Genome-Wide Identification and Expression Profiling of the Invertase Genes Involved in Sugar Metabolism and Accumulation in Actinidia arguta
Abstract
1. Introduction
2. Results
2.1. Genome-Wide Identification of INVs in A. arguta
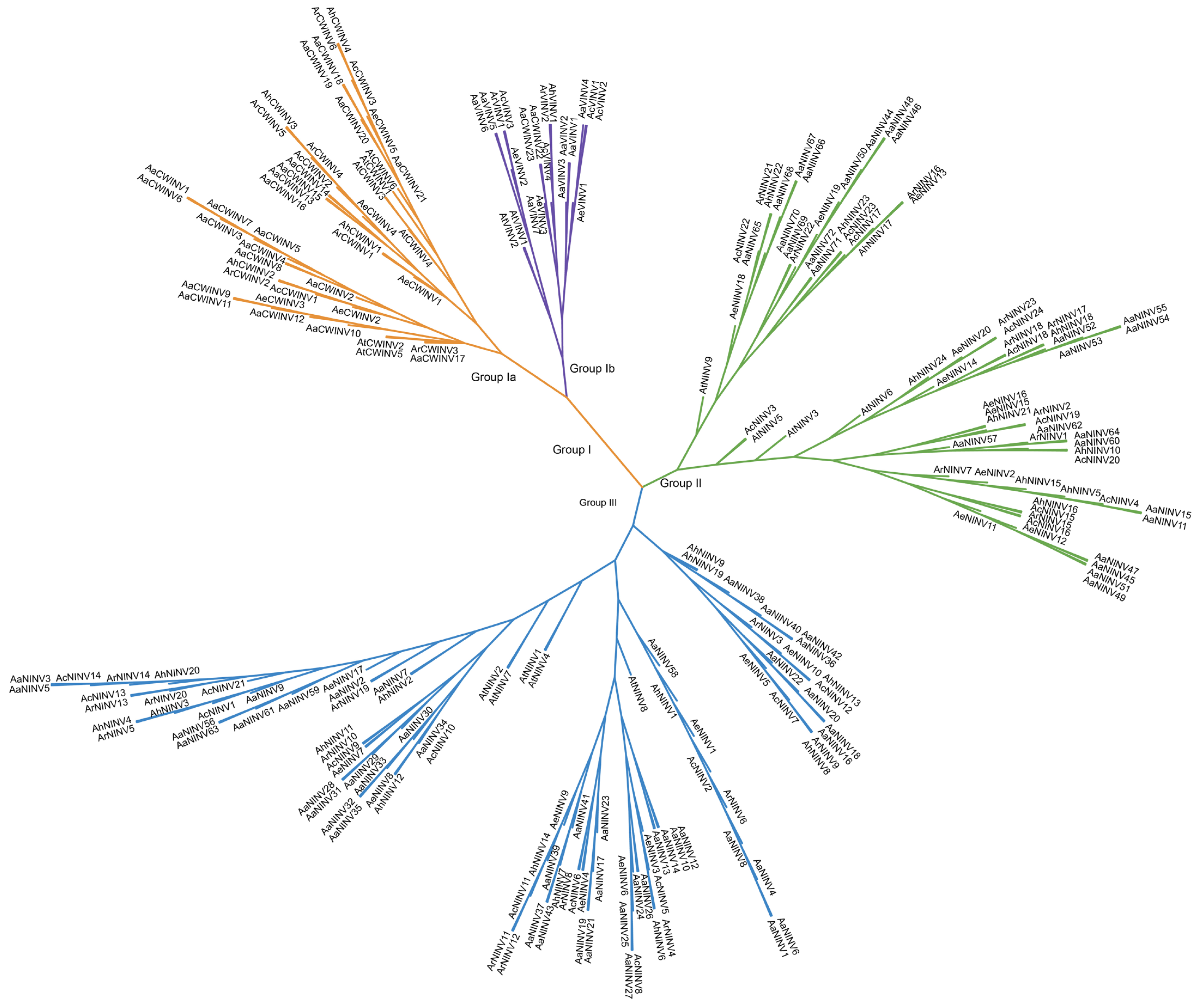
2.2. Phylogenetic Tree Construction
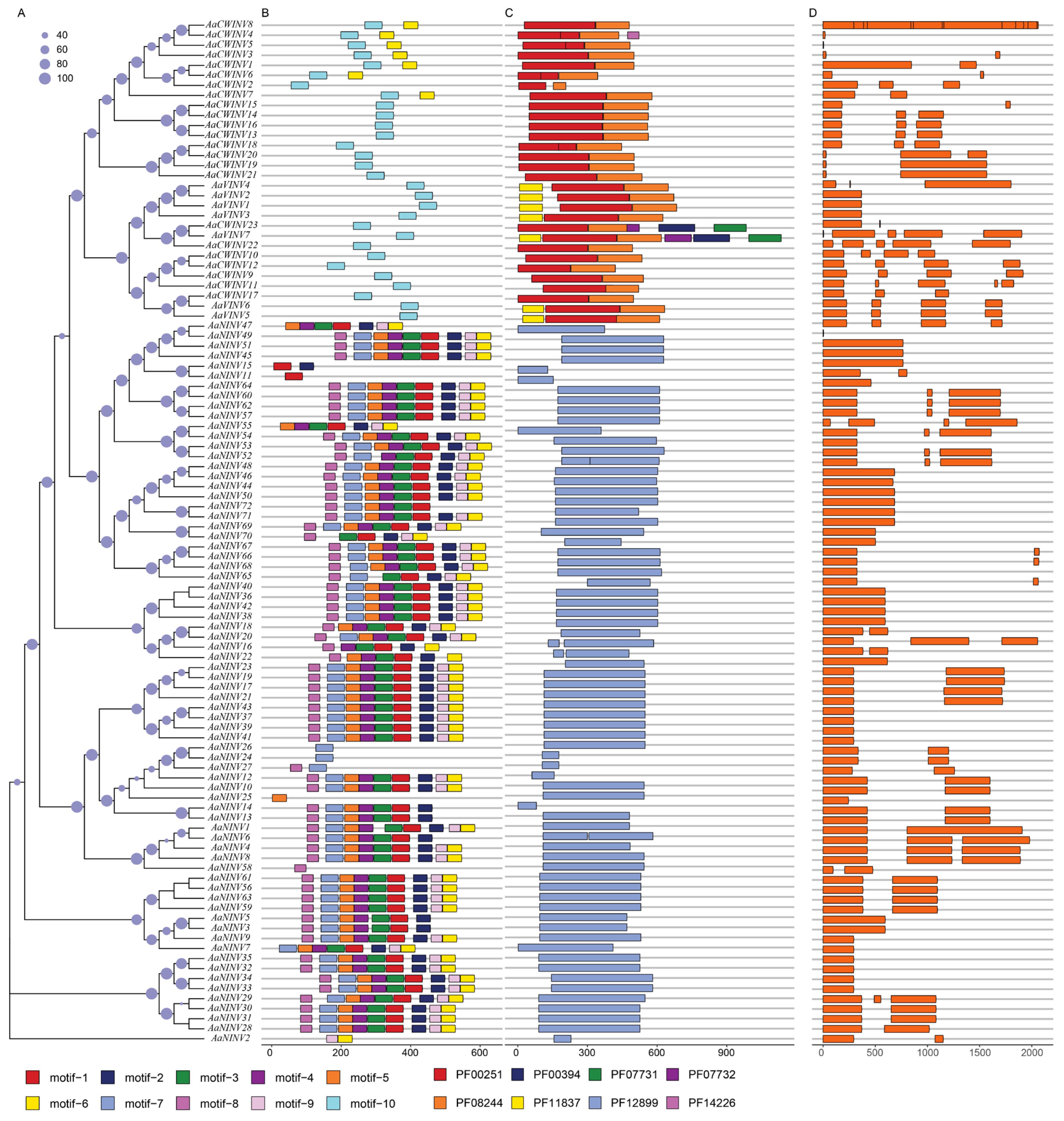
2.3. Gene Structure and Conserved Motif of AaINVs
2.4. Chromosomal Distribution and Gene Duplication Analyses of the AaINV Gene Family
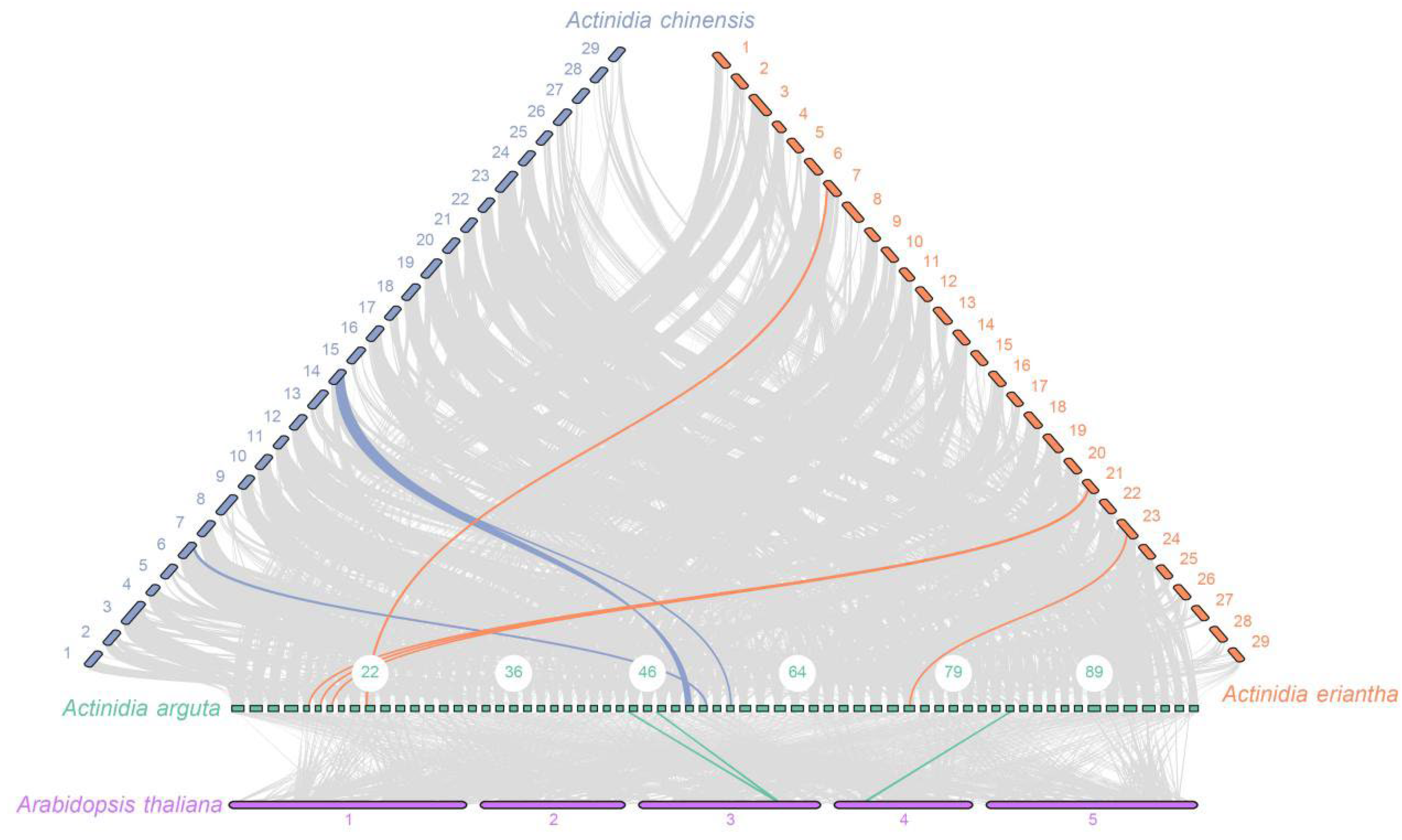
2.5. Collinearity Analysis and Selective Pressure
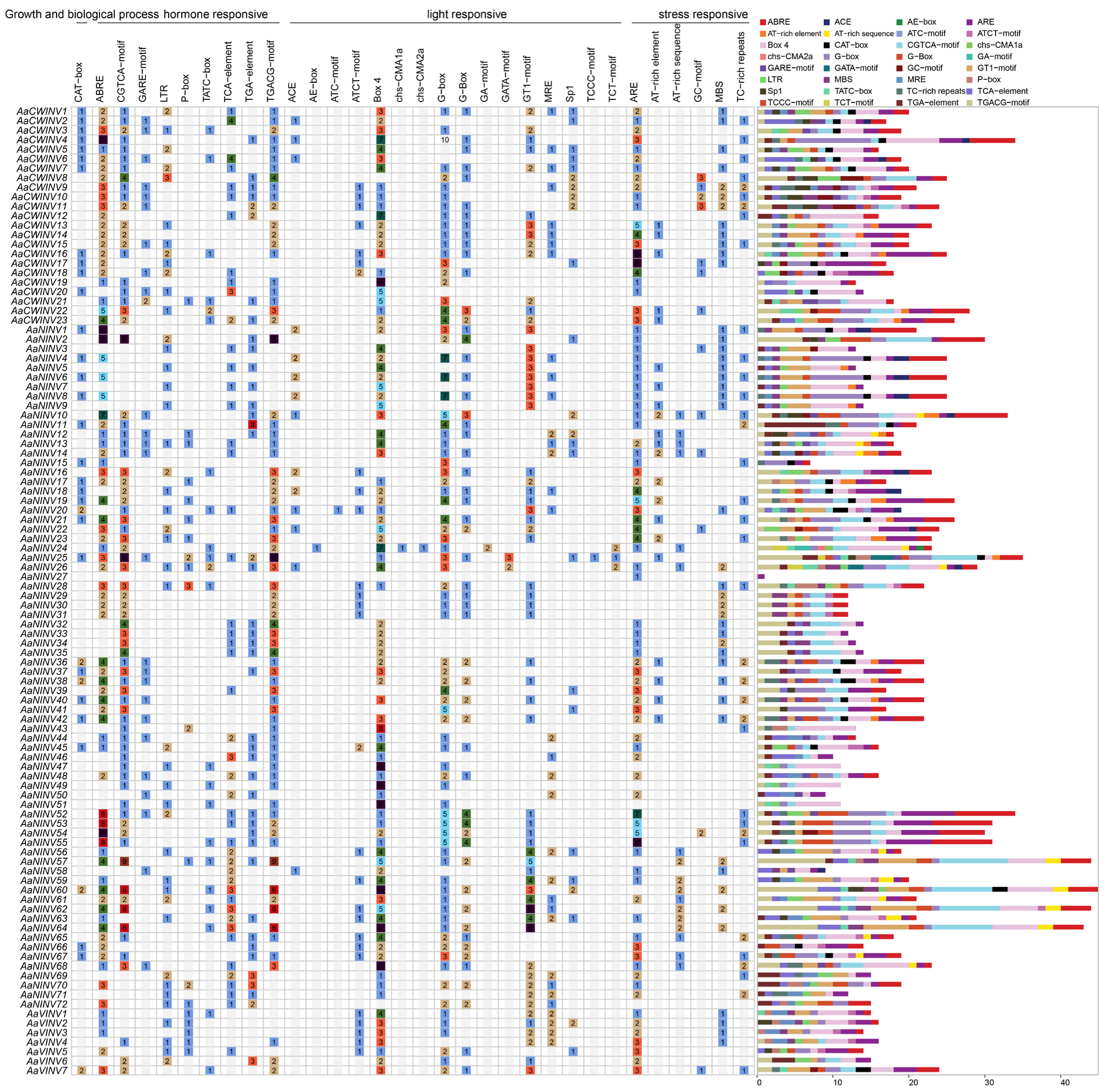
2.6. Cis-Acting Element Analysis of AaINV Gene Family
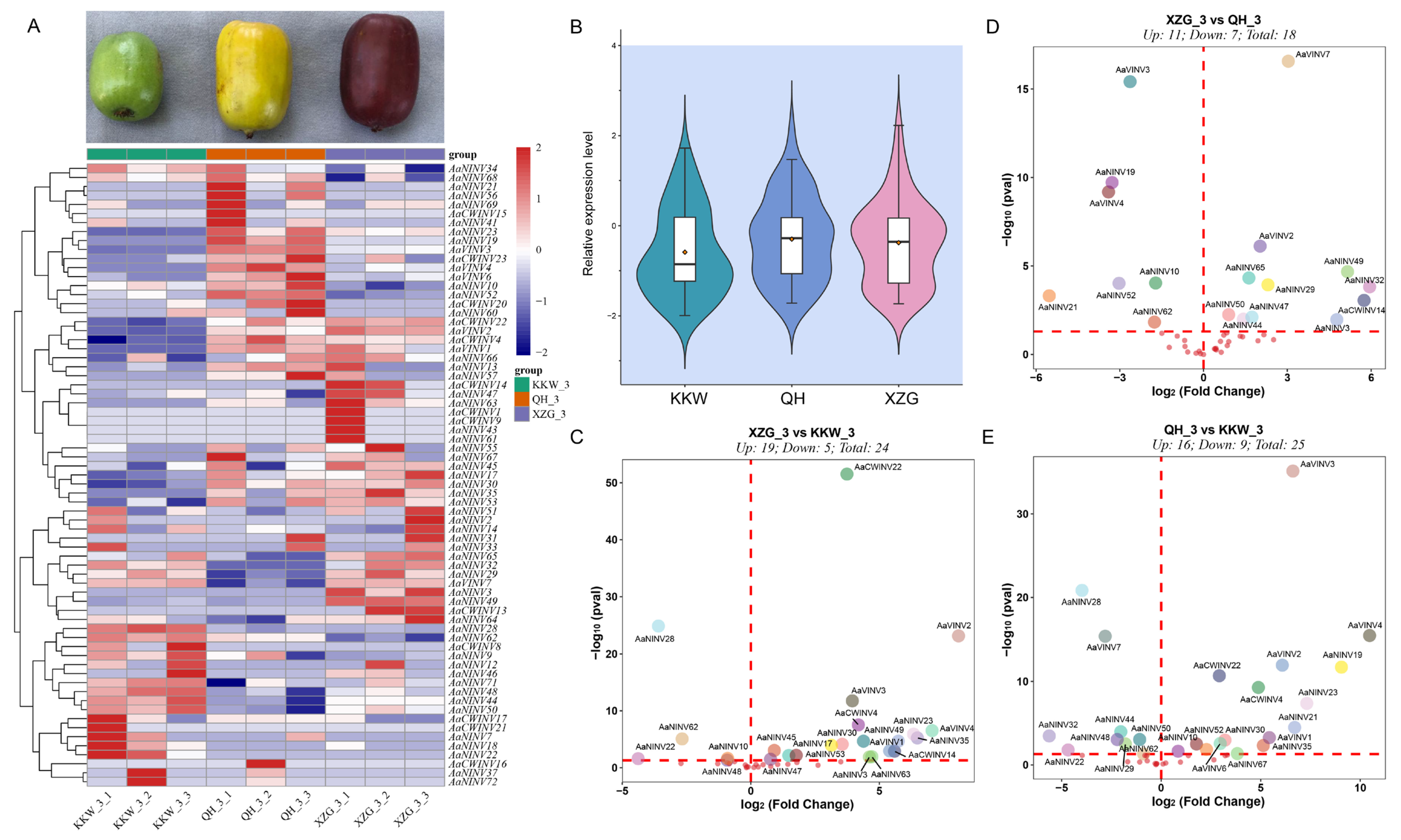
2.7. Genome-Wide Expression Analysis of AaINV Genes in Different Varieties
2.8. GO Functional Annotation of AaINV Genes
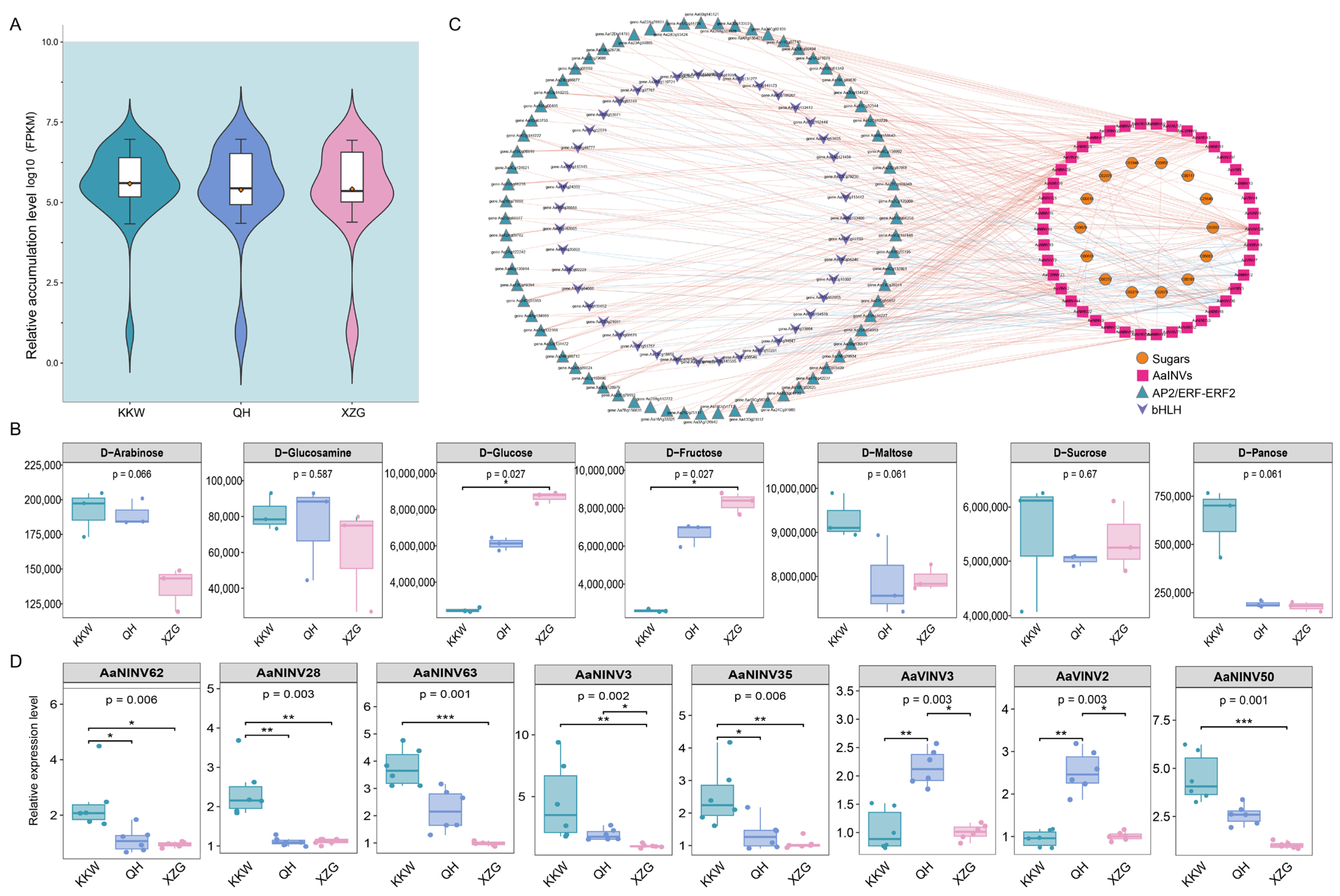
2.9. Correlation Analysis Between AaINVs Expression and Sugar Content in Kiwifruit
2.10. Validation of AaINV Expression by RT-qPCR
3. Discussion
4. Materials and Methods
4.1. Identification of INVs in A. arguta Genome
4.2. Phylogenetic Tree Analysis
4.3. Chromosomal Location, Gene Duplication and Collinearity Analyses
4.4. Gene Structure, Conserved Motif and Domain Analysis of AaINV Members
4.5. Expression Profiles Analysis of AaINVs in A. arguta Based on RNA-Seq Data
4.6. Experimental Validation of AaINV Gene Expression Levels by qRT-PCR
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patrick, W.J.; Botha, C.F.; Birch, G.R. Metabolic engineering of sugars and simple sugar derivatives in plants. Plant Biotechnol. J. 2013, 11, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hu, B.; Liu, J.; Zhou, Y.; Liu, S. Identification and characterization of tonoplast sugar transporter (TST) gene family in cucumber. Hortic. Plant J. 2020, 6, 145–157. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, J.T.; Qin, Y.Q.; Qin, Y.H.; Hu, G.B. Molecular cloning, characterization and expression profile of the sucrose synthase gene family in Litchi chinensis. Hortic. Plant J. 2021, 7, 520–528. [Google Scholar] [CrossRef]
- Cheng, L.; Jin, J.; He, X.; Luo, Z.; Wang, Z.; Yang, J.; Xu, X. Genome-wide identification and analysis of the invertase gene family in tobacco (Nicotiana tabacum) reveals NtNINV10 participating the sugar metabolism. Front. Plant Sci. 2023, 14, 1164296. [Google Scholar] [CrossRef] [PubMed]
