Synthesis of the Titanium Oxides Using a New Microwave Discharge Method
Abstract
1. Introduction
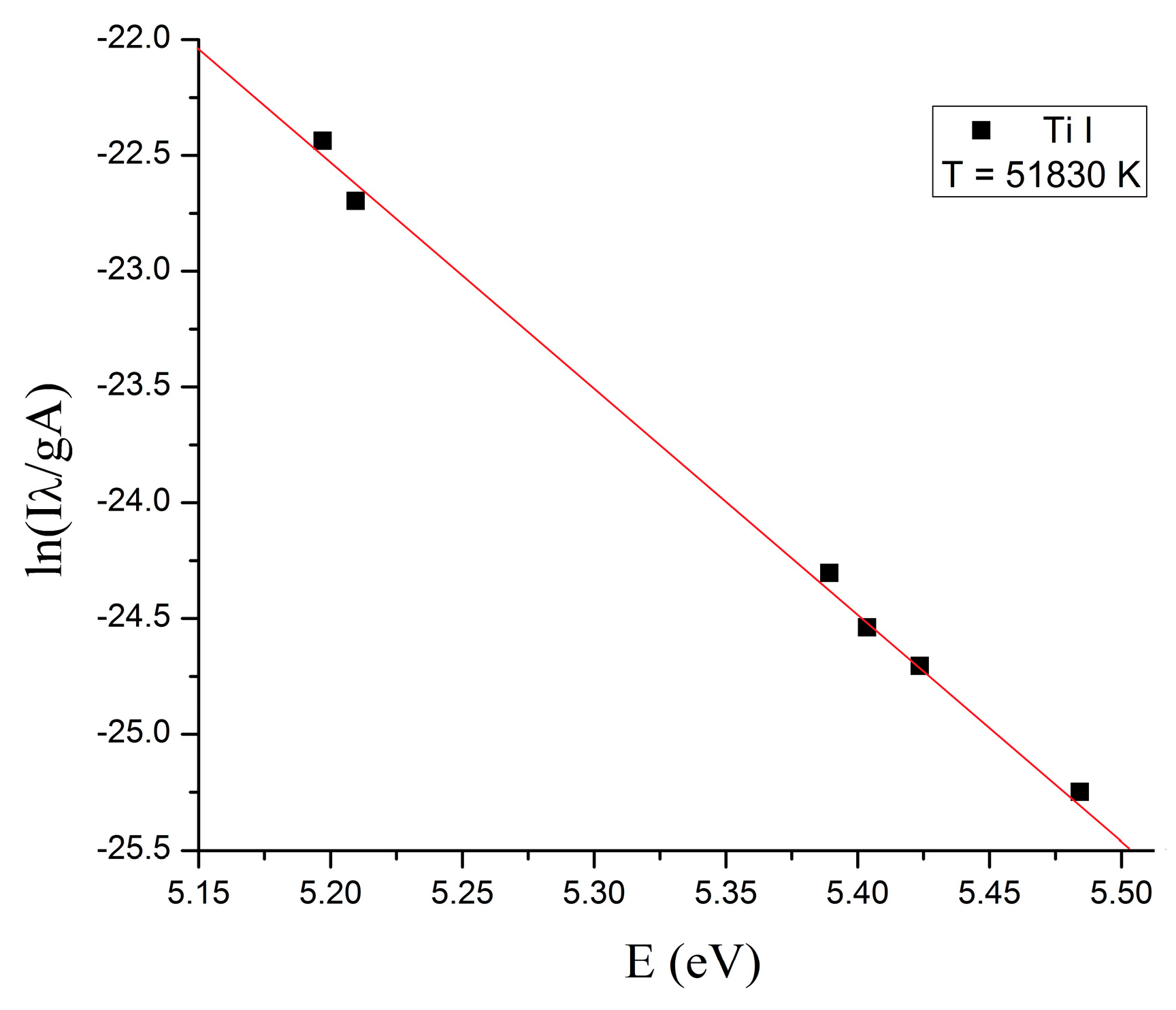
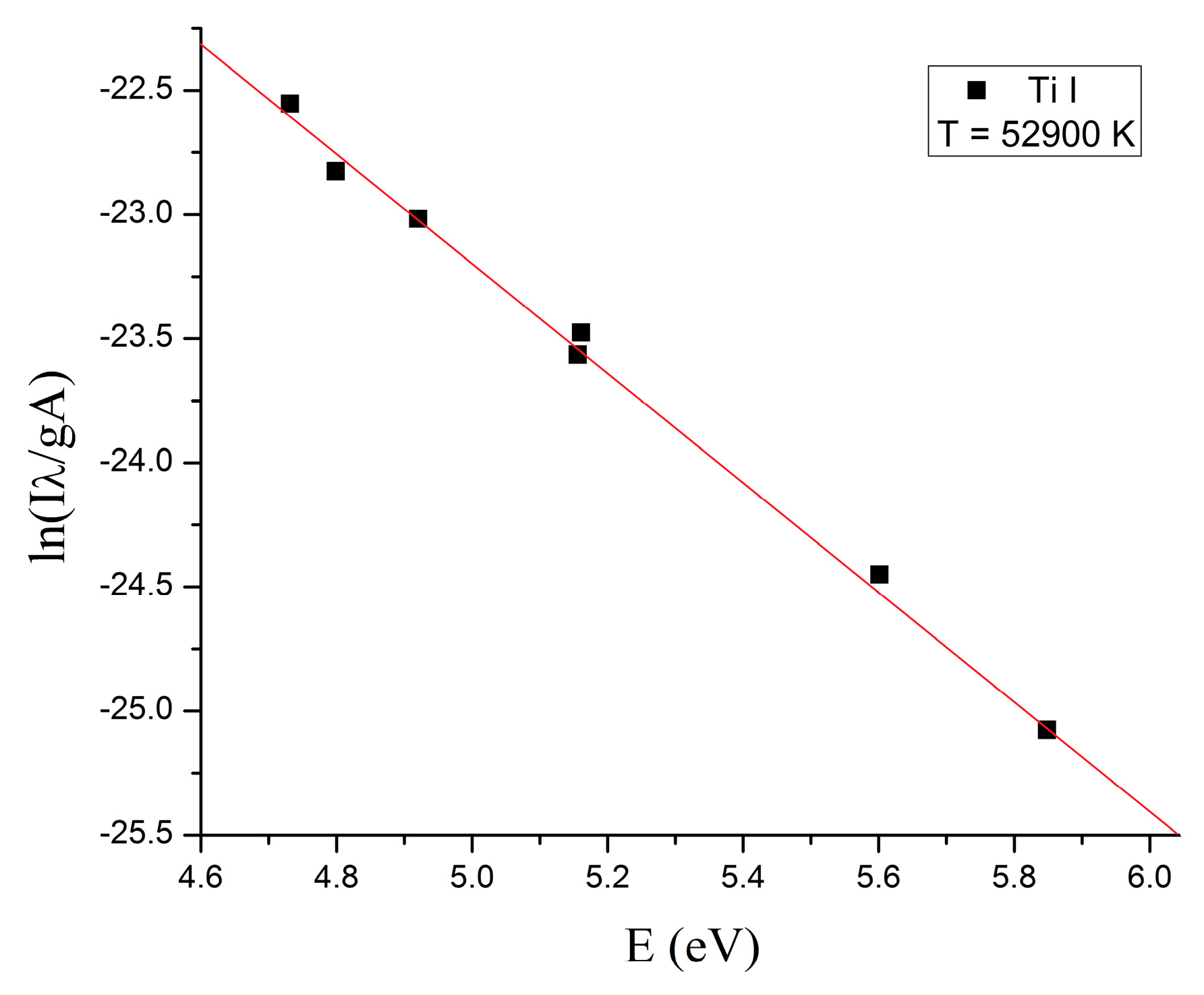
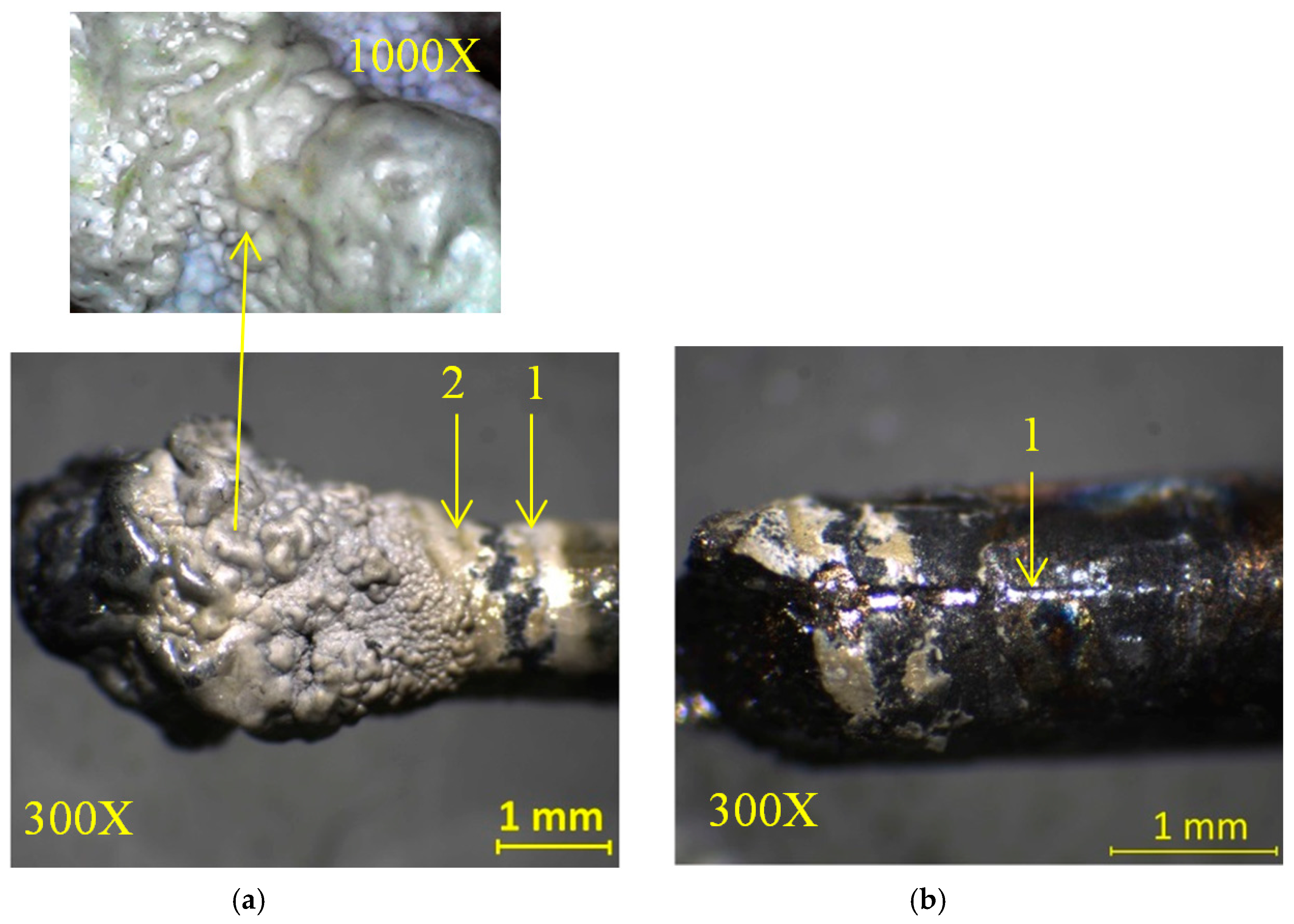
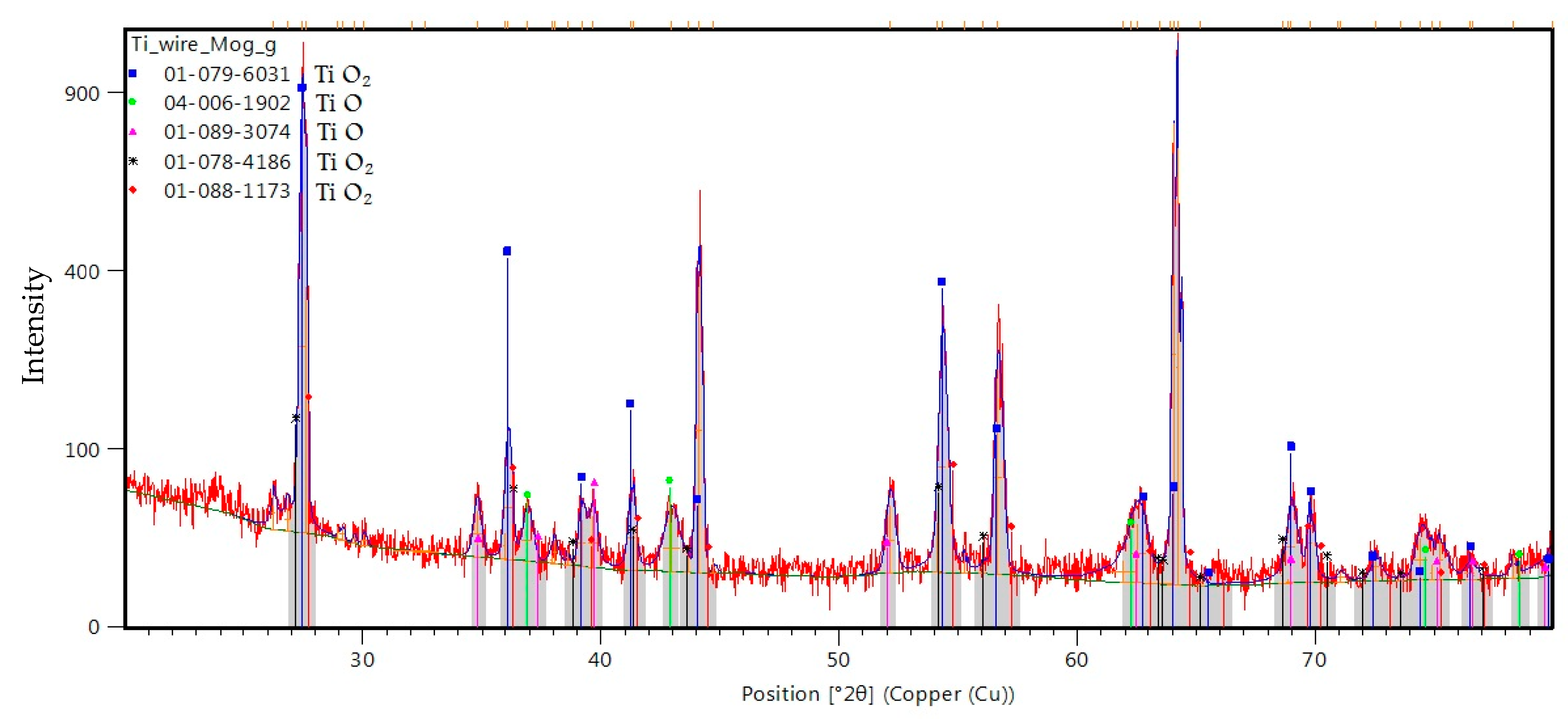
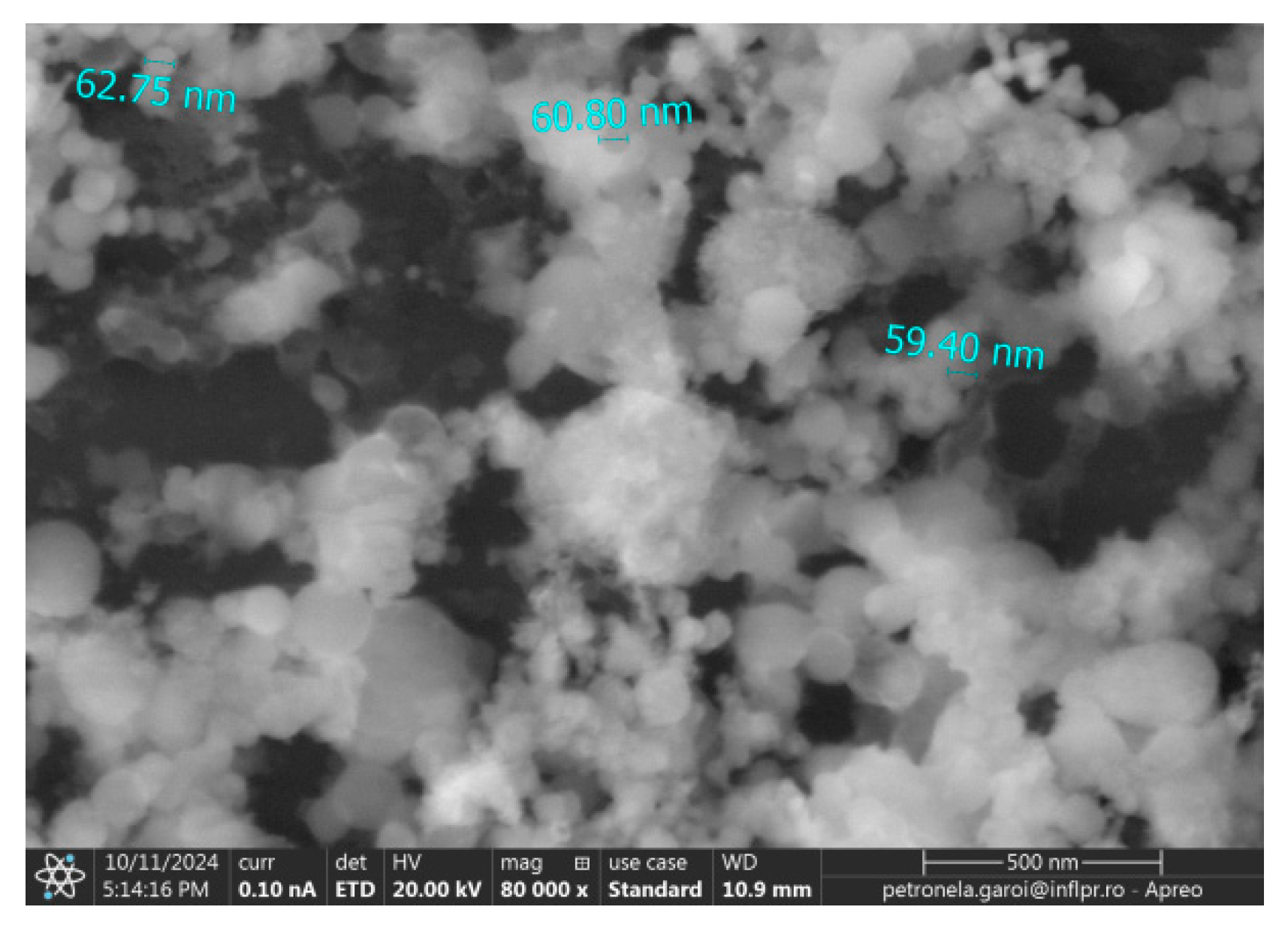
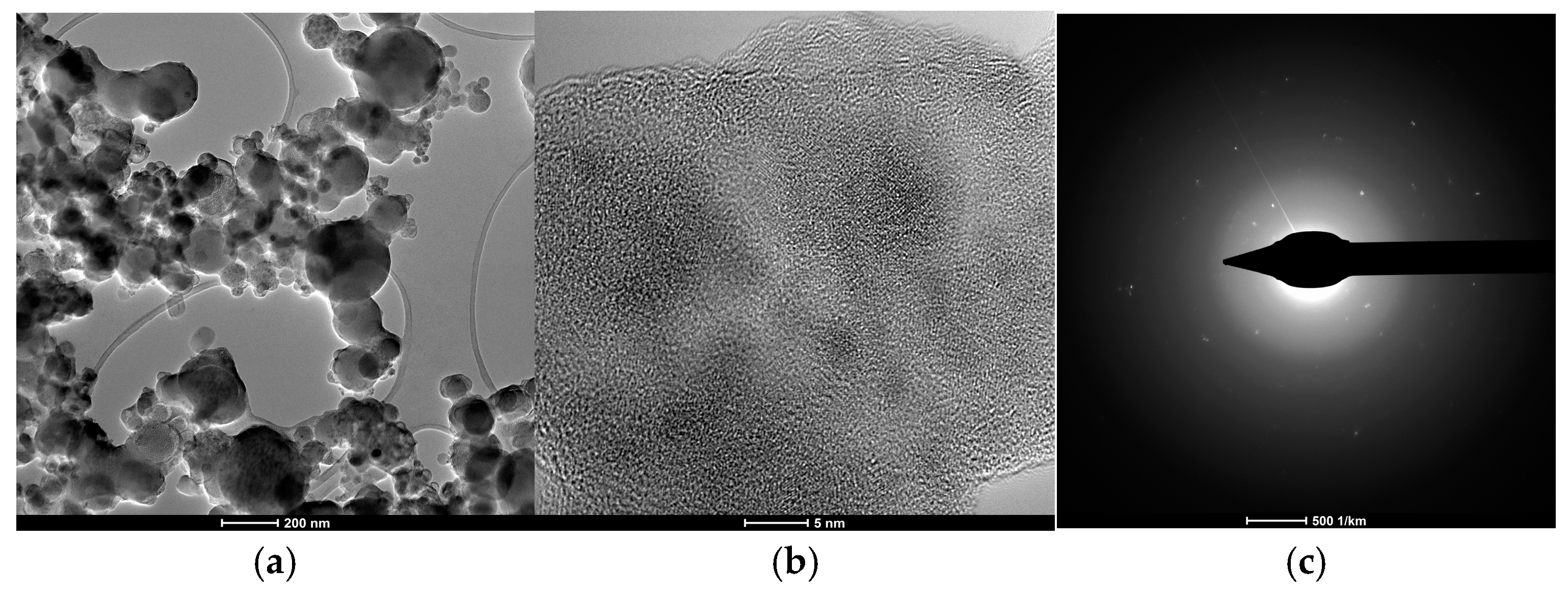
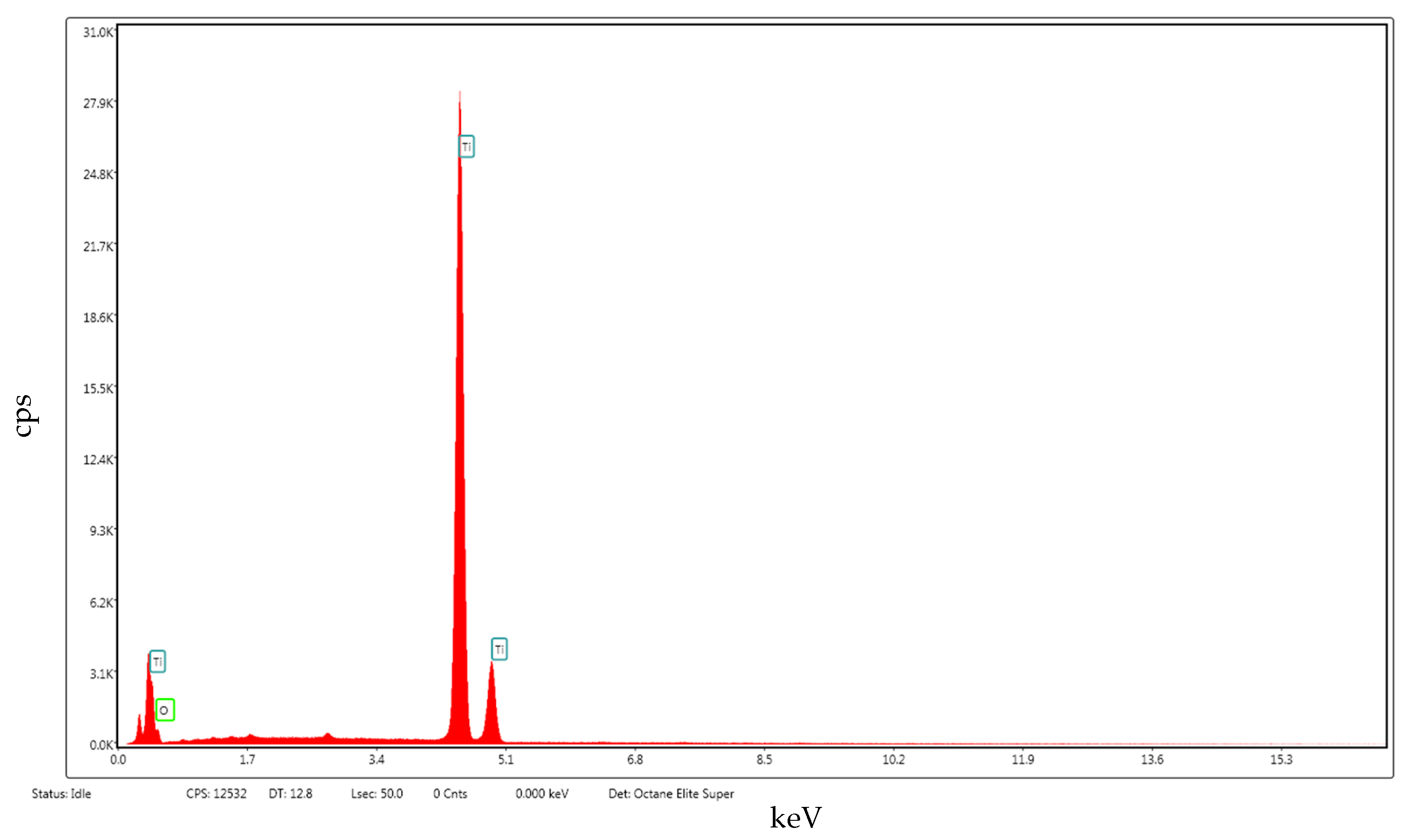
2. Results
3. Discussion
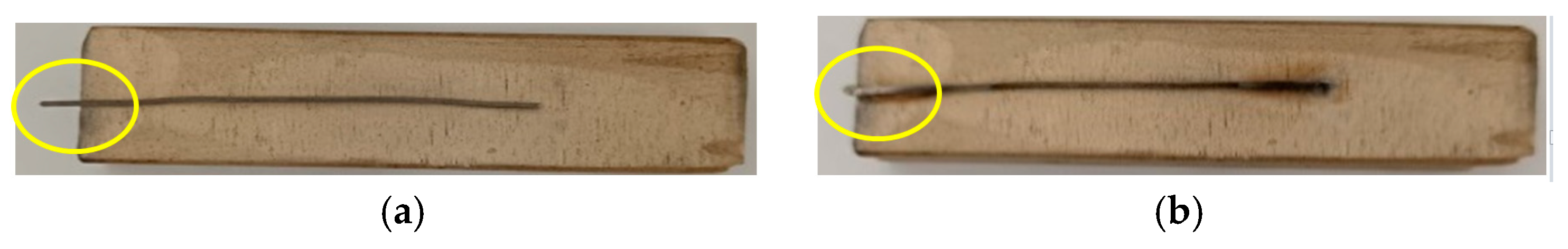
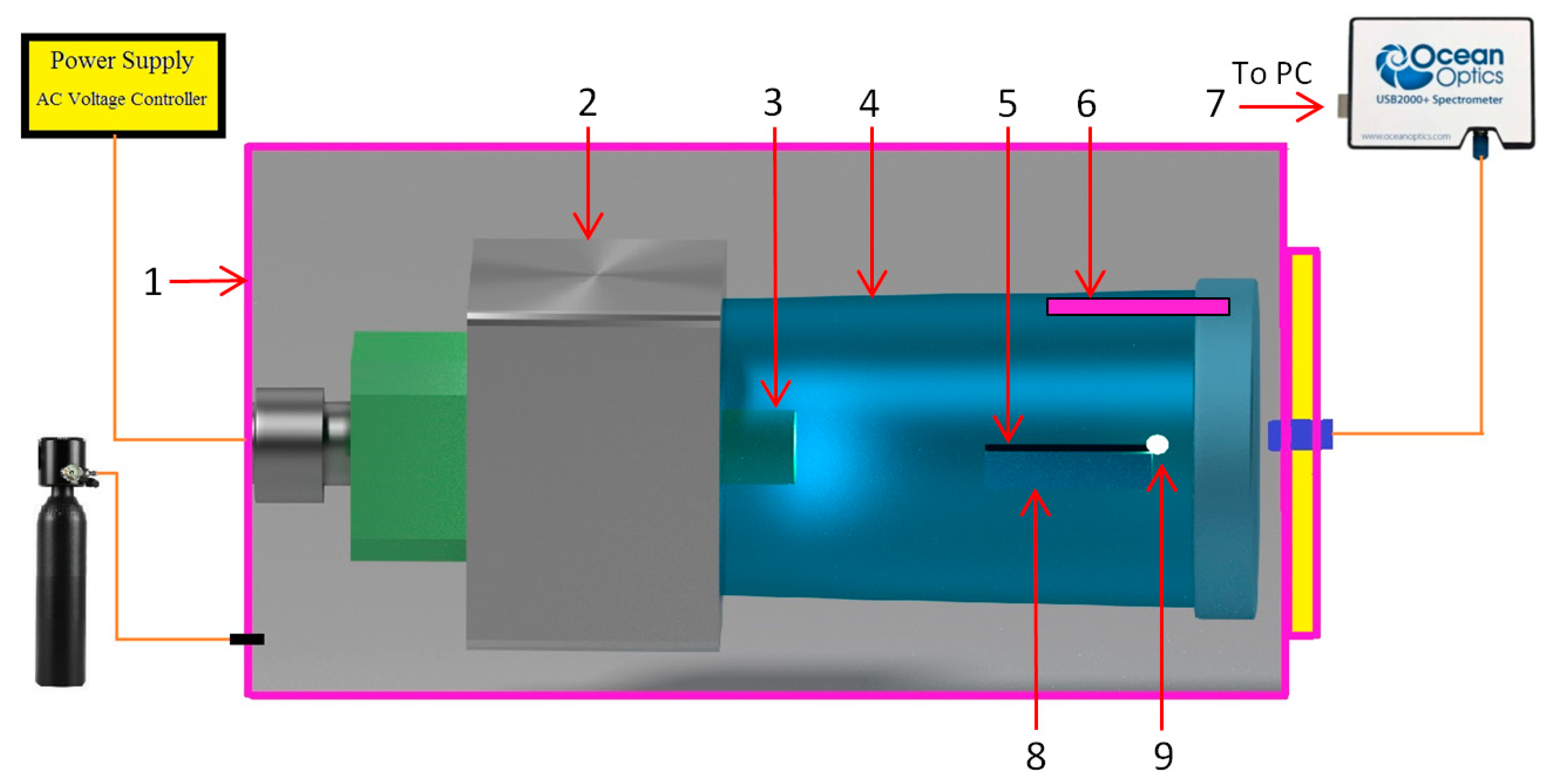
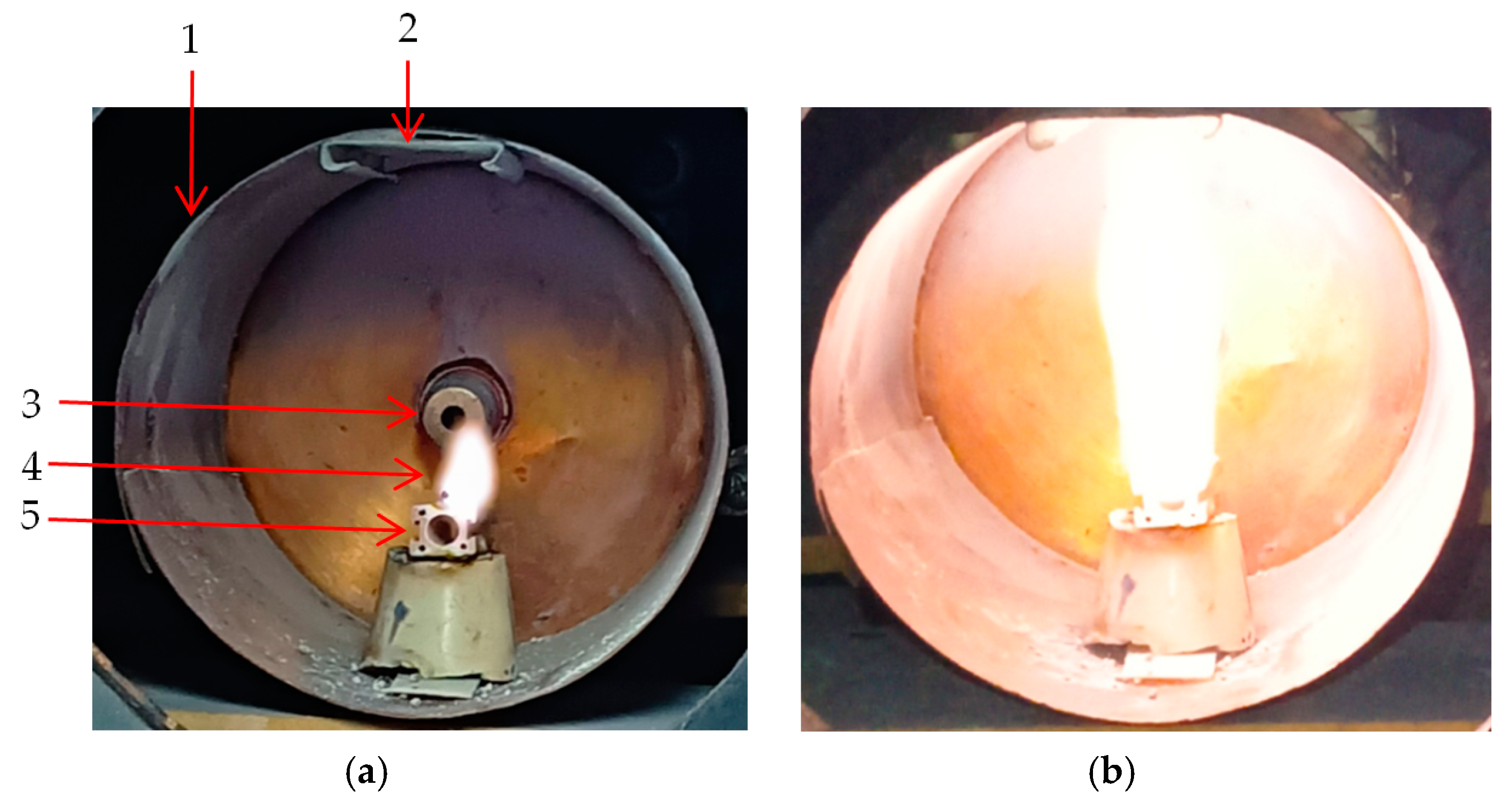
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Azizi-Lalabadi, M.; Ehsani, A.; Divband, B.; Alizadeh-Sani, M. Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci. Rep. 2019, 9, 17439. [Google Scholar] [CrossRef]
- Assim, E.M. Optical constants of titanium monoxide TiO thin films. J. Alloys Compd. 2008, 465, 1–7. [Google Scholar] [CrossRef]
