Isolation, Characterization, and In Vitro Cell Studies of Plant-Based Exosome-like Nanovesicles for Treatment of Early Osteoarthritis
Abstract
1. Introduction
2. Results
2.1. Protein Quantification (BCA) and Nanoparticle Tracking Analysis (NTA)
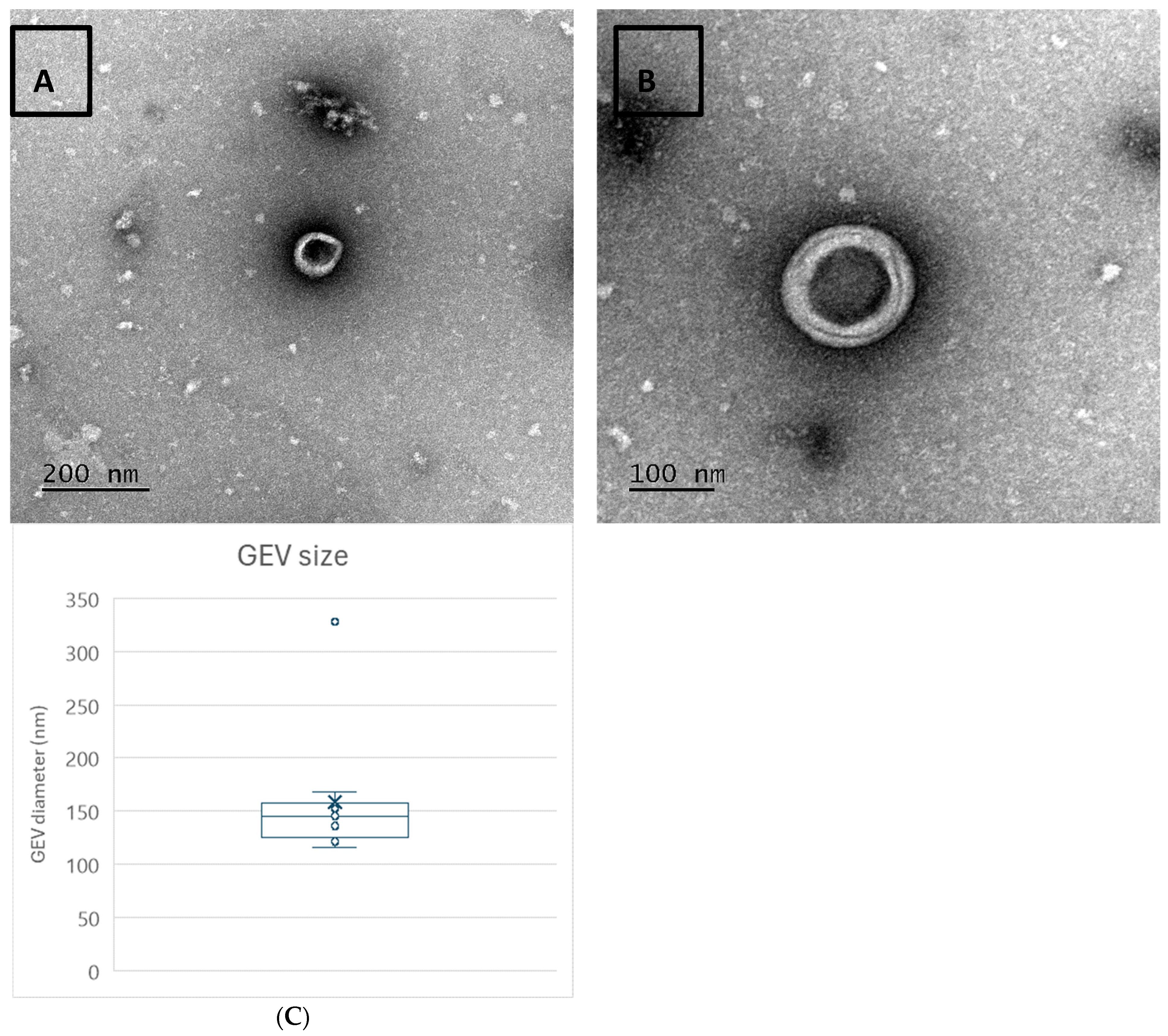
2.2. Transmission Electron Microscopy
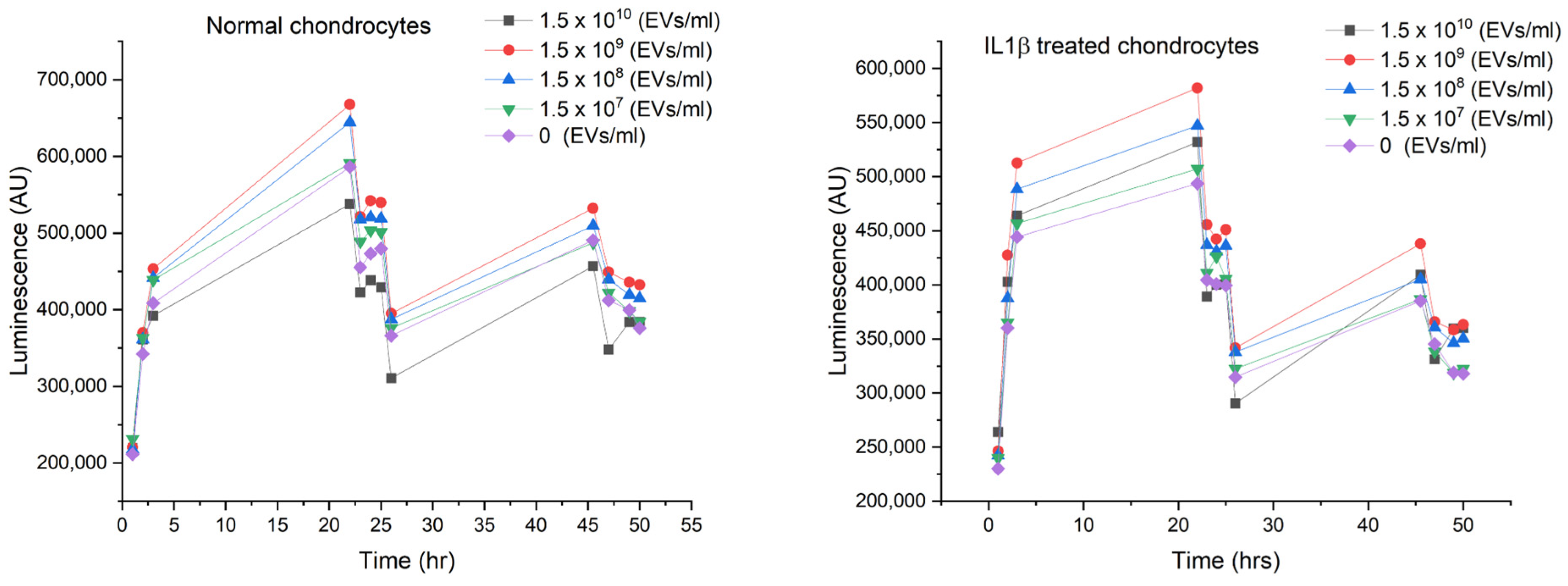
2.3. Dosing
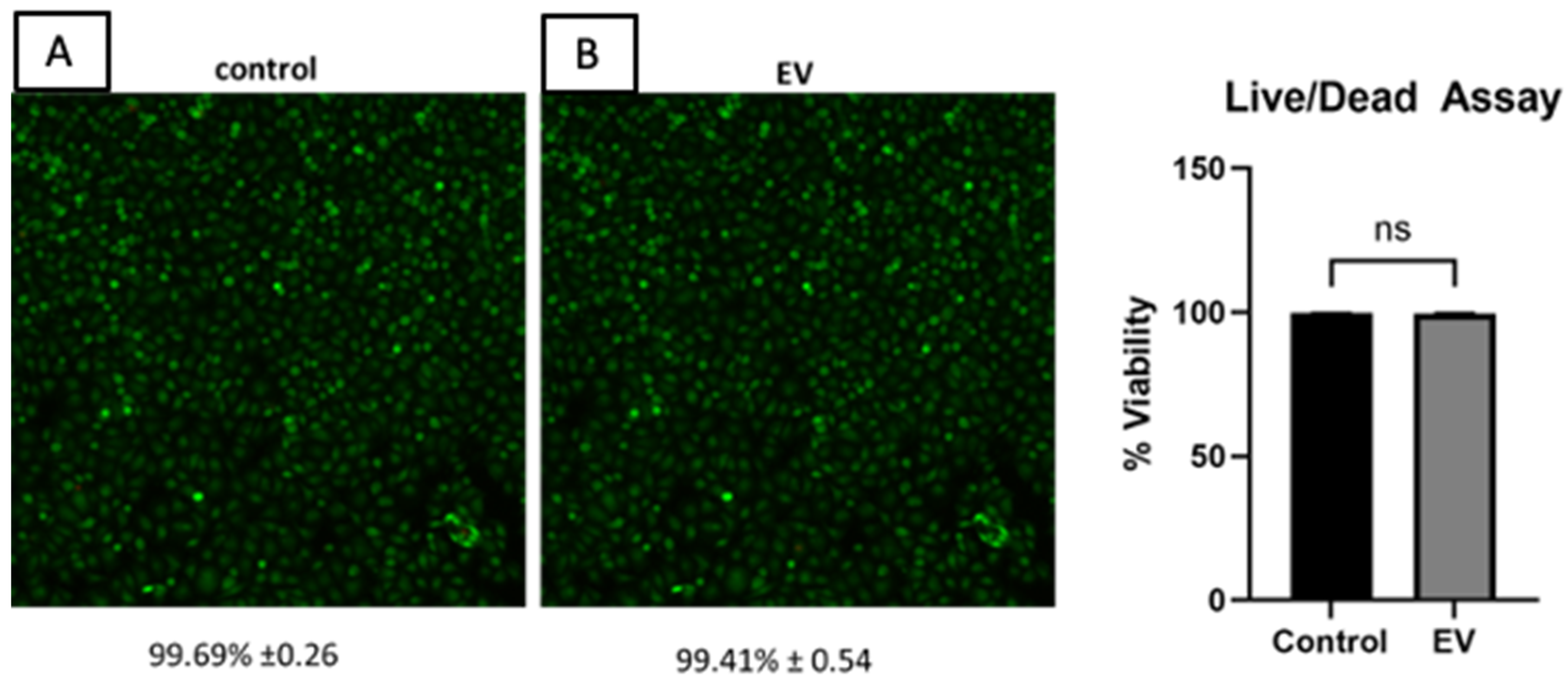
2.4. Cell Viability
2.5. Cellular Uptake
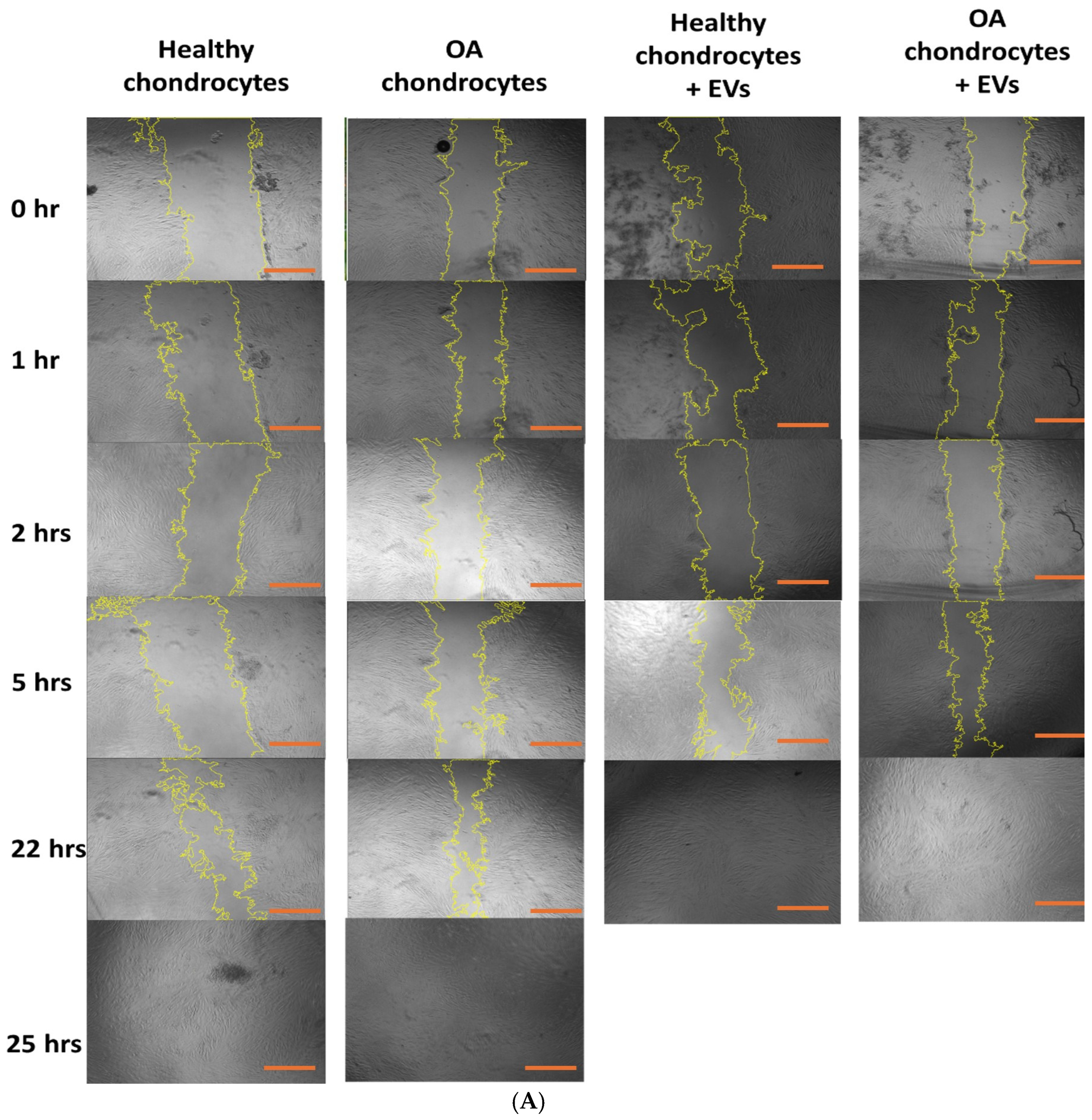
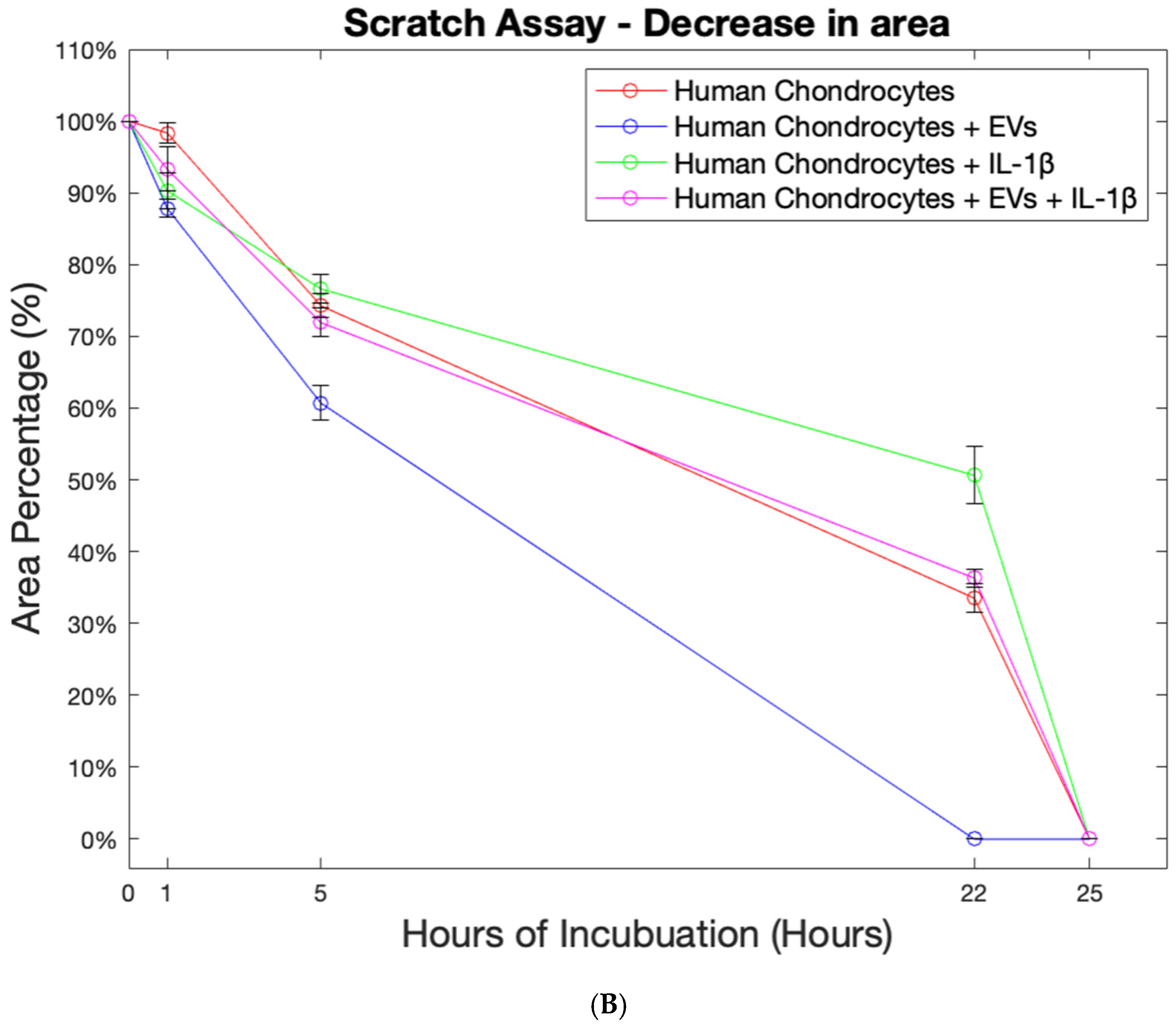
2.6. Effect of EVs on Cell Migration
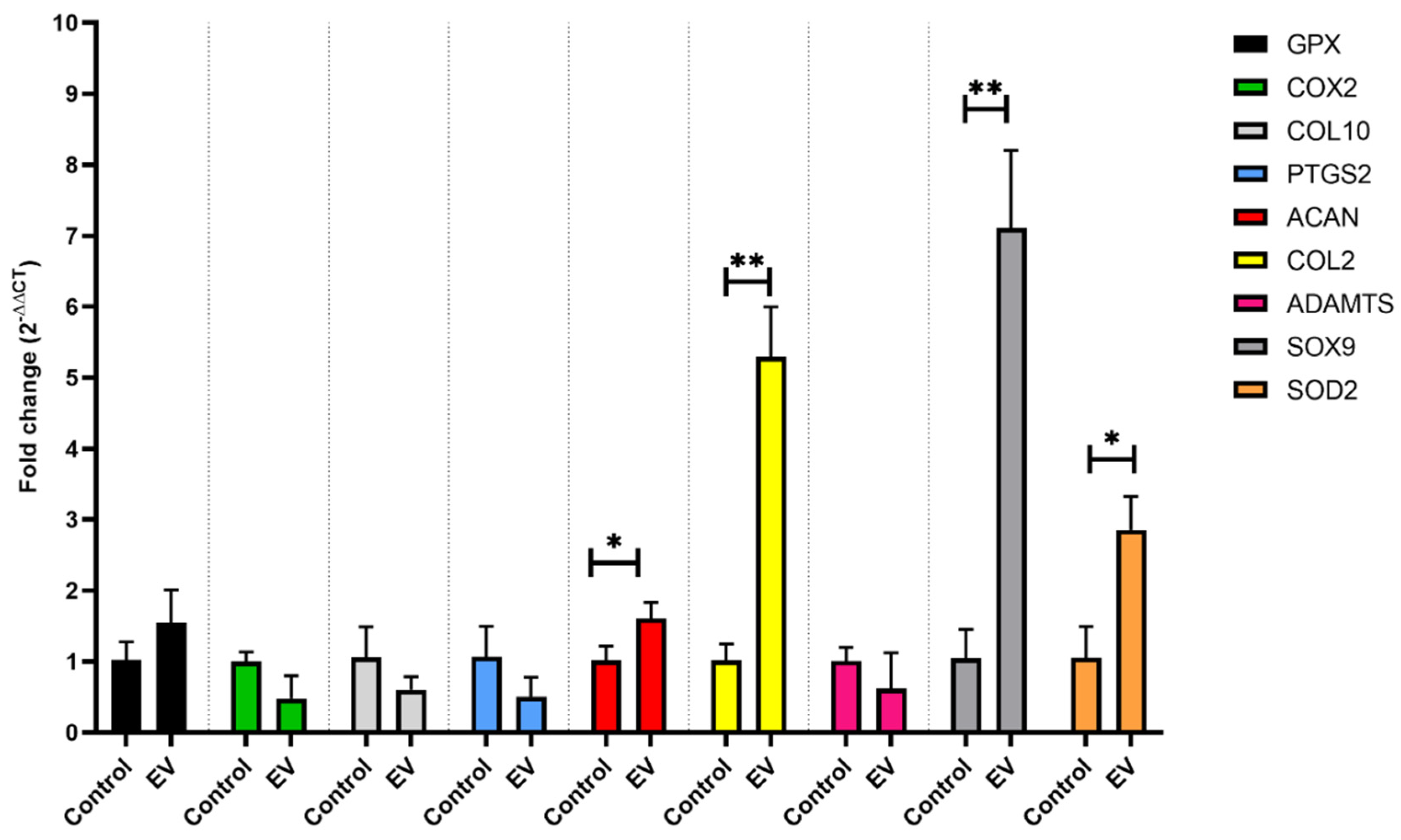
2.7. Gene Expression
3. Discussion
Limitations and Future Direction
4. Materials and Methods
4.1. Isolation and Purification of ELVs
4.2. EVs Freeze Drying
4.3. Physical Characterization of GEVs
4.3.1. Nanoparticle Tracking Analysis (NTA) for the Measurement of GEV Size and Concentration
4.3.2. Protein Quantification
4.3.3. Transmission Electron Microscopy (TEM)
4.4. Cell Experiments
4.4.1. Human Chondrocyte Culture and In Vitro Model of OA-like Chondrocyte
4.4.2. Cell Viability
4.4.3. In Vitro Cellular Uptake of GEVs
4.4.4. Gene Expression-RT PCR
4.4.5. Cell Migration Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, A.E. Osteoarthritis Year in Review 2017: Clinical. Osteoarthr. Cartil. 2018, 26, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Tamaddon, M.; Gilja, H.; Wang, L.; Oliveira, J.M.; Sun, X.; Tan, R.; Liu, C. Osteochondral Scaffolds for Early Treatment of Cartilage Defects in Osteoarthritic Joints: From Bench to Clinic. Biomater. Transl. 2020, 1, 3–17. [Google Scholar]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef]