- Coculo, D.; Lionetti, V. The plant Invertase/Pectin Methylesterase Inhibitor superfamily. Front. Plant Sci. 2022, 13, 863892. [Google Scholar] [CrossRef]
- Veillet, F.; Gaillard, C.; Coutos-Thevenot, P.; La Camera, S. Targeting the AtCWIN1 gene to explore the role of invertases in sucrose transport in roots and during Botrytis cinerea infection. Front. Plant Sci. 2016, 7, 1899. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, K.; Li, G.; Li, Y.; Gao, Z. Identification and expression analyses of invertase genes in moso bamboo reveal their potential drought stress functions. Front. Genet. 2021, 12, 696300. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyer, J.S. Sugar input, metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef]
- Stitt, M.; von Schaewen, A.; Willmitzer, L. “Sink” regulation of photosynthetic metabolism in transgenic tobacco plants expressing yeast invertase in their cell wall involves a decrease of the Calvin-cycle enzymes and an increase of glycolytic enzymes. Planta 1991, 183, 40–50. [Google Scholar] [CrossRef]
- Sturm, A. Invertases. Primary structures, functions, and roles in plant development and sucrose partitioning. Plant Physiol. 1999, 121, 1–8. [Google Scholar] [CrossRef]
- Tamoi, M.; Tabuchi, T.; Demuratani, M.; Otori, K.; Tanabe, N.; Maruta, T.; Shigeoka, S. Point mutation of a plastidic invertase inhibits development of the photosynthetic apparatus and enhances nitrate assimilation in sugar-treated Arabidopsis seedlings. J. Biol. Chem. 2010, 285, 15399–15407. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.E.; Martin, M.V.; Lechner, L.; Martínez-Noël, G.M.A.; Salerno, G.L. The riddle of mitochondrial alkaline/neutral invertases: A novel Arabidopsis isoform mainly present in reproductive tissues and involved in root ROS production. PLoS ONE 2017, 12, e0185286. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Van Den Ende, W.; Van Laere, A.; Cheng, S.; Bennett, J. Structure, evolution, and expression of the two invertase gene families of rice. J. Mol. Evol. 2005, 60, 615–634. [Google Scholar] [CrossRef] [PubMed]
- Greiner, S.; Krausgrill, S.; Rausch, T. Cloning of a tobacco apoplasmic invertase inhibitor. Proof of function of the recombinant protein and expression analysis during plant development. Plant Physiol. 1998, 116, 733–742. [Google Scholar] [CrossRef]
