Characterization of the Temporal Dynamics of the Endothelial–Mesenchymal-like Transition Induced by Soluble Factors from Dengue Virus Infection in Microvascular Endothelial Cells
Abstract
1. Introduction
2. Results
2.1. CMDVs Increase Endothelial Permeability
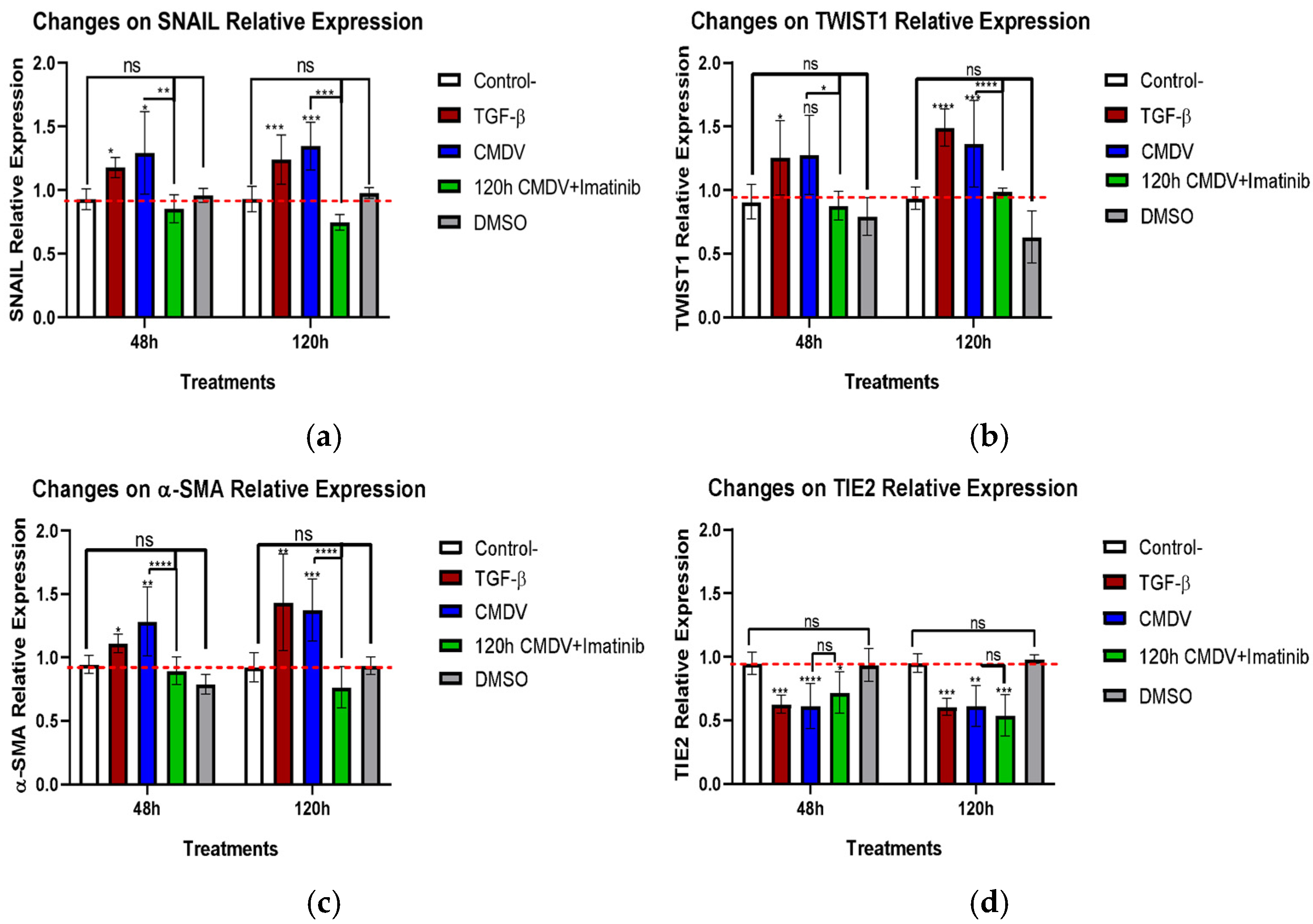
2.2. CMDVs Modulate the Expression of the Proteins Associated with EndMT
2.3. The CMDV Increase Endothelial Cell Stiffness
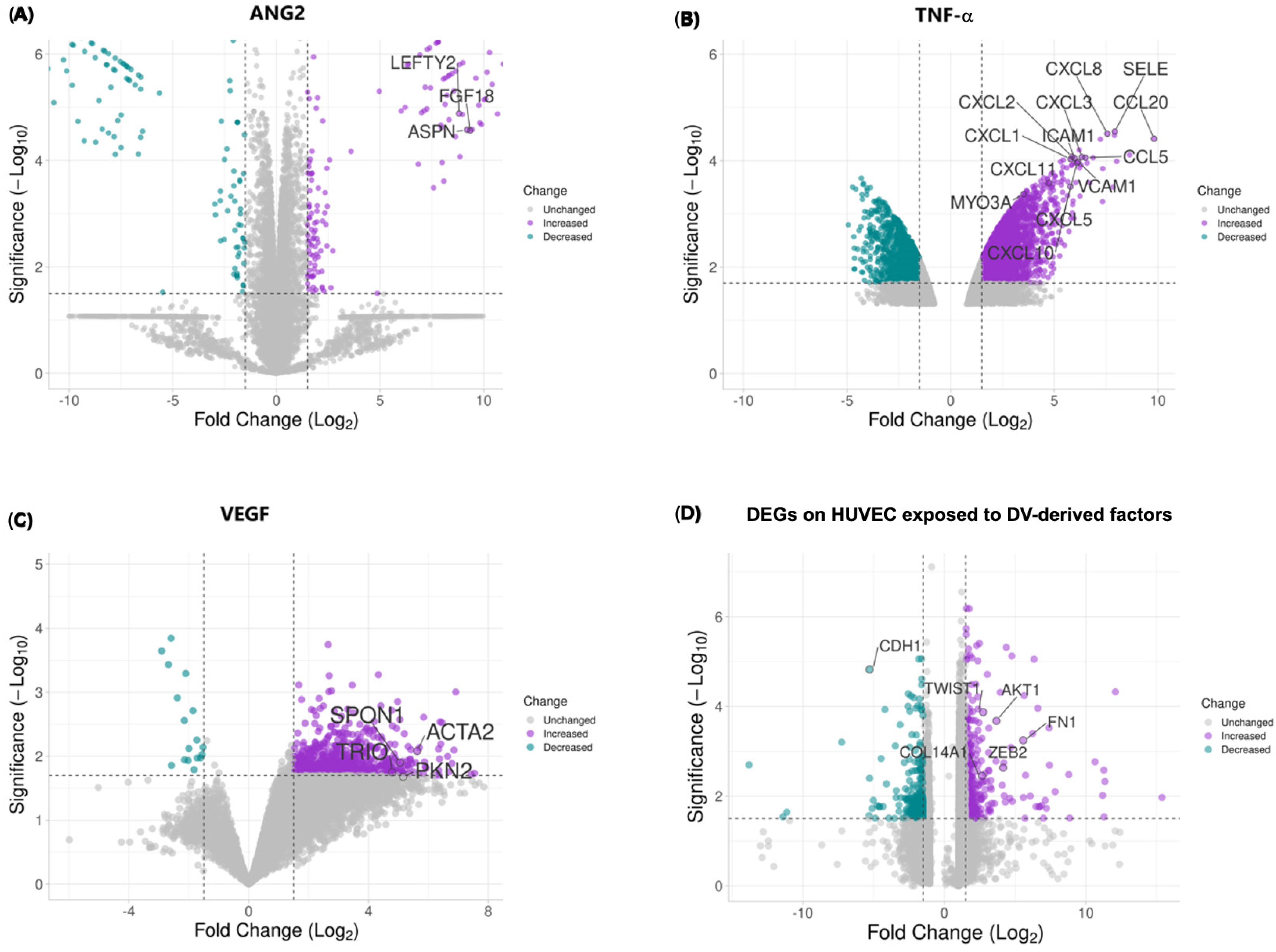
2.4. DV-Induced Soluble Factors Promote Expression of Host Proteins Involved in EndMT
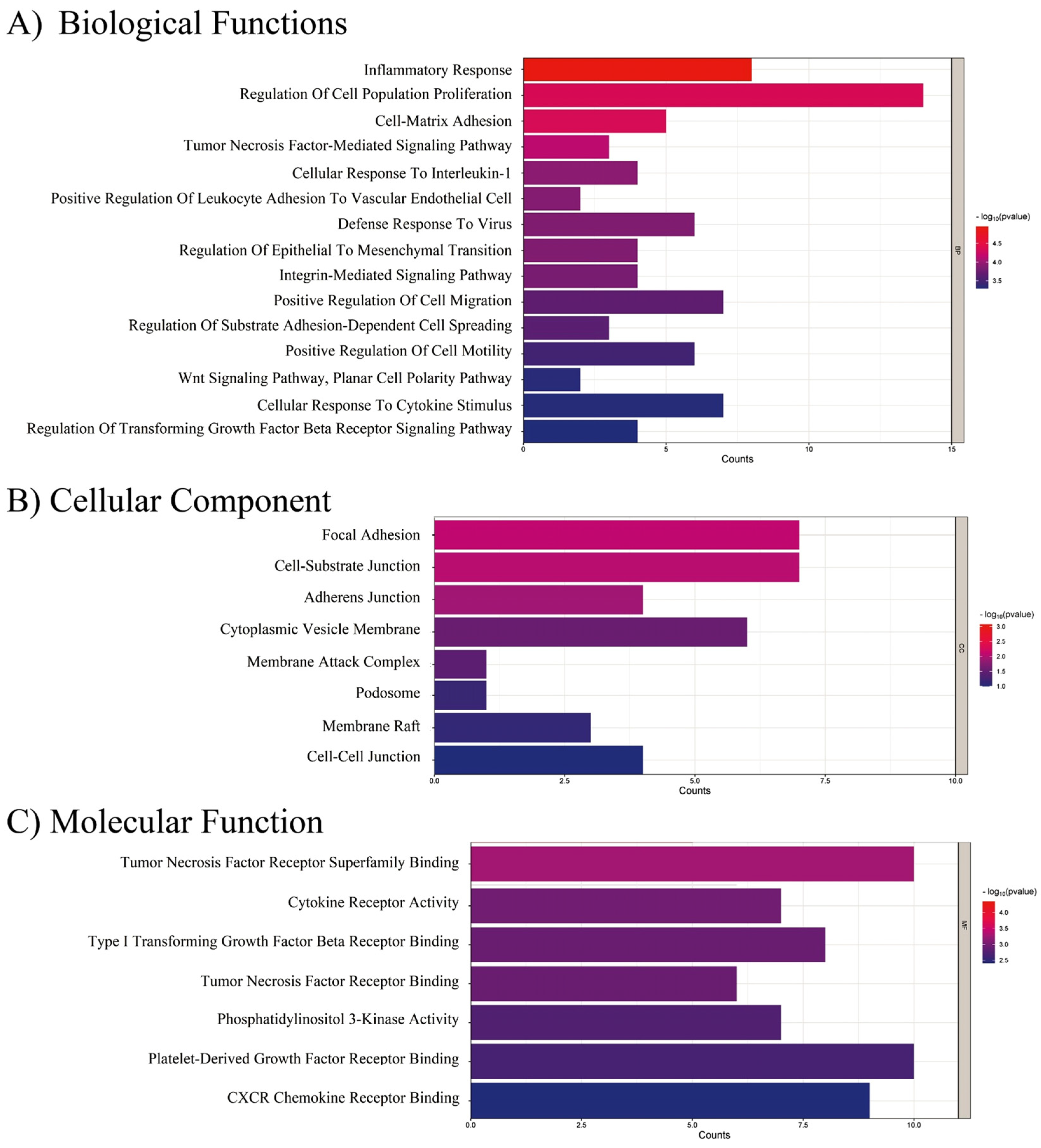
2.5. EndMT-Associated Transcriptional Changes in Response to CMDV and Individual Stimuli
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Viral Infections
4.2. Conditioned Media
4.3. Transendothelial Electrical Resistance (TEER)
4.4. In-Cell Western Assay (ICW)
4.5. AFM Force Spectroscopy
4.6. Bioinformatic Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madewell, Z.J. Arboviruses and Their Vectors. South. Med. J. 2020, 113, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The Current and Future Global Distribution and Population at Risk of Dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef]
- Soneja, S.; Tsarouchi, G.; Lumbroso, D.; Tung, D.K. A Review of Dengue’s Historical and Future Health Risk from a Changing Climate. Curr. Environ. Health Rep. 2021, 8, 245–265. [Google Scholar] [CrossRef]
- Deng, S.Q.; Yang, X.; Wei, Y.; Chen, J.T.; Wang, X.J.; Peng, H.J. A Review on Dengue Vaccine Development. Vaccines 2020, 8, 63. [Google Scholar] [CrossRef]
- Mishra, R.; Lata, S.; Ali, A.; Banerjea, A.C. Dengue Haemorrhagic Fever: A Job Done via Exosomes? Emerg. Microbes Infect. 2019, 8, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Paranavitane, S.A.; Gomes, L.; Kamaladasa, A.; Adikari, T.N.; Wickramasinghe, N.; Jeewandara, C.; Shyamali, N.L.A.; Ogg, G.S.; Malavige, G.N. Dengue NS1 Antigen as a Marker of Severe Clinical Disease. BMC Infect. Dis. 2014, 14, 570. [Google Scholar] [CrossRef]
- Bandara, S.M.R.; Herath, H.M.M.T.B. Corticosteroid Actions on Dengue Immune Pathology; A Review Article. Clin. Epidemiol. Glob. Health 2020, 8, 486–494. [Google Scholar] [CrossRef]
- Malavige, G.N.; Ogg, G.S. Pathogenesis of Vascular Leak in Dengue Virus Infection. Immunology 2017, 151, 261–269. [Google Scholar] [CrossRef]
- Halstead, S. Recent Advances in Understanding Dengue. F1000Research 2019, 8, 1279. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Sabeena, S.P.; Varma, M.; Arunkumar, G. Current Understanding of the Pathogenesis of Dengue Virus Infection. Curr. Microbiol. 2021, 78, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Srikiatkhachorn, A.; Kelley, J.F. Endothelial Cells in Dengue Hemorrhagic Fever. Antivir. Res. 2014, 109, 160–170. [Google Scholar] [CrossRef]
- Piera-Velazquez, S.; Jimenez, S.A. Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases. Physiol. Rev. 2019, 99, 1281–1324. [Google Scholar] [CrossRef]