- López-Huerta, F.; Cervantes, B.; González, O.; Hernández-Torres, J.; García-González, L.; Vega, R.; Herrera-May, A.L.; Soto, E. Biocompatibility and Surface Properties of TiO2 Thin Films Deposited by DC Magnetron Sputtering. Materials 2014, 7, 4105–4117. [Google Scholar] [CrossRef] [PubMed]
- Gázquez, M.J.; Bolívar, J.P.; Garcia-Tenorio, R.; Vaca, F. A Review of the Production Cycle of Titanium Dioxide Pigment. Mater. Sci. Appl. 2014, 5, 441–458. [Google Scholar] [CrossRef]
- Blundell, R.; Butterworth, P.; Charlier, A.; Daurio, D.; Degenhardt, M.; Harris, D.; Hancock, B.; Johnston, M.; Kasina, R.; Kaye, J.; et al. The Role of Titanium Dioxide (E171) and the Requirements for Replacement Materials in Oral Solid Dosage Forms: An IQ Consortium Working Group Review. J. Pharm. Sci. 2022, 111, 2943–2954. [Google Scholar] [CrossRef]
- Manurung, P.; Putri, Y.; Simanjuntak, W.; Low, I. Synthesis and characterisation of chemical bath deposited TiO2 thin-films. Ceram. Int. 2013, 39, 255–259. [Google Scholar] [CrossRef]
