Vitamin D and Type 2 Diabetes Mellitus: Molecular Mechanisms and Clinical Implications—A Narrative Review
Abstract
1. Introduction
2. Diabetes
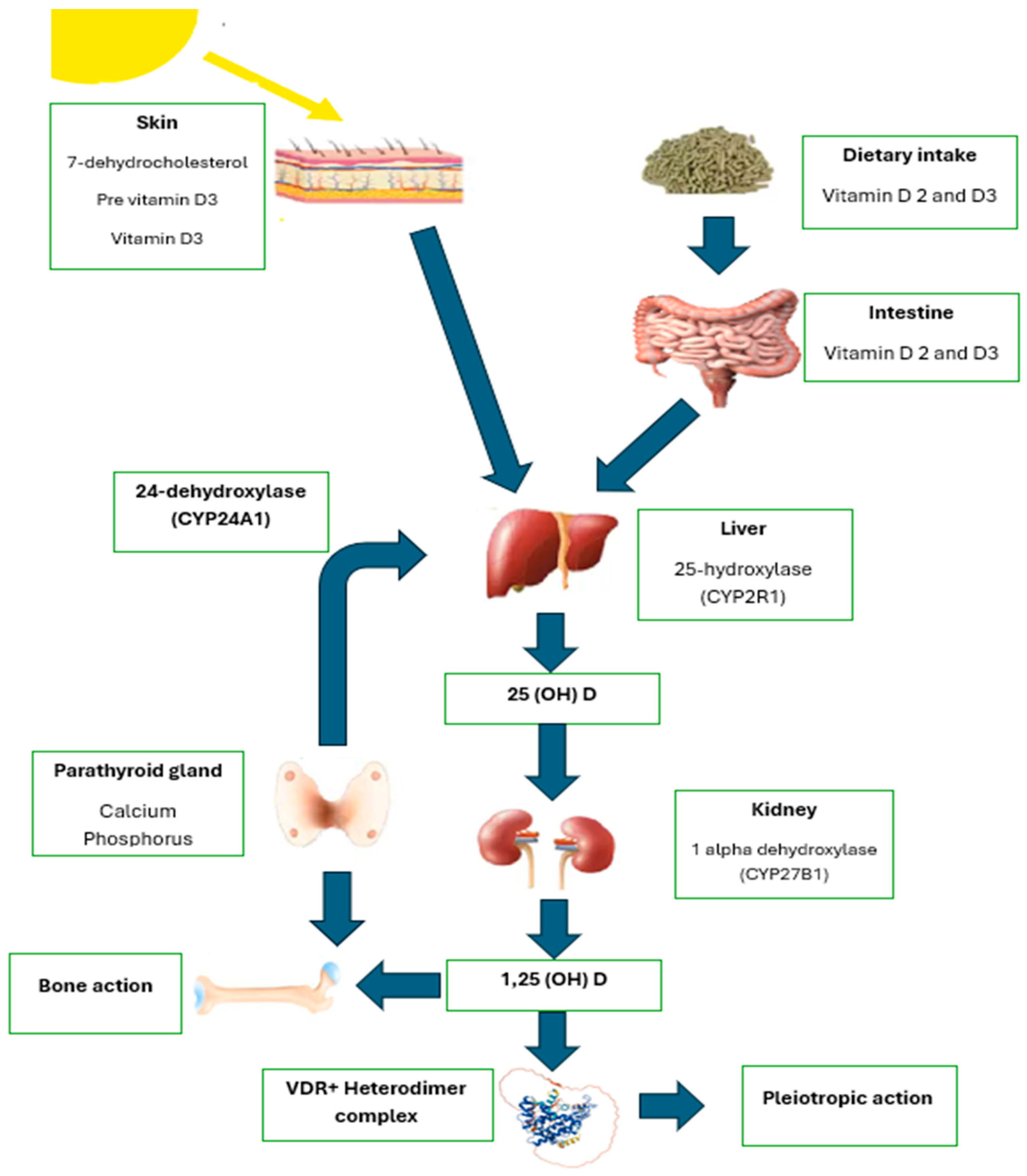
3. Vitamin D
3.1. Glucose Metabolism and Insulin Sensitivity
3.2. Vitamin D and HbA1c
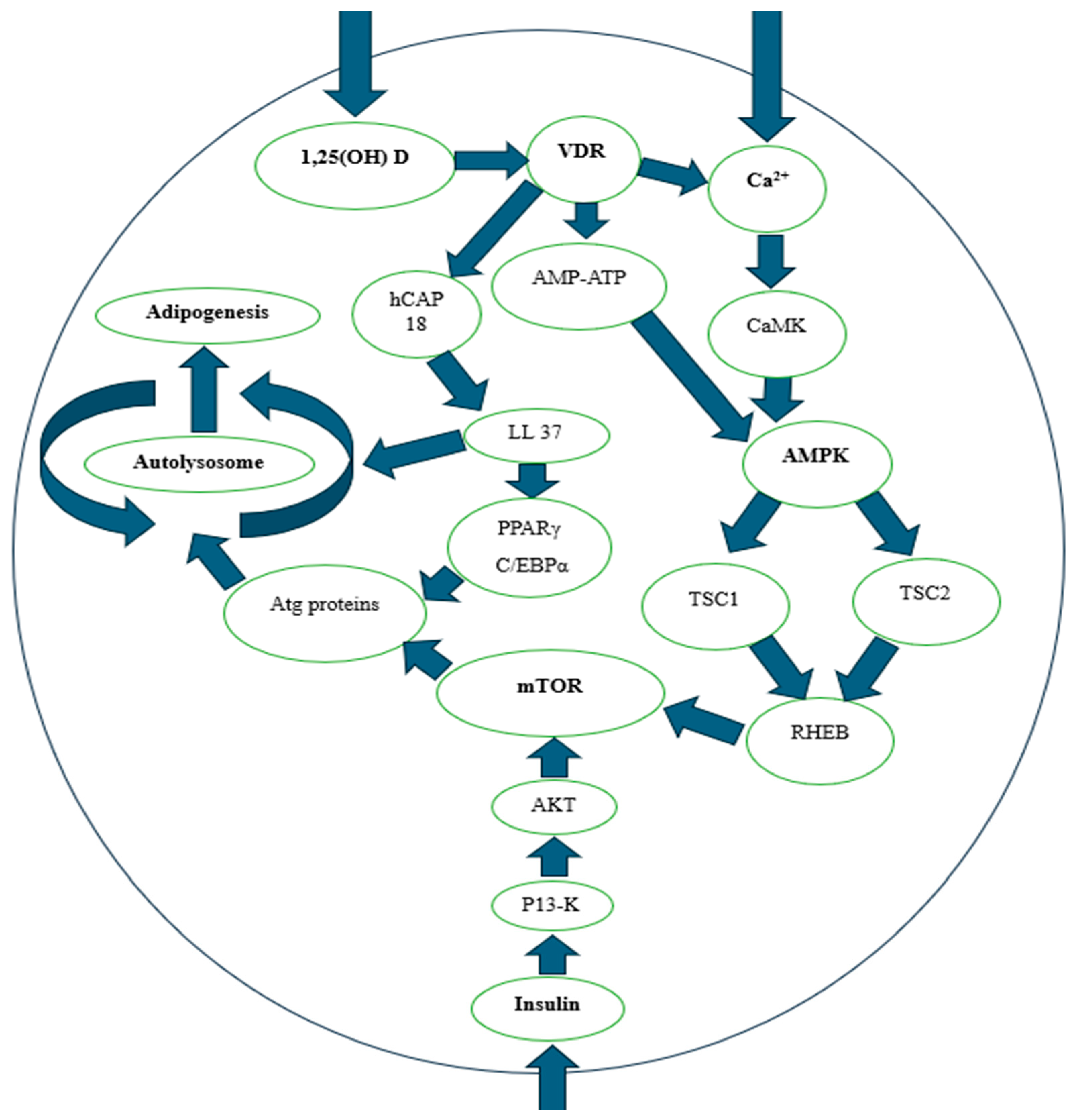
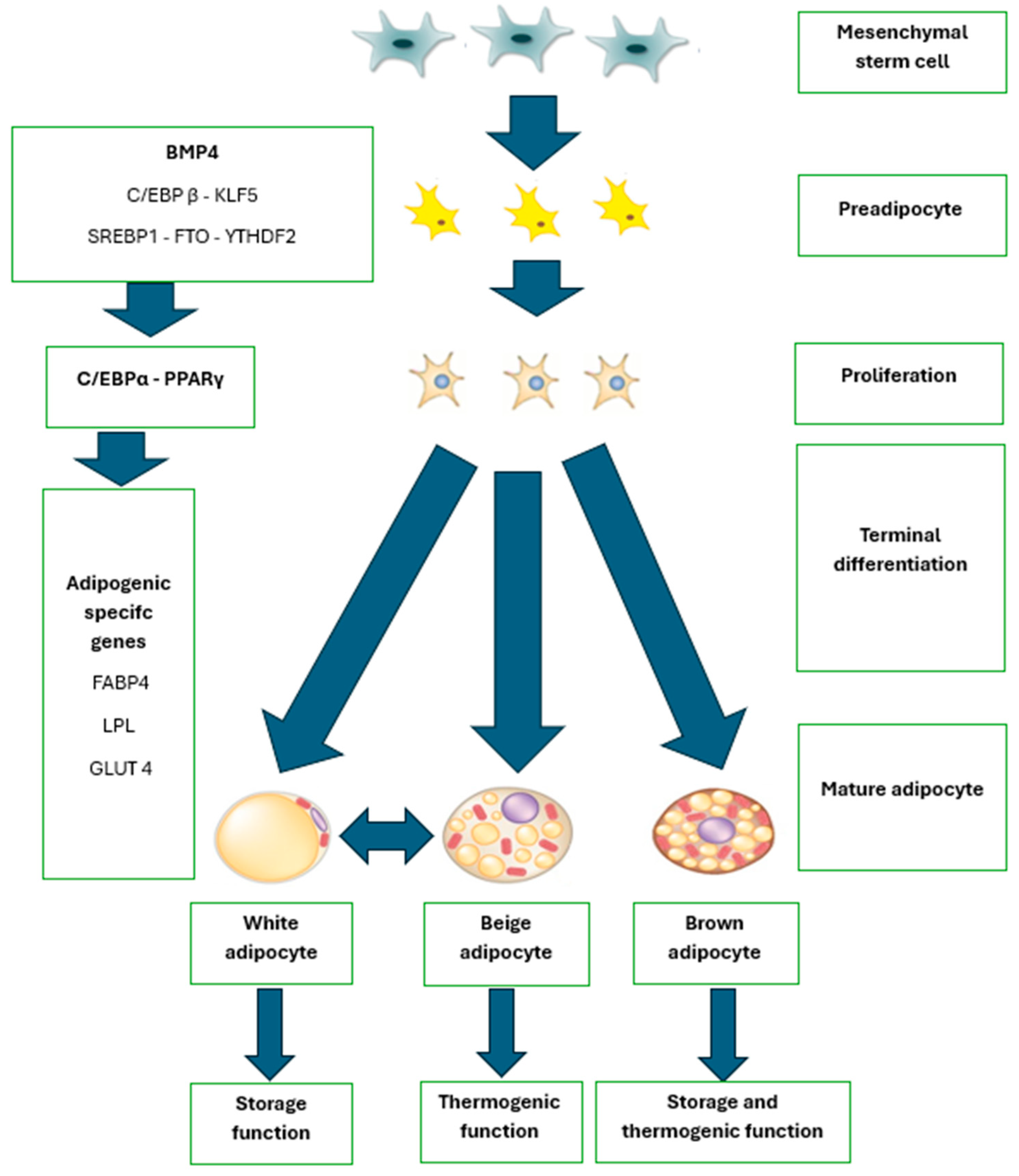
3.3. Adipogenesis
3.4. Vitamin D Levels
3.5. Recommendations for Vitamin D Intake and Considerations for Vulnerable Populations
4. Limitations of the Current Evidence
5. Clinical Implications and Future Research Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pittas, A.G.; Kawahara, T.; Jorde, R.; Dawson-Hughes, B.; Vickery, E.M.; Angellotti, E.; Nelson, J.; Trikalinos, T.A.; Balk, E.M. Vitamin D and Risk for Type 2 Diabetes in People with Prediabetes: A Systematic Review and Meta-analysis of In-dividual Participant Data From 3 Randomized Clinical Trials. Ann. Intern. Med. 2023, 176, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Demay, M.B.; Pittas, A.G.; Bikle, D.D.; Diab, D.L.; Kiely, M.E.; Lazaretti-Castro, M.; Lips, P.; Mitchell, D.M.; Murad, M.H.; Powers, S.; et al. Vitamin D for the Prevention of Disease: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2024, 109, 1907–1947. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. on behalf of the American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, J.; Ni, Y.; Yi, C.; Fang, Y.; Ning, Q.; Shen, B.; Zhang, K.; Liu, Y.; Yang, L.; et al. Global Prevalence of Overweight and Obesity in Children and Adolescents: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2024, 178, 800–813. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, H.; Tang, J.; Li, J.; Chong, W.; Hai, Y.; Feng, Y.; Lunsford, L.D.; Xu, P.; Jia, D.; et al. Effects of Vitamin D Supplementation on Prevention of Type 2 Diabetes in Patients with Prediabetes: A Systematic Review and Meta-Analysis. Diabetes Care 2020, 43, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Pittas, A.G.; Kawahara, T.; Jorde, R.; Dawson-Hughes, B.; Vickery, E.M.; Angellotti, E.; Nelson, J.; Trikalinos, T.A.; Balk, E.M. Summary for Patients: Vitamin D and Risk for Type 2 Diabetes in People with Prediabetes. Ann. Intern. Med. 2023, 176, I22. [Google Scholar] [CrossRef] [PubMed]
- Hands, J.M.; Patrick, R.; Frame, L.A. Vitamin D and Risk for Type 2 Diabetes in People with Prediabetes. Ann. Intern. Med. 2023, 176, 230201. [Google Scholar] [CrossRef]
- Lucato, P.; Solmi, M.; Maggi, S.; Bertocco, A.; Bano, G.; Trevisan, C.; Manzato, E.; Sergi, G.; Schofield, P.; Kouidrat, Y.; et al. Low Vitamin D Levels Increase the Risk of Type 2 Diabetes in Older Adults: A Systematic Review and Meta-Analysis. Maturitas 2017, 100, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Oussaada, S.M.; Akkermans, I.; Chohan, S.; Limpens, J.; Twisk, J.W.R.; Winkler, C.; Karalliedde, J.; Gallagher, J.C.; Romijn, J.A.; Serlie, M.J.; et al. The Effect of Active Vitamin D Supplementation on Body Weight and Composition: A Meta-Analysis of Individual Participant Data. Clin. Nutr. 2024, 43, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Max, F.; Gažová, A.; Smaha, J.; Jankovský, M.; Tesař, T.; Jackuliak, P.; Kužma, M.; Payer, J.; Kyselovič, J. High Doses of Vitamin D and Specific Metabolic Parameters in Type 2 Diabetes Patients: Systematic Review. Nutrients 2024, 16, 3903. [Google Scholar] [CrossRef] [PubMed]