- Andreani, L.; Giuntoli, M.; Addevico, F.; Aringhieri, G.; Cosottini, M.; Marchetti, S. The Effect of Viscosupplementation on Early-Stage Knee Osteoarthritis: Clinical Evaluation and Assessment of Cartilage In Vivo with 7 T MRI. J. Clin. Orthop. Trauma 2021, 19, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Wong, K.L.; Zhang, S.; Wang, M.; Ren, X.; Afizah, H.; Lai, R.C.; Lim, S.K.; Lee, E.H.; Hui, J.H.P.; Toh, W.S. Intra-Articular Injections of Mesenchymal Stem Cell Exosomes and Hyaluronic Acid Improve Structural and Mechanical Properties of Repaired Cartilage in a Rabbit Model. Arthroscopy 2020, 36, 2215–2228.e2. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liang, Y.; Li, X.; Ouyang, K.; Wang, M.; Cao, T.; Li, W.; Liu, J.; Xiong, J.; Li, B.; et al. Exosome-Mediated Delivery of Kartogenin for Chondrogenesis of Synovial Fluid-Derived Mesenchymal Stem Cells and Cartilage Regeneration. Biomaterials 2021, 269, 120539. [Google Scholar] [CrossRef]
- Dad, H.A.; Gu, T.-W.; Zhu, A.-Q.; Huang, L.-Q.; Peng, L.-H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef]
- Peng, L.-H.; Wang, M.-Z.; Chu, Y.; Zhang, L.; Niu, J.; Shao, H.-T.; Yuan, T.-J.; Jiang, Z.-H.; Gao, J.-Q.; Ning, X.-H. Engineering Bacterial Outer Membrane Vesicles as Transdermal Nanoplatforms for Photo-TRAIL–Programmed Therapy against Melanoma. Sci. Adv. 2020, 6, eaba2735. [Google Scholar] [CrossRef]
