Single-Cell Transcriptional Profiling Reveals Cell Type-Specific Sex-Dependent Molecular Patterns of Schizophrenia
Abstract
1. Introduction
2. Results
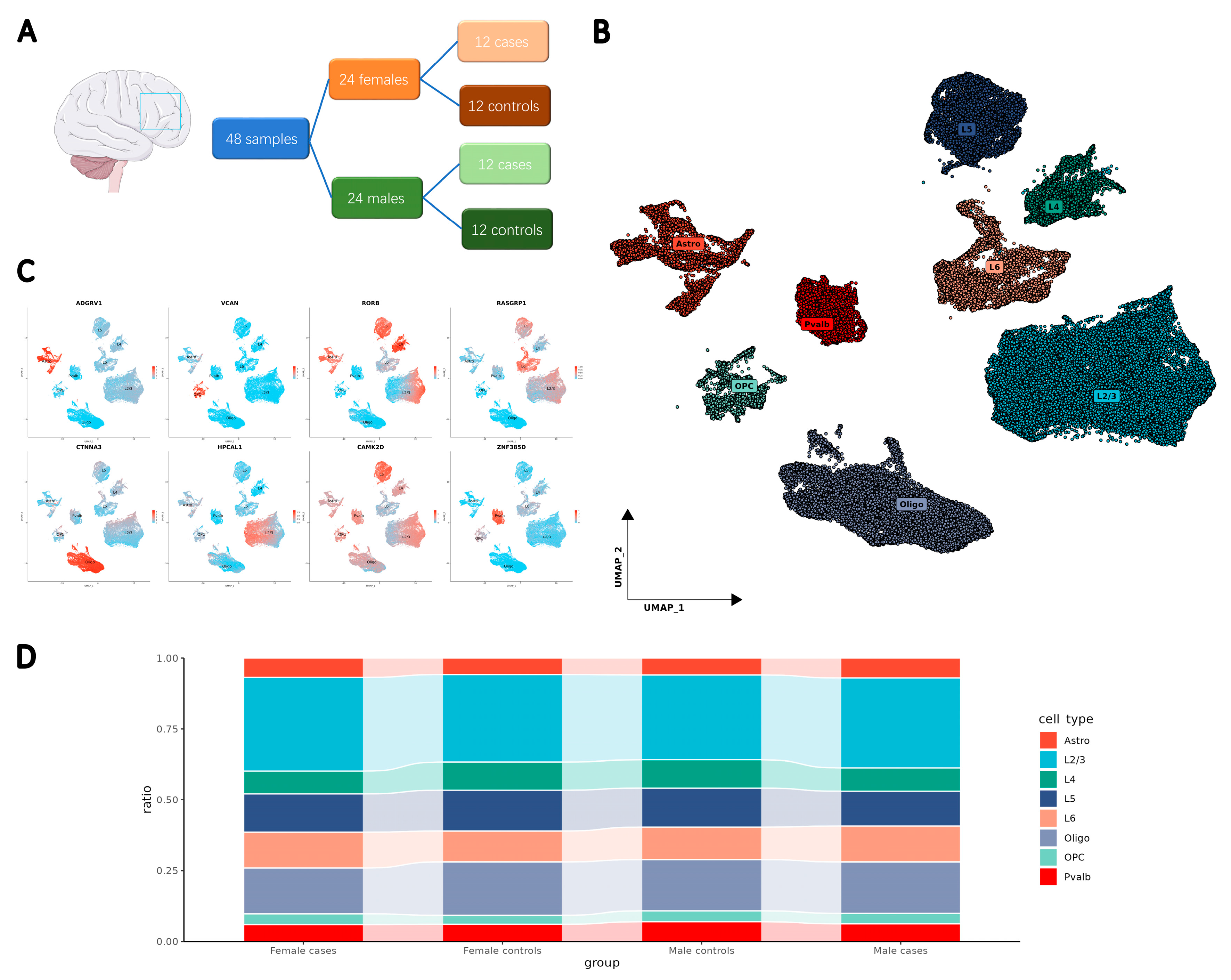
2.1. Integration of Clinical Data to Develop a Single-Cell Resolution Transcriptomic Atlas
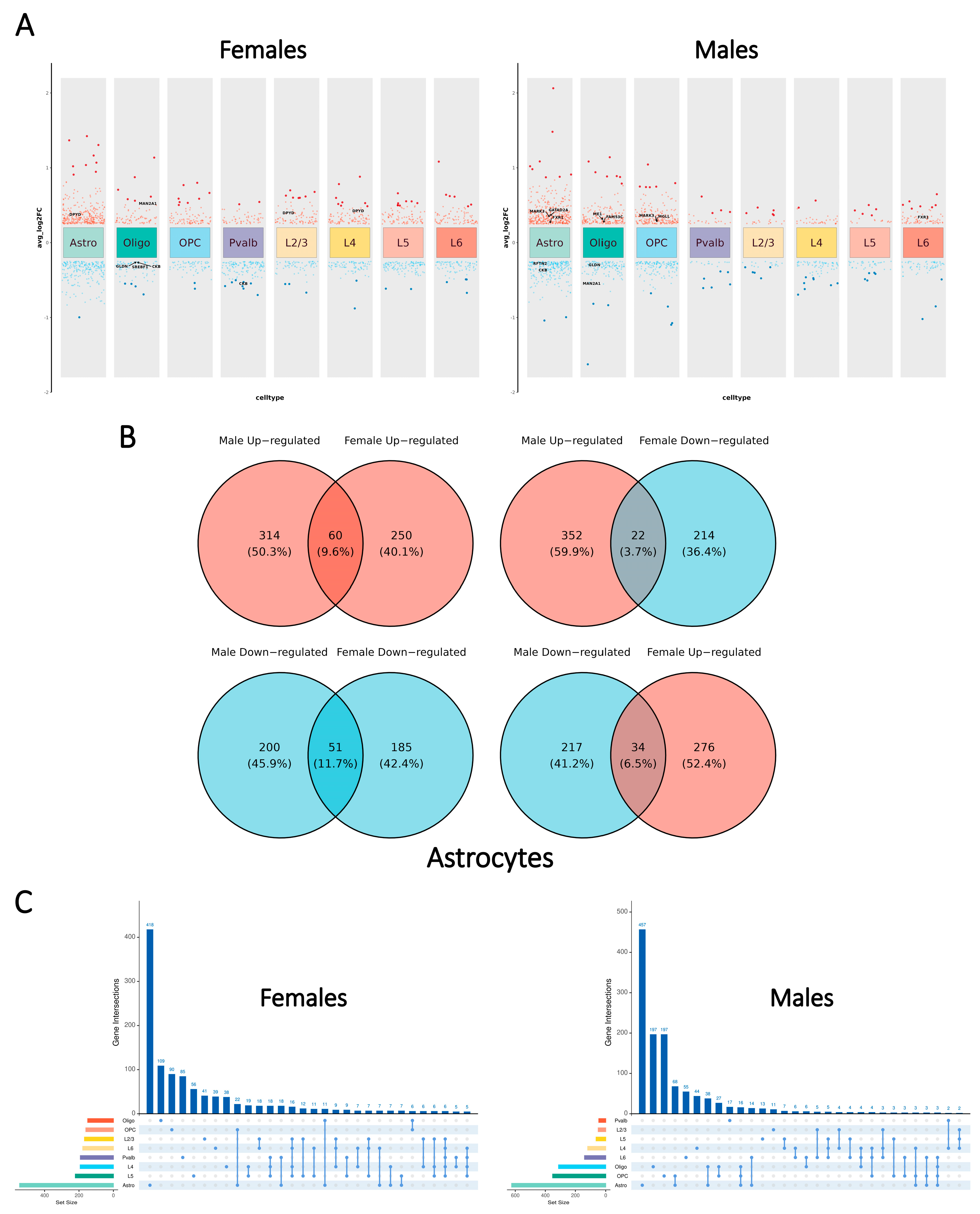
2.2. Global Patterns of Cell Type-Specific Transcriptomic Changes Display Disparities Between the Sexes
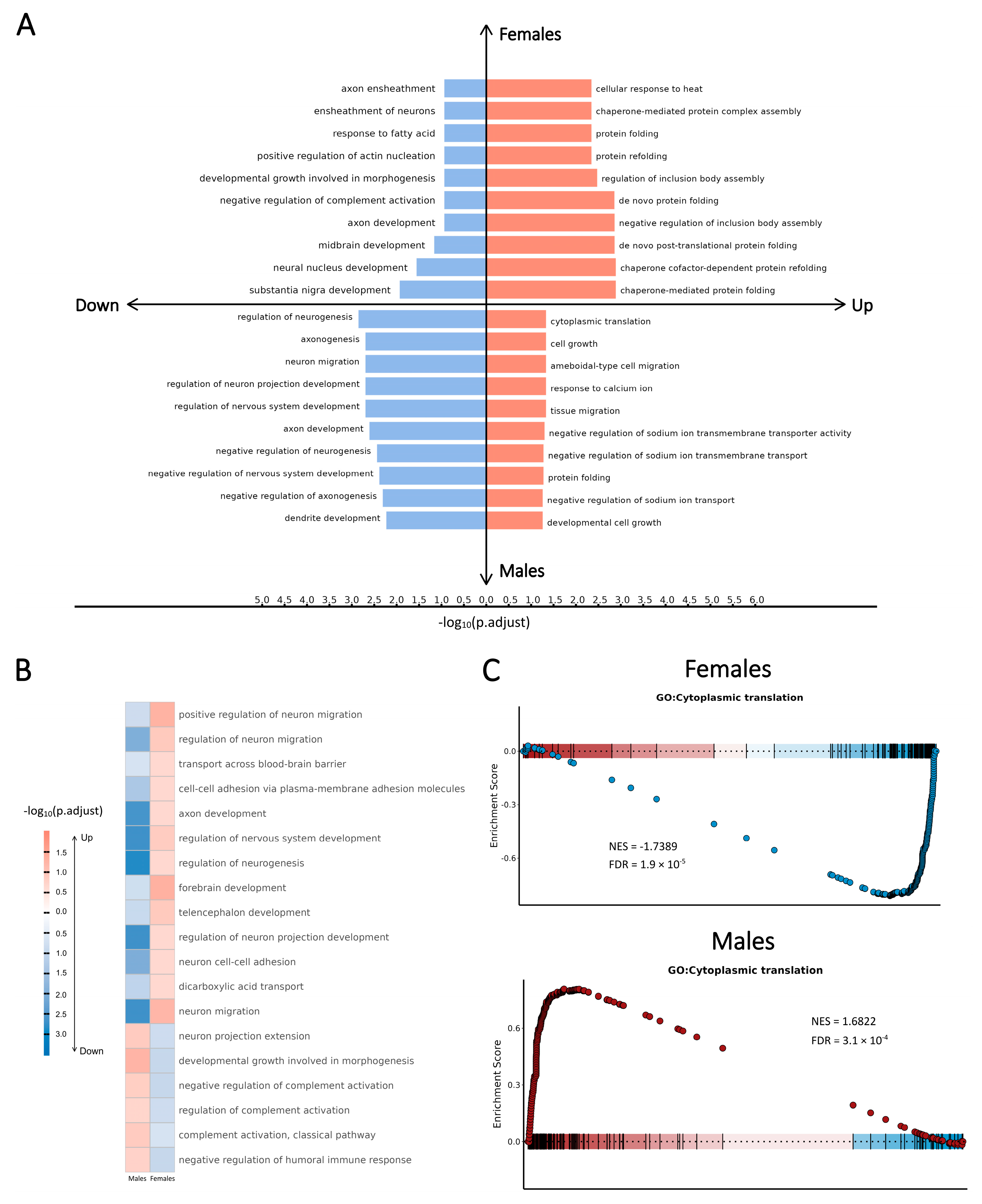
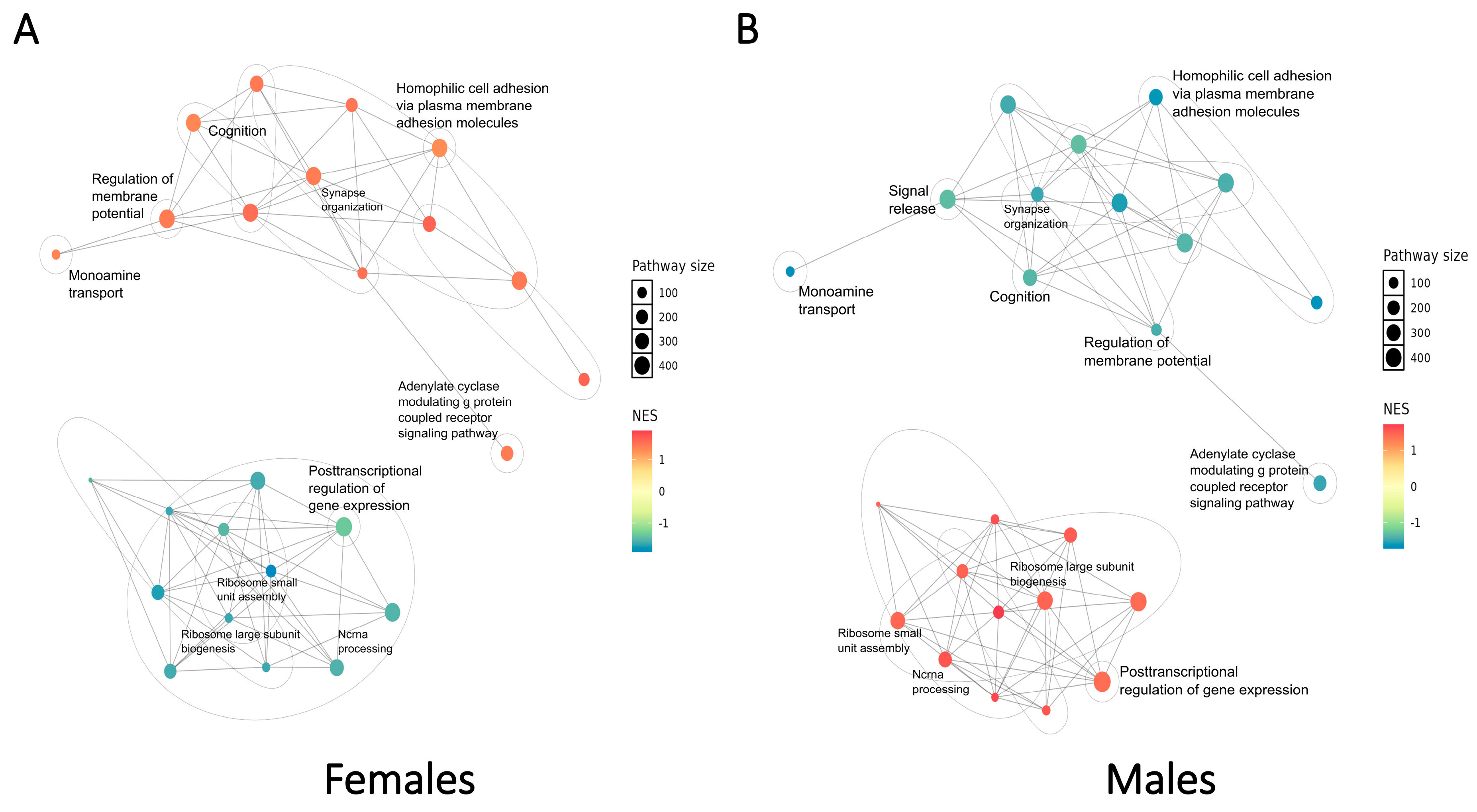
2.3. SCZ-Associated Pathway-Level Patterns of Perturbations Revealed by Cell Type-Specific Transcriptomic Changes in Each Sex
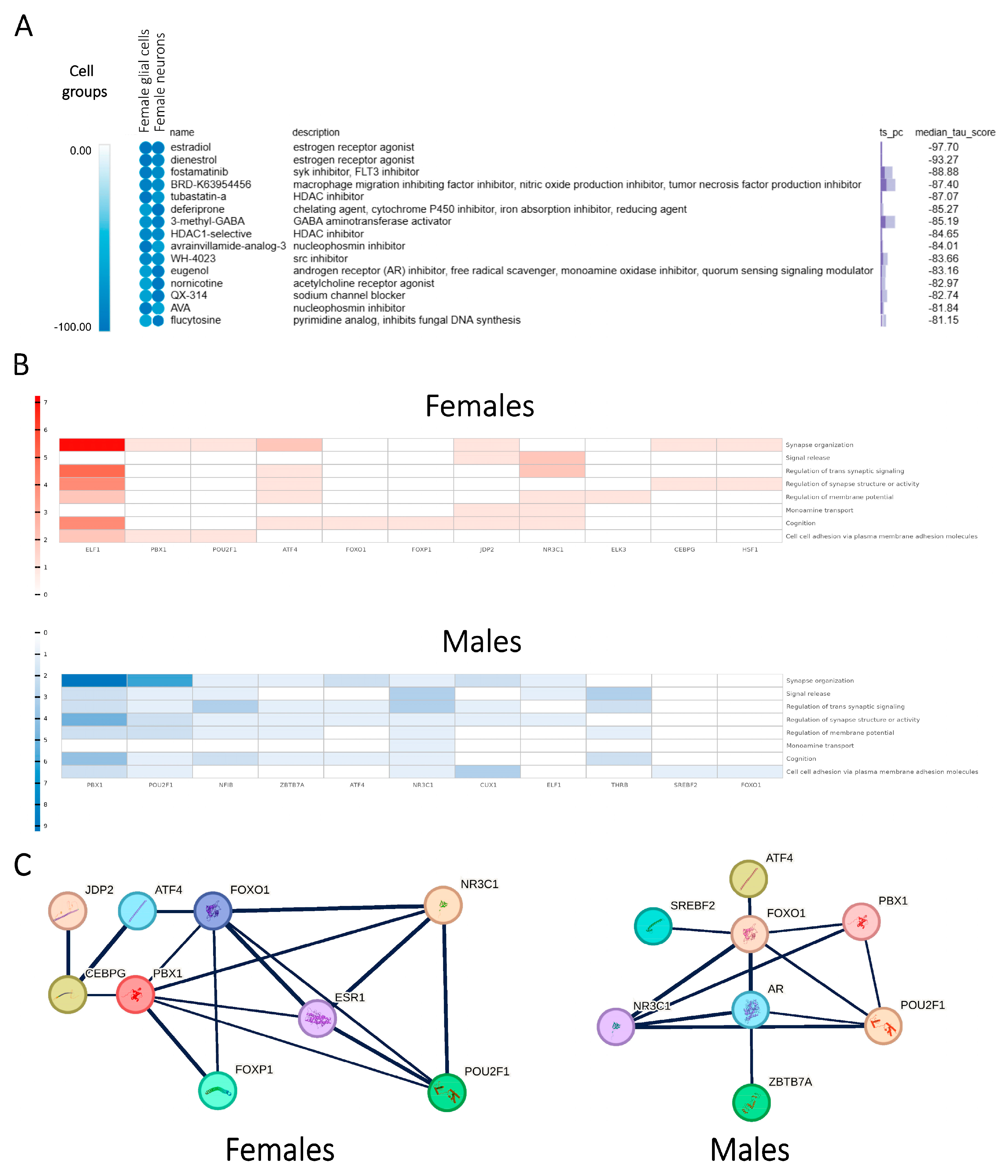
2.4. Regulatory Network Analysis Identifies the Transcriptional Regulators Driving the SCZ-Associated Sex-Dependent Disparities
2.5. Protein–Protein Interaction Networks Determine the Functional Modules Involved in SCZ-Associated Sex-Dependent Disparities
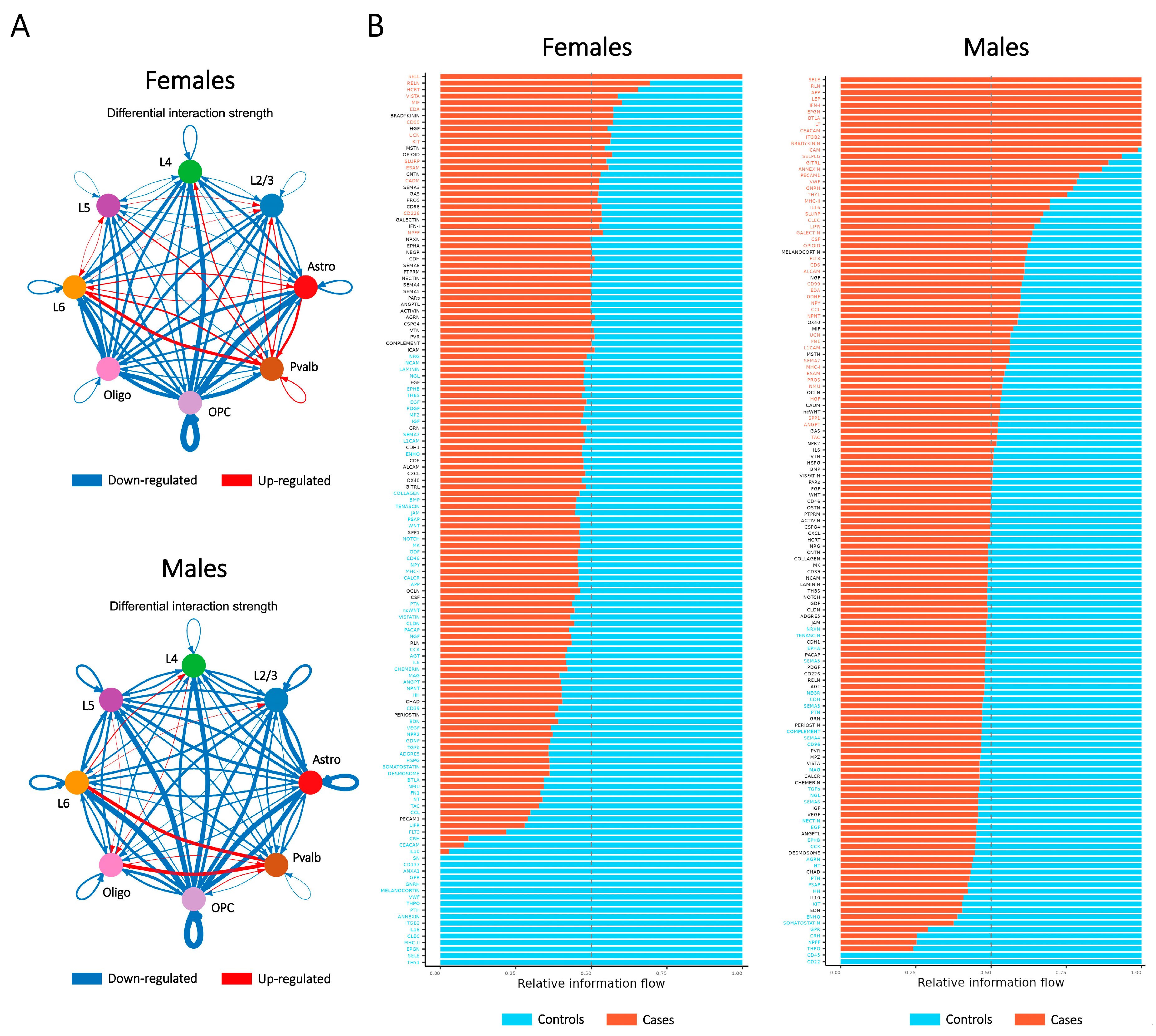
2.6. Ligand–Receptor Interaction Assessment Illustrates the Relationship Between Major Cell Types Contributing to SCZ-Associated Dysregulation in Both Sexes
2.7. In Silico Drug Screening Process Suggests Potential Small-Molecule Modulators Involved in Sex-Specific Treatment of SCZ
2.8. Profiling of Sex Hormone-Related TF Cores Illustrates Sex-Dependent Regulatory Mechanisms Associated with SCZ
2.9. Integrated SCZ-Associated Sex-Dependent Dysregulation Model Demonstrates the Regulatory Roles of Sex Hormones in the Pathology of SCZ
3. Discussion
4. Materials and Methods
4.1. Obtaining and Processing Data of Single-Nucleus RNA Sequencing
4.2. Dimensionality Reduction and Data Integration
4.3. Cell Clustering and Annotation
4.4. Differential Gene Expression Analysis
4.5. Function Enrichment of the DEG Profiling
4.6. Constructing Cell Type-Specific and Integrated GRNs
4.7. Assessing PPI Networks to Determine SCZ-Associated Sex-Dependent Modules
4.8. Ligand–Receptor Interaction Assessment
4.9. In Silico Drug Screening Process with the CMap
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DEG | Differentially expressed gene |
| GO | Gene Ontology |
| GRN | Gene regulatory network |
| GSEA | Gene set enrichment analysis |
| PFC | Prefrontal cortex |
| PPI | Protein–protein interaction |
| SCZ | Schizophrenia |
| snRNA-seq | Single-nucleus RNA sequencing |
| TF | Transcription factor |
References
- Jauhar, S.; Johnstone, M.; McKenna, P.J. Schizophrenia. Lancet 2022, 399, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, D.R. Future of Days Past: Neurodevelopment and Schizophrenia. Schizophr. Bull. 2017, 43, 1164–1168. [Google Scholar] [CrossRef]
- Schwarz, E.; Izmailov, R.; Liò, P.; Meyer-Lindenberg, A. Protein Interaction Networks Link Schizophrenia Risk Loci to Synaptic Function. Schizophr. Bull. 2016, 42, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Nanou, E.; Catterall, W.A. Calcium Channels, Synaptic Plasticity, and Neuropsychiatric Disease. Neuron 2018, 98, 466–481. [Google Scholar] [CrossRef]
