ZmCaM2-1, a Calmodulin Gene, Negatively Regulates Drought Tolerance in Transgenic Arabidopsis Through the ABA-Independent Pathway
Abstract
1. Introduction
2. Results
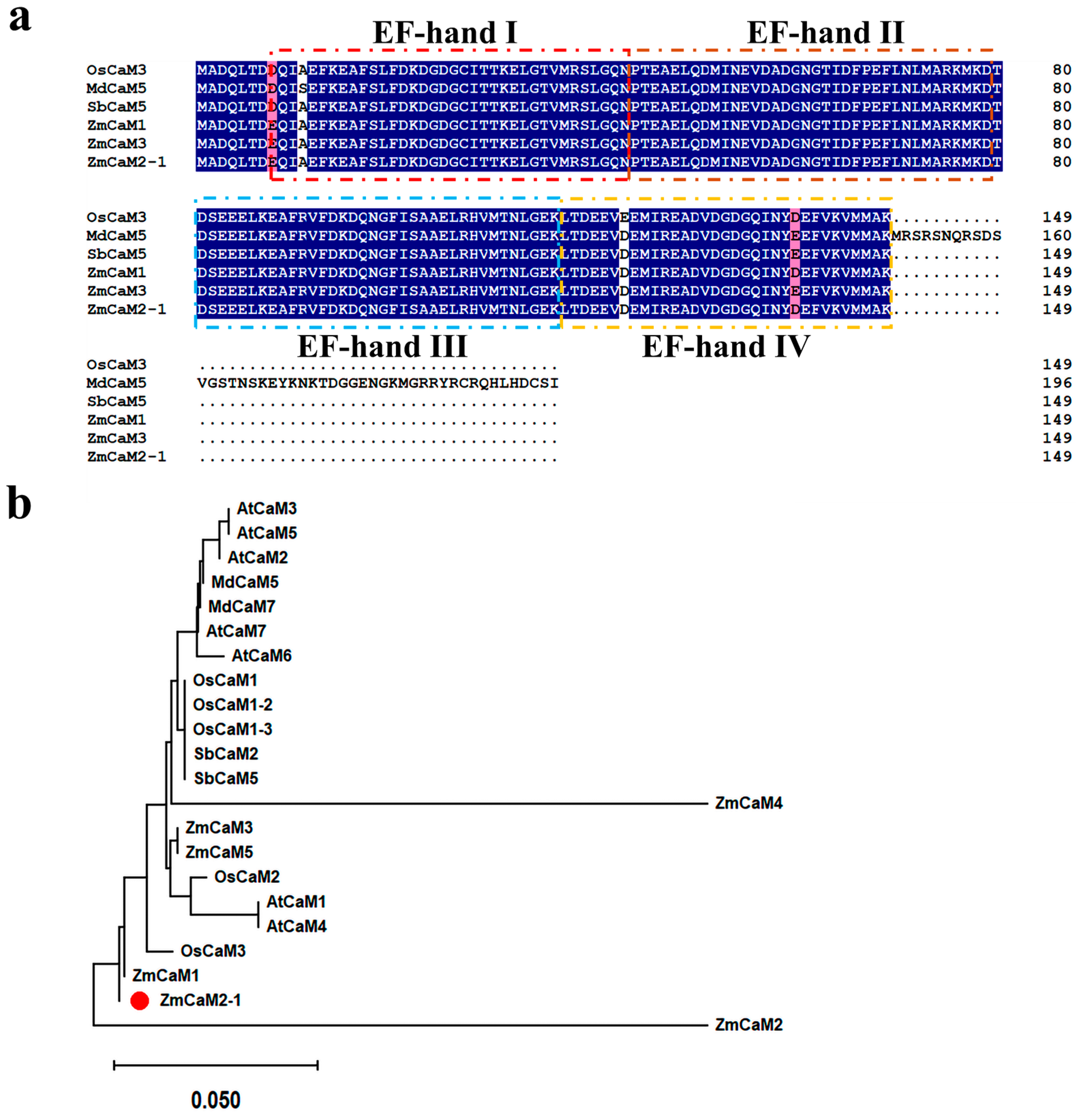
2.1. Gene Cloning and Sequence Analysis of ZmCaM2-1
2.2. Expression Profiling of ZmCaM2-1 Under Various Treatments
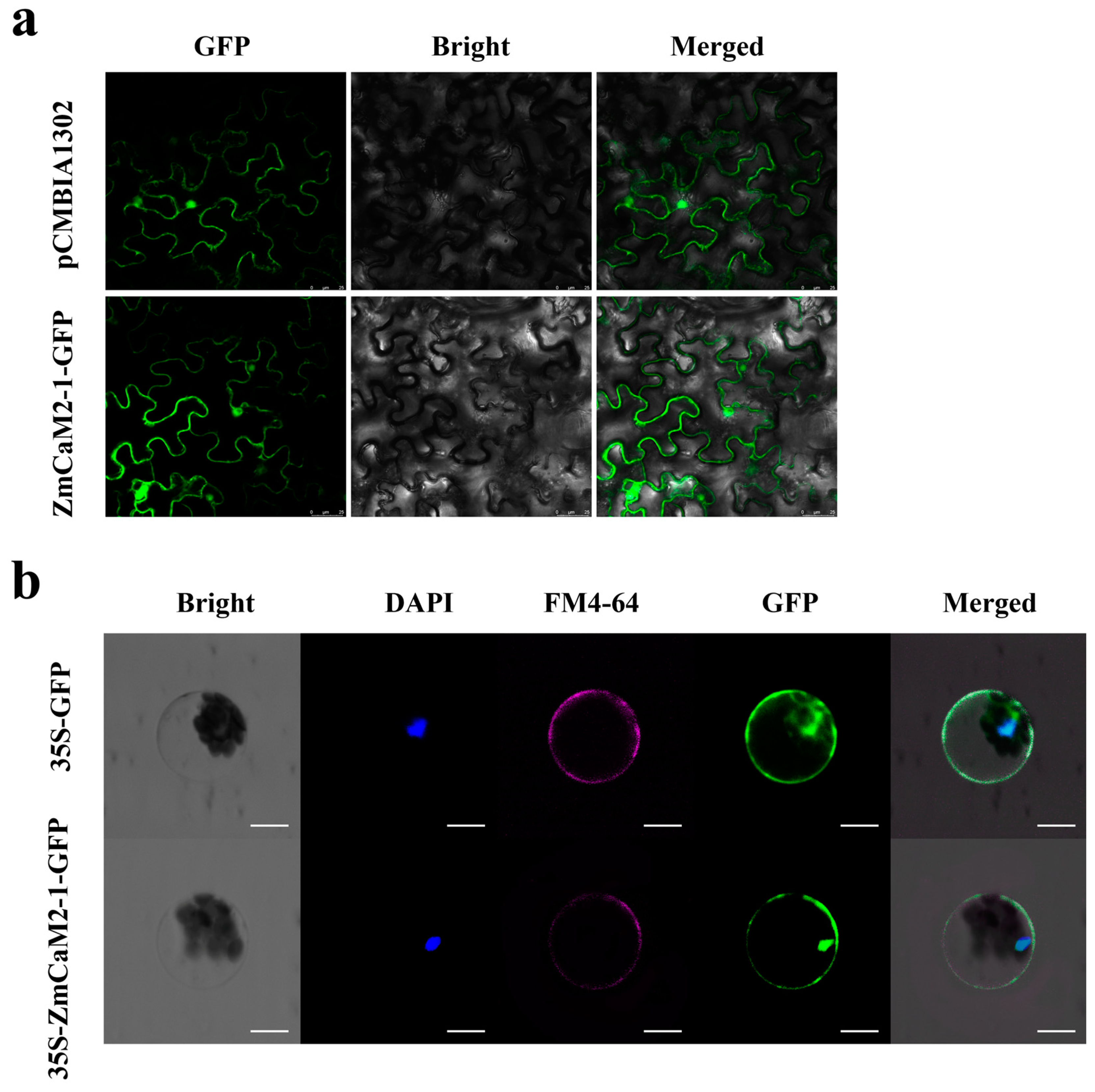
2.3. The ZmCaM2-1 Protein Is Located in the Cell Nucleus and Membrane
2.4. ZmCaM2-1 Is Able to Bind to Ca2+
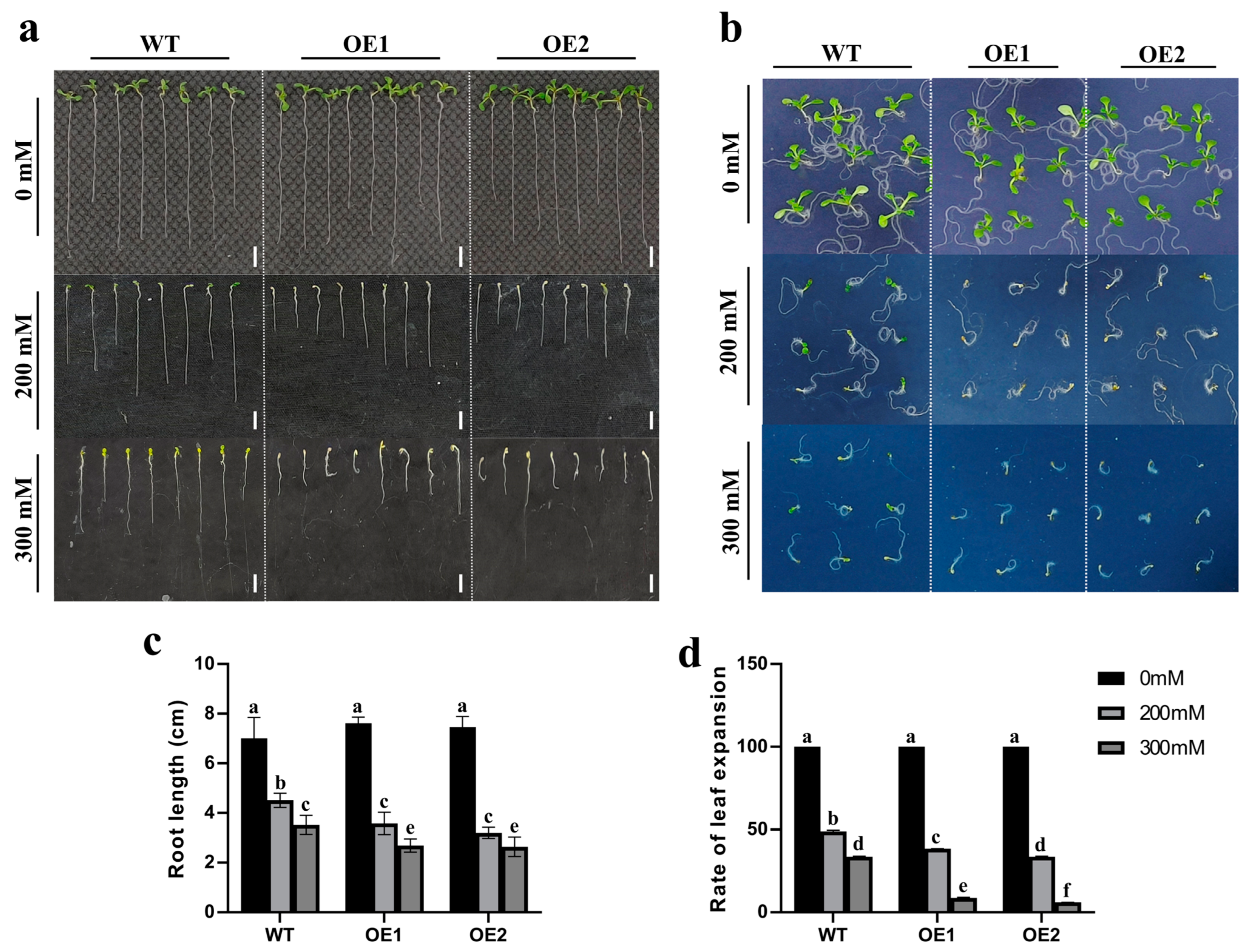
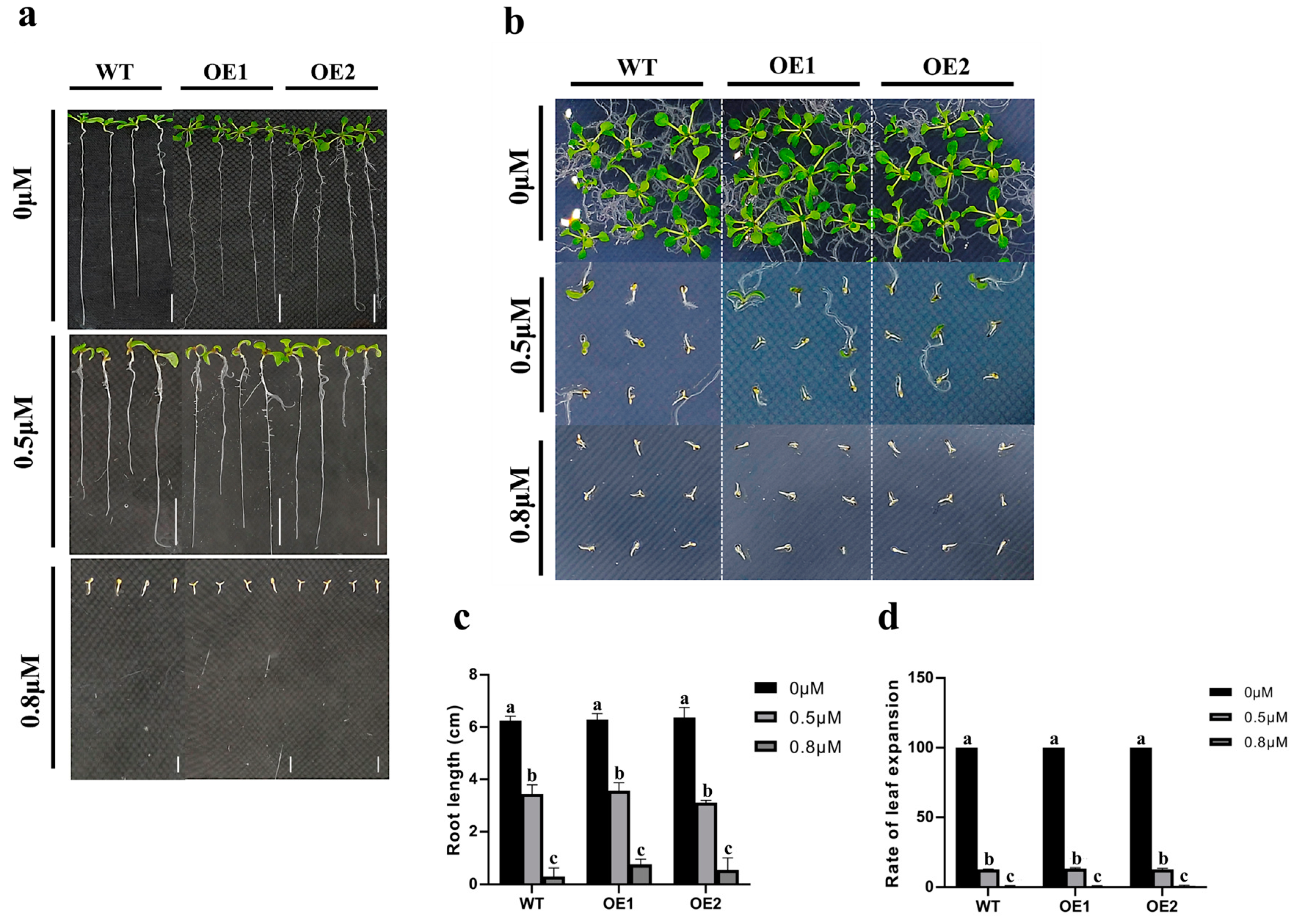
2.5. Overexpression of ZmCaM2-1 Decreases the Tolerance of Arabidopsis to Drought Stress
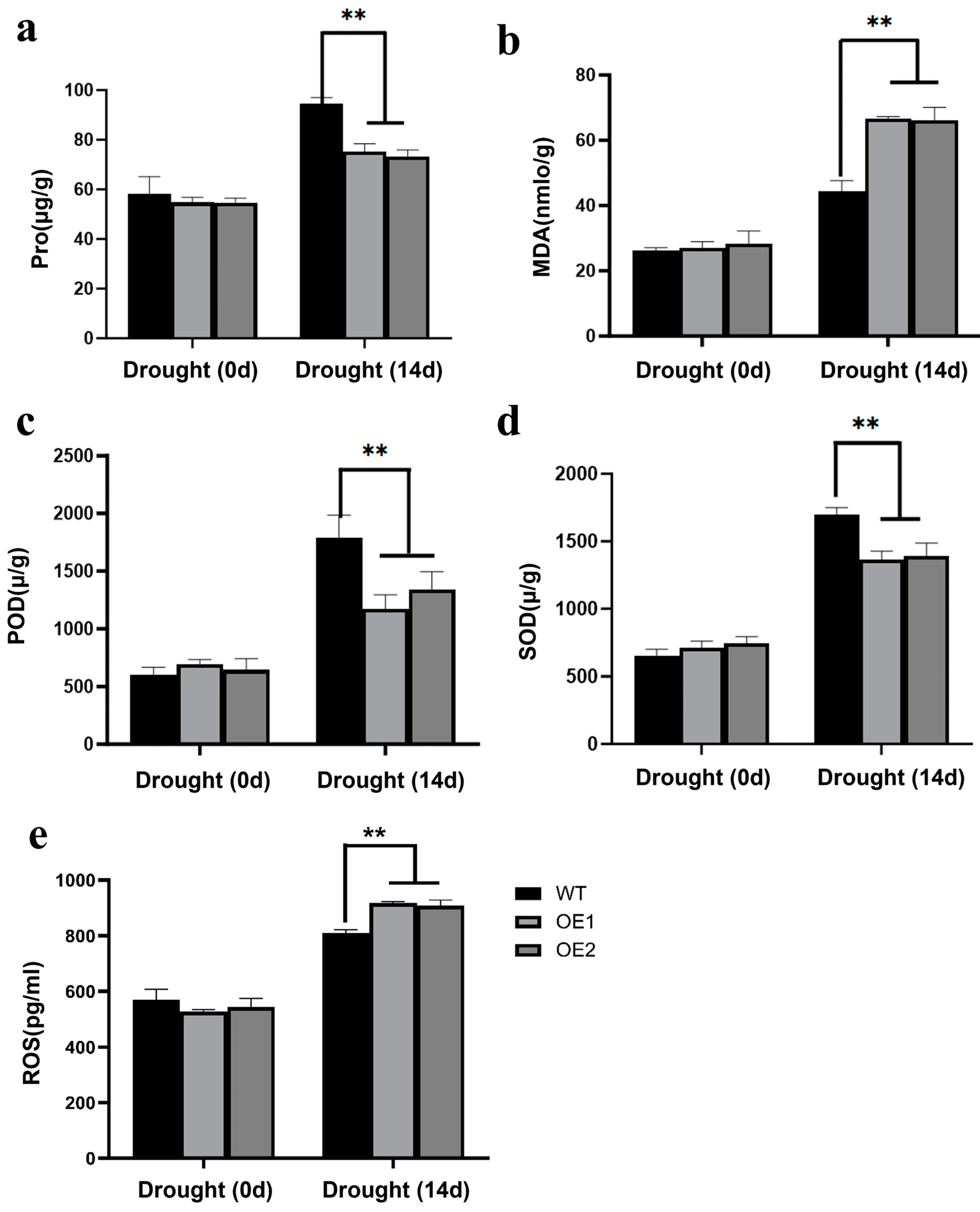
2.6. Overexpression of ZmCaM2-1 Decreases Drougth Stress Tolerance Through Increasing ROS Accumulation
2.7. The Transgenic Arabidopsis Shows Normal Sensitivity to ABA Thanks to the Overexpression of the ZmCaM2-1
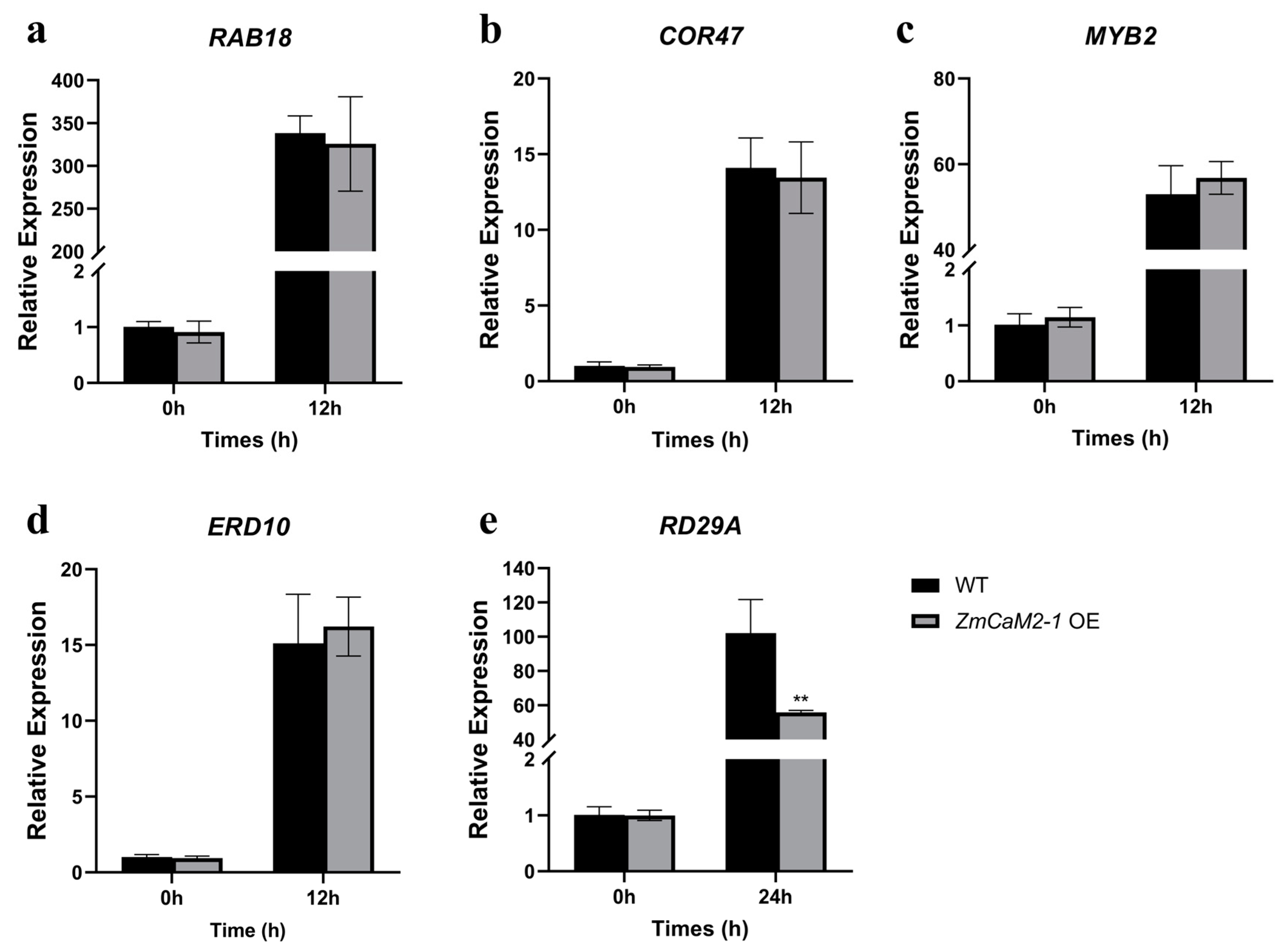
2.8. Overexpression of ZmCaM2-1 Reduces the Expression of Drought-Related Genes but Has No Effect on ABA-Related Genes
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Stress Treatments
4.2. RNA Extraction and qRT-PCR
4.3. ZmCaM2-1 Cloning and Bioinformatics Analysis
4.4. Purification of ZmCaM2-1 Protein and Ca2+ Binding Assay
4.5. Subcellular Localization of ZmCaM2-1
4.6. Gene Transformation and Drought Tolerance Assay
4.7. Physiological Index Detection
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ca2+ | Calcium ion |
| CaM | Calmodulin |
| CML | Calmodulin-like protein |
| CBL | Calcineurin B-Like |
| CDPK | Calcium-dependent protein kinase |
| ABA | Abscisic acid |
| ROS | Reactive oxygen species |
| CDS | Coding sequence |
| qRT-PCR | Quantitative Real-Time PCR |
| GFP | Green fluorescent protein |
| h | Hour |
| EGTA | Ethylene glycol tetraacetic acid |
| WT | Arabidopsis thaliana (Columbia, Col-0) |
| OE | ZmCaM2-1-Overexpressing Arabidopsis |
| d | Day |
| TBA | Thiobarbituric acid |
| Pro | Proline |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
References