- Berger, E.; Colosetti, P.; Jalabert, A.; Meugnier, E.; Wiklander, O.P.B.; Jouhet, J.; Errazurig-Cerda, E.; Chanon, S.; Gupta, D.; Rautureau, G.J.P.; et al. Use of Nanovesicles from Orange Juice to Reverse Diet-Induced Gut Modifications in Diet-Induced Obese Mice. Mol. Ther.-Methods Clin. Dev. 2020, 18, 880–892. [Google Scholar] [CrossRef]
- Reiner, A.T.; Somoza, V. Extracellular Vesicles as Vehicles for the Delivery of Food Bioactives. J. Agric. Food Chem. 2019, 67, 2113–2119. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, D.; Chen, X.; Li, D.; Zhu, L.; Zhang, Y.; Li, J.; Bian, Z.; Liang, X.; Cai, X.; et al. Exogenous Plant MIR168a Specifically Targets Mammalian LDLRAP1: Evidence of Cross-Kingdom Regulation by MicroRNA. Cell Res. 2012, 22, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, M.; Merlin, D. Advances in Plant-Derived Edible Nanoparticle-Based Lipid Nano-Drug Delivery Systems as Therapeutic Nanomedicines. J. Mater. Chem. B 2018, 6, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Pocsfalvi, G.; Turiák, L.; Ambrosone, A.; del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vékey, K. Protein Biocargo of Citrus Fruit-Derived Vesicles Reveals Heterogeneous Transport and Extracellular Vesicle Populations. J. Plant Physiol. 2018, 229, 111–121. [Google Scholar] [CrossRef]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhuang, X.; Deng, Z.-B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted Drug Delivery to Intestinal Macrophages by Bioactive Nanovesicles Released from Grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.-B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies Communication between Plant and Mouse Gut Host Cells through Edible Plant Derived Exosome-like Nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible Ginger-Derived Nanoparticles: A Novel Therapeutic Approach for the Prevention and Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef]