- Cao, H.; Zhou, H.; Cannon, T.D. Functional Connectome-Wide Associations of Schizophrenia Polygenic Risk. Mol. Psychiatry 2021, 26, 2553–2561. [Google Scholar] [CrossRef]
- Toulopoulou, T.; Zhang, X.; Cherny, S.; Dickinson, D.; Berman, K.F.; Straub, R.E.; Sham, P.; Weinberger, D.R. Polygenic Risk Score Increases Schizophrenia Liability through Cognition-Relevant Pathways. Brain 2019, 142, 471–485. [Google Scholar] [CrossRef]
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Primers 2015, 1, 15067. [Google Scholar] [CrossRef]
- Giordano, G.M.; Bucci, P.; Mucci, A.; Pezzella, P.; Galderisi, S. Gender Differences in Clinical and Psychosocial Features Among Persons With Schizophrenia: A Mini Review. Front. Psychiatry 2021, 12, 789179. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Ferreira, H.; Martins, J.; Gonçalves, J.; Castelo-Branco, M. Male Sex Bias in Early and Late Onset Neurodevelopmental Disorders: Shared Aspects and Differences in Autism Spectrum Disorder, Attention Deficit/Hyperactivity Disorder, and Schizophrenia. Neurosci. Biobehav. Rev. 2022, 135, 104577. [Google Scholar] [CrossRef]
- Aleman, A.; Kahn, R.S.; Selten, J.-P. Sex Differences in the Risk of Schizophrenia. Arch. Gen. Psychiatry 2003, 60, 565. [Google Scholar] [CrossRef]
- Taylor, R.; Langdon, R. Understanding Gender Differences in Schizophrenia: A Review of the Literature. Curr. Psychiatry Rev. 2006, 2, 255–265. [Google Scholar] [CrossRef]
- Ochoa, S.; Usall, J.; Cobo, J.; Labad, X.; Kulkarni, J. Gender Differences in Schizophrenia and First-Episode Psychosis: A Comprehensive Literature Review. Schizophr. Res. Treat. 2012, 2012, 916198. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.; Riedel, A.; de Castella, A.R.; Fitzgerald, P.B.; Rolfe, T.J.; Taffe, J.; Burger, H. Estrogen—A Potential Treatment for Schizophrenia. Schizophr. Res. 2001, 48, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Lindamer, L.A.; Lohr, J.B.; Harris, M.J.; Jeste, D.V. Gender, Estrogen, and Schizophrenia. Focus 2004, 2, 138–145. [Google Scholar] [CrossRef]
- Olsen, L.; Hansen, T.; Jakobsen, K.D.; Djurovic, S.; Melle, I.; Agartz, I.; Hall, H.; Ullum, H.; Timm, S.; Wang, A.G.; et al. The Estrogen Hypothesis of Schizophrenia Implicates Glucose Metabolism: Association Study in Three Independent Samples. BMC Med. Genet. 2008, 9, 39. [Google Scholar] [CrossRef]
- Van der Leeuw, C.; Habets, P.; Gronenschild, E.; Domen, P.; Michielse, S.; van Kroonenburgh, M.; van Os, J.; Marcelis, M. Testing the Estrogen Hypothesis of Schizophrenia: Associations between Cumulative Estrogen Exposure and Cerebral Structural Measures. Schizophr. Res. 2013, 150, 114–120. [Google Scholar] [CrossRef]
- Gogos, A.; Sbisa, A.M.; Sun, J.; Gibbons, A.; Udawela, M.; Dean, B. A Role for Estrogen in Schizophrenia: Clinical and Preclinical Findings. Int. J. Endocrinol. 2015, 2015, 615356. [Google Scholar] [CrossRef]
