Euphraticanoids N–T: Aromadendrane-Type Diterpenes and Sesquiterpenes with Fungicidal Activities from Populus euphratica Resins
Abstract
1. Introduction
2. Results and Discussion
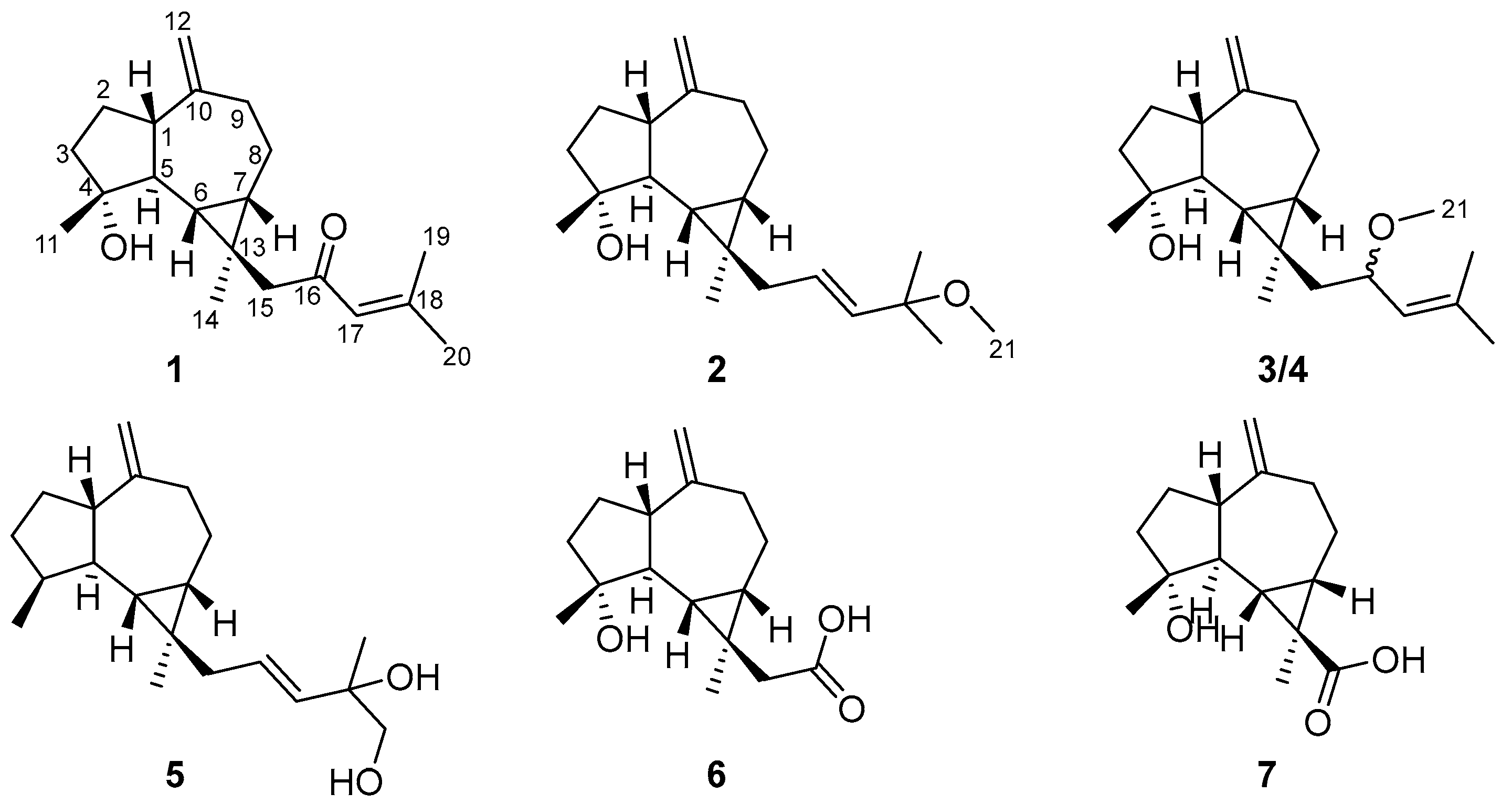
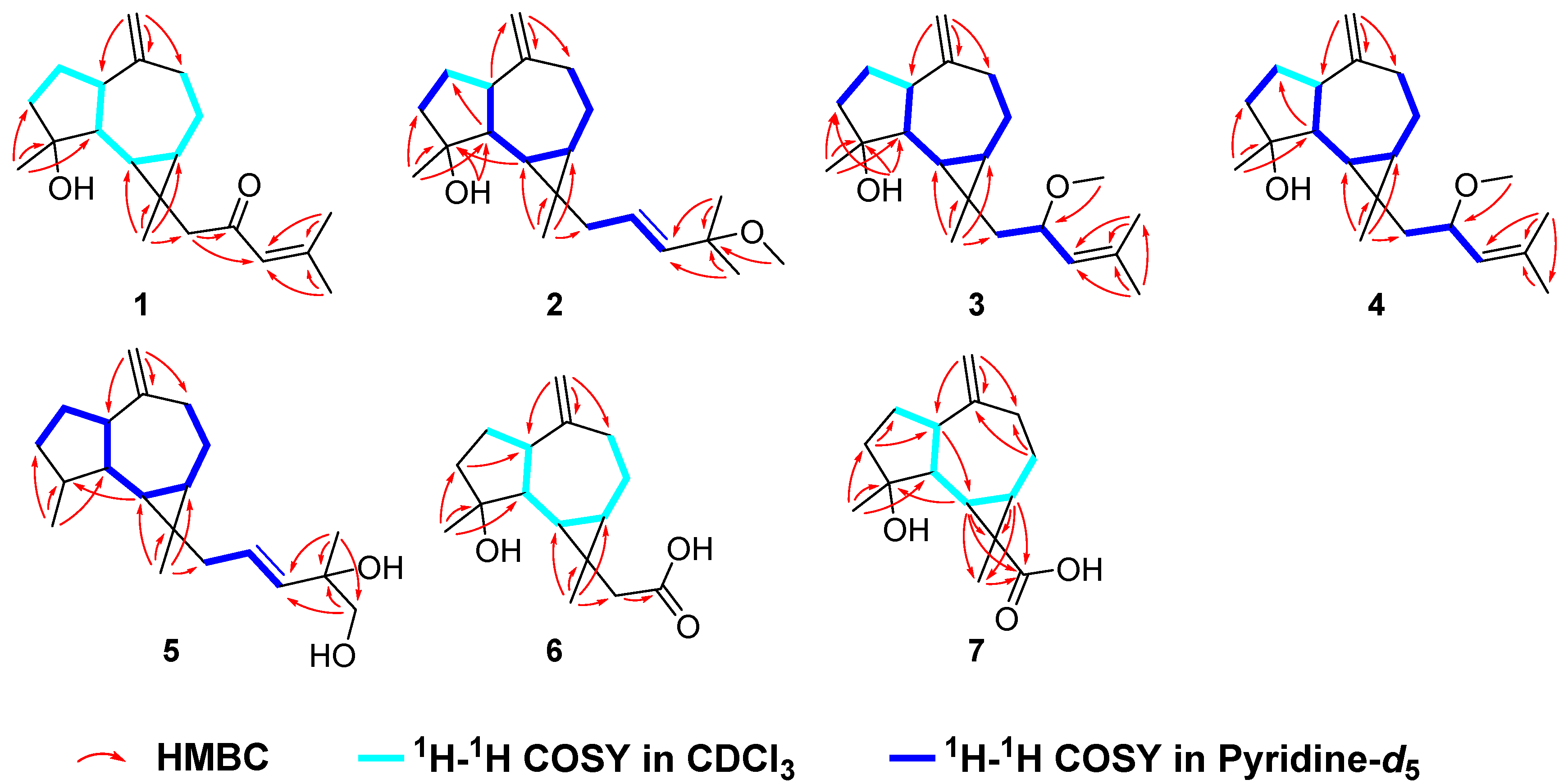
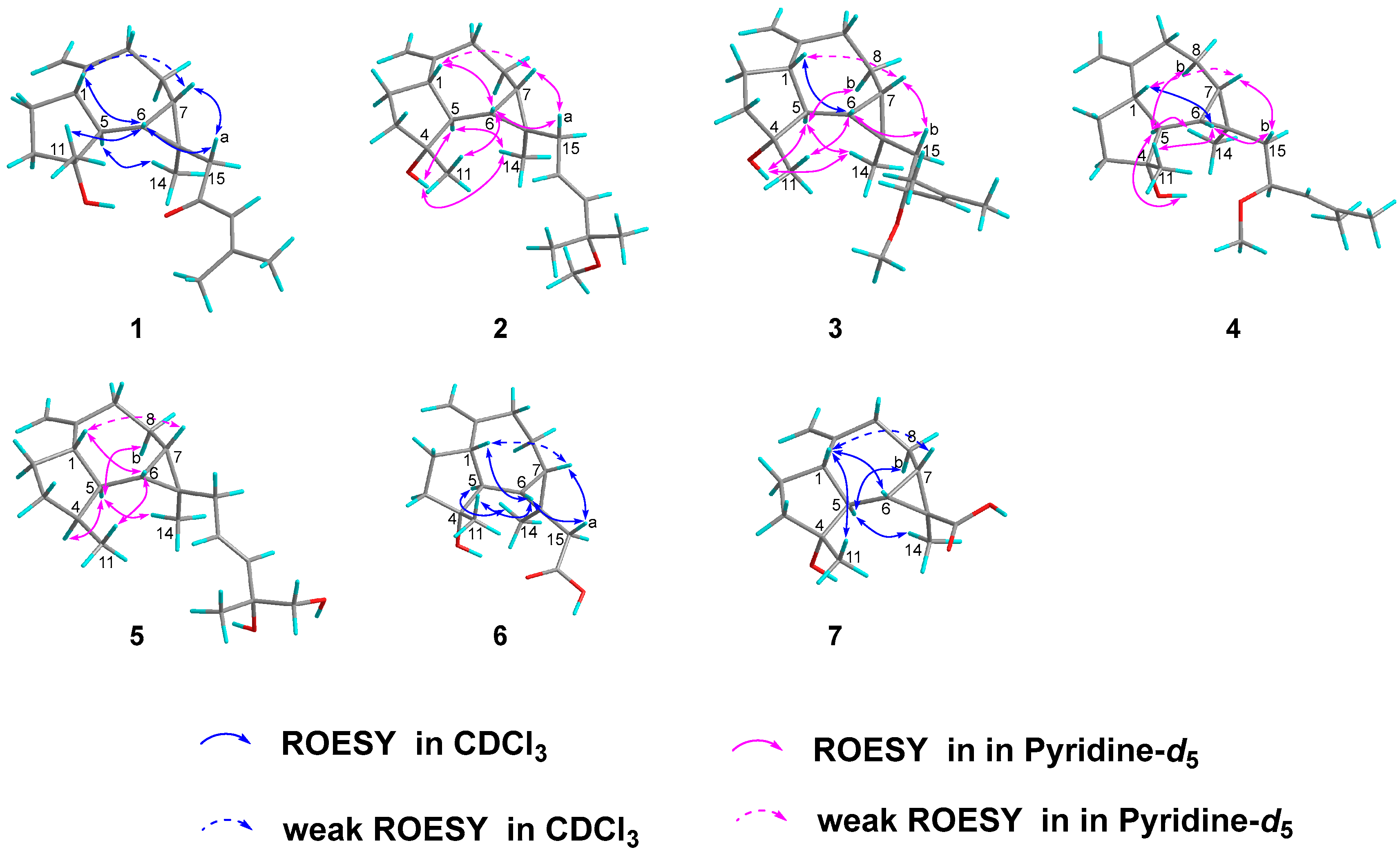
2.1. Compound Structure Elucidation
2.2. Biological Activity
3. Experimental Section
3.1. Fungal Material
3.2. Extraction and Isolation
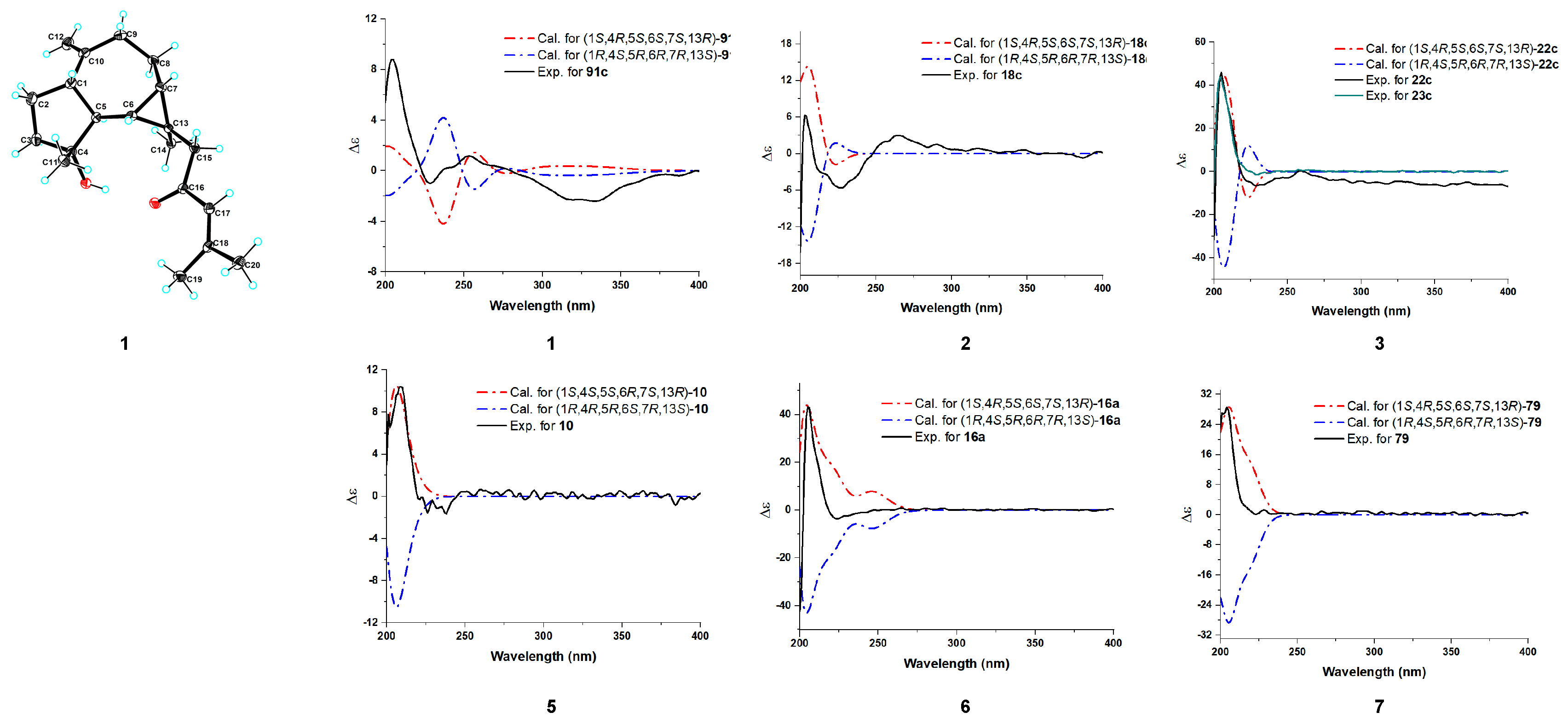
3.3. Crystal Structure of 1
3.4. ECD Calculations for Compounds 1–7
3.5. Fungicidal Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaudhary, G.; Ewoldt, R.H.; Thiffeault, J.L. Unravelling hagfish slime. J. R. Soc. Interface 2019, 16, 20180710. [Google Scholar] [CrossRef]
- Macleod, R.; Sinding, M.S.; Olsen, M.T.; Collins, M.J.; Rowland, S.J. DNA preserved in jetsam whale ambergris. Biol. Lett. 2020, 16, 20190819. [Google Scholar] [CrossRef] [PubMed]
- Frank, E.T.; Kesner, L.; Liberti, J.; Helleu, Q.; LeBoeuf, A.C.; Dascalu, A.; Sponsler, D.B.; Azuma, F.; Economo, E.P.; Waridel, P.; et al. Targeted treatment of injured nestmates with antimicrobial compounds in an ant society. Nat. Commun. 2023, 14, 8446. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, G.; Liu, J. Isolation and screening of fungi for enhanced agarwood formation in Aquilaria sinensis trees. PLoS ONE 2024, 19, e0304946. [Google Scholar] [CrossRef] [PubMed]