- Pinto, M.T.; Covas, D.T.; Kashima, S.; Rodrigues, C.O. Endothelial Mesenchymal Transition: Comparative Analysis of Different Induction Methods. Biol. Proced. Online 2016, 18, 10. [Google Scholar] [CrossRef]
- Zhang, B.; Niu, W.; Dong, H.Y.; Liu, M.L.; Luo, Y.; Li, Z.C. Hypoxia Induces Endothelial-Mesenchymal Transition in Pulmonary Vascular Remodeling. Int. J. Mol. Med. 2018, 42, 270–278. [Google Scholar] [CrossRef]
- Echeverría, C.; Montorfano, I.; Tapia, P.; Riedel, C.; Cabello-Verrugio, C.; Simon, F. Endotoxin-Induced Endothelial Fibrosis Is Dependent on Expression of Transforming Growth Factors Β1 and Β2. Infect. Immun. 2014, 82, 3678–3686. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Pan, L.; Pu, H.; Wang, Y.; Zhang, X.; Li, C.; Yang, Z. Loss of Caveolin-1 Promotes Endothelial-Mesenchymal Transition during Sepsis: A Membrane Proteomic Study. Int. J. Mol. Med. 2013, 32, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Saito, A. EMT and EndMT: Regulated in Similar Ways? J. Biochem. 2013, 153, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Naipauer, J.; Mesri, E.A. The Kaposi’s Sarcoma Progenitor Enigma: KSHV-Induced MEndT–EndMT Axis. Trends Mol. Med. 2023, 29, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Ciszewski, W.M.; Woźniak, L.A.; Sobierajska, K. Diverse Roles of SARS-CoV-2 Spike and Nucleocapsid Proteins in EndMT Stimulation through the TGF-β-MRTF Axis Inhibited by Aspirin. Cell Commun. Signal. 2024, 22, 296. [Google Scholar] [CrossRef]
- Álvarez-Díaz, D.A.; Gutiérrez-díaz, A.A.; Orozco-garcía, E.; Puerta-gonzález, A.; Bermúdez-santana, C.I.; Gallego-gómez, J.C. Dengue Virus Potentially Promotes Migratory Responses on Endothelial Cells by Enhancing Pro-Migratory Soluble Factors and MiRNAs. Virus Res. 2019, 259, 68–76. [Google Scholar] [CrossRef]
- Roa Linares, V.C.; Gallego Gómez, J.C. La Pérdida de Función de La Quinasa Dependiente de Ciclina 5 (CDK5) Altera El Citoesqueleto y Reduce La Infección in Vitro Por El Virus Del Dengue 2. Acta Biol. Colomb. 2019, 24, 474–485. [Google Scholar] [CrossRef]
- Suttitheptumrong, A.; Mahutchariyakul, T.; Rawarak, N.; Reamtong, O.; Boonnak, K.; Pattanakitsakul, S.N. Altered Moesin and Actin Cytoskeleton Protein Rearrangements Affect Transendothelial Permeability in Human Endothelial Cells upon Dengue Virus Infection and Tnf-α Treatment. Viruses 2021, 13, 2042. [Google Scholar] [CrossRef]
- Dalrymple, N.A.; MacKow, E.R. Roles for Endothelial Cells in Dengue Virus Infection. Adv. Virol. 2012, 2012, 840654. [Google Scholar] [CrossRef] [PubMed]
- Rathi, K.R.; Arora, M.M.; Sahai, K.; Tripathi, S.; Singh, S.P.; Raman, D.K.; Anand, K.B. Autopsy Findings in Fatal Dengue Haemorrhagic Fever—06 Cases. Med. J. Armed Forces India 2013, 69, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Póvoa, T.F.; Alves, A.M.B.; Oliveira, C.A.B.; Nuovo, G.J.; Chagas, V.L.A.; Paes, M.V. The Pathology of Severe Dengue in Multiple Organs of Human Fatal Cases: Histopathology, Ultrastructure and Virus Replication. PLoS ONE 2014, 9, e83386. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Wang, X.; Li, S.; Tang, L. Substrate Stiffness Drives Epithelial to Mesenchymal Transition and Proliferation through the Neat1-Wnt/β-Catenin Pathway in Liver Cancer. Int. J. Mol. Sci. 2021, 22, 12066. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, A.; Jiao, T.; Tratsiakovich, Y.; Wernly, B.; Yang, J.; Östenson, C.G.; Danser, A.H.J.; Pernow, J.; Zhou, Z. Therapeutic Potential of Sunitinib in Ameliorating Endothelial Dysfunction in Type 2 Diabetic Rats. Pharmacology 2022, 107, 160–166. [Google Scholar] [CrossRef]
- Escudero-Flórez, M.; Torres-Hoyos, D.; Miranda-Brand, Y.; Boudreau, R.L.; Gallego-Gómez, J.C.; Vicente-Manzanares, M. Dengue Virus Infection Alters Inter-Endothelial Junctions and Promotes Endothelial–Mesenchymal-Transition-like Changes in Human Microvascular Endothelial Cells. Viruses 2023, 15, 1437, Erratum in Viruses 2023, 15, 2252. [Google Scholar] [CrossRef]
- Siavashi, V.; Nassiri, S.M.; Rahbarghazi, R.; Mohseni, Z.; Sharifi, A.M. Distinct Tie2 Tyrosine Phosphorylation Sites Dictate Phenotypic Switching in Endothelial Progenitor Cells. J. Cell. Physiol. 2019, 234, 6209–6219. [Google Scholar] [CrossRef] [PubMed]
- Vitali, R.; Mancini, C.; Cesi, V.; Tanno, B.; Mancuso, M.; Bossi, G.; Zhang, Y.; Martinez, R.V.; Calabretta, B.; Dominici, C.; et al. Slug (SNAI2) down-Regulation by Interference Facilitates Apoptosis and Inhibits Invasive Growth in Neuroblastoma Preclinical Models. Clin. Cancer Res. 2008, 14, 4622–4630. [Google Scholar] [CrossRef] [PubMed]
- Anupriya, M.G.; Singh, S.; Hulyalkar, N.V.; Sreekumar, E. Sphingolipid Signaling Modulates Trans-Endothelial Cell Permeability in Dengue Virus Infected HMEC-1 Cells. Prostaglandins Other Lipid Mediat. 2018, 136, 44–54. [Google Scholar] [CrossRef]
- Velandia-Romero, M.L.; Calderón-Peláez, M.A.; Castellanos, J.E. In Vitro Infection with Dengue Virus Induces Changes in the Structure and Function of the Mouse Brain Endothelium. PLoS ONE 2016, 11, e0157786. [Google Scholar] [CrossRef]
- Velandia-Romero, M.L.; Caldern-Pelaez, M.A.; Balbas-Tepedino, A.; Alejandro Marquez-Ortiz, R.; Madroñero, L.J.; Prieto, A.B.; Castellanos, J.E. Extracellular Vesicles of U937 Macrophage Cell Line Infected with DENV-2 Induce Activation in Endothelial Cells EA.Hy926. PLoS ONE 2020, 15, e0227030. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhang, J.L.; Chen, W.; Xu, X.F.; Gao, N.; Fan, D.Y.; An, J. Roles of Small GTPase RAc1 in the Regulation of Actin Cytoskeleton during Dengue Virus Infection. PLoS Negl. Trop. Dis. 2010, 4, e809. [Google Scholar] [CrossRef]
- Singh, S.; Anupriya, M.G.; Sreekumar, E. Comparative Whole Genome Analysis of Dengue Virus Serotype-2 Strains Differing in Trans-Endothelial Cell Leakage Induction in Vitro. Infect. Genet. Evol. 2017, 52, 34–43. [Google Scholar] [CrossRef]
- Dewi, B.E.; Takasaki, T.; Kurane, I. In Vitro Assessment of Human Endothelial Cell Permeability: Effects of Inflammatory Cytokines and Dengue Virus Infection. J. Virol. Methods 2004, 121, 171–180. [Google Scholar] [CrossRef]