- Qin, W.; Liu, C.; Sodhi, M.; Lu, H. Meta-Analysis of Sex Differences in Gene Expression in Schizophrenia. BMC Syst. Biol. 2016, 10, S9. [Google Scholar] [CrossRef]
- Hoffman, G.E.; Ma, Y.; Montgomery, K.S.; Bendl, J.; Jaiswal, M.K.; Kozlenkov, A.; Peters, M.A.; Dracheva, S.; Fullard, J.F.; Chess, A.; et al. Sex Differences in the Human Brain Transcriptome of Cases With Schizophrenia. Biol. Psychiatry 2022, 91, 92–101. [Google Scholar] [CrossRef]
- Carceller, H.; Hidalgo, M.R.; Escartí, M.J.; Nacher, J.; de la Iglesia-Vayá, M.; García-García, F. The Impact of Sex on Gene Expression in the Brain of Schizophrenic Patients: A Systematic Review and Meta-Analysis of Transcriptomic Studies. Biol. Sex Differ. 2024, 15, 59. [Google Scholar] [CrossRef]
- Velmeshev, D.; Schirmer, L.; Jung, D.; Haeussler, M.; Perez, Y.; Mayer, S.; Bhaduri, A.; Goyal, N.; Rowitch, D.H.; Kriegstein, A.R. Single-Cell Genomics Identifies Cell Type–Specific Molecular Changes in Autism. Science 2019, 364, 685–689. [Google Scholar] [CrossRef]
- Aldridge, S.; Teichmann, S.A. Single Cell Transcriptomics Comes of Age. Nat. Commun. 2020, 11, 4307. [Google Scholar] [CrossRef]
- Maitra, M.; Mitsuhashi, H.; Rahimian, R.; Chawla, A.; Yang, J.; Fiori, L.M.; Davoli, M.A.; Perlman, K.; Aouabed, Z.; Mash, D.C.; et al. Cell Type Specific Transcriptomic Differences in Depression Show Similar Patterns between Males and Females but Implicate Distinct Cell Types and Genes. Nat. Commun. 2023, 14, 2912. [Google Scholar] [CrossRef] [PubMed]
- Emani, P.S.; Liu, J.J.; Clarke, D.; Jensen, M.; Warrell, J.; Gupta, C.; Meng, R.; Lee, C.Y.; Xu, S.; Dursun, C.; et al. Single-Cell Genomics and Regulatory Networks for 388 Human Brains. Science 2024, 384, eadi5199. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, W.B.; Mohammadi, S.; Fullard, J.F.; Davila-Velderrain, J.; Subburaju, S.; Tso, D.R.; Hourihan, M.; Jiang, S.; Lee, H.-C.; Bendl, J.; et al. Single-Cell Multi-Cohort Dissection of the Schizophrenia Transcriptome. Science 2024, 384, eadg5136. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, W.B.; Mohammadi, S.; Davila-Velderrain, J.; Subburaju, S.; Tso, D.R.; Hourihan, M.; Kellis, M. Single-Cell Dissection of Schizophrenia Reveals Neurodevelopmental-Synaptic Axis and Transcriptional Resilience. MedRxiv 2020. [Google Scholar] [CrossRef]
- McKenzie, A.T.; Wang, M.; Hauberg, M.E.; Fullard, J.F.; Kozlenkov, A.; Keenan, A.; Hurd, Y.L.; Dracheva, S.; Casaccia, P.; Roussos, P.; et al. Brain Cell Type Specific Gene Expression and Co-Expression Network Architectures. Sci. Rep. 2018, 8, 8868. [Google Scholar] [CrossRef]