- Ching, Y.H.; Lin, F.M.; Chen, H.C.; Hsu, C.Y.; P’ng, S.Y.; Lin, T.N.; Wang, Y.C.; Lin, C.J.; Chen, Y.C.; Ho, T.J.; et al. Hypoglycemic effects of dracorhodin and dragon blood crude extract from Daemonorops draco. Bot. Stud. 2024, 65, 8. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Guo, T.; Zhou, D.; Meng, Q.; Fang, M.; Chen, G.; Lin, B.; Hou, Y.; Li, N. Structure-guided isolation of anti-neuroinflammatory sesquiterpene coumarins from Ferula sinkiangensis. Chin. J. Nat. Med. 2024, 22, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Sura, M.B.; Cheng, Y.X. Medicinal plant resin natural products: Structural diversity and biological activities. Nat. Prod. Rep. 2024, 41, 1471–1542. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Huang, D.L.; Dong, Y.; Qin, D.P.; Yan, Y.M.; Cheng, Y.X. Neuroprotective norsesquiterpenoids and triterpenoids from Populus euphratica resins. Molecules 2019, 24, 4379. [Google Scholar] [CrossRef] [PubMed]
- Anjaneyulu, A.S.R.; Krishnamurthy, M.V.R.; Rao, G.V. Rare Aromadendrane diterpenoids from a new soft coral speciesof Sinularia Genus of the indian ccean. Tetrahedron 1997, 53, 9301–9312. [Google Scholar] [CrossRef]
