Impact of Saharan Dust and SERPINA1 Gene Variants on Bacterial/Fungal Balance in Asthma Patients
Abstract
1. Introduction
2. Results
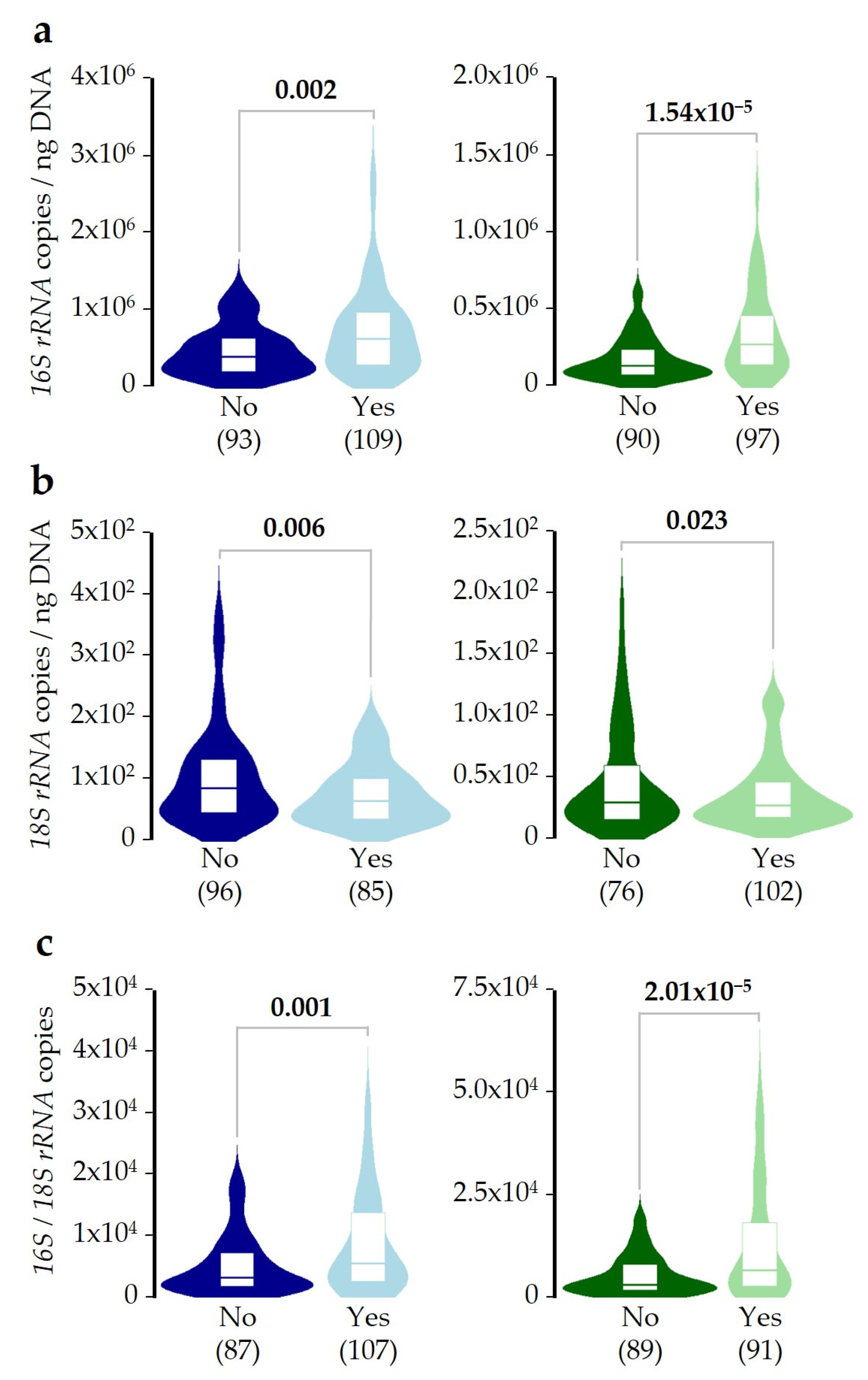
2.1. Microbial DNA Concentrations in the Study Population
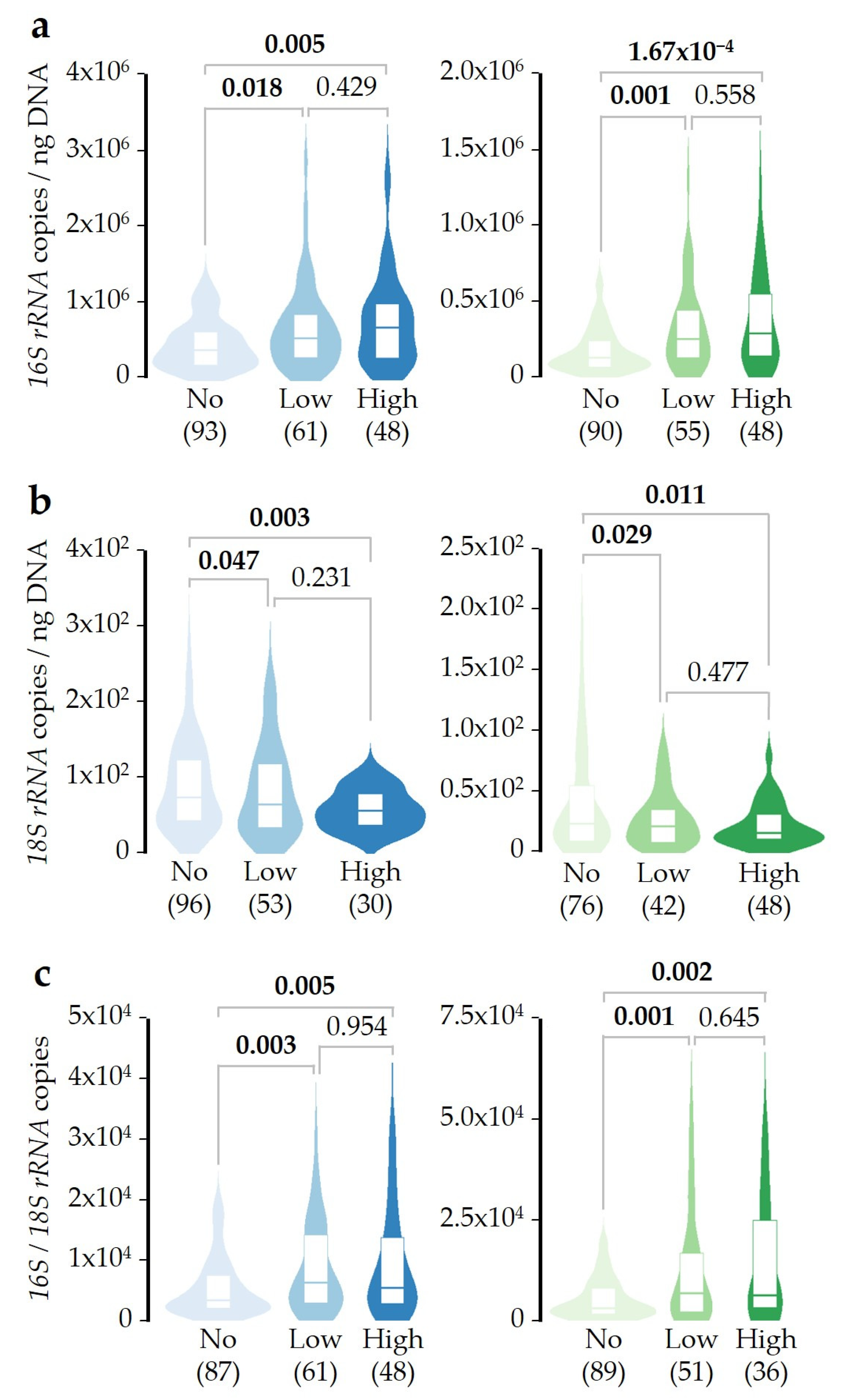
2.2. Bacterial/Fungal DNA Ratio Is Associated with SDI Exposure
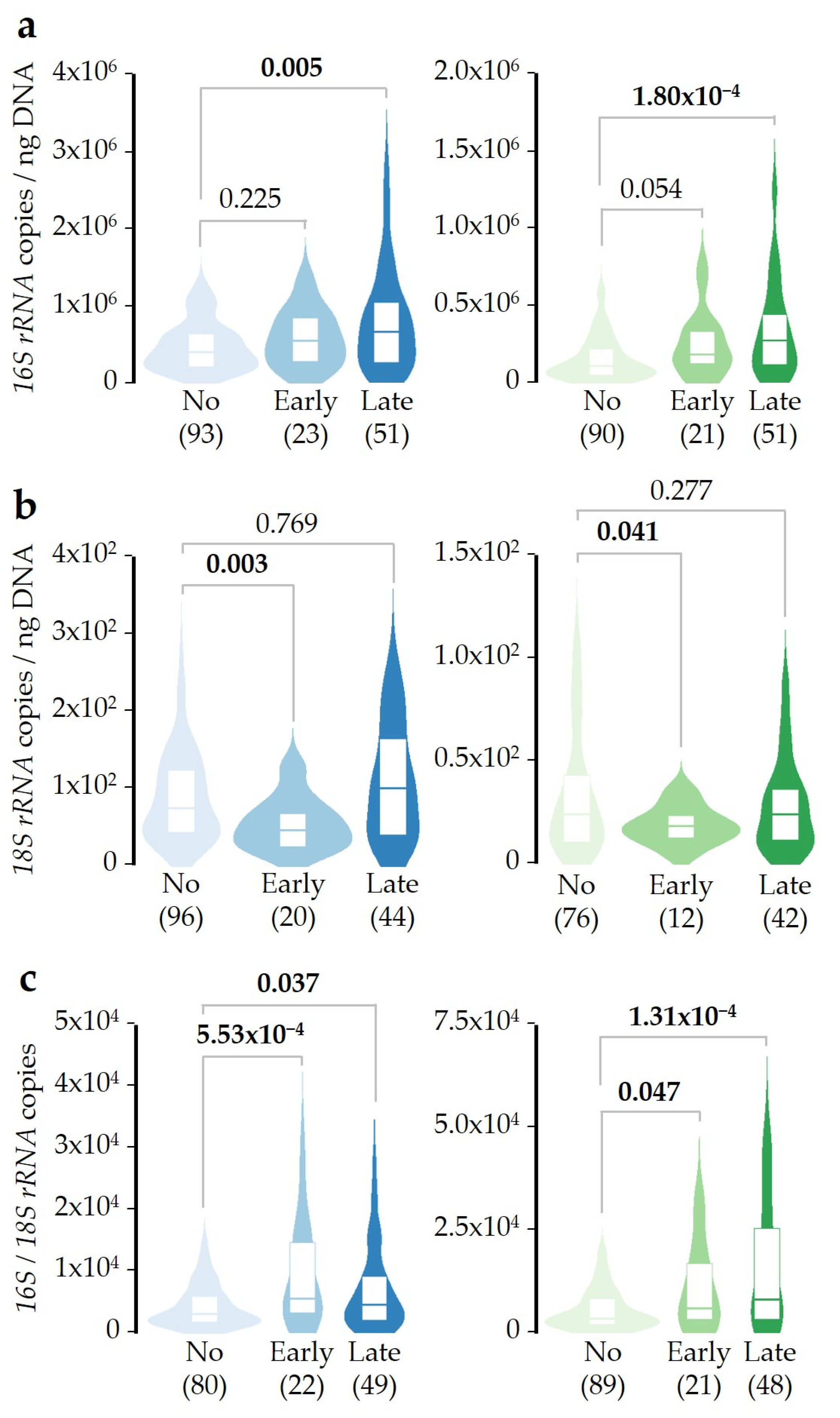
2.3. SDI Exposure Time-Course Effect over Microbial DNA Concentrations
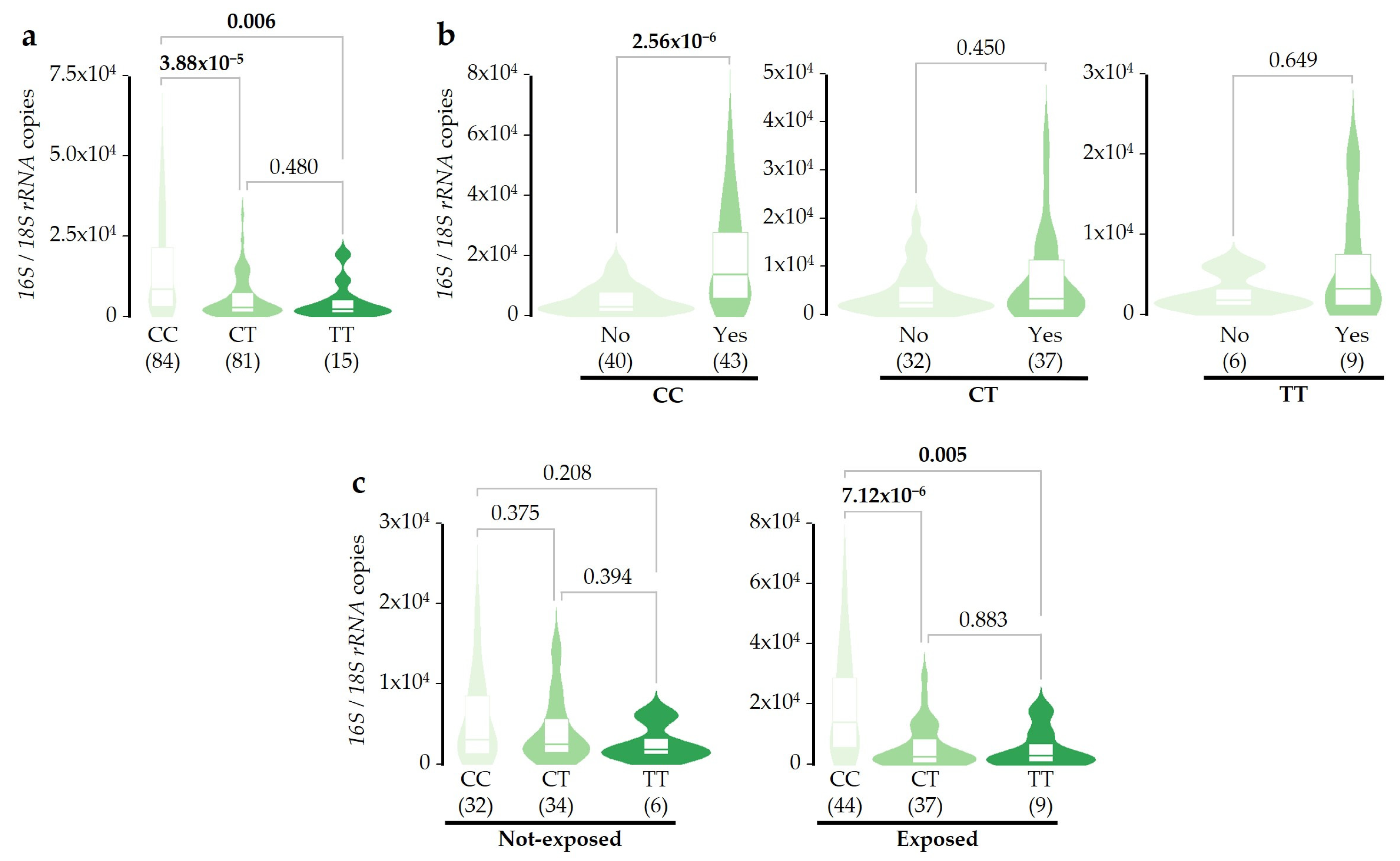
2.4. Associations of SERPINA1 Gene Variants with Microbial DNA Concentrations
3. Discussion
4. Materials and Methods
4.1. Patients Included in This Study
4.2. Definition of Saharan Dust Intrusions
4.3. Bacterial and Fungal DNA Quantification
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wild, C.P. The Exposome: From Concept to Utility. Int. J. Epidemiol. 2012, 41, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulou, V.E.; Taskou, C.; Diamanti, A.; Beka, D.; Papalexis, P.; Trakas, N.; Spandidos, D.A. Saharan Dust and Respiratory Health: Understanding the Link between Airborne Particulate Matter and Chronic Lung Diseases (Review). Exp. Ther. Med. 2024, 28, 460. [Google Scholar] [CrossRef]