- Dewi, B.E.; Takasaki, T.; Kurane, I. Peripheral Blood Mononuclear Cells Increase the Permeability of Dengue Virus-Infected Endothelial Cells in Association with Downregulation of Vascular Endothelial Cadherin. J. Gen. Virol. 2008, 89, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Luplerdlop, N.; Missé, D.; Bray, D.; Deleuze, V.; Gonzalez, J.P.; Leardkamolkarn, V.; Yssel, H.; Veas, F. Dengue-Virus-Infected Dendritic Cells Trigger Vascular Leakage through Metalloproteinase Overproduction. EMBO Rep. 2006, 7, 1176–1181. [Google Scholar] [CrossRef]
- Bok, K.; Castagnaro, N.; Borsa, A.; Nates, S.; Espul, C.; Fay, O.; Fabri, A.; Grinstein, S.; Miceli, I.; Matson, D.O.; et al. Plasma Concentrations of SVCAM-1 and Severity of Dengue Infections. J. Med. Virol. 2001, 65, 97–104. [Google Scholar] [CrossRef]
- Kelley, J.F.; Kaufusi, P.; Nerurkar, V. Dengue Hemorrhagic Fever-Associated Immunomediators Induced via Maturation of Dengue Virus Nonstructural 4B Protein in Monocytes Modulate Endothelial Cell Adhesion Molecules and Human Microvascular Endothelial Cells Permeability. Virology 2012, 422, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Warke, R.V.; Xhaja, K.; Martin, K.J.; Fournier, M.V.; Shaw, S.K.; Brizuela, N.; de Bosch, N.; Lapointe, D.; Ennis, F.A.; Rothman, A.L.; et al. Dengue Virus Induces Novel Changes in Gene Expression of Human Umbilical Vein Endothelial Cells. J. Virol. 2004, 78, 4947. [Google Scholar] [CrossRef]
- Fiestas Solórzano, V.E.; de Lima, R.C.; de Azeredo, E.L. The Role of Growth Factors in the Pathogenesis of Dengue: A Scoping Review. Pathogens 2022, 11, 1179. [Google Scholar] [CrossRef]
- Fiestas Solórzano, V.E.; da Costa Faria, N.R.; dos Santos, C.F.; Corrêa, G.; Cipitelli, M.d.C.; Dornelas Ribeiro, M.; de Souza, L.J.; Damasco, P.V.; da Cunha, R.V.; dos Santos, F.B.; et al. Different Profiles of Cytokines, Chemokines and Coagulation Mediators Associated with Severity in Brazilian Patients Infected with Dengue Virus. Viruses 2021, 13, 1789. [Google Scholar] [CrossRef] [PubMed]
- Jeewandara, C.; Gomes, L.; Wickramasinghe, N.; Gutowska-Owsiak, D.; Waithe, D.; Paranavitane, S.A.; Shyamali, N.L.A.; Ogg, G.S.; Malavige, G.N. Platelet Activating Factor Contributes to Vascular Leak in Acute Dengue Infection. PLoS Negl. Trop. Dis. 2015, 9, e0003459. [Google Scholar] [CrossRef]
- Puerta-Guardo, H.; Glasner, D.R.; Harris, E. Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog. 2016, 12, e1005738. [Google Scholar] [CrossRef]
- Glasner, D.R.; Puerta-Guardo, H.; Beatty, P.; Harris, E. The Good, the Bad, and the Shocking: The Multiple Roles of Dengue Virus Nonstructural Protein 1 in Protection and Pathogenesis. Annu. Rev. Virol. 2018, 5, 227–253. [Google Scholar] [CrossRef] [PubMed]
- Barbachano-Guerrero, A.; Endy, T.P.; King, C.A. Dengue Virus Non-Structural Protein 1 Activates the P38 MAPK Pathway to Decrease Barrier Integrity in Primary Human Endothelial Cells. J. Gen. Virol. 2020, 101, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Chanthick, C.; Suttitheptumrong, A.; Rawarak, N.; Pattanakitsakul, S.N. Transcytosis Involvement in Transport System and Endothelial Permeability of Vascular Leakage during Dengue Virus Infection. Viruses 2018, 10, 69. [Google Scholar] [CrossRef]