- Bessergenev, V.; Khmelinskii, I.; Pereira, R.; Krisuk, V.; Turgambaeva, A.; Igumenov, I. Preparation of TiO2 films by CVD method and its electrical, structural and optical properties. Vacuum 2002, 64, 275–279. [Google Scholar] [CrossRef]
- Fisher, P.; Maksimov, O.; Du, H.; Heydemann, V.D.; Skowronski, M.; Salvador, P.A. Growth, structure, and morphology of TiO2 films deposited by molecular beam epitaxy in pure ozone ambients. Microelectron. J. 2006, 37, 1493–1497. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Rahman, K.H.; Wu, C.-C.; Chen, K.-C. A Review on the Pathways of the Improved Structural Characteristics and Photocatalytic Performance of Titanium Dioxide (TiO2) Thin Films Fabricated by the Magnetron-Sputtering Technique. Catalysts 2020, 10, 598. [Google Scholar] [CrossRef]
- György, E.; Socol, G.; Axente, E.; Mihailescu, I.; Ducu, C.; Ciuca, S. Anatase phase TiO2 thin films obtained by pulsed laser deposition for gas sensing applications. Appl. Surf. Sci. 2005, 247, 429–433. [Google Scholar] [CrossRef]
- Solomon, A. Synthesis, Modification, Applications and Challenges of Titanium Dioxide Nanoparticles. Res. J. Nanosci. Eng. 2019, 3, 10–22. [Google Scholar] [CrossRef]
- Szczyglewska, P.; Feliczak-Guzik, A.; Nowak, I. Nanotechnology–General Aspects: A Chemical Reduction Approach to the Synthesis of Nanoparticles. Molecules 2023, 28, 4932. [Google Scholar] [CrossRef] [PubMed]
- Chandoliya, R.; Sharma, S.; Sharma, V.; Joshi, R.; Sivanesan, I. Titanium Dioxide Nanoparticle: A Comprehensive Review on Synthesis, Applications and Toxicity. Plants 2024, 13, 2964. [Google Scholar] [CrossRef]
- Uhm, H.S.; Hong, Y.C.; Shin, D.H. A microwave plasma torch and its applications. Plasma Sources Sci. Technol. 2006, 15, S26–S34. [Google Scholar] [CrossRef]