- Gusev, A.; Mancuso, N.; Won, H.; Kousi, M.; Finucane, H.K.; Reshef, Y.; Song, L.; Safi, A.; McCarroll, S.; Neale, B.M.; et al. Transcriptome-Wide Association Study of Schizophrenia and Chromatin Activity Yields Mechanistic Disease Insights. Nat. Genet. 2018, 50, 538–548. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the Unification of Biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Aleksander, S.A.; Balhoff, J.; Carbon, S.; Cherry, J.M.; Drabkin, H.J.; Ebert, D.; Feuermann, M.; Gaudet, P.; Harris, N.L.; Hill, D.P.; et al. The Gene Ontology Knowledgebase in 2023. Genetics 2023, 224, iyad031. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Sharma, D.; Kalia, K.; Tiwari, V. Crosstalk between Endoplasmic Reticulum Stress and Oxidative Stress in Schizophrenia: The Dawn of New Therapeutic Approaches. Neurosci. Biobehav. Rev. 2017, 83, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Scott, M.R.; Meador-Woodruff, J.H. Abnormal Expression of ER Quality Control and ER Associated Degradation Proteins in the Dorsolateral Prefrontal Cortex in Schizophrenia. Schizophr. Res. 2018, 197, 484–491. [Google Scholar] [CrossRef]
- Kim, P.; Scott, M.R.; Meador-Woodruff, J.H. Dysregulation of the Unfolded Protein Response (UPR) in the Dorsolateral Prefrontal Cortex in Elderly Patients with Schizophrenia. Mol. Psychiatry 2021, 26, 1321–1331. [Google Scholar] [CrossRef]
- Aibar, S.; González-Blas, C.B.; Moerman, T.; Huynh-Thu, V.A.; Imrichova, H.; Hulselmans, G.; Rambow, F.; Marine, J.-C.; Geurts, P.; Aerts, J.; et al. SCENIC: Single-Cell Regulatory Network Inference and Clustering. Nat. Methods 2017, 14, 1083–1086. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein–Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Guerrero-Juarez, C.F.; Zhang, L.; Chang, I.; Ramos, R.; Kuan, C.-H.; Myung, P.; Plikus, M.V.; Nie, Q. Inference and Analysis of Cell-Cell Communication Using CellChat. Nat. Commun. 2021, 12, 1088. [Google Scholar] [CrossRef]
- Subramanian, A.; Narayan, R.; Corsello, S.M.; Peck, D.D.; Natoli, T.E.; Lu, X.; Gould, J.; Davis, J.F.; Tubelli, A.A.; Asiedu, J.K.; et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell 2017, 171, 1437–1452.e17. [Google Scholar] [CrossRef]
- Brand, B.A.; de Boer, J.N.; Marcelis, M.C.; Grootens, K.P.; Luykx, J.J.; Sommer, I.E. The Direct and Long-Term Effects of Raloxifene as Adjunctive Treatment for Schizophrenia-Spectrum Disorders: A Double-Blind, Randomized Clinical Trial. Schizophr. Bull. 2023, 49, 1579–1590. [Google Scholar] [CrossRef]
- Begemann, M.J.H.; Dekker, C.F.; van Lunenburg, M.; Sommer, I.E. Estrogen Augmentation in Schizophrenia: A Quantitative Review of Current Evidence. Schizophr. Res. 2012, 141, 179–184. [Google Scholar] [CrossRef]
- Kulkarni, J.; Gavrilidis, E.; Worsley, R.; Hayes, E. Role of Estrogen Treatment in the Management of Schizophrenia. CNS Drugs 2012, 26, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.; Butler, S.; Riecher-Rössler, A. Estrogens and SERMS as Adjunctive Treatments for Schizophrenia. Front. Neuroendocr. 2019, 53, 100743. [Google Scholar] [CrossRef]
