A Novel 6-bp Repeat Unit (6-bp RU) of the 13th Intron Within the Conserved EPAS1 Gene in Plateau Pika Is Capable of Altering Enhancer Activity
Abstract
1. Introduction
2. Results
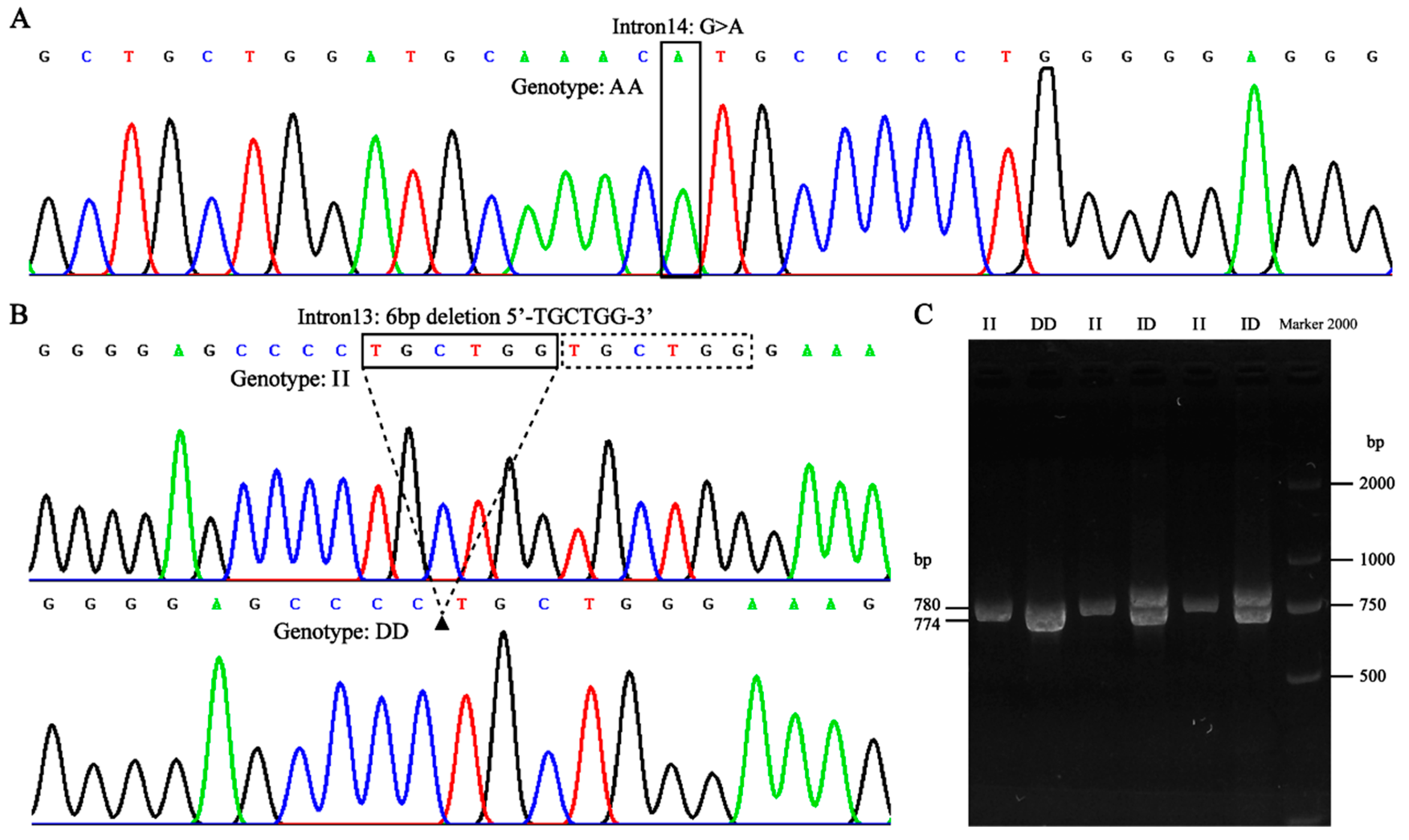
2.1. Detection and Genotyping of Mutations in the Pl-Pika EPAS1 Gene
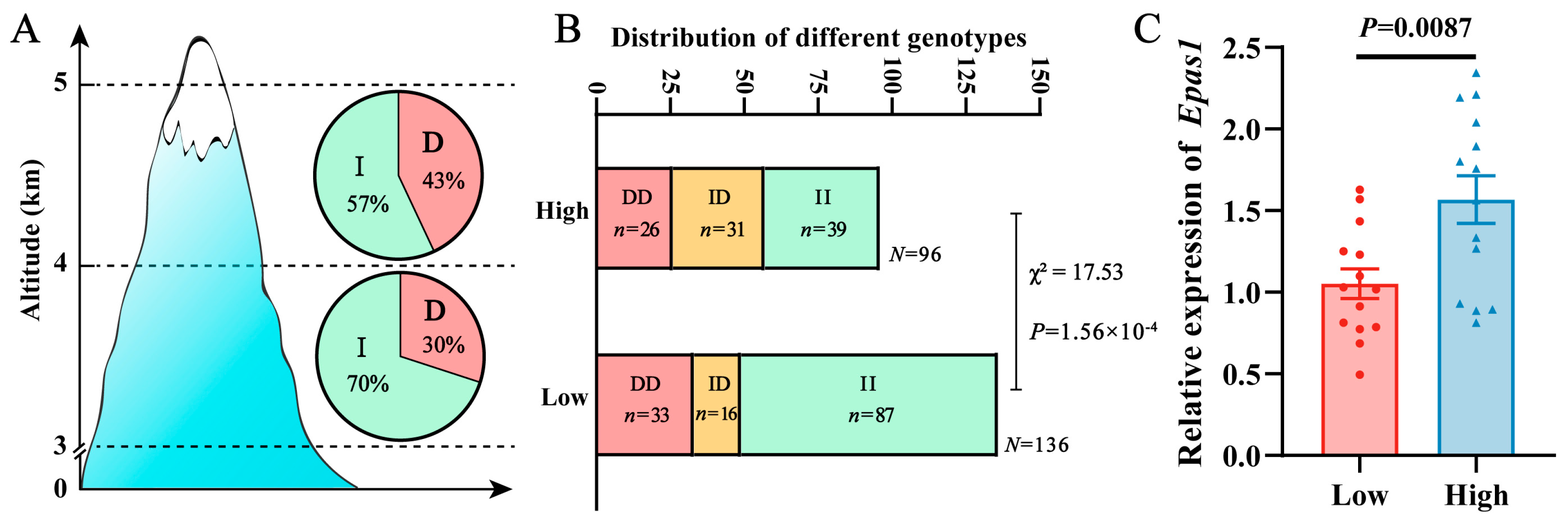
2.2. Distribution of 6-bp Indel in Pl-Pika EPAS1 Gene at Different Altitude Gradients
2.3. The Effect of the 6-bp Repeat Unit Within the EPAS1 Gene on Enhancer Activity
3. Discussion
4. Materials and Methods
4.1. Samples and Data Collection
4.2. DNA Extraction
4.3. Primer Designing, Polymerase Chain Reaction (PCR), and Sequencing
4.4. Transient Transfection and Luciferase Assay
4.5. Transcription Factor Prediction
4.6. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
4.7. Data Statistics and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, S.; Morilak, D.A. Chronic Intermittent Cold Stress Sensitises the Hypothalamic-Pituitary-Adrenal Response to a Novel Acute Stress by Enhancing Noradrenergic Influence in the Rat Paraventricular Nucleus. J. Neuroendocrinol. 2005, 17, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, R.; Huang, C.; Wang, Z.; Liu, X.; Hou, J.; Liu, J.; Cai, L.; Li, N.; Zhang, S.; et al. Cold Adaptive Thermogenesis in Small Mammals from Different Geographical Zones of China. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 129, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-M.; Zhang, Y.-M.; Wang, D.-H. Seasonal Thermogenesis and Body Mass Regulation in Plateau Pikas (Ochotona Curzoniae). Oecologia 2006, 149, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Wuren, T.; Liu, S.; Han, S.; Chen, L.; McClain, D.; Ge, R.-L. Intermittent Cold Exposure Results in Visceral Adipose Tissue “Browning” in the Plateau Pika (Ochotona Curzoniae). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 184, 171–178. [Google Scholar] [CrossRef]