- Whittaker, A.G.; Mingos, D.M.P. Arcing and other microwave characteristics of metal powders in liquid systems. J. Chem. Soc. Dalton Trans. 2000, 9, 1521–1526. [Google Scholar] [CrossRef]
- Cheng, J.; Roy, R.; Agrawal, D. Experimental proof of major role of magnetic field losses in microwave heating of metal and metallic composites. J. Mater. Sci. Lett. 2001, 20, 1561–1563. [Google Scholar] [CrossRef]
- Tanabe, E.; McEuen, A.; Trail, M.; Meddaugh, G.; Bandy, S. Field emission in microwave cavity. Appl. Surf. Sci. 1994, 76–77, 16–20. [Google Scholar] [CrossRef]
- Mogildea, M.; Mogildea, G. Microwave field influence on the EMI 9656B photomultiplier tubes and NaI(Tl) crystal scintillator. Optoelectron. Adv. Mater.–Rapid Commun. 2018, 12, 713–718. [Google Scholar]
- Popescu, S.; Jerby, E.; Meir, Y.; Barkay, Z.; Ashkenazi, D.; Mitchell, J.B.A.; Le Garrec, J.-L.; Narayanan, T. Plasma column and nano-powder generation from solid titanium by localized microwaves in air. J. Appl. Phys. 2015, 118, 023302. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, W.; Wang, Y.; Sun, J.; Zhang, C.; Shahzad, Q.; Mao, Y.; Zhao, X.; Song, Z. Experimental study of destruction of acetone in exhaust gas using microwave-induced metal discharge. Sci. Total. Environ. 2018, 645, 788–795. [Google Scholar] [CrossRef]
- Mogildea, G.; Mogildea, M.; Popa, C.; Chiritoi, G. The Assessment of Carbon Dioxide Dissociation Using a Single-Mode Microwave Plasma Generator. Molecules 2020, 25, 1558. [Google Scholar] [CrossRef] [PubMed]
- Mogîldea, G.; Mogîldea, M.; I Zgura, S.; Craciun, D.; Popa, C.; Prepelita, P.; Bazavan, M.C.; Craciun, V. The assessment of the atmospheric air breakdown voltage generated by the interaction between microwaves and metallic wires. Phys. Scr. 2023, 98, 045508. [Google Scholar] [CrossRef]
- USB2000+ Data Sheet. Available online: https://ph208.edu.physics.uoc.gr/pdfs/OEM-Data-Sheet-USB2000-.pdf (accessed on 27 January 2025).