- Qu, M.; Tang, F.; Wang, L.; Yan, H.; Han, Y.; Yan, J.; Yue, W.; Zhang, D. Associations of ATF4 Gene Polymorphisms with Schizophrenia in Male Patients. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2008, 147B, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Mitsuda, T.; Omi, T.; Tanimukai, H.; Sakagami, Y.; Tagami, S.; Okochi, M.; Kudo, T.; Takeda, M. Sigma-1Rs Are Upregulated via PERK/EIF2α/ATF4 Pathway and Execute Protective Function in ER Stress. Biochem. Biophys. Res. Commun. 2011, 415, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Trinh, M.A.; Kaphzan, H.; Wek, R.C.; Pierre, P.; Cavener, D.R.; Klann, E. Brain-Specific Disruption of the EIF2α Kinase PERK Decreases ATF4 Expression and Impairs Behavioral Flexibility. Cell Rep. 2012, 1, 676–688. [Google Scholar] [CrossRef]
- Gu, S.; Cui, F.; Yin, J.; Fang, C.; Liu, L. Altered MRNA Expression Levels of Autophagy- and Apoptosis-Related Genes in the FOXO Pathway in Schizophrenia Patients Treated with Olanzapine. Neurosci. Lett. 2021, 746, 135669. [Google Scholar] [CrossRef]
- Bacon, C.; Rappold, G.A. The Distinct and Overlapping Phenotypic Spectra of FOXP1 and FOXP2 in Cognitive Disorders. Hum. Genet. 2012, 131, 1687–1698. [Google Scholar] [CrossRef]
- Siper, P.M.; De Rubeis, S.; Trelles, M.D.P.; Durkin, A.; Di Marino, D.; Muratet, F.; Frank, Y.; Lozano, R.; Eichler, E.E.; Kelly, M.; et al. Prospective Investigation of FOXP1 Syndrome. Mol. Autism 2017, 8, 57. [Google Scholar] [CrossRef]
- Sinclair, D.; Webster, M.J.; Fullerton, J.M.; Weickert, C.S. Glucocorticoid Receptor MRNA and Protein Isoform Alterations in the Orbitofrontal Cortex in Schizophrenia and Bipolar Disorder. BMC Psychiatry 2012, 12, 84. [Google Scholar] [CrossRef]
- Palma-Gudiel, H.; Córdova-Palomera, A.; Leza, J.C.; Fañanás, L. Glucocorticoid Receptor Gene (NR3C1) Methylation Processes as Mediators of Early Adversity in Stress-Related Disorders Causality: A Critical Review. Neurosci. Biobehav. Rev. 2015, 55, 520–535. [Google Scholar] [CrossRef]
- Watkeys, O.J.; Kremerskothen, K.; Quidé, Y.; Fullerton, J.M.; Green, M.J. Glucocorticoid Receptor Gene (NR3C1) DNA Methylation in Association with Trauma, Psychopathology, Transcript Expression, or Genotypic Variation: A Systematic Review. Neurosci. Biobehav. Rev. 2018, 95, 85–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, J.; Qing, L.; Li, J.; Yang, H.; Ji, A.; Yan, M.; Hu, L.; Nie, S. DNA Methylation Analysis of the NR3C1 Gene in Patients with Schizophrenia. J. Mol. Neurosci. 2020, 70, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Golonzhka, O.; Nord, A.; Tang, P.L.F.; Lindtner, S.; Ypsilanti, A.R.; Ferretti, E.; Visel, A.; Selleri, L.; Rubenstein, J.L.R. Pbx Regulates Patterning of the Cerebral Cortex in Progenitors and Postmitotic Neurons. Neuron 2015, 88, 1192–1207. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Park, J.K.; Kim, S.K.; Cho, A.-R.; Kim, J.W.; Yim, S.-V.; Chung, J.-H. Association of Polymorphism in the Promoter of the Melatonin Receptor 1A Gene with Schizophrenia and with Insomnia Symptoms in Schizophrenia Patients. J. Mol. Neurosci. 2011, 45, 304–308. [Google Scholar] [CrossRef]