- Jin, M.; Wang, H.; Liu, G.; Lu, J.; Yuan, Z.; Li, T.; Liu, E.; Lu, Z.; Du, L.; Wei, C. Whole-Genome Resequencing of Chinese Indigenous Sheep Provides Insight into the Genetic Basis Underlying Climate Adaptation. Genet. Sel. Evol. 2024, 56, 26. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Cui, C.; He, Y.; Ouzhuluobu; Zhang, H.; Yang, D.; Zhang, Q.; Bianbazhuoma; Yang, L.; He, Y.; et al. Down-Regulation of EPAS1 Transcription and Genetic Adaptation of Tibetans to High-Altitude Hypoxia. Mol. Biol. Evol. 2017, 34, 818–830. [Google Scholar] [CrossRef]
- Shridhar, P.; Glennon, M.S.; Pal, S.; Waldron, C.J.; Chetkof, E.J.; Basak, P.; Clavere, N.G.; Banerjee, D.; Gingras, S.; Becker, J.R. MDM2 Regulation of HIF Signaling Causes Microvascular Dysfunction in Hypertrophic Cardiomyopathy. Circulation 2023, 148, 1870–1886. [Google Scholar] [CrossRef]
- Tian, S.; Pirri, D.; Tardajos-Ayllon, B.; Fragiadaki, M.; Evans, P.C. BS2 Flow-Regulated EPAS1 Limits Atherosclerosis via Metabolic Control of Endothelial Proliferation. Basic Sci. 2023, 109, A247. [Google Scholar]
- Zhang, J.; Han, C.; Dai, H.; Hou, J.; Dong, Y.; Cui, X.; Xu, L.; Zhang, M.; Xia, Q. Hypoxia-Inducible Factor-2α Limits Natural Killer T Cell Cytotoxicity in Renal Ischemia/Reperfusion Injury. J. Am. Soc. Nephrol. 2016, 27, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Xi, Q.; Zhao, F.; Hu, J.; Wang, J.; Liu, X.; Dang, P.; Luo, Y.; Li, S. Expression and Variations in EPAS1 Associated with Oxygen Metabolism in Sheep. Genes 2022, 13, 1871. [Google Scholar] [CrossRef]
- Ivy, C.M.; Velotta, J.P.; Cheviron, Z.A.; Scott, G.R. Genetic Variation in HIF-2α Attenuates Ventilatory Sensitivity and Carotid Body Growth in Chronic Hypoxia in High-Altitude Deer Mice. J. Physiol. 2022, 600, 4207–4225. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Li, Y.; Pan, J.; Wang, D.; Chen, W.; Zheng, Z.; He, X.; Zhao, Q.; Pu, Y.; et al. EPAS1 Gain-of-Function Mutation Contributes to High-Altitude Adaptation in Tibetan Horses. Mol. Biol. Evol. 2019, 36, 2591–2603. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Tian, H.; Yu, Z.; Zhao, H.; Li, W.; Sang, D.; Lin, K.; Cui, Y.; Liao, M.; Xu, Z.; et al. A Highland-Adaptation Mutation of the Epas1 Protein Increases Its Stability and Disrupts the Circadian Clock in the Plateau Pika. Cell Rep. 2022, 39, 110816. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Liu, J.; Chen, B. Life-History Traits and Fitness of Plateau Pika (Ochotona curzoniae) in Alpine Meadow Ecosystem. Ecol. Evol. 2022, 12, e8548. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Réale, D.; Fletcher, Q.E.; Zhang, Y. Among-Population Divergence in Personality Is Linked to Altitude in Plateau Pikas (Ochotona curzoniae). Front. Zool. 2019, 16, 26. [Google Scholar] [CrossRef]
- Zhu, H.; Zhong, L.; Li, J.; Wang, S.; Qu, J. Differential Expression of Metabolism-Related Genes in Plateau Pika (Ochotona curzoniae) at Different Altitudes on the Qinghai-Tibet Plateau. Front. Genet. 2021, 12, 784811. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Fu, L.; Zou, X.-Y.; Li, S.; Ma, X.-D.; Xie, L.; Pang, B.; Ma, J.-B.; Wang, Y.-J.; Du, Y.-R.; et al. Lung Transcriptome Analysis for the Identification of Genes Involved in the Hypoxic Adaptation of Plateau Pika (Ochotona curzoniae). Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 41, 100943. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, X.; Xiao, J.; Liu, J.; Fan, X.; Fan, F.; Lei, H. Genetic Changes in the EPAS1 Gene between Tibetan and Han Ethnic Groups and Adaptation to the Plateau Hypoxic Environment. PeerJ 2019, 7, e7943. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Wang, H.; Liu, G.; Zhao, F.; Kijas, J.W.; Ma, Y.; Lu, J.; Zhang, L.; Cao, J.; Wu, M.; et al. Genome-Wide Analysis Reveals Adaptation to High Altitudes in Tibetan Sheep. Sci. Rep. 2016, 6, 26770. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Zhang, H.; He, Y.; Duren, Z.; Bai, C.; Chen, L.; Luo, X.; Yan, D.-S.; Zhang, C.; Zhu, X.; et al. Chromatin Accessibility Landscape and Regulatory Network of High-Altitude Hypoxia Adaptation. Nat. Commun. 2020, 11, 4928. [Google Scholar] [CrossRef]