- Pięnkowska, A.; Janicka, J.; Duda, M.; Dzwonnik, K.; Lip, K.; Mędza, A.; Szlagatys-Sidorkiewicz, A.; Brzeziński, M. Controversial Impact of Vitamin D Supplementation on Reducing Insulin Resistance and Prevention of Type 2 Diabetes in Patients with Prediabetes: A Systematic Review. Nutrients 2023, 15, 983. [Google Scholar] [CrossRef] [PubMed]
- Vernia, F.; Valvano, M.; Longo, S.; Cesaro, N.; Viscido, A.; Latella, G. Vitamin D in Inflammatory Bowel Diseases: Mechanisms of Action and Therapeutic Implications. Nutrients 2022, 14, 269. [Google Scholar] [CrossRef]
- Triantos, C.; Aggeletopoulou, I.; Mantzaris, G.J.; Mouzaki, A. Molecular Basis of Vitamin D Action in Inflammatory Bowel Disease. Autoimmun. Rev. 2022, 21, 103136. [Google Scholar] [CrossRef] [PubMed]
- Nimitphong, H.; Park, E.; Lee, M.J. Vitamin D Regulation of Adipogenesis and Adipose Tissue Functions. Nutr. Res. Pract. 2020, 14, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Han, S.N. The Role of Vitamin D in Adipose Tissue Biology: Adipocyte Differentiation, Energy Metabolism, and Inflammation. J. Lipid Atheroscler. 2021, 10, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Lacerda de Lucena, L.; Silva, A.S.; Nascimento, R.A.F.D.; Persuhn, D.C.; Neves, J.P.R.; Costa, M.J.C.; Queiroz, D.J.M.; de Lima, R.L.F.C.; Ataíde de Lima, R.P.; de Paiva, M.P.; et al. Relationship Between BsmI Polymorphism and VDR Gene Methylation Profile, Gender, Metabolic Profile, Oxidative Stress, and Inflammation in Adolescents. Nutr. Hosp. 2021, 38, 911–918. [Google Scholar] [PubMed]
- Faghfouri, A.H.; Faghfuri, E.; Maleki, V.; Payahoo, L.; Balmoral, A.; Khaje Bishak, Y. A Comprehensive Insight into the Potential Roles of VDR Gene Polymorphism in Obesity: A Systematic Review. Arch. Physiol. Biochem. 2022, 128, 1645–1657. [Google Scholar] [CrossRef]
- Bhutia, S.K. Vitamin D in Autophagy Signaling for Health and Diseases: Insights on Potential Mechanisms and Future Perspectives. J. Nutr. Biochem. 2022, 99, 108841. [Google Scholar] [CrossRef] [PubMed]
- Orsatti, C.L.; Orsatti, F.L.; de Souza, J.P.E.A.; Nahas, E.A.P. Impact of Vitamin D Supplementation on Modulating Heat-Shock Proteins in Postmenopausal Women: A Randomized, Double-Blind, Placebo-Controlled Study. Menopause 2023, 30, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pan, W.; Yang, G.Z.; Di, Y.N.; Zhao, F.; Zhu, L.Y.; Jiang, Z.H. Proteome Analysis of Differential Protein Expression in Brain of Rats with Type 1 Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2011, 119, 265–270. [Google Scholar] [CrossRef]
- Park, C.Y.; Shin, S.; Han, S.N. Multifaceted Roles of Vitamin D for Diabetes: From Immunomodulatory Functions to Metabolic Regulations. Nutrients 2024, 16, 3185. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Health Estimates. Available online: https://www.who.int/ (accessed on 18 January 2025).