- Le Hellard, S.; Mühleisen, T.W.; Djurovic, S.; Fernø, J.; Ouriaghi, Z.; Mattheisen, M.; Vasilescu, C.; Raeder, M.B.; Hansen, T.; Strohmaier, J.; et al. Polymorphisms in SREBF1 and SREBF2, Two Antipsychotic-Activated Transcription Factors Controlling Cellular Lipogenesis, Are Associated with Schizophrenia in German and Scandinavian Samples. Mol. Psychiatry 2010, 15, 463–472. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.; Liu, D.; Yu, S.; Cong, E.; Li, Y.; Wu, H.; Yue, Y.; Zuo, S.; Wang, Y.; et al. Association between SREBF2 Gene Polymorphisms and Metabolic Syndrome in Clozapine-Treated Patients with Schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 56, 136–141. [Google Scholar] [CrossRef]
- Steen, V.M.; Skrede, S.; Polushina, T.; López, M.; Andreassen, O.A.; Fernø, J.; Hellard, S. Le Genetic Evidence for a Role of the SREBP Transcription System and Lipid Biosynthesis in Schizophrenia and Antipsychotic Treatment. Eur. Neuropsychopharmacol. 2017, 27, 589–598. [Google Scholar] [CrossRef]
- Palha, J.A.; Goodman, A.B. Thyroid Hormones and Retinoids: A Possible Link between Genes and Environment in Schizophrenia. Brain Res. Rev. 2006, 51, 61–71. [Google Scholar] [CrossRef]
- Shigekawa, T.; Ijichi, N.; Ikeda, K.; Horie-Inoue, K.; Shimizu, C.; Saji, S.; Aogi, K.; Tsuda, H.; Osaki, A.; Saeki, T.; et al. FOXP1, an Estrogen-Inducible Transcription Factor, Modulates Cell Proliferation in Breast Cancer Cells and 5-Year Recurrence-Free Survival of Patients with Tamoxifen-Treated Breast Cancer. Horm. Cancer 2011, 2, 286–297. [Google Scholar] [CrossRef]
- Constantinou, C.; Spella, M.; Chondrou, V.; Patrinos, G.P.; Papachatzopoulou, A.; Sgourou, A. The Multi-Faceted Functioning Portrait of LRF/ZBTB7A. Hum. Genom. 2019, 13, 66. [Google Scholar] [CrossRef]
- Ma, Y.; Brewer, J.W.; Alan Diehl, J.; Hendershot, L.M. Two Distinct Stress Signaling Pathways Converge Upon the CHOP Promoter During the Mammalian Unfolded Protein Response. J. Mol. Biol. 2002, 318, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.P.; Zhang, Y.; Zeng, H.; Novoa, I.; Lu, P.D.; Calfon, M.; Sadri, N.; Yun, C.; Popko, B.; Paules, R.; et al. An Integrated Stress Response Regulates Amino Acid Metabolism and Resistance to Oxidative Stress. Mol. Cell 2003, 11, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Boyce, M.; Yuan, J. Cellular Response to Endoplasmic Reticulum Stress: A Matter of Life or Death. Cell Death Differ. 2006, 13, 363–373. [Google Scholar] [CrossRef]
- Li, B.B.; Qian, C.; Gameiro, P.A.; Liu, C.-C.; Jiang, T.; Roberts, T.M.; Struhl, K.; Zhao, J.J. Targeted Profiling of RNA Translation Reveals MTOR-4EBP1/2-Independent Translation Regulation of MRNAs Encoding Ribosomal Proteins. Proc. Natl. Acad. Sci. USA 2018, 115. [Google Scholar] [CrossRef]
- Mortimer, A.M. Relationship between Estrogen and Schizophrenia. Expert Rev. Neurother. 2007, 7, 45–55. [Google Scholar] [CrossRef]
- Brzezinski-Sinai, N.A.; Brzezinski, A. Schizophrenia and Sex Hormones: What Is the Link? Front. Psychiatry 2020, 11. [Google Scholar] [CrossRef]