- López-Villarrubia, E.; Iñiguez, C.; Costa, O.; Ballester, F. Acute Effects of Urban Air Pollution on Respiratory Emergency Hospital Admissions in the Canary Islands. Air Qual. Atmos. Health 2016, 9, 713–722. [Google Scholar] [CrossRef]
- Dominguez-Rodriguez, A.; Baez-Ferrer, N.; Rodríguez, S.; Avanzas, P.; Abreu-Gonzalez, P.; Terradellas, E.; Cuevas, E.; Basart, S.; Werner, E. Saharan Dust Events in the Dust Belt -Canary Islands- and the Observed Association with in-Hospital Mortality of Patients with Heart Failure. J. Clin. Med. 2020, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- von Suchodoletz, H.; Glaser, B.; Thrippleton, T.; Broder, T.; Zang, U.; Eigenmann, R.; Kopp, B.; Reichert, M.; Ludwig, Z. The Influence of Saharan Dust Deposits on La Palma Soil Properties (Canary Islands, Spain). CATENA 2013, 103, 44–52. [Google Scholar] [CrossRef]
- Schweitzer, M.D.; Calzadilla, A.S.; Salamo, O.; Sharifi, A.; Kumar, N.; Holt, G.; Campos, M.; Mirsaeidi, M. Lung Health in Era of Climate Change and Dust Storms. Environ. Res. 2018, 163, 36–42. [Google Scholar] [CrossRef]