- Pludowski, P.; Takacs, I.; Boyanov, M.; Belaya, Z.; Diaconu, C.C.; Mokhort, T.; Zherdova, N.; Rasa, I.; Payer, J.; Pilz, S. Clinical Practice in the Prevention, Diagnosis and Treatment of Vitamin D Deficiency: A Central and Eastern European Expert Consensus Statement. Nutrients 2022, 14, 1483. [Google Scholar] [CrossRef] [PubMed]
- Harreiter, J.; Roden, M. Diabetes Mellitus: Definition, Classification, Diagnosis, Screening, and Prevention (Update 2023). Wien. Klin. Wochenschr. 2023, 135, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Bielka, W.; Przezak, A.; Molęda, P.; Pius-Sadowska, E.; Machaliński, B. Double Diabetes—When Type 1 Diabetes Meets Type 2 Diabetes: Definition, Pathogenesis, and Recognition. Cardiovasc. Diabetol. 2024, 23, 62. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, E.; Gerdes, C.; Petersmann, A.; Müller-Wieland, D.; Müller, U.A.; Freckmann, G.; Heinemann, L.; Nauck, M.; Landgraf, R. Definition, Classification, and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2022, 130, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Antar, S.A.; Ashour, N.A.; Sharaky, M.; Khattab, M.; Ashour, N.A.; Zaid, R.T.; Roh, E.J.; Elkamhawy, A.; Al-Karmalawy, A.A. Diabetes Mellitus: Classification, Mediators, and Complications; A Gate to Identify Potential Targets for the Development of New Effective Treatments. Biomed. Pharmacother. 2023, 168, 115734. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J. Epidemiology, Pathophysiology, Diagnosis, and Treatment of Heart Failure in Diabetes. Diabetes Metab. J. 2021, 45, 146–157. [Google Scholar] [CrossRef]
- Dharmarajan, S.; Carrillo, C.; Qi, Z.; Wilson, J.M.; Baucum, A.J., II; Sorenson, C.M.; Sheibani, N.; Belecky-Adams, T.L. Retinal Inflammation in Murine Models of Type 1 and Type 2 Diabetes with Diabetic Retinopathy. Diabetologia 2023, 66, 2170–2185. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Bahadoran, Z.; Ghasemi, A.; Hosseinpanah, F. Type 2 Diabetes and Cancer: An Overview of Epidemiological Evidence and Potential Mechanisms. Crit. Rev. Oncog. 2019, 24, 223–233. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, R.; Zou, H.; Xie, L.; Zhou, Z.; Xiao, Y. Latent Autoimmune Diabetes in Adults (LADA): From Immunopathogenesis to Immunotherapy. Front. Endocrinol. 2022, 13, 917169. [Google Scholar] [CrossRef]
- Piccioni, A.; Rosa, F.; Mannucci, S.; Manca, F.; Merra, G.; Chiloiro, S.; Candelli, M.; Covino, M.; Gasbarrini, A.; Franceschi, F. Gut Microbiota, LADA, and Type 1 Diabetes Mellitus: An Evolving Relationship. Biomedicines 2023, 11, 707. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.I.G.; DeVries, J.H.; Hess-Fischl, A.; Hirsch, I.B.; Kirkman, M.S.; Klupa, T.; Ludwig, B.; Nørgaard, K.; Pettus, J.; Renard, E.; et al. The Management of Type 1 Diabetes in Adults: A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2021, 64, 2609–2652. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, N.G.; Misra, A.; Mohan, V.; Taylor, R.; Yancy, W. Dietary and Nutritional Approaches for Prevention and Management of Type 2 Diabetes. BMJ 2018, 361, k2234. [Google Scholar] [CrossRef] [PubMed]
- Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Evidence-Based European Recommendations for the Dietary Management of Diabetes. Diabetologia 2023, 66, 965–985. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022: A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, 145–157. [Google Scholar] [CrossRef]
- Purnell, J.Q. What Is Obesity? Definition as a Disease, With Implications for Care. Gastroenterol. Clin. N. Am. 2023, 52, 261–275. [Google Scholar] [CrossRef]
- Kanaley, J.A.; Colberg, S.R.; Corcoran, M.H.; Malin, S.K.; Rodriguez, N.R.; Crespo, C.J.; Kirwan, J.P.; Zierath, J.R. Exercise/Physical Activity in Individuals with Type 2 Diabetes: A Consensus Statement from the American College of Sports Medicine. Med. Sci. Sports Exerc. 2022, 54, 353–368. [Google Scholar] [CrossRef]
- Chung, J.; Miller, B.J. Meta-Analysis of Comorbid Diabetes and Family History of Diabetes in Non-Affective Psychosis. Schizophr. Res. 2020, 216, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Kautzky-Willer, A.; Leutner, M.; Harreiter, J. Sex Differences in Type 2 Diabetes. Diabetologia 2023, 66, 986–1002. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Nan, F.; Liang, H.; Shu, P.; Fan, X.; Song, X.; Hou, Y.; Zhang, D. Excessive Intake of Sugar: An Accomplice of Inflammation. Front. Immunol. 2022, 13, 988481. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Uricoechea, H.; Cáceres-Acosta, M.F. Blood Pressure Control and Impact on Cardiovascular Events in Patients with Type 2 Diabetes Mellitus: A Critical Analysis of the Literature. Clin. Investig. Arterioscler. 2019, 31, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Perego, C.; Da Dalt, L.; Pirillo, A.; Galli, A.; Catapano, A.L.; Norata, G.D. Cholesterol Metabolism, Pancreatic β-Cell Function and Diabetes. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Albarri, E.M.A.; Alnuaimi, A.S.; Abdelghani, D. Effectiveness of Vitamin D2 Compared with Vitamin D3 Replacement Therapy in a Primary Healthcare Setting: A Retrospective Cohort Study. Qatar Med. J. 2022, 2022, 29. [Google Scholar]
- Benedik, E. Sources of Vitamin D for Humans. Int. J. Vitam. Nutr. Res. 2022, 92, 118–125. [Google Scholar] [CrossRef]
- Fuentes-Barría, H.; Aguilera-Eguía, R.; Urbano-Cerda, S.; Vera-Aguirre, V.; González-Wong, C. The role of vitamin D in preventing falls in subjects with sarcopenia part II: Older adult’s vitamin D requirements. Rev. Chil. Nutr. 2020, 47, 830–835. [Google Scholar] [CrossRef]
- Argano, C.; Natoli, G.; Mularo, S.; Nobili, A.; Monaco, M.L.; Mannucci, P.M.; Perticone, F.; Pietrangelo, A.; Corrao, S. Impact of Diabetes Mellitus and Its Comorbidities on Elderly Patients Hospitalized in Internal Medicine Wards: Data from the RePoSi Registry. Healthcare 2022, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Riek, A.E.; Oh, J.; Darwech, I.; Moynihan, C.E.; Bruchas, R.R.; Bernal-Mizrachi, C. 25(OH) vitamin D suppresses macrophage adhesion and migration by downregulation of ER stress and scavenger receptor A1 in type 2 diabetes. J. Steroid Biochem. Mol. Biol. 2014, 144, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Lin, B.; Deng, X.; Huang, K.; Zhang, Y.; Wang, N. VDR activation attenuates osteoblastic ferroptosis and senescence by stimulating the Nrf2/GPX4 pathway in age-related osteoporosis. Free Radic. Biol. Med. 2022, 193, 720–735. [Google Scholar] [CrossRef]
- El-Atifi, M.; Dreyfus, M.; Berger, F.; Wion, D. Expression of CYP2R1 and VDR in human brain pericytes: The neurovascular vitamin D autocrine/paracrine model. Neuroreport 2015, 26, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Zali, A.; Hajyani, S.; Salari, M.; Tajabadi-Ebrahimi, M.; Mortazavian, A.M.; Pakpour, B. Co-administration of probiotics and vitamin D reduced disease severity and complications in patients with Parkinson’s disease: A randomized controlled clinical trial. Psychopharmacology 2024, 241, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Muresan, G.C.; Hedesiu, M.; Lucaciu, O.; Boca, S.; Petrescu, N. Effect of Vitamin D on Bone Regeneration: A Review. Medicina 2022, 58, 1337. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, R.; Ma, H.; Chen, S.; Sun, J.; Qi, J.; Pang, Y.; An, J.; Su, Z. Dietary Magnesium Intake Level Modifies the Association Between Vitamin D and Insulin Resistance: A Large Cross-Sectional Analysis of American Adults. Front. Nutr. 2022, 9, 878665. [Google Scholar] [CrossRef]