- Grigoriadis, S.; Seeman, M. V The Role of Estrogen in Schizophrenia: Implications for Schizophrenia Practice Guidelines for Women. Can. J. Psychiatry 2002, 47, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Chua, W.L.L.; Izquierdo de Santiago, A.; Kulkarni, J.; Mortimer, A. Estrogen for Schizophrenia. Cochrane Database Syst. Rev. 2005. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J. Estrogen—A Key Neurosteroid in the Understanding and Treatment of Mental Illness in Women. Psychiatry Res. 2023, 319, 114991. [Google Scholar] [CrossRef]
- Ellis, A.J.; Hendrick, V.M.; Williams, R.; Komm, B.S. Selective Estrogen Receptor Modulators in Clinical Practice: A Safety Overview. Expert Opin. Drug Saf. 2015, 14, 921–934. [Google Scholar] [CrossRef]
- Ji, E.; Weickert, C.S.; Lenroot, R.; Kindler, J.; Skilleter, A.J.; Vercammen, A.; White, C.; Gur, R.E.; Weickert, T.W. Adjunctive Selective Estrogen Receptor Modulator Increases Neural Activity in the Hippocampus and Inferior Frontal Gyrus during Emotional Face Recognition in Schizophrenia. Transl. Psychiatry 2016, 6, e795. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Wang, Z.; Kong, L.; Liu, L.; Li, L.; Tang, Y. Estradiol and Raloxifene as Adjunctive Treatment for Women with Schizophrenia: A Meta-analysis of Randomized, Double-blind, Placebo-controlled Trials. Acta Psychiatr. Scand. 2023, 147, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Brand, B.A.; de Boer, J.N.; Dazzan, P.; Sommer, I.E. Towards Better Care for Women with Schizophrenia-Spectrum Disorders. Lancet Psychiatry 2022, 9, 330–336. [Google Scholar] [CrossRef]
- Huerta-Ramos, E.; Iniesta, R.; Ochoa, S.; Cobo, J.; Miquel, E.; Roca, M.; Serrano-Blanco, A.; Teba, F.; Usall, J. Effects of Raloxifene on Cognition in Postmenopausal Women with Schizophrenia: A Double-Blind, Randomized, Placebo-Controlled Trial. Eur. Neuropsychopharmacol. 2014, 24, 223–231. [Google Scholar] [CrossRef]
- Weickert, T.W.; Weinberg, D.; Lenroot, R.; Catts, S.V.; Wells, R.; Vercammen, A.; O’Donnell, M.; Galletly, C.; Liu, D.; Balzan, R.; et al. Adjunctive Raloxifene Treatment Improves Attention and Memory in Men and Women with Schizophrenia. Mol. Psychiatry 2015, 20, 685–694. [Google Scholar] [CrossRef]
- Gurvich, C.; Hudaib, A.; Gavrilidis, E.; Worsley, R.; Thomas, N.; Kulkarni, J. Raloxifene as a Treatment for Cognition in Women with Schizophrenia: The Influence of Menopause Status. Psychoneuroendocrinology 2019, 100, 113–119. [Google Scholar] [CrossRef]
- Vahdani, B.; Armani Kian, A.; Esmaeilzadeh, A.; Zenoozian, S.; Yousefi, V.; Mazloomzadeh, S. Adjunctive Raloxifene and Isradipine Improve Cognitive Functioning in Patients With Schizophrenia. J. Clin. Psychopharmacol. 2020, 40, 457–463. [Google Scholar] [CrossRef] [PubMed]
- RCoreTeam. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Hao, Y.; Hao, S.; Andersen-Nissen, E.; Mauck, W.M.; Zheng, S.; Butler, A.; Lee, M.J.; Wilk, A.J.; Darby, C.; Zager, M.; et al. Integrated Analysis of Multimodal Single-Cell Data. Cell 2021, 184, 3573–3587.e29. [Google Scholar] [CrossRef]