- Raimondo, S.; Saieva, L.; Cristaldi, M.; Monteleone, F.; Fontana, S.; Alessandro, R. Label-Free Quantitative Proteomic Profiling of Colon Cancer Cells Identifies Acetyl-CoA Carboxylase Alpha as Antitumor Target of Citrus limon-Derived Nanovesicles. J. Proteom. 2018, 173, 1–11. [Google Scholar] [CrossRef]
- Yang, M.; Liu, X.; Luo, Q.; Xu, L.; Chen, F. An Efficient Method to Isolate Lemon Derived Extracellular Vesicles for Gastric Cancer Therapy. J. Nanobiotechnol. 2020, 18, 100. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape Exosome-like Nanoparticles Induce Intestinal Stem Cells and Protect Mice from DSS-Induced Colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Sahin, F.; Koçak, P.; Günes, M.Y.; Özkan, I.; Yildirim, E.; Kala, E.Y. In Vitro Wound Healing Activity of Wheat-Derived Nanovesicles. Appl. Biochem. Biotechnol. 2019, 188, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Deng, Z.-B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.-G. Ginger-Derived Nanoparticles Protect against Alcohol-Induced Liver Damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, B. Plant-Derived XenomiRs and Cancer: Cross-Kingdom Gene Regulation. Saudi J. Biol. Sci. 2021, 28, 2408–2422. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, S.; Fu, Z.; Wang, Y.; Wang, N.; Liu, Y.; Zhao, C.; Wu, J.; Hu, Y.; Zhang, J.; et al. Effective Detection and Quantification of Dietetically Absorbed Plant MicroRNAs in Human Plasma. J. Nutr. Biochem. 2015, 26, 505–512. [Google Scholar] [CrossRef]