- Chen, X.; Wan, Z.; Geng, T.; Zhu, K.; Li, R.; Lu, Q.; Lin, X.; Liu, S.; Chen, L.; Guo, Y.; et al. Vitamin D Status, Vitamin D Receptor Polymorphisms, and Risk of Microvascular Complications Among Individuals with Type 2 Diabetes: A Prospective Study. Diabetes Care 2023, 46, 270–277. [Google Scholar] [CrossRef]
- Argano, C.; Mirarchi, L.; Amodeo, S.; Orlando, V.; Torres, A.; Corrao, S. The Role of Vitamin D and Its Molecular Bases in Insulin Resistance, Diabetes, Metabolic Syndrome, and Cardiovascular Disease: State of the Art. Int. J. Mol. Sci. 2023, 24, 15485. [Google Scholar] [CrossRef]
- Melo, T.L.; Esper, P.L.G.; Zambrano, L.I.; Ormanji, M.S.; Rodrigues, F.G.; Heilberg, I.P. Expression of vitamin D receptor, CYP27B1 and CYP24A1 hydroxylases and 1,25-dihydroxyvitamin D3 levels in stone formers. Urolithiasis 2020, 48, 19–26. [Google Scholar] [CrossRef]
- Fakhfakh, R.; Feki, S.; Elleuch, A.; Neifar, M.; Marzouk, S.; Elloumi, N.; Hachicha, H.; Abida, O.; Bahloul, Z.; Ayadi, F.; et al. Vitamin D status and CYP27B1-1260 promoter polymorphism in Tunisian patients with systemic lupus erythematosus. Mol. Genet. Genom. Med. 2021, 9, e1618. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Witt, E.E.; Schubert, S.; Sotirchos, E.; Bhargava, P.; Mowry, E.M.; Sachs, K.; Bilen, B.; Steinman, L.; Awani, A.; et al. Peripheral T-Cells, B-Cells, and Monocytes from Multiple Sclerosis Patients Supplemented with High-Dose Vitamin D Show Distinct Changes in Gene Expression Profiles. Nutrients 2022, 14, 4737. [Google Scholar] [CrossRef]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Komisarenko, Y.I.; Bobryk, M.I. Vitamin D Deficiency and Immune Disorders in Combined Endocrine Pathology. Front. Endocrinol. 2018, 9, 600. [Google Scholar] [CrossRef] [PubMed]
- He, L.P.; Song, Y.X.; Zhu, T.; Gu, W.; Liu, C.W. Progress in the Relationship between Vitamin D Deficiency and the Incidence of Type 1 Diabetes Mellitus in Children. J. Diabetes Res. 2022, 2022, 5953562. [Google Scholar] [CrossRef]
- Wu, J.; Atkins, A.; Downes, M.; Wei, Z. Vitamin D in Diabetes: Uncovering the Sunshine Hormone’s Role in Glucose Metabolism and Beyond. Nutrients 2023, 15, 1997. [Google Scholar] [CrossRef]
- Wang, S.; Cai, B.; Han, X.; Gao, Y.; Zhang, X.; Wang, R.; Zhang, Y.; Chen, Q. Vitamin D supplementation for nonalcoholic fatty liver disease in type 2 diabetes mellitus: A protocol for a systematic review and meta-analysis. Medicine 2020, 99, e20148. [Google Scholar] [CrossRef]
- Sindhughosa, D.A.; Wibawa, I.D.N.; Mariadi, I.K.; Somayana, G. Additional treatment of vitamin D for improvement of insulin resistance in non-alcoholic fatty liver disease patients: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 7716. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Jeppesen, P.B. Vitamin D Deficiency Is Inversely Associated with Homeostatic Model Assessment of Insulin Resistance. Nutrients 2021, 13, 4358. [Google Scholar] [CrossRef] [PubMed]
- Mo, M.; Shao, B.; Xin, X.; Luo, W.; Si, S.; Jiang, W.; Wang, S.; Shen, Y.; Wu, J.; Yu, Y. The Association of Gene Variants in the Vitamin D Metabolic Pathway and Its Interaction with Vitamin D on Gestational Diabetes Mellitus: A Prospective Cohort Study. Nutrients 2021, 13, 4220. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, Z.; Hu, Y.; Wang, Y.; Wu, Y.; Lian, F.; Li, H.; Yang, J.; Xu, X. The Effects of Vitamin D Supplementation on Glycemic Control and Maternal-Neonatal Outcomes in Women with Established Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Clin. Nutr. 2021, 40, 3148–3157. [Google Scholar] [CrossRef]
- Huang, S.; Fu, J.; Zhao, R.; Wang, B.; Zhang, M.; Li, L.; Shi, C. The Effect of Combined Supplementation with Vitamin D and Omega-3 Fatty Acids on Blood Glucose and Blood Lipid Levels in Patients with Gestational Diabetes. Ann. Palliat. Med. 2021, 10, 5652–5658. [Google Scholar] [CrossRef]
- He, L.P.; Li, C.P.; Liu, C.W.; Gu, W. The Regulatory Effect of Vitamin D on Pancreatic Beta Cell Secretion in Patients with Type 2 Diabetes. Curr. Med. Chem. 2024, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Shi, D.; He, C.; Xia, G. Vitamin D Alleviates Type 2 Diabetes Mellitus by Mitigating Oxidative Stress-Induced Pancreatic β-Cell Impairment. Exp. Clin. Endocrinol. Diabetes 2023, 131, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Liu, X.; Cai, X.; Zou, F. Vitamin D Supplementation Induces CatG-Mediated CD4+ T Cell Inactivation and Restores Pancreatic β-Cell Function in Mice with Type 1 Diabetes. Am. J. Physiol. Endocrinol. Metab. 2022, 322, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Shi, Y.; Shang, Y.; Chen, X.; Xia, F. MicroRNA-378d Inhibits Glut4 by Targeting Rsbn1 in Vitamin D Deficient Ovarian Granulosa Cells. Mol. Med. Rep. 2021, 23, 369. [Google Scholar] [CrossRef] [PubMed]
- Ammar, M.; Heni, S.; Tira, M.S.; Khalij, Y.; Hamdouni, H.; Amor, D.; Ksibi, S.; Omezzine, A.; Bouslama, A. Variability in response to vitamin D supplementation according to vitamin D metabolism related gene polymorphisms in healthy adults. Eur. J. Clin. Nutr. 2022, 77, 189–194. [Google Scholar] [CrossRef]
- Hu, Z.; Tao, S.; Liu, H.; Pan, G.; Li, B.; Zhang, Z.; Hu, Z.; Tao, S.; Liu, H.; Pan, G.; et al. The Association between Polymorphisms of Vitamin D Metabolic-Related Genes and Vitamin D3 Supplementation in Type 2 Diabetic Patients. J. Diabetes Res. 2019, 2019, 8289741. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Achari, A.E.; Jain, S.K. Vitamin D Supplementation Inhibits Oxidative Stress and Upregulates SIRT1/AMPK/GLUT4 Cascade in High Glucose-Treated 3T3L1 Adipocytes and in Adipose Tissue of High Fat Diet-Fed Diabetic Mice. Arch. Biochem. Biophys. 2017, 615, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Konečná, M.; Poráčová, J.; Nagy, M.; Majherová, M.; Gaľová, J.; Gogaľová, Z.; Vašková, H.; Mydlárová Blaščáková, M.; Gruľová, D.; Sedlák, V. Level of Biochemical Parameters in Patients with Type 2 Diabetes Mellitus Depending on the Genotype of the FokI Polymorphism in the Vitamin D3 Receptor (VDR Gene). Cent. Eur. J. Public Health 2023, 31, S69–S74. [Google Scholar] [CrossRef]
- Farahmand, M.A.; Daneshzad, E.; Fung, T.T.; Zahidi, F.; Muhammadi, M.; Bellissimo, N.; Azadbakht, L. What is the Impact of Vitamin D Supplementation on Glycemic Control in People with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. BMC Endocr. Disord. 2023, 23, 15. [Google Scholar] [CrossRef]
- Afraie, M.; Bahrami, P.; Kohnepoushi, P.; Khateri, S.; Majidi, L.; Saed, L.; Zamani, K.; Baharm, H.M.; Moradi, Y.; Moradpour, F. The Effect of Vitamin D Supplementation on Glycemic Control and Cardiovascular Risk Factors in Type 2 Diabetes: An Updated Systematic Review and Meta-Analysis of Clinical Trials. J. Diabetes Res. 2024, 2024, 9960656. [Google Scholar] [CrossRef]
- Chen, W.; Liu, L.; Hu, F. Efficacy of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Obes. Metab. 2024, 26, 5713–5726. [Google Scholar] [CrossRef]
- Lu, S.; Cao, Z.B. Interplay Between Vitamin D and Adipose Tissue: Implications for Adipogenesis and Adipose Tissue Function. Nutrients 2023, 15, 4832. [Google Scholar] [CrossRef] [PubMed]
- Bantulà, M.; Tubita, V.; Roca-Ferrer, J.; Mullol, J.; Valero, A.; Bobolea, I.; Pascal, M.; de Hollanda, A.; Vidal, J.; Picado, C.; et al. Weight Loss and Vitamin D Improve Hyporesponsiveness to Corticosteroids in Obese Asthma. J. Investig. Allergol. Clin. Immunol. 2023, 33, 464–473. [Google Scholar] [CrossRef]