- Wang, Y.G.; Ren, J.; Wang, A.G.; Yang, J.B.; Ji, T.F.; Ma, Q.G.; Tian, J.; Su, Y.L. Hepatoprotective prenylaromadendrane-type diterpenes from the gum resin of Boswellia carterii. J. Nat. Prod. 2013, 76, 2074–2079. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xu, W.; Song, H.; Liu, Y.; Zhang, J.; Wang, Q. Synthesis and antiviral/fungicidal/insecticidal activities study of novel chiral indole diketopiperazine derivatives containing acylhydrazone moiety. J. Agric. Food Chem. 2020, 68, 5555–5571. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.; Li, L.; Wang, T.; Lu, A.; Wang, Z.; Wang, Q. Discovery of phytoalexin camalexin and its derivatives as novel antiviral and antiphytopathogenic-fungus agents. J. Agric. Food Chem. 2022, 70, 2554–2563. [Google Scholar] [CrossRef] [PubMed]
- He, H.W.; Xu, D.; Wu, K.H.; Lu, Z.Y.; Liu, X.; Xu, G. Discovery of novel salicylaldehyde derivatives incorporating an α-methylene-γ-butyrolactone moiety as fungicidal agents. Pest Manag. Sci. 2023, 79, 5015–5028. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H.; Jiang, J.; Liu, X.; Zhou, T.; Li, J.Q.; Xiao, Y.; Qin, Z. Discovery of triphenylphosphonium (TPP)-conjugated N-(1,1′-biphenyl)-2-yl aliphatic amides as excellent fungicidal candidates. Pest Manag. Sci. 2023, 79, 2920–2933. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]

| No | 1 a | 2 b | 3 b | 4 b |
|---|---|---|---|---|
| 1 | 2.28, m | 2.27, overlap | 2.26, overlap | 2.26, overlap |
| 2 | Ha: 1.94, m | Ha: 2.27, overlap | Ha: 2.26, overlap | Ha: 2.26, overlap |