- Reca, I.B.; Brutus, A.; D’Avino, R.; Villard, C.; Bellincampi, D.; Giardina, T. Molecular cloning, expression and characterization of a novel apoplastic invertase inhibitor from tomato (Solanum lycopersicum) and its use to purify a vacuolar invertase. Biochimie 2008, 90, 1611–1623. [Google Scholar] [CrossRef]
- Ahiakpa, J.K.; Magdy, M.; Karikari, B.; Munir, S.; Mumtaz, M.A.; Tamim, S.A.; Mahmood, S.; Liu, G.Z.; Chen, W.F.; Wang, Y.; et al. Genome-wide identification and expression profiling of tomato invertase genes indicate their response to stress and phytohormones. J. Plant Growth Regul. 2021, 41, 1481–1498. [Google Scholar] [CrossRef]
- Sherson, S.M.; Alford, H.L.; Forbes, S.M.; Wallace, G.; Smith, S.M. Roles of cell-wall invertases and monosaccharide transporters in the growth and development of Arabidopsis. J. Exp. Bot. 2003, 54, 525–531. [Google Scholar] [CrossRef]
- Chourey, P.S.; Jain, M.; Li, Q.B.; Carlson, S.J. Genetic control of cell wall invertases in developing endosperm of maize. Planta 2006, 223, 159–167. [Google Scholar] [CrossRef]
- Proels, R.K.; Hückelhoven, R. Cell-wall invertases, key enzymes in the modulation of plant metabolism during defence responses. Mol. Plant Pathol. 2014, 15, 858–864. [Google Scholar] [CrossRef]
- Liu, J.; Han, L.; Huai, B.; Zheng, P.; Chang, Q.; Guan, T.; Li, D.; Huang, L.; Kang, Z. Down-regulation of a wheat alkaline/neutral invertase correlates with reduced host susceptibility to wheat stripe rust caused by Puccinia striiformis. J. Exp. Bot. 2015, 66, 7325–7338. [Google Scholar] [CrossRef]
- Yu, X.; Wang, X.; Zhang, W.; Qian, T.; Tang, G.; Guo, Y.; Zheng, C.C. Antisense suppression of an acid invertase gene (MAI1) in muskmelon alters plant growth and fruit development. J. Exp. Bot. 2008, 59, 2969–2977. [Google Scholar] [CrossRef] [PubMed]
- Roitsch, T.; González, M.C. Function and regulation of plant invertases: Sweet sensations. Trends Plant Sci. 2004, 9, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Gou, J.Y.; Xue, H.W. PIP5K9, an arabidopsis phosphatidylinositol monophosphate kinase, interacts with a cytosolic invertase to negatively regulate sugar-mediated root growth. Plant Cell 2007, 19, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.L.; Lechner, L.; Zabaleta, E.J.; Salerno, G.L. A mitochondrial alkaline/neutral invertase isoform (A/N-InvC) functions in developmental energy-demanding processes in arabidopsis. Planta 2013, 237, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Feng, F.; Cheng, L. Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development. PLoS ONE 2012, 7, e33055. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Yang, W.; Li, J.; Tang, W.; Gong, R. Transcriptomic and metabolomic analysis of quality changes during sweet cherry fruit development and mining of related genes. Int. J. Mol. Sci. 2022, 23, 7402. [Google Scholar] [CrossRef]
- Wang, R.; Shu, P.; Zhang, C.; Zhang, J.; Chen, Y.; Zhang, Y.; Du, K.; Xie, Y.; Li, M.; Ma, T.; et al. Integrative analyses of metabolome and genome-wide transcriptome reveal the regulatory network governing flavor formation in kiwifruit (Actinidia chinensis). New Phytol. 2022, 233, 373–389. [Google Scholar] [CrossRef]
- Wu, S.; Wu, D.; Song, J.; Zhang, Y.; Tan, Q.; Yang, T.; Yang, J.; Wang, S.; Xu, J.; Xu, W.; et al. Metabolomic and transcriptomic analyses reveal new insights into the role of abscisic acid in modulating mango fruit ripening. Hortic. Res. 2022, 9, uhac102. [Google Scholar] [CrossRef]
- Kim, J.Y.; Mahé, A.; Brangeon, J.; Prioul, J.L. A maize vacuolar invertase, IVR2, is induced by water stress. Organ/tissue specificity and diurnal modulation of expression. Plant Physiol. 2000, 124, 71–84. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhen, X.H.; Zhou, Y.J.; Wang, Y.L.; Hou, J.Y.; Wang, X.; Li, R.M.; Liu, J.; Hu, X.W.; Geng, M.T.; et al. MeNINV1: An alkaline/neutral invertase gene of manihot esculenta, enhanced sucrose catabolism and promoted plant vegetative growth in transgenic Arabidopsis. Plants 2022, 11, 946. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Y.; Zhu, F. Kiwifruit (Actinidia spp.): A review of chemical diversity and biological activities. Food Chem. 2021, 350, 128469. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.M.; Man, Y.P.; Lei, R.; Liu, Y.B.; Wu, J.H.; Wang, Y.C. Structural analysis of Actinidia arguta natural populations and preliminary application in association mapping of fruit traits. Sci. Hortic. 2022, 304, 111306. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, Y.; Zhang, L.; Wang, F.; Yu, G.; Wang, Y.; Kang, X.; Wu, Y. Characterization and analysis of the full-Length transcriptome provide insights into fruit quality formation in kiwifruit cultivar Actinidia arguta cv. Qinziyu. Agronomy 2023, 13, 143. [Google Scholar] [CrossRef]