- Nimitphong, H.; Guo, W.; Holick, M.F.; Fried, S.K.; Lee, M.J. Vitamin D Inhibits Adipokine Production and Inflammatory Signaling Through the Vitamin D Receptor in Human Adipocytes. Obesity 2021, 29, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Lee, H.; Lim, Y. Effect of Vitamin D3 Supplementation on Hepatic Lipid Dysregulation Associated with Autophagy Regulatory AMPK/Akt-mTOR Signaling in Type 2 Diabetic Mice. Exp. Biol. Med. 2021, 246, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Lu, L.; Guo, K.; Lu, J.; Chen, H. Vitamin D Protects Against High Glucose-Induced Pancreatic β-Cell Dysfunction via AMPK-NLRP3 Inflammasome Pathway. Mol. Cell. Endocrinol. 2022, 547, 111596. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, H.; Tang, S.; Wu, X.; Wang, J.; Yi, B.; Liu, J.; Li, Z.; Liao, Q.; Li, A.; et al. Vitamin D-VDR (Vitamin D Receptor) Alleviates Glucose Metabolism Reprogramming in Lipopolysaccharide-Induced Acute Kidney Injury. Front. Physiol. 2023, 14, 1083643. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Yi, B.; Han, H.; Yang, S.; Hu, Z.; Zheng, L.; Wang, J.; Liao, Q.; Zhang, H. Vitamin D-VDR (Vitamin D Receptor) Regulates Defective Autophagy in Renal Tubular Epithelial Cells in Streptozotocin-Induced Diabetic Mice via the AMPK Pathway. Autophagy 2022, 18, 877–890. [Google Scholar] [CrossRef]
- An, J.H.; Cho, D.H.; Lee, G.Y.; Kang, M.S.; Kim, S.J.; Han, S.N. Effects of Vitamin D Supplementation on CD4+ T Cell Subsets and mTOR Signaling Pathway in High-Fat-Diet-Induced Obese Mice. Nutrients 2021, 13, 796. [Google Scholar] [CrossRef] [PubMed]
- Khodir, S.A.; Samaka, R.M.; Ameen, O. Autophagy and mTOR Pathways Mediate the Potential Renoprotective Effects of Vitamin D on Diabetic Nephropathy. Int. J. Nephrol. 2020, 2020, 7941861. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.J.; Li, X.N.; Xiao, Z.; Li, C.Y.; Jia, L.H. Low Vitamin D During Pregnancy Is Associated with Infantile Eczema by Up-Regulation of PI3K/AKT/mTOR Signaling Pathway and Affecting FOXP3 Expression: A Bidirectional Cohort Study. J. Nutr. Biochem. 2024, 124, 109516. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.T.; Halim, S.A.; Santos, J.M.; Khan, F.; Khan, A.; Rahman, M.M.; Hussain, F.; Al-Harrasi, A.; Gollahon, L.S.; Rahman, S.M. A High Dose of Calcitriol Inhibits Glycolysis and M2 Macrophage Polarization in the Tumor Microenvironment by Repressing mTOR Activation: In Vitro and Molecular Docking Studies. Cell. Physiol. Biochem. 2023, 57, 105–122. [Google Scholar] [PubMed]
- Yuan, Q.; Zhang, R.; Sun, M.; Guo, X.; Yang, J.; Bian, W.; Xie, C.; Miao, D.; Mao, L. Sirt1 Mediates Vitamin D Deficiency-Driven Gluconeogenesis in the Liver via mTorc2/Akt Signaling. J. Diabetes Res. 2022, 2022, 1755563. [Google Scholar] [CrossRef]
- Mutt, S.J.; Raza, G.S.; Mäkinen, M.J.; Keinänen-Kiukaanniemi, S.; Järvelin, M.R.; Herzig, K.H. Vitamin D Deficiency Induces Insulin Resistance and Re-Supplementation Attenuates Hepatic Glucose Output via the PI3K-AKT-FOXO1 Mediated Pathway. Mol. Nutr. Food Res. 2020, 64, 1900728. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qin, R. Vitamin D3 Affects Browning of White Adipocytes by Regulating Autophagy via PI3K/Akt/mTOR/p53 Signaling In Vitro and In Vivo. Apoptosis 2022, 27, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Provvisiero, D.P.; Negri, M.; Amatrudo, F.; Patalano, R.; Montò, T.; de Angelis, C.; Graziadio, C.; Pugliese, G.; de Alteriis, G.; Colao, A.; et al. 1,25-Dihydroxyvitamin D3 Mitigates the Adipogenesis Induced by Bisphenol A in 3T3-L1 and hAMSC Through miR-27-3p Regulation. Int. J. Obes. 2024, 48, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, A.F.; Ashfaq, F.; Alsayegh, A.A.; Bajahzer, M.; Khan, M.I.; Beg, M.M.A. MiRNA-200a and MiRNA-200b Expression, and Vitamin-D Level: Prognostic Significance in Obese Non-Diabetic and Obese Type 2 Diabetes Mellitus Individuals. World J. Clin. Cases 2024, 12, 6916–6925. [Google Scholar] [CrossRef]
- Sheane, B.J.; Smyth, P.; Scott, K.; Aziz, R.; Buckley, M.; Lodge, E.; Kiely, N.; Kingston, M.; McGovern, E.; Healy, M.; et al. An Association Between MicroRNA-21 Expression and Vitamin D Deficiency in Coronary Artery Disease. Microrna 2015, 4, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; John, S.M.; Carrera-Bastos, P.; Schmitz, G. MicroRNA-21-Enriched Exosomes as Epigenetic Regulators in Melanomagenesis and Melanoma Progression: The Impact of Western Lifestyle Factors. Cancers 2020, 12, 2111. [Google Scholar] [CrossRef]
- Mohamed Mekawy, D.; Eissa, M.; Adly Sadik, N.; Mohamed Abd-Elrahman, R.; Fawzy, A.; Fathy Amer, M. Vitamin D and miRNA-155 in Behçet’s Disease: Possible Association with the Disease and Disease Activity. Rep. Biochem. Mol. Biol. 2023, 12, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, W.; Sun, T.; Huang, Y.; Wang, Y.; Deb, D.K.; Yoon, D.; Kong, J.; Thadhani, R.; Li, Y.C. 1,25-Dihydroxyvitamin D Promotes Negative Feedback Regulation of TLR Signaling via Targeting MicroRNA-155-SOCS1 in Macrophages. J. Immunol. 2013, 190, 3687–3695. [Google Scholar] [CrossRef]
- Wan Nik, W.N.F.H.; Zulkeflee, H.A.; Ab Rahim, S.N.; Tuan Ismail, T.S. Association of vitamin D and magnesium with insulin sensitivity and their influence on glycemic control. World J. Diabetes 2023, 14, 26–34. [Google Scholar] [CrossRef]
- Pires, C. Superfoods for Type 2 Diabetes: A Narrative Review and Proposal for New International Recommendations. Medicina 2023, 59, 1184. [Google Scholar] [CrossRef]
- Abdo, B.; Abdullah, M.; AlShoaibi, I.A.; Ahmed, F.; Alawdi, R.; Alzanen, K.; Algaadi, K. Relationship Between Glycated Hemoglobin (HbA1c) and Vitamin D Levels in Type 2 Diabetes Patients: A Retrospective Cross-Sectional Study. Cureus 2024, 16, 62468. [Google Scholar] [CrossRef] [PubMed]
- Abukanna, A.M.A.; Alanazi, R.F.A.; Alruwaili, F.S.; Alayyashi, A.Z.M.; Alanzi, F. Vitamin D Deficiency as a Risk Factor for Diabetes and Poor Glycemic Control in Saudi Arabia: A Systematic Review. Cureus 2023, 15, 48577. [Google Scholar] [CrossRef]
- Alqahtani, R.M.; Alsulami, E.F. The Association Between Glycated Hemoglobin (HbA1c) Level and Vitamin D Level in Diabetes Mellitus Patients: A Cross-Sectional Study. Cureus 2023, 15, 47166. [Google Scholar] [CrossRef]
- Akhter, A.; Alouffi, S.; Shahab, U.; Akasha, R.; Fazal-Ur-Rehman, M.; Ghoniem, M.E.; Ahmad, N.; Kaur, K.; Pandey, R.P.; Alshammari, A.; et al. Vitamin D supplementation modulates glycated hemoglobin (HBA1c) in diabetes mellitus. Arch. Biochem. Biophys. 2024, 753, 109911. [Google Scholar] [CrossRef]
- Felício, J.S.; de Rider Britto, H.A.; Cortez, P.C.; de Souza Resende, F.; de Lemos, M.N.; de Moraes, L.V.; de Aquino, V.T.; de Souza Parente, F.; de Queiroz, N.N.M.; Abrahão Neto, J.F.; et al. Association Between 25(OH)Vitamin D, HbA1c and Albuminuria in Diabetes Mellitus: Data from a Population-Based Study (VIDAMAZON). Front. Endocrinol. 2021, 12, 723502. [Google Scholar] [CrossRef]
- Lu, L.; Lu, Q.; Chen, W.; Li, J.; Li, C.; Zheng, Z. Vitamin D3 Protects Against Diabetic Retinopathy by Inhibiting High-Glucose-Induced Activation of the ROS/TXNIP/NLRP3 Inflammasome Pathway. J. Diabetes Res. 2018, 2018, 8193523. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, S.; Guo, H. Active Vitamin D and Vitamin D Receptor Help Prevent High Glucose-Induced Oxidative Stress of Renal Tubular Cells via AKT/UCP2 Signaling Pathway. Biomed. Res. Int. 2019, 2019, 9013904. [Google Scholar] [CrossRef]
