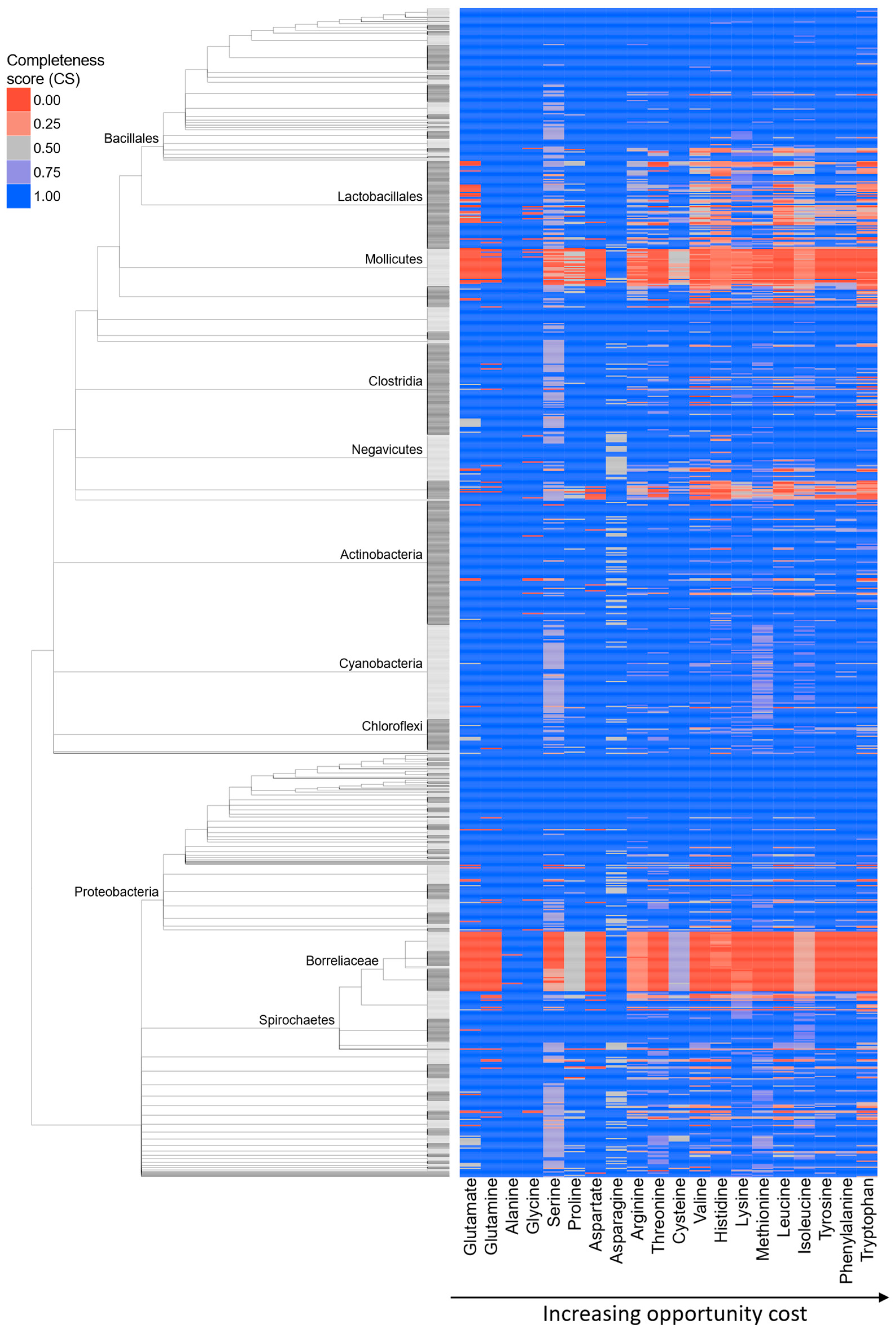
2.1. Global Trends in Bacterial AA Auxotrophies
2.2. Expensive AAs Are More Commonly Lost
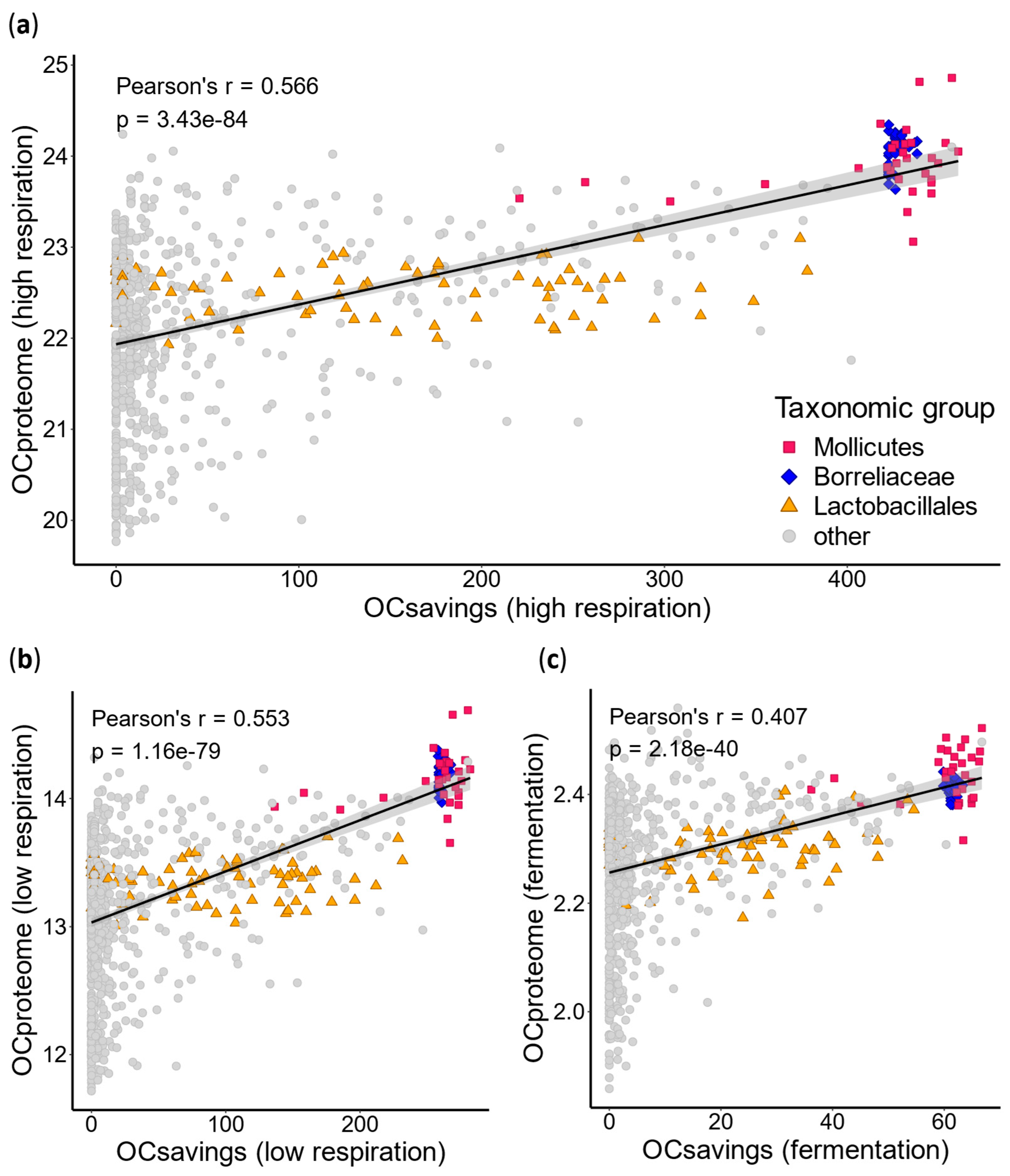
2.3. Energy Savings via AA Auxotrophies Enable Costlier Proteomes
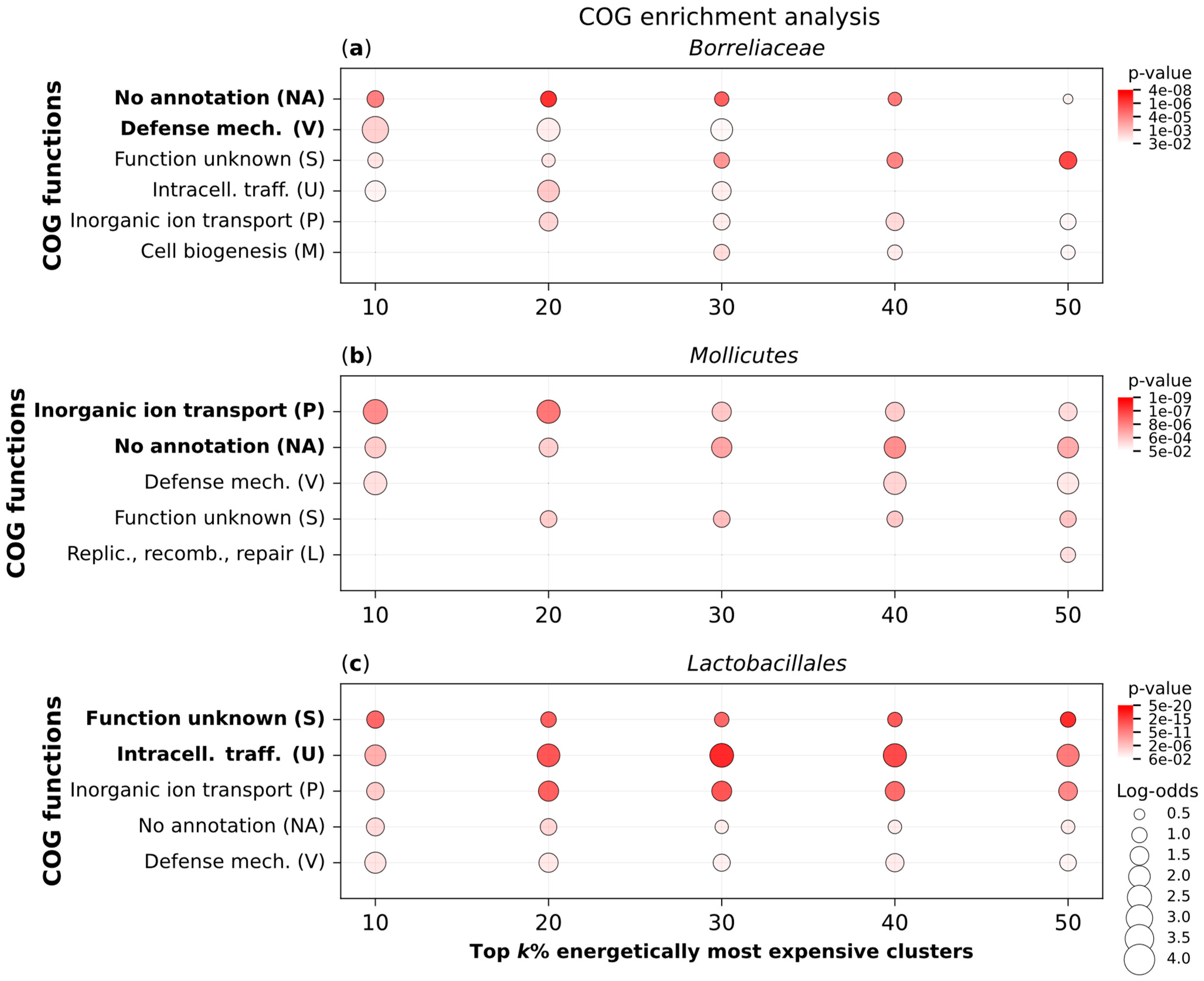
2.4. Expensive Proteins Have Ecologically Relevant Functions
3. Discussion
4. Materials and Methods
4.1. Databases, Completeness Score and Auxotrophy Index
4.2. Opportunity Cost Measures
4.3. COG Functions Enrichment Analyses
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Domazet-Lošo, M.; Široki, T.; Šimičević, K.; Domazet-Lošo, T. Macroevolutionary Dynamics of Gene Family Gain and Loss along Multicellular Eukaryotic Lineages. Nat. Commun. 2024, 15, 2663. [Google Scholar] [CrossRef] [PubMed]
- Kasalo, N.; Domazet-Lošo, M.; Domazet-Lošo, T. Massive Outsourcing of Energetically Costly Amino Acids at the Origin of Animals. bioRxiv 2024. [Google Scholar] [CrossRef]
- Payne, S.H.; Loomis, W.F. Retention and Loss of Amino Acid Biosynthetic Pathways Based on Analysis of Whole-Genome Sequences. Eukaryot. Cell 2006, 5, 272–276. [Google Scholar] [CrossRef]
- Ramoneda, J.; Jensen, T.B.N.; Price, M.N.; Casamayor, E.O.; Fierer, N. Taxonomic and Environmental Distribution of Bacterial Amino Acid Auxotrophies. Nat. Commun. 2023, 14, 7608. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.J.; Fozouni, P.; Eisen, M.B.; King, N. Gene Family Innovation, Conservation and Loss on the Animal Stem Lineage. eLife 2018, 7, e34226. [Google Scholar] [CrossRef]
- Guedes, R.; Prosdocimi, F.; Fernandes, G.; Moura, L.; Ribeiro, H.; Ortega, J. Amino Acids Biosynthesis and Nitrogen Assimilation Pathways: A Great Genomic Deletion during Eukaryotes Evolution. BMC Genom. 2011, 12, S2. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Waschina, S.; Pande, S.; Bohl, K.; Kaleta, C.; Kost, C. Less Is More: Selective Advantages CAN Explain the Prevalent Loss of Biosynthetic Genes in Bacteria. Evolution 2014, 68, 2559–2570. [Google Scholar] [CrossRef]