- Li, Z.Z.; Man, Y.P.; Lan, X.Y.; Wang, Y.C. Ploidy and phenotype variation of a natural Actinidia arguta population in the east of Daba Mountain located in a region of Shanxi. Sci. Hortic. 2013, 161, 259–265. [Google Scholar] [CrossRef]
- Wang, Y.H.; Xu, F.X.; Feng, X.Q.; Macarthur, R.L. Modulation of Actinidia arguta fruit ripening by three ethylene biosynthesis inhibitors. Food Chem. 2015, 173, 405–413. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, J.; Gao, N.; Gong, E.S.; Xin, G.; Liu, C.; Si, X.; Sun, X.; Li, B. Assessment of the phytochemical profile and antioxidant activities of eight kiwi berry (Actinidia arguta (Siebold & zuccarini) miquel) varieties in China. Food Sci. Nutr. 2021, 9, 5616–5625. [Google Scholar]
- Sawicki, T.; Błaszczak, W.; Latocha, P. In vitro anticholinergic and antiglycaemic properties of frost-hardy Actinidia fruit extracts and their polyphenol profile, L-ascorbic acid content and antioxidant capacity. Food Res. Int. 2023, 173, 113324. [Google Scholar] [CrossRef]
- Xu, H.R.; Pan, S.; Wang, J.B.; Ye, T.C.; Yan, M.; Liang, X.M.; Qian, G.L.; Yan, T.C.; Xin, G. Comparative characterization of volatile organic compounds and aroma profiles in 10 Actinidia arguta cultivars by gas chromatography-mass spectrometry (GC-MS), sensory analysis, and odor activity value (OAV) combined with chemometrics. J. Food Compos. Anal. 2024, 133, 106450. [Google Scholar] [CrossRef]
- Kim, J.G.; Beppu, K.; Kataoka, I. Physical and compositional characteristics of ‘mitsuko’ and local hardy kiwifruits in Japan. Hortic. Environ. Biotechnol. 2012, 53, 1–8. [Google Scholar] [CrossRef]
- Lin, Y.; Tang, H.; Zhao, B.; Lei, D.; Zhou, X.; Yao, W.; Fan, J.; Zhang, Y.; Chen, Q.; Wang, Y.; et al. Comparative changes of health-promoting phytochemicals and sugar metabolism of two hardy kiwifruit (Actinidia arguta) cultivars during fruit development and maturity. Front. Plant Sci. 2022, 13, 1087452. [Google Scholar] [CrossRef]