- Kandler, K.; Benker, N.; Bundke, U.; Cuevas, E.; Ebert, M.; Knippertz, P.; Rodríguez, S.; Schütz, L.; Weinbruch, S. Chemical Composition and Complex Refractive Index of Saharan Mineral Dust at Izaña, Tenerife (Spain) Derived by Electron Microscopy. Atmos. Environ. 2007, 41, 8058–8074. [Google Scholar] [CrossRef]
- Hu, P.; Sharaby, Y.; Gu, J.-D.; Radian, A.; Lang-Yona, N. Environmental Processes and Health Implications Potentially Mediated by Dust-Borne Bacteria. Environ. Microbiol. Rep. 2024, 16, e13222. [Google Scholar] [CrossRef]
- Navarro, A.; del Moral, A.; Weber, B.; Weber, J.; Molinero, A.; Delgado, R.; Párraga, J.; Martínez-Checa, F. Microbial Composition of Saharan Dust Plumes Deposited as Red Rain in Granada (Southern Spain). Sci. Total Environ. 2024, 913, 169745. [Google Scholar] [CrossRef] [PubMed]
- Lwin, K.S.; Tobias, A.; Chua, P.L.; Yuan, L.; Thawonmas, R.; Ith, S.; Htay, Z.W.; Yu, L.S.; Yamasaki, L.; Roqué, M.; et al. Effects of Desert Dust and Sandstorms on Human Health: A Scoping Review. GeoHealth 2023, 7, e2022GH000728. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.W. Atmospheric Movement of Microorganisms in Clouds of Desert Dust and Implications for Human Health. Clin. Microbiol. Rev. 2007, 20, 459–477. [Google Scholar] [CrossRef]