- Yang, J.; Hotz, T.; Broadnax, L.; Yarmarkovich, M.; Elbaz-Younes, I.; Hirschi, K.D. Anomalous Uptake and Circulatory Characteristics of the Plant-Based Small RNA MIR2911. Sci. Rep. 2016, 6, 26834. [Google Scholar] [CrossRef]
- Malemud, C.J. MicroRNAs and Osteoarthritis. Cells 2018, 7, 92. [Google Scholar] [CrossRef]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-Derived Exosome-like Nanoparticles Prevent Oxidative Stress in Human Mesenchymal Stromal Cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef]
- Takov, K.; He, Z.; Johnston, H.E.; Timms, J.F.; Guillot, P.V.; Yellon, D.M.; Davidson, S.M. Small Extracellular Vesicles Secreted from Human Amniotic Fluid Mesenchymal Stromal Cells Possess Cardioprotective and Promigratory Potential. Basic Res. Cardiol. 2020, 115, 26. [Google Scholar] [CrossRef]
- Ansari, F.J.; Ahmadi Tafti, H.; Amanzadeh, A.; Rabbani, S.; Shokrgozar, M.A.; Heidari, R.; Behroozi, J.; Eyni, H.; Uversky, V.N.; Ghanbari, H. Comparison of the Efficiency of Ultrafiltration, Precipitation, and Ultracentrifugation Methods for Exosome Isolation. Biochem. Biophys. Rep. 2024, 38, 101668. [Google Scholar] [CrossRef]
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized Exosome Isolation Protocol for Cell Culture Supernatant and Human Plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Bondeson, J.; Wainwright, S.; Hughes, C.; Caterson, B. The Regulation of the ADAMTS4 and ADAMTS5 Aggrecanases in Osteoarthritis: A Review. Clin. Exp. Rheumatol. 2008, 26, 139–145. [Google Scholar] [PubMed]