- Wang, C.; Puerta-Guardo, H.; Biering, S.; Glasner, D.R.; Tran, E.B.; Patana, M.; Gomberg, T.A.; Malvar, C.; Lo, N.T.N.; Espinosa, D.; et al. Endocytosis of Flavivirus NS1 Is Required for NS1-Mediated Endothelial Hyperpermeability and Is Abolished by a Single N-Glycosylation Site Mutation. PLoS Pathog. 2019, 15, e1007938. [Google Scholar] [CrossRef]
- Dalrymple, N.A.; Mackow, E. Productive Dengue Virus Infection of Human Endothelial Cells Is Directed by Heparan Sulfate-Containing Proteoglycan Receptors. J. Virol. 2011, 85, 9478–9485. [Google Scholar] [CrossRef] [PubMed]
- Modhiran, N.; Watterson, D.; Muller, D.; Panetta, A.K.; Sester, D.; Liu, L.; Hume, D.; Stacey, K.; Young, P. Dengue Virus NS1 Protein Activates Cells via Toll-like Receptor 4 and Disrupts Endothelial Cell Monolayer Integrity. Sci. Transl. Med. 2015, 7, 304ra142. [Google Scholar] [CrossRef] [PubMed]
- Modhiran, N.; Watterson, D.; Blumenthal, A.; Baxter, A.; Young, P.; Stacey, K. Dengue Virus NS1 Protein Activates Immune Cells via TLR4 but Not TLR2 or TLR6. Immunol. Cell Biol. 2017, 95, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Sabbineni, H.; Verma, A.; Somanath, P.R. Isoform-Specific Effects of Transforming Growth Factor β on Endothelial-to-Mesenchymal Transition. J. Cell. Physiol. 2018, 233, 8418–8428. [Google Scholar] [CrossRef]
- Dong, W.; Kong, M.; Zhu, Y.; Shao, Y.; Wu, D.; Lu, J.; Guo, J.; Xu, Y. Activation of TWIST Transcription by Chromatin Remodeling Protein BRG1 Contributes to Liver Fibrosis in Mice. Front. Cell Dev. Biol. 2020, 8, 340. [Google Scholar] [CrossRef]
- Ladak, S.; McQueen, L.; Tomkova, K.; Adebayo, A.; Suleiman, S.; George, S.; Murphy, G.; Zakkar, M. Dexamethasone Modulate TWIST Mediated EndMT Changes in Venous EC under Acute Shear Stress. Implications for Vein Grafts Disease. medRxiv 2023, 1–74. [Google Scholar] [CrossRef]
- Kryczka, J.; Przygodzka, P.; Bogusz, H.; Boncela, J. HMEC-1 Adopt the Mixed Amoeboid-Mesenchymal Migration Type during EndMT. Eur. J. Cell Biol. 2017, 96, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, M.; Gawryś, K.; Popielarski, M.; Bednarek, R.; Studzian, M.; Sitkiewicz, E.; Szemraj, J.; Świątkowska, M. Differential Quantitative Proteomics of Human Microvascular Endothelial Cells 1 by ITRAQ Reveals Palladin to Be a New Biomarker During TGF-Β1 Induced Endothelial Mesenchymal Transition. J. Proteom. Bioinform. 2017, 10, 236–245. [Google Scholar] [CrossRef]
- Islam, S.; Boström, K.I.; Di Carlo, D.; Simmons, C.A.; Tintut, Y.; Yao, Y.; Hsu, J.J. The Mechanobiology of Endothelial-to-Mesenchymal Transition in Cardiovascular Disease. Front. Physiol. 2021, 12, 734215. [Google Scholar] [CrossRef] [PubMed]