- An, X.; Mao, L.; Wang, Y.; Xu, Q.; Liu, X.; Zhang, S.; Qiao, Z.; Li, B.; Li, F.; Kuang, Z.; et al. Genomic Structural Variation Is Associated with Hypoxia Adaptation in High-Altitude Zokors. Nat. Ecol. Evol. 2024, 8, 339–351. [Google Scholar] [CrossRef]
- Spitz, F.; Furlong, E.E.M. Transcription Factors: From Enhancer Binding to Developmental Control. Nat. Rev. Genet. 2012, 13, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Jeyarajah, M.J.; Jaju Bhattad, G.; Kelly, R.D.; Baines, K.J.; Jaremek, A.; Yang, F.-H.P.; Okae, H.; Arima, T.; Dumeaux, V.; Renaud, S.J. The Multifaceted Role of GCM1 during Trophoblast Differentiation in the Human Placenta. Proc. Natl. Acad. Sci. USA 2022, 119, e2203071119. [Google Scholar] [CrossRef]
- Lu, X.; He, Y.; Zhu, C.; Wang, H.; Chen, S.; Lin, H.-Y. Twist1 Is Involved in Trophoblast Syncytialization by Regulating GCM1. Placenta 2016, 39, 45–54. [Google Scholar] [CrossRef]
- Takeda, K.; Ho, V.C.; Takeda, H.; Duan, L.-J.; Nagy, A.; Fong, G.-H. Placental but Not Heart Defects Are Associated with Elevated Hypoxia-Inducible Factor Alpha Levels in Mice Lacking Prolyl Hydroxylase Domain Protein 2. Mol. Cell Biol. 2006, 26, 8336–8346. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-H.; Yang, M.-R.; Wang, L.-J.; Chen, M.-H.; Chang, G.-D.; Chen, H. New Insights into the Regulation of Placental Growth Factor Gene Expression by the Transcription Factors GCM1 and DLX3 in Human Placenta. J. Biol. Chem. 2018, 293, 9801–9811. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Chen, C.-P.; Lee, Y.-S.; Ng, P.-S.; Chang, G.-D.; Pao, Y.-H.; Lo, H.-F.; Peng, C.-H.; Cheong, M.-L.; Chen, H. Functional Antagonism between ΔNp63α and GCM1 Regulates Human Trophoblast Stemness and Differentiation. Nat. Commun. 2022, 13, 1626. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Zhang, Y.; Yue, L.; Ren, H.; Pan, C. Ssc-miR-429 Expression Proliles and Functions on Inducing Leydig Cells Apoptosis. Theriogenology 2024, 216, 62–68. [Google Scholar] [CrossRef]

| Sampling Time | Place | Longitude | Latitude | Elevation | Sample Size |
|---|---|---|---|---|---|
| August 2019 and November 2019 | Kunlun Mountain Pass | 100°18′ E | 38°33′ N | 4700 m | 23 |