| Hb: 1.68, overlap | Hb: 1.68, m | Hb: 1.68, m | Hb: 1.67, overlap | |
| 3 | Ha: 1.84, m | Ha: 2.05, m | Ha: 2.04, m | Ha: 2.05, m |
| Hb: 1.68, overlap | Hb: 1.67, m | Hb: 1.67, m | Hb: 1.67, overlap | |
| 5 | 1.43, t (11.0) | 1.74, t (10.5) | 1.72, t (10.4) | 1.77, t (10.3) |
| 6 | 0.60, t-like (11.0) | 0.64, t-like (10.5) | 0.61, t (10.4) | 0.58, t (10.4) |
| 7 | 0.77, td (11.0, 6.6) | 0.77, td (10.5, 6.1) | 0.79, td (10.4, 6.4) | 0.94, td (10.4, 6.4) |
| 8 | Ha: 2.02, m | Ha: 1.97, m | Ha: 1.93, dt (13.0, 6.4) | Ha: 2.03, m |
| Hb: 0.99, m | Hb: 1.13, m | Hb: 1.08, q-like (13.0) | Hb: 1.15, q-like (12.8) | |
| 9 | Ha: 2.43, dd (13.0, 6.5) | Ha: 2.48, dd (13.2, 5.9) | Ha: 2.48, dd (13.0, 6.4) | Ha: 2.48, dd (12.8, 6.0) |
| Hb: 1.98, m | Hb: 2.14, t (13.2) | Hb: 2.12, t (13.0) | Hb: 2.13, t (12.8) | |
| 11 | 1.24, s | 1.54, s | 1.51, s | 1.54, s |
| 12 | Ha: 4.73, br s | Ha: 4.89, br s | Ha: 4.88, br s | Ha: 4.89, br s |
| Hb: 4.69, br s | Hb: 4.80, br s | Hb: 4.81, br s | Hb: 4.81, br s | |
| 14 | 1.03, s | 1.17, s | 1.21, s | 1.28, s |
| 15 | Ha: 2.83, d (17.9) | Ha: 2.06, m | Ha: 2.02, dd (13.7, 6.6) | Ha: 1.63, dd (14.3, 4.1) |
| Hb: 1.95, d (17.9) | Hb: 2.03, m | Hb: 1.27, dd (13.7, 6.6) | Hb: 1.57, dd (14.3, 8.4) | |
| 16 | 5.77, td (15.7, 7.1) | 4.21, dt (9.4, 6.6) | 4.23, td (8.4, 4.1) | |
| 17 | 6.00, br s | 5.66, d (15.7) | 5.14, d (9.4) | 5.20, br d (8.4) |