- Vergadi, E.; Rouva, G.; Angeli, M.; Galanakis, E. Infectious Diseases Associated with Desert Dust Outbreaks: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 6907. [Google Scholar] [CrossRef] [PubMed]
- Oduber, F.; Calvo, A.I.; Blanco-Alegre, C.; Castro, A.; Nunes, T.; Alves, C.; Sorribas, M.; Fernández-González, D.; Vega-Maray, A.M.; Valencia-Barrera, R.M.; et al. Unusual Winter Saharan Dust Intrusions at Northwest Spain: Air Quality, Radiative and Health Impacts. Sci. Total Environ. 2019, 669, 213–228. [Google Scholar] [CrossRef]
- Rodríguez González, S.; López-Darias, J. Emerging Extreme Saharan-Dust Events Expand Northward over the Atlantic and Europe Prompting Record-Breaking PM10 and PM2.5 Episodes 2024. Atmos. Chem. Phys. 2024, 24, 12031–12053. [Google Scholar] [CrossRef]
- Valderrama, A.; Ortiz-Hernández, P.; Agraz-Cibrián, J.M.; Tabares-Guevara, J.H.; Gómez, D.M.; Zambrano-Zaragoza, J.F.; Taborda, N.A.; Hernandez, J.C. Particulate Matter (PM10) Induces in Vitro Activation of Human Neutrophils, and Lung Histopathological Alterations in a Mouse Model. Sci. Rep. 2022, 12, 7581. [Google Scholar] [CrossRef] [PubMed]