| Maduo County | 98°23′ E | 34°88′ N | 4400 m | 13 | |
| Guozhuoyi Mountain Pass | 98°60′ E | 35°46′ N | 4300 m | 19 | |
| Gouli Township | 98°13′ E | 35°75′ N | 3900 m | 22 | |
| Reshui Township | 98°46′ E | 36°01′ N | 3600 m | 21 | |
| Haibei Autonomous Prefecture | 100°90′ E | 36°95′ N | 3300 m | 17 | |
| Haibei Autonomous Prefecture | 100°90′ E | 36°95′ N | 3100 m | 23 | |
| August 2021 | Kunlun Mountain Pass | 100°18′ E | 38°33′ N | 4800 m | 21 |
| Maduo County | 98°23′ E | 34°88′ N | 4200 m | 19 | |
| Guide County | 101°41′ E | 36°36′ N | 3700 m | 19 | |
| Maqin County | 100°27′ E | 34°45′ N | 3700 m | 17 | |
| Guinan County | 100°66′ E | 35°50′ N | 3300 m | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Q.; Xu, Y.; Song, Q.; Cao, S.; Li, Y.; Lan, X.; Zhang, L.; Pan, C. A Novel 6-bp Repeat Unit (6-bp RU) of the 13th Intron Within the Conserved EPAS1 Gene in Plateau Pika Is Capable of Altering Enhancer Activity. Int. J. Mol. Sci. 2025, 26, 2163. https://doi.org/10.3390/ijms26052163
Tang Q, Xu Y, Song Q, Cao S, Li Y, Lan X, Zhang L, Pan C. A Novel 6-bp Repeat Unit (6-bp RU) of the 13th Intron Within the Conserved EPAS1 Gene in Plateau Pika Is Capable of Altering Enhancer Activity. International Journal of Molecular Sciences. 2025; 26(5):2163. https://doi.org/10.3390/ijms26052163
Chicago/Turabian StyleTang, Qi, Yuhui Xu, Qingchuan Song, Siqi Cao, Yang Li, Xianyong Lan, Liangzhi Zhang, and Chuanying Pan. 2025. "A Novel 6-bp Repeat Unit (6-bp RU) of the 13th Intron Within the Conserved EPAS1 Gene in Plateau Pika Is Capable of Altering Enhancer Activity" International Journal of Molecular Sciences 26, no. 5: 2163. https://doi.org/10.3390/ijms26052163
APA StyleTang, Q., Xu, Y., Song, Q., Cao, S., Li, Y., Lan, X., Zhang, L., & Pan, C. (2025). A Novel 6-bp Repeat Unit (6-bp RU) of the 13th Intron Within the Conserved EPAS1 Gene in Plateau Pika Is Capable of Altering Enhancer Activity. International Journal of Molecular Sciences, 26(5), 2163. https://doi.org/10.3390/ijms26052163