- Mee, M.T.; Wang, H.H. Engineering Ecosystems and Synthetic Ecologies. Mol. BioSyst. 2012, 8, 2470. [Google Scholar] [CrossRef]
- Price, M.N.; Zane, G.M.; Kuehl, J.V.; Melnyk, R.A.; Wall, J.D.; Deutschbauer, A.M.; Arkin, A.P. Filling Gaps in Bacterial Amino Acid Biosynthesis Pathways with High-Throughput Genetics. PLoS Genet. 2018, 14, e1007147. [Google Scholar] [CrossRef]
- D’Souza, G.; Kost, C. Experimental Evolution of Metabolic Dependency in Bacteria. PLoS Genet. 2016, 12, e1006364. [Google Scholar] [CrossRef]
- Mee, M.T.; Collins, J.J.; Church, G.M.; Wang, H.H. Syntrophic Exchange in Synthetic Microbial Communities. Proc. Natl. Acad. Sci. USA 2014, 111, E2149–E2156. [Google Scholar] [CrossRef]
- Kaleta, C.; Schäuble, S.; Rinas, U.; Schuster, S. Metabolic Costs of Amino Acid and Protein Production in Escherichia coli. Biotechnol. J. 2013, 8, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Zengler, K.; Zaramela, L.S. The Social Network of Microorganisms—How Auxotrophies Shape Complex Communities. Nat. Rev. Microbiol. 2018, 16, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Starke, S.; Harris, D.M.M.; Zimmermann, J.; Schuchardt, S.; Oumari, M.; Frank, D.; Bang, C.; Rosenstiel, P.; Schreiber, S.; Frey, N.; et al. Amino Acid Auxotrophies in Human Gut Bacteria Are Linked to Higher Microbiome Diversity and Long-Term Stability. ISME J. 2023, 17, 2370–2380. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.; Kaleta, C.; Waschina, S. Gapseq: Informed Prediction of Bacterial Metabolic Pathways and Reconstruction of Accurate Metabolic Models. Genome Biol. 2021, 22, 81. [Google Scholar] [CrossRef]