- Verma, P.; Dalal, K. ADAMTS-4 and ADAMTS-5: Key Enzymes in Osteoarthritis. J. Cell. Biochem. 2011, 112, 3507–3514. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E. Pain Management in Osteoarthritis: The Role of COX-2 Inhibitors. J. Rheumatol. Suppl. 1997, 49, 20–24. [Google Scholar] [PubMed]
- Scott, J.L.; Gabrielides, C.; Davidson, R.K.; Swingler, T.E.; Clark, I.M.; Wallis, G.A.; Boot-Handford, R.P.; Kirkwood, T.B.; Taylor, R.W.; Young, D.A. Superoxide Dismutase Downregulation in Osteoarthritis Progression and End-Stage Disease. Ann. Rheum. Dis. 2010, 69, 1502–1510. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/Oxidative Stress Signaling in Osteoarthritis. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative Stress and Inflammation in Osteoarthritis Pathogenesis: Role of Polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Lian, C.; Wang, X.; Qiu, X.; Wu, Z.; Gao, B.; Liu, L.; Liang, G.; Zhou, H.; Yang, X.; Peng, Y.; et al. Collagen Type II Suppresses Articular Chondrocyte Hypertrophy and Osteoarthritis Progression by Promoting Integrin β1−SMAD1 Interaction. Bone Res. 2019, 7, 8. [Google Scholar] [CrossRef]
- Stanly, C.; Alfieri, M.; Ambrosone, A.; Leone, A.; Fiume, I.; Pocsfalvi, G. Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line. Cells 2020, 9, 2722. [Google Scholar] [CrossRef]
- Savcı, Y.; Kırbaş, O.K.; Bozkurt, B.T.; Abdik, E.A.; Taşlı, P.N.; Şahin, F.; Abdik, H. Grapefruit-Derived Extracellular Vesicles as a Promising Cell-Free Therapeutic Tool for Wound Healing. Food Funct. 2021, 12, 5144–5156. [Google Scholar] [CrossRef]