- Ciszewski, W.M.; Sobierajska, K.; Wawro, M.E.; Klopocka, W.; Chefczyńska, N.; Muzyczuk, A.; Siekacz, K.; Wujkowska, A.; Niewiarowska, J. The ILK-MMP9-MRTF Axis Is Crucial for EndMT Differentiation of Endothelial Cells in a Tumor Microenvironment. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 2283–2296. [Google Scholar] [CrossRef]
- Mimouni, M.; Lajoix, A.D.; Desmetz, C. Experimental Models to Study Endothelial to Mesenchymal Transition in Myocardial Fibrosis and Cardiovascular Diseases. Int. J. Mol. Sci. 2024, 25, 382. [Google Scholar] [CrossRef] [PubMed]
- Van Meeteren, L.A.; Ten Dijke, P. Regulation of Endothelial Cell Plasticity by TGF-β. Cell Tissue Res. 2012, 347, 177–186. [Google Scholar] [CrossRef]
- Yoshimatsu, Y.; Watabe, T. Emerging Roles of Inflammation-Mediated Endothelial–Mesenchymal Transition in Health and Disease. Inflamm. Regen. 2022, 42, 9. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, J.C.; Kishta, F.; Xu, Y.; Baker, A.H. Endothelial to Mesenchymal Transition: At the Axis. Cardiovasc. Res. 2024, 120, 223–236. [Google Scholar] [CrossRef]
- Chislock, E.M.; Ring, C.; Pendergast, A.M. Abl Kinases Are Required for Vascular Function, Tie2 Expression, and Angiopoietin-1-Mediated Survival. Proc. Natl. Acad. Sci. USA 2013, 110, 12432–12437. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Wang, Z.; Gao, C.; Wu, J.; Wu, Q. Trajectory Modeling of Endothelial-to-Mesenchymal Transition Reveals Galectin-3 as a Mediator in Pulmonary Fibrosis. Cell Death Dis. 2021, 12, 327. [Google Scholar] [CrossRef]
- Di Benedetto, P.; Ruscitti, P.; Berardicurti, O.; Vomero, M.; Navarini, L.; Dolo, V.; Cipriani, P.; Giacomelli, R. Endothelial-to-Mesenchymal Transition in Systemic Sclerosis. Clin. Exp. Immunol. 2021, 205, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Aono, Y.; Nishioka, Y.; Inayama, M.; Ugai, M.; Kishi, J.; Uehara, H.; Izumi, K.; Sone, S. Imatinib as a Novel Antifibrotic Agent in Bleomycin-Induced Pulmonary Fibrosis in Mice. Am. J. Respir. Crit. Care Med. 2005, 171, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Akhmetshina, A.; Venalis, P.; Dees, C.; Busch, N.; Zwerina, J.; Schett, G.; Distler, O.; Distler, J.H.W. Treatment with Imatinib Prevents Fibrosis in Different Preclinical Models of Systemic Sclerosis and Induces Regression of Established Fibrosis. Arthritis Rheum. 2009, 60, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Touret, F.; Baronti, C.; Goethals, O.; Van Loock, M.; de Lamballerie, X.; Querat, G. Phylogenetically Based Establishment of a Dengue Virus Panel, Representing All Available Genotypes, as a Tool in Dengue Drug Discovery. Antivir. Res. 2019, 168, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Vedagiri, D.; Gupta, D.; Mishra, A.; Krishna, G.; Bhaskar, M.; Sah, V.; Basu, A. Retinoic Acid-Inducible Gene I-Like Receptors Activate Snail To Limit RNA Viral Infections. J. Virol. 2021, 95, 01216–01221. [Google Scholar] [CrossRef]
- Alfaro-García, J.P.; Granados-Alzate, M.C.; Vicente-Manzanares, M.; Gallego-Gómez, J.C. An Integrated View of Virus-Triggered Cellular Plasticity Using Boolean Networks. Cells 2021, 10, 2863. [Google Scholar] [CrossRef] [PubMed]