- Lu, X.M.; Yu, X.F.; Li, G.Q.; Qu, M.H.; Wang, H.; Liu, C.; Man, Y.P.; Jiang, X.H.; Li, M.Z.; Wang, J.; et al. Genome assembly of autotetraploid Actinidia arguta highlights adaptive evolution and enables dissection of important economic traits. Plant Commun. 2024, 5, 100856. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ni, D.A.; Ruan, Y.L. Posttranslational elevation of Cell Wall invertase activity by silencing its inhibitor in tomato delays leaf senescence and increases seed weight and fruit hexose level. Plant Cell 2009, 21, 2072–2089. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Zhu, Z.; Wang, W.; Cai, J.; Chen, Y.; Li, L.; Tian, S. A tomato vacuolar invertase inhibitor mediates sucrose metabolism and influences fruit ripening. Plant Physiol. 2016, 172, 1596–1611. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Takei-Hoshi, R.; Yoshikawa, I.; Nishida, K.; Kobayashi, M.; Kusano, M.; Lu, Y.; Ariizumi, T.; Ezura, H.; Otagaki, S.; et al. Functional disruption of cell wall invertase inhibitor by genome editing increases sugar content of tomato fruit without decrease fruit weight. Sci. Rep. 2021, 11, 21534. [Google Scholar] [CrossRef]
- Pressey, R. Separation and properties of potato invertase and invertase inhibitor. Arch. Biochem. Biophys. 1966, 113, 667–674. [Google Scholar] [CrossRef]
- Juarez-Colunga, S.; Lopez-Gonzalez, C.; Morales-Elıas, N.C.; Massange-Sánchez, J.A.; Trachsel, S.; Tiessen, A. Genome-wide analysis of the invertase gene family from maize. Plant Mol. Biol. 2018, 97, 385–406. [Google Scholar] [CrossRef]
- Jaillon, O.; Aury, J.M.; Noel, B.; Policriti, A.; Clepet, C.; Casagrande, A.; Choisne, N.; Aubourg, S.; Vitulo, N.; Jubin, C.; et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 2007, 449, 463–467. [Google Scholar]
- Peng, Z.; Lu, Y.; Li, L.; Zhao, Q.; Feng, Q.; Gao, Z.; Lu, H.; Hu, T.; Yao, N.; Liu, K.; et al. The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla). Nat. Genet. 2017, 45, 456–461. [Google Scholar] [CrossRef]
- Alberto, F.; Bignon, C.; Sulzenbacher, G.; Henrissat, B.; and Czjzek, M. The three-dimensional structure of invertase (beta-fructosidase) from Thermotoga maritima reveals a bimodular arrangement and an evolutionary relationship between retaining and inverting glycosidases. J. Biol. Chem. 2004, 279, 18903–18910. [Google Scholar] [CrossRef]