- Mocayar Marón, F.J.; Ferder, L.; Reiter, R.J.; Manucha, W. Daily and Seasonal Mitochondrial Protection: Unraveling Common Possible Mechanisms Involving Vitamin D and Melatonin. J. Steroid Biochem. Mol. Biol. 2020, 199, 105595. [Google Scholar] [CrossRef]
- Shan, Z.; Fa, W.H.; Tian, C.R.; Yuan, C.S.; Jie, N. Mitophagy and Mitochondrial Dynamics in Type 2 Diabetes Mellitus Treatment. Aging 2022, 14, 2902–2919. [Google Scholar] [CrossRef]
- Phillips, E.A.; Hendricks, N.; Bucher, M.; Maloyan, A. Vitamin D Supplementation Improves Mitochondrial Function and Reduces Inflammation in Placentae of Obese Women. Front. Endocrinol. 2022, 13, 893848. [Google Scholar] [CrossRef]
- Liu, J.; Li, N.; Zhu, Z.; Kiang, K.M.; Ng, A.C.K.; Dong, C.M.; Leung, G.K. Vitamin D Enhances Hematoma Clearance and Neurologic Recovery in Intracerebral Hemorrhage. Stroke 2022, 53, 2058–2068. [Google Scholar] [CrossRef]
- Sadie-Van Gijsen, H. The Regulation of Marrow Fat by Vitamin D: Molecular Mechanisms and Clinical Implications. Curr. Osteoporos. Rep. 2019, 17, 405–415. [Google Scholar] [CrossRef]
- Xu, W.; Hu, X.; Qi, X.; Zhu, R.; Li, C.; Zhu, Y.; Yin, S.; Cheng, L.; Zhu, R. Vitamin D Ameliorates Angiotensin II-Induced Human Endothelial Progenitor Cell Injury via the PPAR-γ/HO-1 Pathway. J. Vasc. Res. 2019, 56, 17–27. [Google Scholar] [CrossRef]
- Di Filippo, L.; De Lorenzo, R.; Giustina, A.; Rovere-Querini, P.; Conte, C. Vitamin D in Osteosarcopenic Obesity. Nutrients 2022, 14, 1816. [Google Scholar] [CrossRef]
- Jing, W.; Xiong, Z.; Cai, X.; Huang, Y.; Li, X.; Yang, X.; Liu, L.; Tang, W.; Lin, Y.; Tian, W. Effects of Gamma-Secretase Inhibition on the Proliferation and Vitamin D3-Induced Osteogenesis in Adipose-Derived Stem Cells. Biochem. Biophys. Res. Commun. 2010, 392, 442–447. [Google Scholar] [CrossRef]
- Cominacini, M.; Fumaneri, A.; Ballerini, L.; Braggio, M.; Valenti, M.T.; Dalle Carbonare, L. Unraveling the Connection: Visceral Adipose Tissue and Vitamin D Levels in Obesity. Nutrients 2023, 15, 4259. [Google Scholar] [CrossRef]
- Mirza, I.; Mohamed, A.; Deen, H.; Balaji, S.; Elsabbahi, D.; Munasser, A.; Naquiallah, D.; Abdulbaseer, U.; Hassan, C.; Masrur, M.; et al. Obesity-Associated Vitamin D Deficiency Correlates with Adipose Tissue DNA Hypomethylation, Inflammation, and Vascular Dysfunction. Int. J. Mol. Sci. 2022, 23, 14377. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Y.; Wang, Q.; Yan, L.; Fu, X.; Xiao, R. Convergent Alteration of the Mesenchymal Stem Cell Heterogeneity in Adipose Tissue During Aging. FASEB J. 2023, 37, 23114. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Hong, C.G.; Hu, W.B.; Chen, M.L.; Duan, R.; Li, H.M.; Yue, T.; Cao, J.; Wang, Z.X.; Chen, C.Y.; et al. Autophagy Receptor OPTN (Optineurin) Regulates Mesenchymal Stem Cell Fate and Bone-Fat Balance During Aging by Clearing FABP3. Autophagy 2021, 17, 2766–2782. [Google Scholar] [CrossRef]
- Amri, E.Z. Beige or Brite Adipocytes of the Adipose Organ: Link with White and Brown Adipocytes. Ann. Endocrinol. 2024, 85, 253–254. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Y.; Song, Y.; Wang, J.; Han, Y.; Yang, N.; Lin, H.; Yin, Y.; Han, X. PPA1 Promotes Adipogenesis by Regulating the Stability of C/EBPs. Cell Death Differ. 2024, 31, 1044–1056. [Google Scholar] [CrossRef]
- An, S.M.; Cho, S.H.; Yoon, J.C. Adipose Tissue and Metabolic Health. Diabetes Metab. J. 2023, 47, 595–611. [Google Scholar] [CrossRef]
- Hafidi, M.E.; Buelna-Chontal, M.; Sánchez-Muñoz, F.; Carbó, R. Adipogenesis: A Necessary but Harmful Strategy. Int. J. Mol. Sci. 2019, 20, 3657. [Google Scholar] [CrossRef]
- Nunn, E.R.; Shinde, A.B.; Zaganjor, E. Weighing in on Adipogenesis. Front. Physiol. 2022, 13, 821278. [Google Scholar] [CrossRef]
- Pant, R.; Firmal, P.; Shah, V.K.; Alam, A.; Chattopadhyay, S. Epigenetic Regulation of Adipogenesis in the Development of Metabolic Syndrome. Front. Cell Dev. Biol. 2020, 8, 619888. [Google Scholar] [CrossRef]
- White, U. Adipose Tissue Expansion in Obesity, Health, and Disease. Front. Cell Dev. Biol. 2023, 11, 1188844. [Google Scholar] [CrossRef]
- Emadzadeh, M.; Rashidmayvan, M.; Sahebi, R.; Sadeghi, R.; Ferns, G.A.; Ghayour-Mobarhan, M. The effect of vitamin D fortified products on anthropometric indices: A systematic review and meta-analysis. Complement. Ther. Clin. Pract. 2020, 41, 101242. [Google Scholar] [CrossRef]
- Musazadeh, V.; Zarezadeh, M.; Ghalichi, F.; Kalajahi, F.H.; Ghoreishi, Z. Vitamin D supplementation positively affects anthropometric indices: Evidence obtained from an umbrella meta-analysis. Front. Nutr. 2022, 9, 980749. [Google Scholar] [CrossRef] [PubMed]
- Perna, S. Is Vitamin D Supplementation Useful for Weight Loss Programs? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina 2019, 55, 368. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Daouk, R.; Azar, J.; Sapudom, J.; Teo, J.C.M.; Abou-Kheir, W.; Al-Sayegh, M. Modeling Adipogenesis: Current and Future Perspective. Cells 2020, 9, 2326. [Google Scholar] [CrossRef]
- Shantavasinkul, P.C.; Nimitphong, H. Vitamin D and Visceral Obesity in Humans: What Should Clinicians Know? Nutrients 2022, 14, 3075. [Google Scholar] [CrossRef] [PubMed]
- Bennour, I.; Haroun, N.; Sicard, F.; Mounien, L.; Landrier, J.F. Recent insights into vitamin D, adipocyte, and adipose tissue biology. Obes. Rev. 2022, 23, 13453. [Google Scholar] [CrossRef] [PubMed]
- Lefterova, M.I.; Haakonsson, A.K.; Lazar, M.A.; Mandrup, S. PPARγ and the Global Map of Adipogenesis and Beyond. Trends Endocrinol. Metab. 2014, 25, 293–302. [Google Scholar] [CrossRef]
- Furuhashi, M. Fatty Acid-Binding Protein 4 in Cardiovascular and Metabolic Diseases. J. Atheroscler. Thromb. 2019, 26, 216–232. [Google Scholar] [CrossRef] [PubMed]
- Baboota, R.K.; Blüher, M.; Smith, U. Emerging Role of Bone Morphogenetic Protein 4 in Metabolic Disorders. Diabetes 2021, 70, 303–312. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, G.L.; Zhu, X.; Peng, T.H.; Lv, Y.C. Critical Roles of FTO-Mediated mRNA m6A Demethylation in Regulating Adipogenesis and Lipid Metabolism: Implications in Lipid Metabolic Disorders. Genes Dis. 2021, 9, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhao, Y.; Wu, R.; Jiang, Q.; Cai, M.; Bi, Z.; Liu, Y.; Yao, Y.; Feng, J.; Wang, Y.; et al. ZFP217 Regulates Adipogenesis by Controlling Mitotic Clonal Expansion in a METTL3-m^6A-Dependent Manner. RNA Biol. 2019, 16, 1785–1793. [Google Scholar] [CrossRef]
- Cai, M.; Liu, Q.; Jiang, Q.; Wu, R.; Wang, X.; Wang, Y. Loss of m6A on FAM134B Promotes Adipogenesis in Porcine Adipocytes Through m^6A-YTHDF2-Dependent Way. IUBMB Life 2019, 71, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, S.; Gerini, G.; Megiorni, F.; Pontecorvi, P.; Messina, E.; Camero, S.; Anastasiadou, E.; Romano, E.; Onesti, M.G.; Napoli, C.; et al. Inhibiting DNA Methylation as a Strategy to Enhance Adipose-Derived Stem Cells Differentiation: Focus on the Role of Akt/mTOR and Wnt/β-Catenin Pathways on Adipogenesis. Front. Cell Dev. Biol. 2022, 10, 926180. [Google Scholar] [CrossRef] [PubMed]
- Bertolio, R.; Napoletano, F.; Mano, M.; Maurer-Stroh, S.; Fantuz, M.; Zannini, A.; Bicciato, S.; Sorrentino, G.; Del Sal, G. Sterol Regulatory Element Binding Protein 1 Couples Mechanical Cues and Lipid Metabolism. Nat. Commun. 2019, 10, 1326. [Google Scholar] [CrossRef]