- Cao, L.; Lu, X.; Wang, G.; Zhang, P.; Fu, J.; Wang, Z.; Wei, L.; Wang, T. Transcriptional regulatory networks in response to drought stress and rewatering in maize (Zea mays L.). Mol. Genet. Genomics 2021, 296, 1203–1219. [Google Scholar] [CrossRef]
- Balassa, G.; Oláh, C.; Balassa, K.; Rácz, I.; Kátay, G.; Kalapos, B.; Boldizsár, I.; Sárvári, É.; Solti, Á.; Pál, M.; et al. Physiological and molecular background of maize cold-tolerance enhancement with S-methylmethionine salicylate. Plant Growth Regul. 2022, 41, 2073–2091. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global Synthesis of Drought Effects on Maize and Wheat Production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, E.; Puccioni, S.; Eichmeier, A.; Natale, R.; Gori, M.; Biricolti, S.; Mattii, G.B. Effect of zeolite and irrigation treatments on grapevine leaves, an interdisciplinary approach. Plant Soil 2024, preprint, 1–20. [Google Scholar] [CrossRef]
- Aldon, D.; Mbengue, M.; Mazars, C.; Galaud, J.P. Calcium signalling in plant biotic interactions. Int. J. Mol. Sci. 2018, 19, 665. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, L.; Singh, A.; Wang, H.; Du, L.; Poovaiah, B.W. Involvement of calmodulin and calmodulin-like proteins in plant responses to abiotic stresses. Front. Plant Sci. 2015, 6, 600. [Google Scholar] [CrossRef]
- McAinsh, M.R.; Brownlee, C.; Hetherington, A.M. Visualizing Changes in Cytosolic-Free Ca2+ during the Response of Stomatal Guard Cells to Abscisic Acid. Plant Cell. 1992, 4, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Bagur, R.; Hajnóczky, G. Intracellular Ca2+ Sensing: Its Role in Calcium Homeostasis and Signaling. Mol. Cell 2017, 66, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Zhang, M.; Zhang, L. Genome-wide identification and expression analysis of calmodulin-like (CML) genes in Chinese cabbage (Brassica rapa L. ssp. pekinensis). BMC Genom. 2017, 18, 842. [Google Scholar] [CrossRef]