- Wang, L.; Ruan, Y.L. New insights into roles of cell wall invertase in early seed development revealed by comprehensive spatial and temporal expression patterns of GhCWIN1 in cotton. Plant Physiol. 2012, 160, 777–787. [Google Scholar] [CrossRef]
- Tauzin, A.S.; Sulzenbacher, G.; Lafond, M.; Desseaux, V.; Reca, I.B.; Perrier, J.; Bellincampi, D.; Fourquet, P.; Lévêque, C.; Giardina, T. Functional characterization of a vacuolar invertase from Solanum lycopersicum: Post-translational regulation by N-glycosylation and a proteinaceous inhibitor. Biochimie 2014, 101, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Qiang, X.; Dong, P.; Ren, T.; Zhang, Y.; Yang, Y. Genome-Wide Identification and Characterization of Sucrose Metabolism Genes Involved in Actinidia rufa and Their Expression Profiling during the Fruit Developmental Stages. Horticulturae 2024, 10, 772. [Google Scholar] [CrossRef]
- Yu, J.Q.; Gu, K.D.; Sun, C.H.; Zhang, Q.Y.; Wang, J.H.; Ma, F.F.; You, C.X.; Hu, D.G.; Hao, Y.J. The apple bHLH transcription factor MdbHLH3 functions in determining the fruit carbohydrates and malate. Plant Biotechnol. J. 2021, 19, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Tournier, B.; Sanchez-Ballesta, M.T.; Jones, B.; Pesquet, E.; Regad, F.; Latché, A.; Pech, J.C.; Bouzayen, M. New members of the tomato ERF family show specific expression pattern and diverse DNA-binding capacity to the GCC box element. FEBS Lett. 2003, 550, 149–154. [Google Scholar] [CrossRef]
- Yin, X.R.; Allan, A.C.; Chen, K.S.; Ferguson, I.B. Kiwifruit EIL and ERF genes involved in regulating fruit ripening. Plant Physiol. 2010, 153, 1280–1292. [Google Scholar] [CrossRef]
- Mistry, J.; Finn, R.D.; Eddy, S.R.; Bateman, A.; Punta, M. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Res. 2013, 41, e121. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss bioinformatics resource portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. Circlize implements and enhances circular visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Krishnakumar, V.; Zeng, X.; Xu, Z.; Taranto, A.; Lomas, J.S.; Zhang, Y.; Huang, Y.; Wang, Y.; Yim, W.C.; et al. JCVI: A Versatile Toolkit for Comparative Genomics Analysis. iMeta 2024, 3, e211. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0: A toolkit incorporating gamma-series methods and sliding window strategies. Genom. Proteom. Bioinf. 2010, 8, 77–80. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Bailey, T.L.; Williams, N.; Misleh, C.; Li, W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef]