- Price, M.N.; Deutschbauer, A.M.; Arkin, A.P. GapMind: Automated Annotation of Amino Acid Biosynthesis. mSystems 2020, 5, e00291-20. [Google Scholar] [CrossRef]
- Steinegger, M.; Söding, J. MMseqs2 Enables Sensitive Protein Sequence Searching for the Analysis of Massive Data Sets. Nat. Biotechnol. 2017, 35, 1026–1028. [Google Scholar] [CrossRef]
- Domazet-Lošo, T.; Brajković, J.; Tautz, D. A Phylostratigraphy Approach to Uncover the Genomic History of Major Adaptations in Metazoan Lineages. Trends Genet. 2007, 23, 533–539. [Google Scholar] [CrossRef]
- Domazet-Loso, T.; Tautz, D. An Evolutionary Analysis of Orphan Genes in Drosophila. Genome Res. 2003, 13, 2213–2219. [Google Scholar] [CrossRef]
- Tautz, D.; Domazet-Lošo, T. The Evolutionary Origin of Orphan Genes. Nat. Rev. Genet. 2011, 12, 692–702. [Google Scholar] [CrossRef]
- Kerstholt, M.; Netea, M.G.; Joosten, L.A.B. Borrelia Burgdorferi Hijacks Cellular Metabolism of Immune Cells: Consequences for Host Defense. Ticks Tick-Borne Dis. 2020, 11, 101386. [Google Scholar] [CrossRef] [PubMed]
- Sirand-Pugnet, P.; Citti, C.; Barré, A.; Blanchard, A. Evolution of Mollicutes: Down a Bumpy Road with Twists and Turns. Res. Microbiol. 2007, 158, 754–766. [Google Scholar] [CrossRef]
- Fisunov, G.Y.; Alexeev, D.G.; Bazaleev, N.A.; Ladygina, V.G.; Galyamina, M.A.; Kondratov, I.G.; Zhukova, N.A.; Serebryakova, M.V.; Demina, I.A.; Govorun, V.M. Core Proteome of the Minimal Cell: Comparative Proteomics of Three Mollicute Species. PLoS ONE 2011, 6, e21964. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Makarova, K.; Slesarev, A.; Wolf, Y.; Sorokin, A.; Mirkin, B.; Koonin, E.; Pavlov, A.; Pavlova, N.; Karamychev, V.; Polouchine, N.; et al. Comparative Genomics of the Lactic Acid Bacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 15611–15616. [Google Scholar] [CrossRef] [PubMed]