- Misiukiewicz-Stepien, P.; Paplinska-Goryca, M. Biological Effect of PM10 on Airway Epithelium-Focus on Obstructive Lung Diseases. Clin. Immunol. 2021, 227, 108754. [Google Scholar] [CrossRef]
- Choi, J.; Sim, J.K.; Oh, J.Y.; Lee, Y.S.; Hur, G.Y.; Lee, S.Y.; Shim, J.J.; Moon, J.; Min, K.H. Relationship between Particulate Matter (PM10) and Airway Inflammation Measured with Exhaled Nitric Oxide Test in Seoul, Korea. Can. Respir. J. 2020, 2020, 1823405. [Google Scholar] [CrossRef]
- Glieca, S.; Quarta, E.; Bottari, B.; Lal, V.C.; Sonvico, F.; Buttini, F. The Role of Airways Microbiota on Local and Systemic Diseases: A Rationale for Probiotics Delivery to the Respiratory Tract. Expert Opin. Drug Deliv. 2024, 21, 991–1005. [Google Scholar] [CrossRef]
- Tian, C.; Li, X.; Yuan, X.; Li, Z. Link between Respiratory Microbiota and Asthma: An Emerging Therapeutic Approach. Am. J. Transl. Res. 2024, 16, 6289–6302. [Google Scholar] [CrossRef] [PubMed]
- 2024 GINA Main Report. Available online: https://ginasthma.org/2024-report/ (accessed on 18 September 2024).