- Sundaram, K.; Mu, J.; Kumar, A.; Behera, J.; Lei, C.; Sriwastva, M.K.; Xu, F.; Dryden, G.W.; Zhang, L.; Chen, S.Y.; et al. Garlic Exosome-like Nanoparticles Reverse High-Fat Diet Induced Obesity via the Gut/Brain Axis. Theranostics 2022, 12, 1220–1246. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y.; Wang, Y. Comparison of Exosomes Secreted by Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells and Synovial Membrane-Derived Mesenchymal Stem Cells for the Treatment of Osteoarthritis. Stem Cell Res. Ther. 2017, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xu, M.; Zhu, H.; Dong, C.; Ji, J.; Liu, Y.; Deng, A.; Gu, Z. Therapeutic Effects of Bone Marrow Mesenchymal Stem Cells-Derived Exosomes on Osteoarthritis. J. Cell. Mol. Med. 2021, 25, 9281–9294. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Yu, J. Exosome-like Nanoparticles from Ginger Rhizomes Inhibited NLRP3 Inflammasome Activation. Mol. Pharm. 2019, 16, 2690–2699. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Xu, Z.; Thakur, A.; Zhang, K.; Liang, Q.; Liu, Y.; Yan, Y. Current Understanding of Plant-Derived Exosome-like Nanoparticles in Regulating the Inflammatory Response and Immune System Microenvironment. Pharmacol. Res. 2023, 190, 106733. [Google Scholar] [CrossRef]
- Patist, A.; Zoerb, H. Preservation Mechanisms of Trehalose in Food and Biosystems. Colloids Surf. B Biointerfaces 2005, 40, 107–113. [Google Scholar] [CrossRef]


| Gene | Forward | Reverse |
|---|---|---|
| COL2A | GGCAATAGCAGGTTCACGTACA | CGATAACAGTCTTGCCCCACTT |