- García-Niño, W.R.; Zazueta, C. New Insights of Krüppel-Like Transcription Factors in Adipogenesis and the Role of Their Regulatory Neighbors. Life Sci. 2021, 265, 118763. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Molina, E.; González, N.Y.; Low-Padilla, E.; Oliveros-Velásquez, J.D.; Mendivelso-Duarte, F.; Gómez-Gómez, O.V.; Castillo, A.N.; Barrero-Garzón, L.I.; Álvarez-Moreno, C.A.; Moscoso-Martínez, E.A. Recommendations for the rational use of the 25-hydroxyvitamin D test Policy Brief. Rev. Colomb. Nefrol. 2019, 6, 179–192. [Google Scholar]
- Jablonski, N.G.; Chaplin, G. The roles of vitamin D and cutaneous vitamin D production in human evolution and health. Int. J. Paleopathol. 2018, 23, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Leary, P.F.; Zamfirova, I.; Au, J.; McCracken, W.H. Effect of Latitude on Vitamin D Levels. J. Am. Osteopath. Assoc. 2017, 117, 433–439. [Google Scholar] [CrossRef]
- Kift, R.C.; Webb, A.R. Globally Estimated UVB Exposure Times Required to Maintain Sufficiency in Vitamin D Levels. Nutrients 2024, 16, 1489. [Google Scholar] [CrossRef] [PubMed]
- Quigley, M.; Rieger, S.; Capobianco, E.; Wang, Z.; Zhao, H.; Hewison, M.; Lisse, T.S. Vitamin D Modulation of Mitochondrial Oxidative Metabolism and mTOR Enforces Stress Adaptations and Anticancer Responses. JBMR Plus 2021, 6, 10572. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Patel, J.; Rait, G.; Shroff, R. Hypervitaminosis D and Nephrocalcinosis: Too Much of a Good Thing? Pediatr. Nephrol. 2022, 37, 2225–2229. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.R. Vitamin D toxicity related to its physiological and unphysiological supply. Trends. Endocrinol. Metab. 2021, 32, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Cojic, M.; Kocic, R.; Klisic, A.; Kocic, G. The Effects of Vitamin D Supplementation on Metabolic and Oxidative Stress Markers in Patients with Type 2 Diabetes: A 6-Month Follow-Up Randomized Controlled Study. Front. Endocrinol. 2021, 12, 610893. [Google Scholar] [CrossRef]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ceglia, L.; Chadha, C.; Chatterjee, R.; et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 520–530. [Google Scholar] [CrossRef]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The Health Effects of Vitamin D Supplementation: Evidence from Human Studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Embleton, N.D.; Moltu, S.J.; Lapillonne, A.; van den Akker, C.H.P.; Carnielli, V.; Fusch, C.; Gerasimidis, K.; van Goudoever, J.B.; Haiden, N.M.; Iacobelli, S.; et al. Enteral Nutrition in Preterm Infants (2022): A Position Paper from the ESPGHAN Committee on Nutrition and Invited Experts. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.M.; Hart, K.H.; Williams, E.L.; Mendis, J.; Lanham-New, S.A.; Botelho, P.B. Vitamin D Supplementation and Sunlight Exposure on Serum Vitamin D Concentrations in 2 Parallel, Double-Blind, Randomized, Placebo-Controlled Trials. J. Nutr. 2021, 151, 3137–3150. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight, UV Radiation, Vitamin D, and Skin Cancer: How Much Sunlight Do We Need? Adv. Exp. Med. Biol. 2020, 1268, 19–36. [Google Scholar]
- Kruger, M.C.; Chan, Y.M.; Lau, C.; Lau, L.T.; Chin, Y.S.; Kuhn-Sherlock, B.; Schollum, L.M.; Todd, J.M. Fortified Milk Supplementation Improves Vitamin D Status, Grip Strength, and Maintains Bone Density in Chinese Premenopausal Women Living in Malaysia. Biores. Open Access 2019, 8, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.S. Lessons Learned from a Randomized Controlled Trial with Vitamin D Fortified Milk in Colombian Adolescents: Importance to Vitamin D Fortification Policies in Latin America. J. Nutr. 2023, 153, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Itkonen, S.T.; Erkkola, M.; Lamberg-Allardt, C.J.E. Vitamin D Fortification of Fluid Milk Products and Their Contribution to Vitamin D Intake and Vitamin D Status in Observational Studies—A Review. Nutrients 2018, 10, 1054. [Google Scholar] [CrossRef] [PubMed]
- Joo, N.S.; Shin, S.H.; Kim, K.N.; Lee, S.H.; Jung, S.; Yeum, K.J. Home Meal Replacement Fortified with Eggshell Powder and Vitamin D Prevents Bone Loss in Postmenopausal Women: A Randomized, Double-Blind, Controlled Study. Nutrients 2024, 16, 1152. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The Vitamin D Deficiency Pandemic: Approaches for Diagnosis, Treatment, and Prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Vasdeki, D.; Tsamos, G.; Dimakakos, E.; Patriarcheas, V.; Koufakis, T.; Kotsa, K.; Cholewka, A.; Stanek, A. Vitamin D Supplementation: Shedding Light on the Role of the Sunshine Vitamin in the Prevention and Management of Type 2 Diabetes and Its Complications. Nutrients 2024, 16, 3651. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Sharma, P.; Girgis, C.M.; Gunton, J.E. Vitamin D and Beta Cells in Type 1 Diabetes: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 14434. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zheng, Y.; Wang, P.; Zhang, Y. The Effect of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Md Isa, Z.; Amsah, N.; Ahmad, N. The Impact of Vitamin D Deficiency and Insufficiency on the Outcome of Type 2 Diabetes Mellitus Patients: A Systematic Review. Nutrients 2023, 15, 2310. [Google Scholar] [CrossRef] [PubMed]
- Sukik, A.; Alalwani, J.; Ganji, V. Vitamin D, Gut Microbiota, and Cardiometabolic Diseases—A Possible Three-Way Axis. Int. J. Mol. Sci. 2023, 24, 940. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Bilezikian, J.P.; Adler, R.A.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Bollerslev, J.; Bouillon, R.; Brandi, M.L.; Casanueva, F.F.; et al. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocr. Rev. 2024, 45, 625–654. [Google Scholar] [CrossRef]
- Naik, P.P.; Farrukh, S.N. Influence of Ethnicities and Skin Color Variations in Different Populations: A Review. Skin Pharmacol. Physiol. 2022, 35, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Sakamoto, Y.; Honda, Y.; Sasaki, T.; Igeta, Y.; Ogishima, D.; Matsuoka, S.; Kim, S.-G.; Ishijima, M.; Miyagawa, K. Estimation of the Vitamin D Status of Pregnant Japanese Women Based on Food Intake and VD Synthesis by Solar UV-B Radiation Using a Questionnaire and UV-B Observations. J. Steroid Biochem. Mol. Biol. 2023, 229, 106272. [Google Scholar] [CrossRef] [PubMed]
- Zalneraitis, B.H.; Huuki, E.; Benavides, L.C.; Benavides, J.M. Relation of Vitamin D Level, BMI, and Location of Lower Extremity Stress Fractures in Military Trainees. Mil. Med. 2023, 188, 1970–1974. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yan, T.; Li, Z.; Zhou, S.; Peng, W.; Cui, W.; Xu, J.; Cao, Z.-B.; Shi, L.; Wang, Y. Effects of Endurance Exercise and Vitamin D Supplementation on Insulin Resistance and Plasma Lipidome in Middle-Aged Adults with Type 2 Diabetes. Nutrients 2023, 15, 3027. [Google Scholar] [CrossRef] [PubMed]