- Frakolaki, E.; Kaimou, P.; Moraiti, M.; Kalliampakou, K.I.; Karampetsou, K.; Dotsika, E.; Liakos, P.; Vassilacopoulou, D.; Mavromara, P.; Bartenschlager, R.; et al. The Role of Tissue Oxygen Tension in Dengue Virus Replication. Cells 2018, 7, 241. [Google Scholar] [CrossRef]
- Hincapie, V.; Gallego-gómez, J.C. Transición Epitelio-Mesénquima Inducida Por Virus. Acta Biol. Colomb. 2020, 26, 105–115. [Google Scholar] [CrossRef]
- Clark, M.J.; Miduturu, C.; Schmidt, A.G.; Jang, J.; Chu, H.; Gray, N.S.; Yang, P.L.; Clark, M.J.; Miduturu, C.; Schmidt, A.G.; et al. GNF-2 Inhibits Dengue Virus by Targeting Abl Kinases and the Viral E Protein Article GNF-2 Inhibits Dengue Virus by Targeting Abl Kinases and the Viral E Protein. Cell Chem. Biol. 2016, 23, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sicinski, P. A Kinase of Many Talents: Non-Neuronal Functions of CDK5 in Development and Disease. Open Biol. 2020, 10, 190287. [Google Scholar] [CrossRef]
- Chislock, E.M.; Pendergast, A.M. Abl Family Kinases Regulate Endothelial Barrier Function In Vitro and in Mice. PLoS ONE 2013, 8, e85231. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wu, Y.F.; Wang, X.; Han, Y.Z. The C-Abl Inhibitor in Parkinson Disease. Neurol. Sci. 2017, 38, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bleher, R.; Wang, L.; Garcia, J.G.N.; Dudek, S.M.; Shekhawat, G.S.; Dravid, V.P. Imatinib Alters Agonists-Mediated Cytoskeletal Biomechanics in Lung Endothelium. Sci. Rep. 2017, 7, 14152. [Google Scholar] [CrossRef]
- Talavera, D.; Castillo, A.M.; Dominguez, M.C.; Escobar Gutierrez, A.; Meza, I. IL8 Release, Tight Junction and Cytoskeleton Dynamic Reorganization Conducive to Permeability Increase Are Induced by Dengue Virus Infection of Microvascular Endothelial Monolayers. J. Gen. Virol. 2004, 85, 1801–1813. [Google Scholar] [CrossRef]
- Lutter, S.; Xie, S.; Tatin, F.; Makinen, T. Smooth Muscle–Endothelial Cell Communication Activates Reelin Signaling and Regulates Lymphatic Vessel Formation. J. Cell Biol. 2012, 197, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Dugina, V.B.; Shagieva, G.S.; Shakhov, A.S. The Cytoplasmic Actins in the Regulation of Endothelial Cell Function. Int. J. Mol. Sci. 2021, 22, 7836. [Google Scholar] [CrossRef] [PubMed]
- Molla, R.; Shimizu, A.; Komeno, M.; Rahman, N.I.A.; Ern, J.; Soh, C.; Kim, L.; Nguyen, C.; Khan, M.R.; Tesega, W.W.; et al. Vascular Smooth Muscle RhoA Counteracts Abdominal Aortic Aneurysm Formation by Modulating MAP4K4 Activity. Commun. Biol. 2022, 5, 1071. [Google Scholar] [CrossRef]
- Ciszewski, W.M.; Wawro, M.E.; Sacewicz-hofman, I.; Sobierajska, K. Cytoskeleton Reorganization in Endmt—The Role in Cancer and Fibrotic Diseases. Int. J. Mol. Sci. 2021, 22, 11607. [Google Scholar] [CrossRef]
- Hawez, A.; Ding, Z.; Taha, D.; Madhi, R. C-Abl Kinase Regulates Neutrophil Extracellular Trap Formation and Lung Injury in Abdominal Sepsis. Nature 2021, 102, 263–271. [Google Scholar] [CrossRef]