| 19 | 1.87, d (0.8) | 1.31, s | 1.75, s | 1.72, br s |
| 20 | 2.14, d (0.8) | 1.31, s | 1.70, s | 1.70, br s |
| 21 | 3.19, s | 3.27, s | 3.28, s | |
| 4-OH | 5.37, s | 5.15, s | 5.29, s |
| No | 1 a | 2 b | 3 b | 4 b | 5 b | 6 a | 7 a |
|---|---|---|---|---|---|---|---|
| 1 | 49.0, CH | 54.3, CH | 53.4, CH | 54.0, CH | 54.0, CH | 49.1, CH | 52.2, CH |
| 2 | 24.2, CH2 | 27.6, CH2 | 27.2, CH2 | 27.5, CH2 | 29.8, CH2 | 24.3, CH2 | 26.8, CH2 |
| 3 | 39.5, CH2 | 43.0, CH2 | 42.5, CH2 | 42.9, CH2 | 35.6, CH2 | 39.5, CH2 | 41.2, CH2 |
| 4 | 79.8, C | 80.3, C | 80.2, C | 80.2, C | 36.2, CH | 80.5, C | 81.2, C |
| 5 | 53.5, CH | 54.3, CH | 54.3, CH | 54.3, CH | 44.1, CH | 53.3, CH | 52.9, CH |
| 6 | 24.7, CH | 29.9, CH | 29.9, CH | 30.0, CH | 28.3, CH | 24.9, CH | 32.4, CH |
| 7 | 26.5, CH | 26.8, CH | 27.3, CH | 27.8, CH | 26.6, CH | 26.9, CH | 31.2, CH |
| 8 | 25.1, CH2 | 25.5, CH2 | 25.6, CH2 | 25.5, CH2 | 25.4, CH2 | 25.2, CH2 | 24.6, CH2 |
| 9 | 39.1, CH2 | 39.6, CH2 | 39.7, CH2 | 39.7, CH2 | 39.6, CH2 | 38.9, CH2 | 38.4, CH2 |
| 10 | 154.3, C | 154.4, C | 154.7, C | 154.6, C | 154.7, C | 154.0, C | 152.3, C |
| 11 | 23.9, CH3 | 27.3, CH3 | 26.6, CH3 | 26.9, CH3 | 18.0, CH3 | 23.9, CH3 | 25.9, CH3 |
| 12 | 106.7, CH2 | 106.7, CH2 | 106.7, CH2 | 106.6, CH2 | 106.4, CH2 | 107.0, CH2 | 107.7, CH2 |