- Rombauts, S.; Déhais, P.; Van Montagu, M.; Rouzé, P. PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res. 1999, 27, 295–296. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible read trimming tool for Illumina NGS data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, Q.; Li, J.; Gong, H.; Fan, X.; Yang, Y.; Liu, X.; Yin, X. Transcriptome co-expression network analysis identifies key genes and regulators of ripening kiwifruit ester biosynthesis. BMC Plant Biol. 2020, 20, 103. [Google Scholar] [CrossRef] [PubMed]
- Kohl, M.; Wiese, S.; Warscheid, B. Cytoscape: Software for visualization and analysis of biological networks. Methods Mol. Biol. 2011, 696, 291–303. [Google Scholar] [PubMed]
- Ampomah-Dwamena, C.; McGhie, T.; Reginald, W.; Montefiori, M.; Hellens, R.P.; Allan, A.C. The kiwifruit lycopene beta-cyclase plays a significant role in carotenoid accumulation in fruit. J. Exp. Bot. 2009, 60, 3765–3779. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiang, X.; Ren, T.; Zhang, Y.; Jia, Y. Genome-Wide Identification and Expression Profiling of the Invertase Genes Involved in Sugar Metabolism and Accumulation in Actinidia arguta. Int. J. Mol. Sci. 2025, 26, 2150. https://doi.org/10.3390/ijms26052150
Qiang X, Ren T, Zhang Y, Jia Y. Genome-Wide Identification and Expression Profiling of the Invertase Genes Involved in Sugar Metabolism and Accumulation in Actinidia arguta. International Journal of Molecular Sciences. 2025; 26(5):2150. https://doi.org/10.3390/ijms26052150
Chicago/Turabian StyleQiang, Xu, Ting Ren, Ying Zhang, and Yun Jia. 2025. "Genome-Wide Identification and Expression Profiling of the Invertase Genes Involved in Sugar Metabolism and Accumulation in Actinidia arguta" International Journal of Molecular Sciences 26, no. 5: 2150. https://doi.org/10.3390/ijms26052150
APA StyleQiang, X., Ren, T., Zhang, Y., & Jia, Y. (2025). Genome-Wide Identification and Expression Profiling of the Invertase Genes Involved in Sugar Metabolism and Accumulation in Actinidia arguta. International Journal of Molecular Sciences, 26(5), 2150. https://doi.org/10.3390/ijms26052150