- Sánchez-Lerma, B.; Morales-Chirivella, F.J.; Peñuelas, I.; Blanco Guerra, C.; Mesa Lugo, F.; Aguinaga-Ontoso, I.; Guillén-Grima, F. High Prevalence of Asthma and Allergic Diseases in Children Aged 6 to [Corrected] 7 Years from the Canary Islands. [Corrected]. J. Investig. Allergol. Clin. Immunol. 2009, 19, 383–390. [Google Scholar]
- Juliá-Serdá, G.; Cabrera-Navarro, P.; Acosta-Fernández, O.; Martín-Pérez, P.; Losada-Cabrera, P.; García-Bello, M.A.; Carrillo-Díaz, T.; Antó-Boqué, J. High Prevalence of Asthma and Atopy in the Canary Islands, Spain. Int. J. Tuberc. Lung Dis. 2011, 15, 536–541. [Google Scholar] [CrossRef]
- Melén, E.; Zar, H.J.; Siroux, V.; Shaw, D.; Saglani, S.; Koppelman, G.H.; Hartert, T.; Gern, J.E.; Gaston, B.; Bush, A.; et al. Asthma Inception: Epidemiologic Risk Factors and Natural History Across the Life Course. Am. J. Respir. Crit. Care Med. 2024, 210, 737–754. [Google Scholar] [CrossRef]
- Martín-González, E.; Hernández-Pérez, J.M.; Pérez, J.A.P.; Pérez-García, J.; Herrera-Luis, E.; González-Pérez, R.; González-González, O.; Mederos-Luis, E.; Sánchez-Machín, I.; Poza-Guedes, P.; et al. Alpha-1 Antitrypsin Deficiency and Pi*S and Pi*Z SERPINA1 Variants Are Associated with Asthma Exacerbations. Pulmonology 2023, 31, 2416870. [Google Scholar] [CrossRef]
- Ortega, V.E.; Tejwani, V.; Shrivastav, A.K.; Pasha, S.; Zein, J.G.; Boorgula, M.; Castro, M.; Denlinger, L.; Erzurum, S.C.; Fahy, J.V.; et al. A1-Antitrypsin Gene Variation Associates with Asthma Exacerbations and Related Health Care Utilization. J. Allergy Clin. Immunol. Pract. 2025. [Google Scholar] [CrossRef] [PubMed]
- Dunlea, D.M.; Fee, L.T.; McEnery, T.; McElvaney, N.G.; Reeves, E.P. The Impact of Alpha-1 Antitrypsin Augmentation Therapy on Neutrophil-Driven Respiratory Disease in Deficient Individuals. J. Inflamm. Res. 2018, 11, 123–134. [Google Scholar] [CrossRef]
- Mazzuca, C.; Vitiello, L.; Travaglini, S.; Maurizi, F.; Finamore, P.; Santangelo, S.; Rigon, A.; Vadacca, M.; Angeletti, S.; Scarlata, S. Immunological and Homeostatic Pathways of Alpha -1 Antitrypsin: A New Therapeutic Potential. Front. Immunol. 2024, 15, 1443297. [Google Scholar] [CrossRef]
- Mornex, J.-F.; Traclet, J.; Guillaud, O.; Dechomet, M.; Lombard, C.; Ruiz, M.; Revel, D.; Reix, P.; Cottin, V. Alpha1-Antitrypsin Deficiency: An Updated Review. La Presse Médicale 2023, 52, 104170. [Google Scholar] [CrossRef]
- Fromme, M.; Payancé, A.; Mandorfer, M.; Thorhauge, K.H.; Pons, M.; Miravitlles, M.; Stolk, J.; van Hoek, B.; Stirnimann, G.; Frankova, S.; et al. Longitudinal Evaluation of Individuals With Severe Alpha-1 Antitrypsin Deficiency (Pi∗ZZ Genotype). Gastroenterology 2024, 68, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Seixas, S.; Marques, P.I. Known Mutations at the Cause of Alpha-1 Antitrypsin Deficiency an Updated Overview of SERPINA1 Variation Spectrum. Appl. Clin. Genet. 2021, 14, 173–194. [Google Scholar] [CrossRef]
- Strange, C. Airway Disease in Alpha-1 Antitrypsin Deficiency. COPD J. Chronic Obstr. Pulm. Dis. 2013, 10, 68–73. [Google Scholar] [CrossRef]
- Jaeger, M.; Dietschmann, A.; Austermeier, S.; Dinçer, S.; Porschitz, P.; Vornholz, L.; Maas, R.J.A.; Sprenkeler, E.G.G.; Ruland, J.; Wirtz, S.; et al. Alpha1-Antitrypsin Impacts Innate Host–Pathogen Interactions with Candida Albicans by Stimulating Fungal Filamentation. Virulence 2024, 15, 2333367. [Google Scholar] [CrossRef] [PubMed]
- Cickovski, T.; Manuel, A.; Mathee, K.; Campos, M.; Narasimhan, G. Effects of Various Alpha-1 Antitrypsin Supplement Dosages on the Lung Microbiome and Metabolome. In Proceedings of the Computational Advances in Bio and Medical Sciences, Virtual, 10–12 December 2020; Măndoiu, I., Murali, T.M., Narasimhan, G., Rajasekaran, S., Skums, P., Zelikovsky, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 90–101. [Google Scholar]