- National Institute of Standards and Technology (NIST). Atomic Spectra Database. Available online: https://physics.nist.gov/ (accessed on 27 January 2025).
- Bousquet, B.; Gardette, V.; Ros, V.M.; Gaudiuso, R.; Dell’Aglio, M.; De Giacomo, A. Plasma excitation temperature obtained with Boltzmann plot method: Significance, precision, trueness and accuracy. Spectrochim. Acta Part B At. Spectrosc. 2023, 204, 106686. [Google Scholar] [CrossRef]
- Mogildea, M.; Mogildea, G.; Craciun, V.; Zgura, S.I. The Effects Induced by Microwave Field upon Tungsten Wires of Different Diameters. Materials 2021, 14, 1036. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, R.; Sun, J.; Liu, C. Oxidation Mechanism of Biomedical Titanium Alloy Surface and Experiment. Int. J. Corros. 2020, 2020, 1036. [Google Scholar] [CrossRef]
- Barreca, F.; Acacia, N.; Barletta, E.; Spadaro, D.; Currò, G.; Neri, F. Titanium oxide nanoparticles prepared by laser ablation in water. Radiat. Eff. Defects Solids 2010, 165, 573–578. [Google Scholar] [CrossRef]
- Ayorinde, T.; Sayes, C.M. An updated review of industrially relevant titanium dioxide and its environmental health effects. J. Hazard. Mater. Lett. 2023, 4, 100085. [Google Scholar] [CrossRef]
- Cochran, D.L. A Comparison of Endosseous Dental Implant Surfaces. J. Periodontol. 1999, 70, 1523–1539. [Google Scholar] [CrossRef]
- Kmetykó, Á.; Mogyorósi, K.; Gerse, V.; Kónya, Z.; Pusztai, P.; Dombi, A.; Hernádi, K. Photocatalytic H2 Production Using Pt-TiO2 in the Presence of Oxalic Acid: Influence of the Noble Metal Size and the Carrier Gas Flow Rate. Materials 2014, 7, 7022–7038. [Google Scholar] [CrossRef] [PubMed]
- Khiabani, N.H.; Yaghmaee, M.S.; Sarani, A.; Shokri, B. Synthesis-Gas Production from CH4-CO2-Ar via Microwave Plasma Torch, Adv. Stud. Theor. Phys. 2012, 6, 1273–1287. Available online: https://www.m-hikari.com/astp/astp2012/astp25-28-2012/yaghmaeeASTP25-28-2012.pdf (accessed on 27 January 2025).
- Bianchi, C.; Datta, A.; Dughiero, F. Mechanistic understanding of plasma arcing in microwave food processing. Chem. Eng. Sci. 2018, 195, 141–158. [Google Scholar] [CrossRef]