- Munir, S.; Khan, M.R.; Song, J.; Munir, S.; Zhang, Y.; Ye, Z.; Wang, T. Genome-wide identification, characterization and expression analysis of calmodulin-like (CML) proteins in tomato (Solanum lycopersicum). Plant Physiol. Biochem. 2016, 102, 167–179. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, L.; Yang, R.; Yi, X.; Zhang, B. Contribution and distribution of inorganic ions and organic compounds to the osmotic adjustment in Halostachys caspica response to salt stress. Sci. Rep. 2015, 5, 13639. [Google Scholar]
- Yin, X.; Huang, L.; Wang, M.; Cui, Y.; Xia, X. OsDSR-1, a calmodulin-like gene, improves drought tolerance through scavenging of reactive oxygen species in rice (Oryza sativa L.). Mol. Breed. 2017, 37, 1–13. [Google Scholar] [CrossRef]
- Yang, S.; Xiong, X.; Arif, S.; Gao, L.; Zhao, L.; Shah, I.H.; Zhang, Y. A calmodulin-like CmCML13 from Cucumis melo improved transgenic Arabidopsis salt tolerance through reduced shoot’s Na+ and also improved drought resistance. Plant Physiol. Biochem. 2020, 155, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.S.; Vadassery, J.; Heyer, M.; Reichelt, M.; Bender, K.W.; Snedden, W.A.; Boland, W.; Mithöfer, A. Mutation of the Arabidopsis calmodulin-like protein CML37 deregulates the jasmonate pathway and enhances susceptibility to herbivory. Mol. Plant. 2014, 7, 1712–1726. [Google Scholar] [CrossRef]
- Wang, T.Z.; Zhang, J.L.; Tian, Q.Y.; Zhao, M.G.; Zhang, W.H. A Medicago truncatula EF-hand family gene, MtCaMP1, is involved in drought and salt stress tolerance. PLoS ONE 2013, 8, e58952. [Google Scholar] [CrossRef]
- Aleynova, O.A.; Kiselev, K.V.; Suprun, A.R.; Ananev, A.A.; Dubrovina, A.S. Involvement of the Calmodulin-like Protein Gene VaCML92 in Grapevine Abiotic Stress Response and Stilbene Production. Int. J. Mol. Sci. 2023, 24, 15827. [Google Scholar] [CrossRef]
- Magnan, F.; Ranty, B.; Charpenteau, M.; Sotta, B.; Galaud, J.P.; Aldon, D. Mutations in AtCML9, a calmodulin-like protein from Arabidopsis thaliana, alter plant responses to abiotic stress and abscisic acid. Plant J. 2008, 56, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Song, S.; Zhang, H.; Li, Y.; Niu, L.; Zhang, J.; Wang, W. Signaling transduction of ABA, ROS, and Ca2+ in plant stomatal closure in response to drought. Int. J. Mol. Sci. 2022, 23, 14824. [Google Scholar] [CrossRef] [PubMed]