- Pilo, P.; Frey, J.; Vilei, E.M. Molecular Mechanisms of Pathogenicity of Mycoplasma Mycoides Subsp. Mycoides SC. Vet. J. 2007, 174, 513–521. [Google Scholar] [CrossRef][Green Version]
- Strnad, M.; Rudenko, N.; Rego, R.O.M. Pathogenicity and Virulence of Borrelia burgdorferi. Virulence 2023, 14, 2265015. [Google Scholar] [CrossRef]
- Waites, K.B.; Talkington, D.F. Mycoplasma pneumoniae and Its Role as a Human Pathogen. Clin. Microbiol. Rev. 2004, 17, 697–728. [Google Scholar] [CrossRef]
- Čorak, N.; Anniko, S.; Daschkin-Steinborn, C.; Krey, V.; Koska, S.; Futo, M.; Široki, T.; Woichansky, I.; Opašić, L.; Kifer, D.; et al. Pleomorphic Variants of Borreliella (Syn. Borrelia) Burgdorferi Express Evolutionary Distinct Transcriptomes. Int. J. Mol. Sci. 2023, 24, 5594. [Google Scholar] [CrossRef]
- Anderson, C.; Brissette, C.A. The Brilliance of Borrelia: Mechanisms of Host Immune Evasion by Lyme Disease-Causing Spirochetes. Pathogens 2021, 10, 281. [Google Scholar] [CrossRef]
- Dulipati, V.; Meri, S.; Panelius, J. Complement Evasion Strategies of Borrelia burgdorferi Sensu Lato. FEBS Lett. 2020, 594, 2645–2656. [Google Scholar] [CrossRef]
- Lambert, L.C.; Trummell, H.Q.; Singh, A.; Cassell, G.H.; Bridges, R.J. Mycoplasma Pulmonis Inhibits Electrogenic Ion Transport across Murine Tracheal Epithelial Cell Monolayers. Infect. Immun. 1998, 66, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wuertz, S. Bacteria and Archaea on Earth and Their Abundance in Biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Knight, R. Global Patterns in Bacterial Diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 11436–11440. [Google Scholar] [CrossRef] [PubMed]