- Souliez, F.; Chianese, S.; Dizac, G.; Micci, M. Low-power microwave arcjet testing—Plasma and plume diagnostics and performance evaluation. In Proceedings of the 35th Joint Propulsion Conference and Exhibit, Los Angeles, CA, USA, 20–24 June 1999. [Google Scholar] [CrossRef]


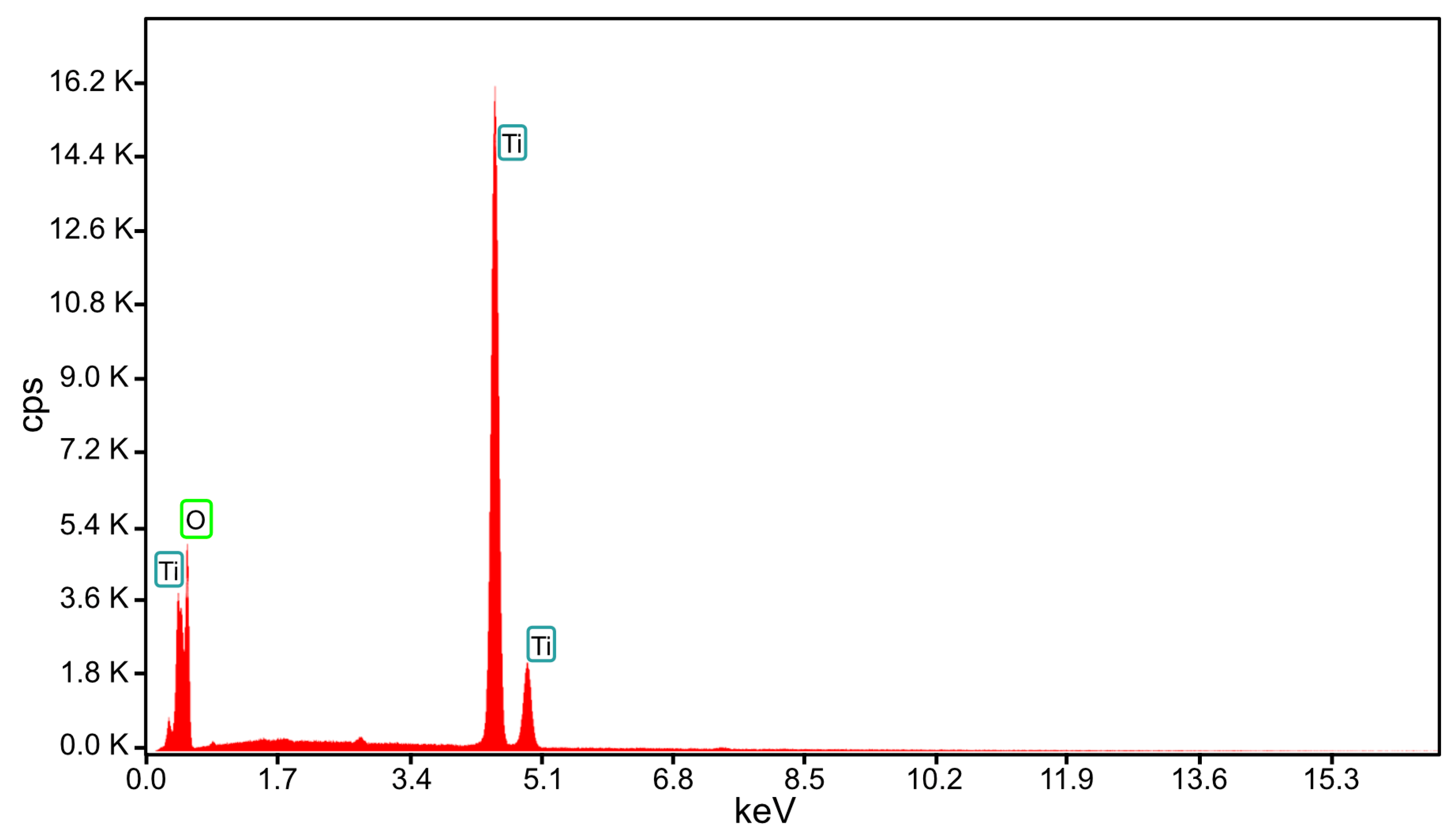

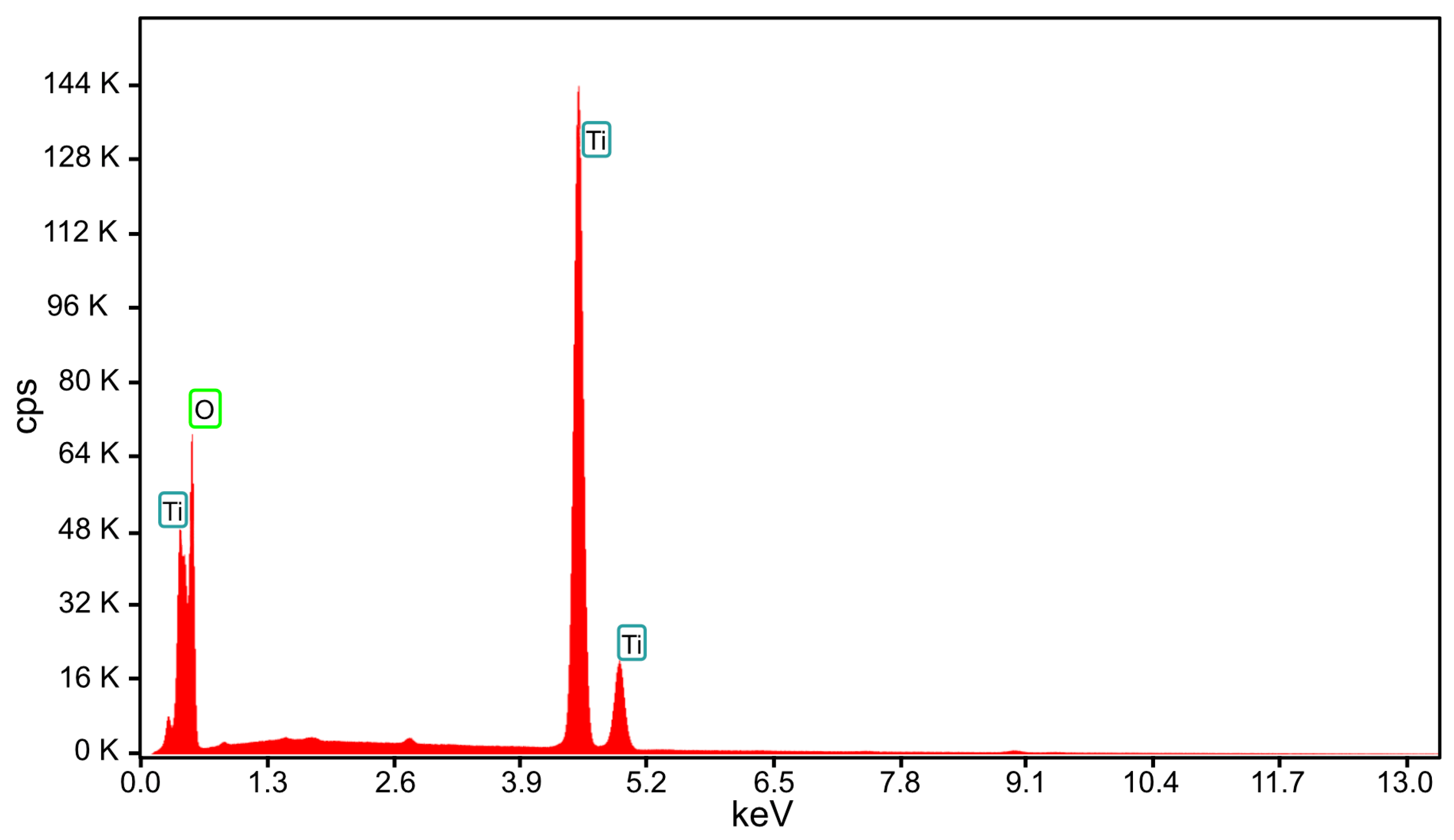


| Element | Weight % | Atomic % |
|---|---|---|
| O K | 36.95 | 63.7 |
| Ti K | 63.05 | 36.3 |
| Element | Weight % | Atomic % |
|---|---|---|
| O K | 46.14 | 71.95 |
| Ti K | 53.86 | 28.05 |
| Element | Weight % | Atomic % |
|---|---|---|
| O K | 4.22 | 11.65 |
| Ti K | 95.78 | 88.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogildea, M.; Mogildea, G.; Zgura, S.I.; Chiritoi, G.; Ionescu, C.; Craciun, V.; Prepelita, P.; Mihailescu, N.; Paraschiv, A.; Vasile, B.S.; et al. Synthesis of the Titanium Oxides Using a New Microwave Discharge Method. Int. J. Mol. Sci. 2025, 26, 2173. https://doi.org/10.3390/ijms26052173
Mogildea M, Mogildea G, Zgura SI, Chiritoi G, Ionescu C, Craciun V, Prepelita P, Mihailescu N, Paraschiv A, Vasile BS, et al. Synthesis of the Titanium Oxides Using a New Microwave Discharge Method. International Journal of Molecular Sciences. 2025; 26(5):2173. https://doi.org/10.3390/ijms26052173
Chicago/Turabian StyleMogildea, Marian, George Mogildea, Sorin I. Zgura, Gabriel Chiritoi, Cristian Ionescu, Valentin Craciun, Petronela Prepelita, Natalia Mihailescu, Alexandru Paraschiv, Bogdan Stefan Vasile, and et al. 2025. "Synthesis of the Titanium Oxides Using a New Microwave Discharge Method" International Journal of Molecular Sciences 26, no. 5: 2173. https://doi.org/10.3390/ijms26052173
APA StyleMogildea, M., Mogildea, G., Zgura, S. I., Chiritoi, G., Ionescu, C., Craciun, V., Prepelita, P., Mihailescu, N., Paraschiv, A., Vasile, B. S., & Constantinescu, C. D. (2025). Synthesis of the Titanium Oxides Using a New Microwave Discharge Method. International Journal of Molecular Sciences, 26(5), 2173. https://doi.org/10.3390/ijms26052173