| 13 | 20.5, C | 24.9, C | 22.7, C | 23.2, C | 24.7, C | 21.0, C | 30.3, C |
| 14 | 14.5, CH3 | 14.6, CH3 | 14.9, CH3 | 15.3, CH3 | 14.0, CH3 | 14.3, CH3 | 23.5, CH3 |
| 15 | 56.6, CH2 | 46.4, CH2 | 49.6, CH2 | 49.8, CH2 | 46.3, CH2 | 46.2, CH2 | 177.4, C |
| 16 | 201.0, C | 128.6, CH | 76.6, CH | 76.9, CH | 126.7, CH | 176.6, C | |
| 17 | 124.1, CH | 137.8, CH | 127.9, CH | 128.2, CH | 138.7, CH | ||
| 18 | 155.8, C | 75.2, C | 135.3, C | 134.9, C | 73.6, C | ||
| 19 | 27.9, CH3 | 26.9, CH3 | 26.2, CH3 | 26.2, CH3 | 25.9, CH3 | ||
| 20 | 21.0, CH3 | 26.2, CH3 | 18.6, CH3 | 18.6, CH3 | 71.5, CH3 | ||
| 21 | 50.5, CH3 | 55.6, CH3 | 55.8, CH3 |
| No | 5 b | 6 a | 7 a |
|---|---|---|---|
| 1 | 2.17, m | 2.30, m | 2.18, td (10.7, 6.6) |
| 2 | Ha: 1.66, m | Ha: 1.95, m | Ha: 1.92, m |
| Hb: 1.57, m | Hb: 1.67, m | Hb: 1.67, m | |
| 3 | Ha: 1.79, ddd (12.6, 6.4, 3.2) | Ha: 1.81, m | Ha: 1.86, m |
| Hb: 1.16, m | Hb: 1.68, m | Hb: 1.57, ddd (12.7, 10.4, 6.5) | |
| 4 | 2.03, m | ||
| 5 | 1.39, q (10.4) | 1.41, t (10.8), | 1.95, t (10.7) |
| 6 | 0.65, t (10.4) | 0.73, t (10.8) | 0.98, t-like (10.7) |
| 7 | 0.74, td (10.4, 6.6) | 0.88, td (10.8, 6.8) | 1.20, td (10.7, 6.1) |
| 8 | Ha: 1.90, dt (12.5, 6.1) | Ha: 2.05, dt (14.6, 6.8) | Ha: 2.03, m |
| Hb: 1.04, m | Hb: 0.98, m | Hb: 1.45, q-like (12.3) | |
| 9 | Ha: 2.42, dd (13.1, 6.1) | Ha: 2.43, dd (13.2, 6.8) | Ha: 2.44, dd (13.6, 6.4) |