- Thacher, T.D.; Dudenkov, D.V.; Mara, K.C.; Maxson, J.A.; Wi, C.I.; Juhn, Y.J. The Relationship of 25-Hydroxyvitamin D Concentrations and Individual-Level Socioeconomic Status. J. Steroid Biochem. Mol. Biol. 2020, 197, 105545. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, M.C.C.; de Castro, R.M.; Mendes, T.V.; do Carmo, M.A.V.; Sampaio, E.S.; Corona, L.P.; Lima, D.B.; Raposo, A.; Alasqah, I.; Alqarawi, N.; et al. Relationship Between Vitamin D Insufficiency and Anemia in Older Adults: An Approach Considering Clinical Aspects and Food Insecurity. Nutrients 2024, 16, 3669. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Veronese, N.; Marrone, E.; Di Palermo, C.; Iommi, C.; Ruggirello, R.; Caffarelli, C.; Gonnelli, S.; Barbagallo, M. Vitamin D and Risk of Incident Type 2 Diabetes in Older Adults: An Updated Systematic Review and Meta-Analysis. Nutrients 2024, 16, 1561. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Daneshvar, M.; Jibril, A.T.; Sluyter, J.D.; Waterhouse, M.; Romero, B.D.; Neale, R.E.; Manson, J.E.; Shab-Bidar, S. Serum 25(OH)D Concentration, Vitamin D Supplementation, and Risk of Cardiovascular Disease and Mortality in Patients with Type 2 Diabetes or Prediabetes: A Systematic Review and Dose-Response Meta-Analysis. Am. J. Clin. Nutr. 2023, 118, 697–707. [Google Scholar] [CrossRef] [PubMed]
| Category | Variable/Factor | Criteria/Description | Ref. |
|---|---|---|---|
| Diagnostic Criteria | Plasma glucose | ≥126 mg/dL. | [35] |
| Oral glucose tolerance | 2 h post-load ≥200 mg/dL. | [35] | |
| Hemoglobin A1c | ≥6.5%. | [35] | |
| Random plasma glucose | ≥200 mg/dL with classic symptoms. | [35] | |
| C-peptide | Relatively preserved. | [35] | |
| Ketoacidosis | Less frequent. | [35] | |
| Risk Factors | Obesity | Excess body fat. | [40] |
| Sedentary lifestyle | Low physical activity. | [41] | |
| Family history | Relatives with type 2 diabetes. | [42] | |
| Age | Predominantly adults (though can present in children). | [43] | |
| Diet high in fats and sugars | Sugary, fatty diet linked to insulin resistance. | [44] | |
| Hypertension | High blood pressure. | [45] | |
| High cholesterol levels | Elevated Low-Density Lipoprotein and Triglycerides. | [46] |
| Gene | Function | Characteristics | Ref. |
|---|---|---|---|
| C/EBPα | Induces PPARγ expression, crucial for early adipocyte differentiation. | Activates genes involved in lipid metabolism. | [113] |
| PPARγ | Regulates fat cell differentiation and adipogenesis. | Activated by lipids, promotes adipocyte differentiation. | [137] |
| FABP4 | Modulates adipogenesis by influencing PPARγ activity. | Links metabolism to inflammation, elevated levels associated with obesity and insulin resistance. | [138] |
| BMP4 | Regulates precursor cell commitment to adipocytes. | Downregulates PDGFRβ, promotes adipogenic differentiation, induces PPARγ expression. | [139] |
| FTO | Modulates mRNA stability, impacting early adipogenesis. | Demethylase activity affects lipid metabolism and adipocyte differentiation. | [140] |
| YTHDF2 | Influences mRNA stability, inhibits adipogenesis. | Degrades m6A-modified mRNAs, affecting cell cycle and differentiation. | [141,142] |
| mTOR | Key regulator of growth and metabolism, affecting adipocyte differentiation. | Coordinates with lysosomes during adipogenesis, regulates energy metabolism and insulin signaling. | [143] |
| SSREBP1 | Regulates lipid synthesis during adipogenesis. | Induces PPARγ expression, involved in lipid accumulation and adipogenesis. | [144] |
| KLF5 | Induces early adipocyte differentiation, works with other transcription factors. | Part of complex networks influenced by growth factors, circadian proteins, and regulatory molecules. | [145] |
| Levels | Netherlands | Institute of Medicine | International Osteoporosis Foundation and American Geriatrics Society | Expert Opinion |
|---|---|---|---|---|
| Severe Deficiency | 10–12 ng/mL 25–30 nmol/L | 10–12 ng/mL 25–30 nmol/L | 10–12 ng/mL 25–30 nmol/L | 10–12 ng/mL 25–30 nmol/L |
| Slight Deficiency | N/A | <20 ng/mL <50 nmol/L | <30 ng/mL <75 nmol/L | <40 ng/mL <100 nmol/L |
| Adequate | >10–12 ng/mL >25–30 nmol/L | >20 ng/mL >50 nmol/L | >30 ng/mL >75 nmol/L | >40 ng/mL >100 nmol/L |
| Years | Institute of Medicine | Deficiency Risk for the Endocrine Society | ||||
|---|---|---|---|---|---|---|
| AI (μg/UI) | EAR (μg/UI) | RDA (μg/IU) | UL (μg/IU) | IU | UL (IU) | |
| 0 to 0.5 | 10/400 | N/A | N/A | 25/1000 | 400 to 1000 | 2000 |
| 0.5 to 1 | 10/400 | N/A | N/A | 38/1500 | 400 to 1000 | 2000 |
| 1 to 3 | N/A | 10/400 | 15/600 | 63/2500 | 600 to 1000 | 4000 |
| 4 to 8 | N/A | 10/400 | 15/600 | 75/3000 | 600 to 1000 | 4000 |
| 9 to 13 | N/A | 10/400 | 15/600 | 100/4000 | 600 to 1000 | 4000 |
| 14 to 18 | N/A | 10/400 | 15/600 | 100/4000 | 600 to 1000 | 4000 |
| 19 to 30 | N/A | 10/400 | 15/600 | 100/4000 | 1500 to 2000 | 10,000 |
| 31 to 50 | N/A | 10/400 | 15/600 | 100/4000 | 1500 to 2000 | 10,000 |
| 51 to 70 | N/A | 10/400 | 15/600 | 100/4000 | 1500 to 2000 | 10,000 |
| >70 | N/A | 10/400 | 20/800 | 100/4000 | 1500 to 2000 | 10,000 |
| Purpose | Hazard Ratio (95% CI) | Ref |
|---|---|---|
| Evaluate whether administration of vitamin D decreases risk for diabetes among people with prediabetes. | 0.85 [95% CI, 0.75 to 0.96] | [1] |
| Assess whether vitamin D supplementation reduces the risk of type 2 diabetes in people with prediabetes. | 0.89 (95% CI 0.80 to 0.99; I2 = 0%) | [5] |
| Investigating whether low serum 25OHD can predict the onset of diabetes in prospective studies among older adults. | 1.31 (95% CI, 1.11–1.54; I2 = 37%) | [8] |
| Examine the therapeutic effects of vitamin D supplementation versus placebo on glycemic control, pregnancy complications, and newborn outcomes in pregnant women diagnosed with Gestational diabetes mellitus. | −10.20 (95% CI, −13.43 to −6.96, I2 = 80%) | [82] |
| Evaluate the effects of oral vitamin D supplementation on glycemic control in type 2 diabetes patients compared with a placebo, and to assess various factors’ influences on supplementation effects. | −0.57 (95%CI: −1.09 to −0.04; I2 = 83%) | [165] |
| Examining whether hypovitaminosis D can predict incident diabetes in prospective longitudinal studies conducted among older adults. | 1.20 (95% CI, 1.06 to 1.35, I2 = 29.9%) | [176] |
| Evaluate the association between vitamin D status and all-cause mortality and cardiovascular disease in people with type 2 diabetes. | 1.36 (95% CI, 1.23 to 1.49, I2 = 57%) | [177] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes-Barría, H.; Aguilera-Eguía, R.; Flores-Fernández, C.; Angarita-Davila, L.; Rojas-Gómez, D.; Alarcón-Rivera, M.; López-Soto, O.; Maureira-Sánchez, J. Vitamin D and Type 2 Diabetes Mellitus: Molecular Mechanisms and Clinical Implications—A Narrative Review. Int. J. Mol. Sci. 2025, 26, 2153. https://doi.org/10.3390/ijms26052153
Fuentes-Barría H, Aguilera-Eguía R, Flores-Fernández C, Angarita-Davila L, Rojas-Gómez D, Alarcón-Rivera M, López-Soto O, Maureira-Sánchez J. Vitamin D and Type 2 Diabetes Mellitus: Molecular Mechanisms and Clinical Implications—A Narrative Review. International Journal of Molecular Sciences. 2025; 26(5):2153. https://doi.org/10.3390/ijms26052153
Chicago/Turabian StyleFuentes-Barría, Héctor, Raúl Aguilera-Eguía, Cherie Flores-Fernández, Lissé Angarita-Davila, Diana Rojas-Gómez, Miguel Alarcón-Rivera, Olga López-Soto, and Juan Maureira-Sánchez. 2025. "Vitamin D and Type 2 Diabetes Mellitus: Molecular Mechanisms and Clinical Implications—A Narrative Review" International Journal of Molecular Sciences 26, no. 5: 2153. https://doi.org/10.3390/ijms26052153
APA StyleFuentes-Barría, H., Aguilera-Eguía, R., Flores-Fernández, C., Angarita-Davila, L., Rojas-Gómez, D., Alarcón-Rivera, M., López-Soto, O., & Maureira-Sánchez, J. (2025). Vitamin D and Type 2 Diabetes Mellitus: Molecular Mechanisms and Clinical Implications—A Narrative Review. International Journal of Molecular Sciences, 26(5), 2153. https://doi.org/10.3390/ijms26052153