- Vanderbeld, B.; Snedden, W.A. Developmental and stimulus-induced expression patterns of Arabidopsis calmodulin-like genes CML37, CML38 and CML39. Plant Mol. Biol. 2007, 64, 683–697. [Google Scholar] [CrossRef]
- Xu, G.Y.; Rocha, P.S.; Wang, M.L.; Xu, M.L.; Cui, Y.C.; Li, L.Y.; Zhu, Y.X.; Xia, X. A novel rice calmodulin-like gene, OsMSR2, enhances drought and salt tolerance and increases ABA sensitivity in Arabidopsis. Planta 2011, 234, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Vadassery, J.; Reichelt, M.; Hause, B.; Gershenzon, J. CML42-mediated calcium signaling coordinates responses to Spodoptera herbivory and abiotic stresses in Arabidopsis. Plant Physiol. 2012, 159, 1159–1175. [Google Scholar]
- Yin, X.M.; Huang, L.F.; Zhang, X.; Wang, M.L.; Xu, G.Y.; Xia, X.J. OsCML4 improves drought tolerance through scavenging of reactive oxygen species in rice. J. Plant Biol. 2015, 58, 68–73. [Google Scholar]
- Du, B.; Chen, N.; Song, L.; Wang, D.; Cai, H.; Yao, L.; Li, X.; Guo, C. Alfalfa (Medicago sativa L.) MsCML46 gene encoding calmodulin-like protein confers tolerance to abiotic stress in tobacco. Plant Cell Rep. 2021, 40, 1907–1922. [Google Scholar] [CrossRef]
- Munir, S.; Liu, H.; Xing, Y.; Hussain, S.; Ouyang, B.; Zhang, Y.; Li, H.; Ye, Z. Overexpression of calmodulin-like (ShCML44) stress-responsive gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses. Sci. Rep. 2016, 6, 31772. [Google Scholar]
- Cui, Y.; Wu, K.; Yao, X. The CDPK-related protein kinase HvCRK2 and HvCRK4 interact with HvCML32 to negatively regulate drought tolerance in transgenic Arabidopsis thaliana. Plant Physiol. Biochem. 2024, 214, 108909. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.F.; Dong, Y.H.; Ren, Z.Z.; Wang, Z.Y.; Su, H.H.; Ku, L.X.; Chen, Y.H. Over-expression of ZmIBH1-1 to Improve Drought Resistance in Maize Seedlings. Sci. Agric. Sin. 2021, 54, 4500–4521. [Google Scholar]