| Hb: 2.05, m | Hb: 1.99, m | Hb: 2.01, m | |
| 11 | 1.01, d (8.6) | 1.29, s | 1.35, s |
| 12 | 4.76, s | Ha: 4.74, br s | Ha: 4.72, br s |
| Hb: 4.71, br s | Hb: 4.69, br s | ||
| 14 | 1.00, s | 1.08, s | 1.35, s |
| 15 | 2.06, m | Ha: 2.68, d (16.8) | |
| Hb: 1.87, d (16.8) | |||
| 16 | 6.18, dt (15.0, 7.1) | ||
| 17 | 6.03, d (15.0) | ||
| 19 | 1.67, (s) | ||
| 20 | 3.98, d (10.4) | ||
| 3.93, d (10.4) |
| Cpd. | FG * | CM | CL | BC | AA | SS | RS |
|---|---|---|---|---|---|---|---|
| 1 | 61.4 ± 4.3 | 80.0 ± 5.3 | 54.5 ± 0.0 | 36.2 ± 7.9 | 53.3 ± 2.9 | 61.4 ± 6.1 | 56.0 ± 9.2 |
| 2 | 55.7 ± 6.5 | 60.0 ± 2.7 | 51.5 ± 2.6 | 55.2 ± 6.0 | 53.3 ± 2.9 | 36.8 ± 13.9 | 52.0 ± 0.0 |
| 3 | 65.7 ± 0.0 | NT ** | 47.0 ± 2.6 | 56.9 ± 6.0 | 51.7 ± 2.9 | 66.7 ± 8.0 | 38.0 ± 3.5 |
| 4 | 58.6 ± 2.5 | NT | NT | 34.5 ± 7.9 | 51.7 ± 5.8 | 49.1 ± 13.2 | 40.0 ± 0.0 |
| 5 | 54.3 ± 6.5 | 72.3 ± 0.0 | NT | 56.9 ± 3.0 | 63.3 ± 2.9 | 40.4 ± 8.0 | 40.0 ± 6.0 |
| 6 | 37.1 ± 4.9 | NT | NT | 5.2 ± 3.0 | 13.3 ± 2.9 | 36.8 ± 0.0 | 20.0 ± 3.5 |
| 7 | NT | NT | NT | 34.5 ± 6.0 | NT | 33.3 ± 3.0 | NT |
| hymexazol | 61.4 ± 0.0 | 50.8 ± 2.7 | 83.3 ± 2.6 | 70.7 ± 3.0 | 66.7 ± 2.9 | 61.4 ± 3.0 | 62.0 ± 3.5 |
| Compound | R2 | Regression Equation (y = ax + b) | EC50 (mg/L) | |
|---|---|---|---|---|
| C. mebaldsii | 1 | 0.943 | y = 1.171x + 3.601 | 15.7 |
| 2 | 0.950 | y = 0.712x + 3.844 | 42.1 | |