- Kaner, Z.; Ochayon, D.E.; Shahaf, G.; Baranovski, B.M.; Bahar, N.; Mizrahi, M.; Lewis, E.C. Acute Phase Protein A1-Antitrypsin Reduces the Bacterial Burden in Mice by Selective Modulation of Innate Cell Responses. J. Infect. Dis. 2015, 211, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Merico, E.; Cesari, D.; Dinoi, A.; Grasso, F.M.; Donateo, A.; Guascito, M.R.; Contini, D. Long-Term Characterisation of African Dust Advection in South-Eastern Italy: Influence on Fine and Coarse Particle Concentrations, Size Distributions, and Carbon Content. Atmos. Res. 2020, 233, 104690. [Google Scholar] [CrossRef]
- Federici, E.; Petroselli, C.; Montalbani, E.; Casagrande, C.; Ceci, E.; Moroni, B.; La Porta, G.; Castellini, S.; Selvaggi, R.; Sebastiani, B.; et al. Airborne Bacteria and Persistent Organic Pollutants Associated with an Intense Saharan Dust Event in the Central Mediterranean. Sci. Total Environ. 2018, 645, 401–410. [Google Scholar] [CrossRef]
- Luo, Y.; Yao, Q.; Ding, P.; Hou, M.; Deng, F.; Wang, Y.; Ding, C.; Li, X.; Wang, D.; Sun, Z.; et al. Health Impacts of an Extreme Dust Event: A Case and Risk Assessment Study on Airborne Bacteria in Beijing, China. Environ. Sci. Eur. 2024, 36, 41. [Google Scholar] [CrossRef]
- Marotz, C.; Morton, J.T.; Navarro, P.; Coker, J.; Belda-Ferre, P.; Knight, R.; Zengler, K. Quantifying Live Microbial Load in Human Saliva Samples over Time Reveals Stable Composition and Dynamic Load. mSystems 2021, 6. [Google Scholar] [CrossRef]
- Props, R.; Kerckhof, F.-M.; Rubbens, P.; De Vrieze, J.; Hernandez Sanabria, E.; Waegeman, W.; Monsieurs, P.; Hammes, F.; Boon, N. Absolute Quantification of Microbial Taxon Abundances. ISME J. 2017, 11, 584–587. [Google Scholar] [CrossRef]
- Perez-Garcia, J.; González-Carracedo, M.; Espuela-Ortiz, A.; Hernández-Pérez, J.M.; González-Pérez, R.; Sardón-Prado, O.; Martin-Gonzalez, E.; Mederos-Luis, E.; Poza-Guedes, P.; Corcuera-Elosegui, P.; et al. The Upper-Airway Microbiome as a Biomarker of Asthma Exacerbations despite Inhaled Corticosteroid Treatment. J. Allergy Clin. Immunol. 2023, 151, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yi, J.; Xiang, J.; Jia, W.; Chen, A.; Chen, L.; Zheng, L.; Zhou, W.; Wu, M.; Yu, Z.; et al. Exploration of Lung Mycobiome in the Patients with Non-Small-Cell Lung Cancer. BMC Microbiol. 2023, 23, 81. [Google Scholar] [CrossRef] [PubMed]
- Lofgren, L.A.; Uehling, J.K.; Branco, S.; Bruns, T.D.; Martin, F.; Kennedy, P.G. Genome-Based Estimates of Fungal rDNA Copy Number Variation across Phylogenetic Scales and Ecological Lifestyles. Mol. Ecol. 2019, 28, 721–730. [Google Scholar] [CrossRef]
- Belvoncikova, P.; Splichalova, P.; Videnska, P.; Gardlik, R. The Human Mycobiome: Colonization, Composition and the Role in Health and Disease. J. Fungi 2022, 8, 1046. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, K.; Zhang, B.; Tu, Q.; Yao, Y.; Cui, B.; Ren, B.; He, J.; Shen, X.; Van Nostrand, J.D.; et al. Salivary Mycobiome Dysbiosis and Its Potential Impact on Bacteriome Shifts and Host Immunity in Oral Lichen Planus. Int. J. Oral Sci. 2019, 11, 13. [Google Scholar] [CrossRef]
- Perez-Garcia, J.; Hernández-Pérez, J.M.; González-Pérez, R.; Sardón, O.; Martin-Gonzalez, E.; Espuela-Ortiz, A.; Mederos-Luis, E.; Callero, A.; Herrera-Luis, E.; Corcuera, P.; et al. The Genomics and Metagenomics of Asthma Severity (GEMAS) Study: Rationale and Design. J. Pers. Med. 2020, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Red de Control y Vigilancia de La Calidad Del Aire de Canarias. Available online: https://www3.gobiernodecanarias.org/medioambiente/calidaddelaire/inicio.do (accessed on 26 August 2024).