- Wang, Z.; Wang, L.H.; Li, J.X.; Yang, W.; Ci, J.B.; Ren, X.J.; Jiang, L.Y.; Yang, W.G. Identification and expression analysis revealed drought stress-responsive Calmodulin and Calmodulin-like genes in maize. J. Plant Interact. 2022, 17, 450–461. [Google Scholar] [CrossRef]
- Garrigos, M.; Deschamps, S.; Viel, A.; Lund, S.; Champeil, P.; Møller, J.V.; le Maire, M. Detection of Ca2+-binding proteins by electrophoretic migration in the presence of Ca2+ combined with 45Ca2+ overlay of protein blots. Anal. Biochem. 1991, 194, 82–88. [Google Scholar] [PubMed]
- Tong, T.; Li, Q.; Jiang, W.; Chen, G.; Xue, D.; Deng, F.; Zeng, F.; Chen, Z.H. Molecular evolution of calcium signaling and transport in plant adaptation to abiotic stress. Int. J. Mol. Sci. 2021, 22, 12308. [Google Scholar] [CrossRef]
- Gong, M.; Chen, S.N.; Song, Y.Q.; Li, Z.G. Effect of calcium and calmodulin on intrinsic heat tolerance in relation to antioxidant systems in maize seedlings. Funct. Plant Biol. 1997, 24, 371–379. [Google Scholar]
- Gong, M.; Li, Y.-J.; Dai, X.; Tian, M.; Li, Z.-G. Involvement of calcium and calmodulin in the acquisition of heat-shock induced thermotolerance in maize seedlings. J. Plant Physiol. 1997, 150, 615–621. [Google Scholar] [CrossRef]
- Yap, K.L.; Ames, J.B.; Swindells, M.B.; Ikura, M. Diversity of conformational states and changes within the EF-hand protein superfamily. Proteins 1999, 37, 499–507. [Google Scholar] [CrossRef]
- Kim, M.C.; Chung, W.S.; Yun, D.J.; Cho, M.J. Calcium and calmodulin-mediated regulation of gene expression in plants. Mol. Plant. 2009, 2, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhang, Y.; Zhang, X.; Pi, E.; Zhu, Y. Analysis of EF-hand proteins in soybean genome suggests their potential roles in environmental and nutritional stress signaling. Front. Plant Sci. 2017, 8, 877. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Su’udi, M.; Kim, W.Y.; Kim, D.R.; Kwak, Y.S.; Son, D. Functional characterization of orchardgrass cytosolic Hsp70 (DgHsp70) and the negative regulation by Ca2+/AtCaM2 binding. Plant Physiol. Biochem. 