| hymexazol | 0.953 | y = 2.314x + 0.537 | 84.8 | |
| F. graminearum | 1 | 0.961 | y = 1.426x + 2.381 | 68.6 |
| 2 | 0.983 | y = 1.369x + 2.410 | 78.0 | |
| hymexazol | 0.961 | y = 1.561x + 2.157 | 66.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Q.; Liu, Y.-Y.; Huang, D.; Cheng, Y.-X. Euphraticanoids N–T: Aromadendrane-Type Diterpenes and Sesquiterpenes with Fungicidal Activities from Populus euphratica Resins. Int. J. Mol. Sci. 2025, 26, 2187. https://doi.org/10.3390/ijms26052187
Jiang Q, Liu Y-Y, Huang D, Cheng Y-X. Euphraticanoids N–T: Aromadendrane-Type Diterpenes and Sesquiterpenes with Fungicidal Activities from Populus euphratica Resins. International Journal of Molecular Sciences. 2025; 26(5):2187. https://doi.org/10.3390/ijms26052187
Chicago/Turabian StyleJiang, Qinbin, Yun-Yun Liu, Danling Huang, and Yong-Xian Cheng. 2025. "Euphraticanoids N–T: Aromadendrane-Type Diterpenes and Sesquiterpenes with Fungicidal Activities from Populus euphratica Resins" International Journal of Molecular Sciences 26, no. 5: 2187. https://doi.org/10.3390/ijms26052187
APA StyleJiang, Q., Liu, Y.-Y., Huang, D., & Cheng, Y.-X. (2025). Euphraticanoids N–T: Aromadendrane-Type Diterpenes and Sesquiterpenes with Fungicidal Activities from Populus euphratica Resins. International Journal of Molecular Sciences, 26(5), 2187. https://doi.org/10.3390/ijms26052187