- Futo, M.; Opašić, L.; Koska, S.; Čorak, N.; Široki, T.; Ravikumar, V.; Thorsell, A.; Lenuzzi, M.; Kifer, D.; Domazet-Lošo, M.; et al. Embryo-Like Features in Developing Bacillus subtilis Biofilms. Mol. Biol. Evol. 2021, 38, 31–47. [Google Scholar] [CrossRef]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness with Single-Copy Orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Midford, P.E.; Ong, W.K.; Paley, S.; Subhraveti, P.; Karp, P.D. The MetaCyc Database of Metabolic Pathways and Enzymes—A 2019 Update. Nucleic Acids Res. 2020, 48, D445–D453. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for Taxonomy-Based Analysis of Pathways and Genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef]
- Kanehisa, M. The KEGG Database. In Novartis Foundation Symposia; Bock, G., Goode, J.A., Eds.; Wiley: Hoboken, NJ, USA, 2002; Volume 247, pp. 91–103. ISBN 978-0-470-84480-9. [Google Scholar]
- Craig, C.L.; Weber, R.S. Selection Costs of Amino Acid Substitutions in ColE1 and ColIa Gene Clusters Harbored by Escherichia Coli. Mol. Biol. Evol. 1998, 15, 774–776. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Li, J.; Chen, H.; He, X.; Zhang, H.; Liang, H.; Lu, J. Biosynthetic Energy Cost for Amino Acids Decreases in Cancer Evolution. Nat. Commun. 2018, 9, 4124. [Google Scholar] [CrossRef]
- Akashi, H.; Gojobori, T. Metabolic Efficiency and Amino Acid Composition in the Proteomes of Escherichia coli and Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2002, 99, 3695–3700. [Google Scholar] [CrossRef]
- Kok, S.; Kozak, B.U.; Pronk, J.T.; Maris, A.J.A. Energy Coupling in Saccharomyces Cerevisiae: Selected Opportunities for Metabolic Engineering. FEMS Yeast Res. 2012, 12, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, R.; Kuepfer, L.; Sauer, U. Systematic Evaluation of Objective Functions for Predicting Intracellular Fluxes in Escherichia coli. Mol. Syst. Biol. 2007, 3, 119. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Cepas, J.; Szklarczyk, D.; Heller, D.; Hernández-Plaza, A.; Forslund, S.K.; Cook, H.; Mende, D.R.; Letunic, I.; Rattei, T.; Jensen, L.J.; et al. eggNOG 5.0: A Hierarchical, Functionally and Phylogenetically Annotated Orthology Resource Based on 5090 Organisms and 2502 Viruses. Nucleic Acids Res. 2019, 47, D309–D314. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and Sensitive Protein Alignment Using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the SciPy Proceedings, Austin, TX, USA, 28 June–3 July 2010; pp. 92–96. [Google Scholar]
- Yu, G.; Smith, D.K.; Zhu, H.; Guan, Y.; Lam, T.T. Ggtree: An Package for Visualization and Annotation of Phylogenetic Trees with Their Covariates and Other Associated Data. Methods Ecol. Evol. 2017, 8, 28–36. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasalo, N.; Domazet-Lošo, T.; Domazet-Lošo, M. Bacterial Amino Acid Auxotrophies Enable Energetically Costlier Proteomes. Int. J. Mol. Sci. 2025, 26, 2285. https://doi.org/10.3390/ijms26052285
Kasalo N, Domazet-Lošo T, Domazet-Lošo M. Bacterial Amino Acid Auxotrophies Enable Energetically Costlier Proteomes. International Journal of Molecular Sciences. 2025; 26(5):2285. https://doi.org/10.3390/ijms26052285
Chicago/Turabian StyleKasalo, Niko, Tomislav Domazet-Lošo, and Mirjana Domazet-Lošo. 2025. "Bacterial Amino Acid Auxotrophies Enable Energetically Costlier Proteomes" International Journal of Molecular Sciences 26, no. 5: 2285. https://doi.org/10.3390/ijms26052285
APA StyleKasalo, N., Domazet-Lošo, T., & Domazet-Lošo, M. (2025). Bacterial Amino Acid Auxotrophies Enable Energetically Costlier Proteomes. International Journal of Molecular Sciences, 26(5), 2285. https://doi.org/10.3390/ijms26052285