2012, 58, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Park, C.Y.; Kim, J.C.; Do Heo, W.; Cheong, M.S.; Park, H.C.; Kim, M.C.; Moon, B.C.; Choi, M.S.; Kang, Y.H.; et al. Direct interaction of a divergent CaM isoform and the transcription factor, MYB2, enhances salt tolerance in Arabidopsis. J. Biol. Chem. 2005, 280, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Perochon, A.; Aldon, D.; Galaud, J.P.; Ranty, B. Calmodulin and calmodulin-like proteins in plant calcium signaling. Biochimie 2011, 93, 2048–2053. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Hou, Q.; Liu, Y.; Wang, J.; Deng, S. Isolation and functional characterization of a salt-responsive calmodulin-like gene MpCML40 from semi- mangrove Millettia pinnata. Int. J. Mol. Sci. 2021, 22, 3475. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Yang, S.; Guan, D.; He, S. CaCML13 acts positively in pepper immunity against Ralstonia solanacearum infection forming feedback loop with CabZIP63. Int. J. Mol. Sci. 2020, 21, 4186. [Google Scholar] [CrossRef] [PubMed]
- Jamra, J.G.; Agarwal, A.; Singh, N.; Sanyal, S.K.; Kumar, A.; Pandey, G.K. Ectopic expression of finger millet calmodulin confers drought and salinity tolerance in Arabidopsis thaliana. Plant Cell Rep. 2021, 40, 2205–2223. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Chung, P.J.; Park, S.H.; Redillas, M.C.F.R.; Kim, Y.S.; Suh, J.W.; Kim, J.K. Overexpression of OsERF48 causes regulation of OsCML16, a calmodulin-like protein gene that enhances root growth and drought tolerance. Plant Biotechnol. J. 2017, 15, 1295–1308. [Google Scholar] [CrossRef]
- Chen, C.; Sun, X.; Duanmu, H.; Zhu, D.; Yu, Y.; Cao, L.; Liu, A.; Jia, B.; Xiao, J.; Zhu, Y. GsCML27, a gene encoding a calcium-binding ef-hand protein from Glycine soja, plays differential roles in plant responses to bicarbonate, salt and osmotic stresses. PLoS ONE 2015, 10, e0141888. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Qiao, Z.; Liu, H.; Acharya, B.R.; Li, C.; Zhang, W. CML20, an Arabidopsis calmodulin-like protein, negatively regulates guard cell ABA signalling and drought stress tolerance. Front. Plant Sci. 2017, 8, 824. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, S.; Zhang, Q.; Cui, M.; Zhao, M.; Li, N.; Wang, S.; Wu, R.; Zhang, L.; Cao, Y.; et al. The interaction of ABA and ROS in plant growth and stress resistances. Front Plant Sci. 2022, 13, 1050132. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cao, J.; Chen, Q.; He, J.; Liu, Z.; Wang, J.; Li, X.; Yang, Y. The kinase CIPK11 functions as a negative regulator in drought stress response in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 2422. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wu, C.; Ye, X.; Zhou, J.; Chen, Y.; Li, L.; Lin, M.; Wang, S.; Liu, S.; Yan, Y.; et al. MePP2C24, a cassava (Manihot esculenta) gene encoding protein phosphatase 2C, negatively regulates drought stress and abscisic acid responses in transgenic Arabidopsis thaliana. Plant Physiol. Biochem. 2024, 206, 108291. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, R.; Ye, X.; Zheng, X.; Tan, B.; Wang, W.; Li, Z.; Li, J.; Cheng, J.; Feng, J. Overexpressing VvWRKY18 from grapevine reduces the drought tolerance in Arabidopsis by increasing leaf stomatal density. J. Plant Physiol. 2022, 275, 153741. [Google Scholar] [CrossRef] [PubMed]
- Mustilli, A.C.; Merlot, S.; Vavasseur, A.; Fenzi, F.; Giraudat, J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 2002, 14, 3089–3099. [Google Scholar] [CrossRef]
- Kimura, S.; Kaya, H.; Kawarazaki, T.; Hiraoka, G.; Senzaki, E.; Michikawa, M.; Kuchitsu, K. Protein phosphorylation is a prerequisite for the Ca2+-dependent activation of Arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2+ and reactive oxygen species. Biochim. Et Biophys. Acta 2012, 1823, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Ravi, B.; Foyer, C.H.; Pandey, G.K. The integration of reactive oxygen species (ROS) and calcium signalling in abiotic stress responses. Plant Cell Environ. 2023, 46, 1985–2006. [Google Scholar] [CrossRef]
- Dar, N.A.; Amin, I.; Wani, W.; Wani, S.A.; Shikari, A.B.; Wani, S.H.; Masoodi, K. Z Abscisic acid: A key regulator of abiotic stress tolerance in plants. Plant Gene 2017, 11, 106–111. [Google Scholar] [CrossRef]