| GAPDH | TCTCCTCTGACTTCAACAGCGAC | CCCTGTTGCTGTAGCCAAATTC |
| ADAMTS-5 | GCAGAACATCGACCAACTCTACTC | CCAGCAATGCCCACCGAAC |
| RPL13A | CTTTCCTCCGCAAGCGG | GTCCGCCAGAAGATGCG |
| ACAN | ACAGATGCTTCCATCCCAGC | TCACATACCTCCTGGTCTGC |
| COX-2 | CAAATTGCTGGCAGGGTTGC | AGGGCTTCAGCATAAAGCGT |
| iNOS | CTGGCAAGCCCAAGGTCTAT, | TCCCCGCAAACATAGAGGTG |
| MMP13 | TCCAGTCTCTCTATGGTCCAGG | TCCAGTCTCTCTATGGTCCAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashidi, N.; Liu, C.; Guillot, P.V.; Tamaddon, M. Isolation, Characterization, and In Vitro Cell Studies of Plant-Based Exosome-like Nanovesicles for Treatment of Early Osteoarthritis. Int. J. Mol. Sci. 2025, 26, 2211. https://doi.org/10.3390/ijms26052211
Rashidi N, Liu C, Guillot PV, Tamaddon M. Isolation, Characterization, and In Vitro Cell Studies of Plant-Based Exosome-like Nanovesicles for Treatment of Early Osteoarthritis. International Journal of Molecular Sciences. 2025; 26(5):2211. https://doi.org/10.3390/ijms26052211
Chicago/Turabian StyleRashidi, Narjes, Chaozong Liu, Pascale V. Guillot, and Maryam Tamaddon. 2025. "Isolation, Characterization, and In Vitro Cell Studies of Plant-Based Exosome-like Nanovesicles for Treatment of Early Osteoarthritis" International Journal of Molecular Sciences 26, no. 5: 2211. https://doi.org/10.3390/ijms26052211
APA StyleRashidi, N., Liu, C., Guillot, P. V., & Tamaddon, M. (2025). Isolation, Characterization, and In Vitro Cell Studies of Plant-Based Exosome-like Nanovesicles for Treatment of Early Osteoarthritis. International Journal of Molecular Sciences, 26(5), 2211. https://doi.org/10.3390/ijms26052211