- Directive-2008/50-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/dir/2008/50/oj (accessed on 26 August 2024).
- Histórico de Informes de Episodios Naturales. Available online: https://www.miteco.gob.es/es/calidad-y-evaluacion-ambiental/temas/atmosfera-y-calidad-del-aire/calidad-del-aire/evaluacion-datos/fuentes-naturales/anuales.html (accessed on 26 August 2024).
- Liu, C.M.; Aziz, M.; Kachur, S.; Hsueh, P.-R.; Huang, Y.-T.; Keim, P.; Price, L.B. BactQuant: An Enhanced Broad-Coverage Bacterial Quantitative Real-Time PCR Assay. BMC Microbiol. 2012, 12, 56. [Google Scholar] [CrossRef]
- Liu, C.M.; Kachur, S.; Dwan, M.G.; Abraham, A.G.; Aziz, M.; Hsueh, P.-R.; Huang, Y.-T.; Busch, J.D.; Lamit, L.J.; Gehring, C.A.; et al. FungiQuant: A Broad-Coverage Fungal Quantitative Real-Time PCR Assay. BMC Microbiol. 2012, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Peirson, S.N.; Butler, J.N.; Foster, R.G. Experimental Validation of Novel and Conventional Approaches to Quantitative Real-time PCR Data Analysis. Nucleic Acids Res. 2003, 31, e73. [Google Scholar] [CrossRef]
- Citing RStudio. Available online: https://support.posit.co/hc/en-us/articles/206212048-Citing-RStudio (accessed on 26 August 2024).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escuela-Escobar, A.; Perez-Garcia, J.; Martín-González, E.; González Martín, C.; Hernández-Pérez, J.M.; González Pérez, R.; Sánchez Machín, I.; Poza Guedes, P.; Mederos-Luis, E.; Pino-Yanes, M.; et al. Impact of Saharan Dust and SERPINA1 Gene Variants on Bacterial/Fungal Balance in Asthma Patients. Int. J. Mol. Sci. 2025, 26, 2158. https://doi.org/10.3390/ijms26052158
Escuela-Escobar A, Perez-Garcia J, Martín-González E, González Martín C, Hernández-Pérez JM, González Pérez R, Sánchez Machín I, Poza Guedes P, Mederos-Luis E, Pino-Yanes M, et al. Impact of Saharan Dust and SERPINA1 Gene Variants on Bacterial/Fungal Balance in Asthma Patients. International Journal of Molecular Sciences. 2025; 26(5):2158. https://doi.org/10.3390/ijms26052158
Chicago/Turabian StyleEscuela-Escobar, Ainhoa, Javier Perez-Garcia, Elena Martín-González, Cristina González Martín, José M. Hernández-Pérez, Ruperto González Pérez, Inmaculada Sánchez Machín, Paloma Poza Guedes, Elena Mederos-Luis, María Pino-Yanes, and et al. 2025. "Impact of Saharan Dust and SERPINA1 Gene Variants on Bacterial/Fungal Balance in Asthma Patients" International Journal of Molecular Sciences 26, no. 5: 2158. https://doi.org/10.3390/ijms26052158
APA StyleEscuela-Escobar, A., Perez-Garcia, J., Martín-González, E., González Martín, C., Hernández-Pérez, J. M., González Pérez, R., Sánchez Machín, I., Poza Guedes, P., Mederos-Luis, E., Pino-Yanes, M., Lorenzo-Díaz, F., González Carracedo, M. A., & Pérez Pérez, J. A. (2025). Impact of Saharan Dust and SERPINA1 Gene Variants on Bacterial/Fungal Balance in Asthma Patients. International Journal of Molecular Sciences, 26(5), 2158. https://doi.org/10.3390/ijms26052158