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 2014, 21, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Y.; Tang, C.; Shen, Q.; Fu, J.; Wang, Q. Maize transcription factor ZmHsf28 positively regulates plant drought tolerance. Int. J. Mol. Sci. 2023, 24, 8079. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Jiang, S.; Pan, J.; Cai, G.; Li, D. Group 5 LEA protein, ZmLEA5C, enhance tolerance to osmotic and low temperature stresses in transgenic tobacco and yeast. Plant Physiol. Biochem. 2014, 84, 22–31. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kokkirala, V.R.; Yonggang, P.; Abbagani, S.; Zhu, Z.; Umate, P. Subcellular localization of proteins of Oryza sativa L. in the model tobacco and tomato plants. Plant Signal. Behav. 2010, 5, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Maehly, A.C. The assay of catalases and peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Liu, M.; Wang, H.; Li, M.; Liu, X.; Zang, Z.; Jiang, L. ZmCaM2-1, a Calmodulin Gene, Negatively Regulates Drought Tolerance in Transgenic Arabidopsis Through the ABA-Independent Pathway. Int. J. Mol. Sci. 2025, 26, 2156. https://doi.org/10.3390/ijms26052156
Wu Z, Liu M, Wang H, Li M, Liu X, Zang Z, Jiang L. ZmCaM2-1, a Calmodulin Gene, Negatively Regulates Drought Tolerance in Transgenic Arabidopsis Through the ABA-Independent Pathway. International Journal of Molecular Sciences. 2025; 26(5):2156. https://doi.org/10.3390/ijms26052156
Chicago/Turabian StyleWu, Zhiqiang, Meiyi Liu, Hanqiao Wang, Mingrui Li, Xiaoyue Liu, Zhenyuan Zang, and Liangyu Jiang. 2025. "ZmCaM2-1, a Calmodulin Gene, Negatively Regulates Drought Tolerance in Transgenic Arabidopsis Through the ABA-Independent Pathway" International Journal of Molecular Sciences 26, no. 5: 2156. https://doi.org/10.3390/ijms26052156
APA StyleWu, Z., Liu, M., Wang, H., Li, M., Liu, X., Zang, Z., & Jiang, L. (2025). ZmCaM2-1, a Calmodulin Gene, Negatively Regulates Drought Tolerance in Transgenic Arabidopsis Through the ABA-Independent Pathway. International Journal of Molecular Sciences, 26(5), 2156. https://doi.org/10.3390/ijms26052156