- Korsunsky, I.; Millard, N.; Fan, J.; Slowikowski, K.; Zhang, F.; Wei, K.; Baglaenko, Y.; Brenner, M.; Loh, P.; Raychaudhuri, S. Fast, Sensitive and Accurate Integration of Single-Cell Data with Harmony. Nat. Methods 2019, 16, 1289–1296. [Google Scholar] [CrossRef]
- Baran, Y.; Bercovich, A.; Sebe-Pedros, A.; Lubling, Y.; Giladi, A.; Chomsky, E.; Meir, Z.; Hoichman, M.; Lifshitz, A.; Tanay, A. MetaCell: Analysis of Single-Cell RNA-Seq Data Using K-Nn Graph Partitions. Genome Biol. 2019, 20, 206. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-24275-0. [Google Scholar]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R Package for the Visualization of Intersecting Sets and Their Properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://bioconductor.org/packages/release/data/annotation/html/org.Hs.eg.db.html (accessed on 30 October 2024).
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. ClusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innov. 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://cran.r-project.org/web/packages/pheatmap/index.html (accessed on 30 October 2024).
- Available online: https://www.biorxiv.org/content/10.1101/060012v3 (accessed on 30 October 2024).
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database Hallmark Gene Set Collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Kerseviciute, I.; Gordevicius, J. APEAR: An R Package for Autonomous Visualisation of Pathway Enrichment Networks. Bioinformatics 2023, 39, btad672. [Google Scholar] [CrossRef] [PubMed]
- Van de Sande, B.; Flerin, C.; Davie, K.; De Waegeneer, M.; Hulselmans, G.; Aibar, S.; Seurinck, R.; Saelens, W.; Cannoodt, R.; Rouchon, Q.; et al. A Scalable SCENIC Workflow for Single-Cell Gene Regulatory Network Analysis. Nat. Protoc. 2020, 15, 2247–2276. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, R.; Zhang, T.; Sun, B. Single-Cell Transcriptional Profiling Reveals Cell Type-Specific Sex-Dependent Molecular Patterns of Schizophrenia. Int. J. Mol. Sci. 2025, 26, 2227. https://doi.org/10.3390/ijms26052227
Zhou R, Zhang T, Sun B. Single-Cell Transcriptional Profiling Reveals Cell Type-Specific Sex-Dependent Molecular Patterns of Schizophrenia. International Journal of Molecular Sciences. 2025; 26(5):2227. https://doi.org/10.3390/ijms26052227
Chicago/Turabian StyleZhou, Runguang, Tianli Zhang, and Baofa Sun. 2025. "Single-Cell Transcriptional Profiling Reveals Cell Type-Specific Sex-Dependent Molecular Patterns of Schizophrenia" International Journal of Molecular Sciences 26, no. 5: 2227. https://doi.org/10.3390/ijms26052227
APA StyleZhou, R., Zhang, T., & Sun, B. (2025). Single-Cell Transcriptional Profiling Reveals Cell Type-Specific Sex-Dependent Molecular Patterns of Schizophrenia. International Journal of Molecular Sciences, 26(5), 2227. https://doi.org/10.3390/ijms26052227