- Amado, J.; Anne, A.; Stalborch, M.D.V.; Valent, E.T.; Nawaz, K.; Bezu, J.V.; Abl-related, A.; Crk-like, C. Depletion of Arg/Abl2 Improves Endothelial Cell Adhesion and Prevents Vascular Leak during Inflammation Acute Respiratory Distress Syndrome. Angiogenesis 2021, 24, 677–693. [Google Scholar] [CrossRef]
- Deville, S.S.; Cordes, N. The Extracellular, Cellular, and Nuclear Stiffness, a Trinity in the Cancer Resistome—A Review. Front. Oncol. 2019, 9, 1376. [Google Scholar] [CrossRef] [PubMed]
- DeWane, G.; Salvi, A.M.; DeMali, K.A. Fueling the Cytoskeleton-Links between Cell Metabolism and Actin Remodeling. J. Cell Sci. 2021, 134, jcs248385. [Google Scholar] [CrossRef]
- Danielsson, F.; Peterson, M.K.; Araújo, H.C.; Lautenschläger, F.; Gad, A.K.B. Vimentin Diversity in Health and Disease. Cells 2018, 7, 147. [Google Scholar] [CrossRef]
- Takaoka, Y.; Uchinomiya, S.; Kobayashi, D.; Endo, M.; Hayashi, T.; Fukuyama, Y.; Hayasaka, H.; Miyasaka, M.; Ueda, T.; Shimada, I.; et al. Endogenous Membrane Receptor Labeling by Reactive Cytokines and Growth Factors to Chase Their Dynamics in Live Cells. Chem 2018, 4, 1451–1464. [Google Scholar] [CrossRef]
- Zhong, A.; Mirzaei, Z.; Simmons, C.A. The Roles of Matrix Stiffness and SS-Catenin Signaling in Endothelial-to-Mesenchymal Transition of Aortic Valve Endothelial Cells. Cardiovasc. Eng. Technol. 2018, 9, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.K.; Wang, N.; Leckband, D.E. Local VE-Cadherin Mechanotransduction Triggers Long-Ranged Remodeling of Endothelial Monolayers. J. Cell Sci. 2015, 128, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as Biomechanical Sensors of the Microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Tian, D.; Zeng, X.; Liu, Y.; Fu, Q.; Liang, A.; Zhang, Y.; Gao, Q.; Cheng, J.; et al. Integrin Β3 Mediates the Endothelial-to-Mesenchymal Transition via the Notch Pathway. Cell. Physiol. Biochem. 2018, 49, 985–997. [Google Scholar] [CrossRef]
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Geng, Z.; Wang, S.; Yu, Z.; Liu, T.; Guan, S.; Du, S.; Zhu, C. The Driving Mechanism and Targeting Value of Mimicry between Vascular Endothelial Cells and Tumor Cells in Tumor Progression. Biomed. Pharmacother. 2023, 165, 115029. [Google Scholar] [CrossRef]
- Rivera, J.; Neira, M.; Parra, E.; Méndez, J.; Sarmiento, L.; Caldas, M.L. Detección de Antígenos Del Virus Del Dengue En Tejidos Post Mórtem. Biomedica 2014, 34, 514–520. [Google Scholar] [CrossRef]
- Rivera, J.A.; Rengifo, A.C.; Parra, E.A.; Castellanos, J.E.; Caldas, M.L. Histopatología Ilustrada de Casos Fatales de Dengue En Colombia. Biomedica 2020, 40, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Vásquez Ochoa, M.; García Cordero, J.; Gutiérrez Castañeda, B.; Santos Argumedo, L.; Villegas Sepúlveda, N.; Cedillo Barrón, L. A Clinical Isolate of Dengue Virus and Its Proteins Induce Apoptosis in HMEC-1 Cells: A Possible Implication in Pathogenesis. Arch. Virol. 2009, 154, 919–928. [Google Scholar] [CrossRef]
- Martinez-Gutierrez, M.; Correa-Londoño, L.A.; Castellanos, J.E.; Gallego-Gómez, J.C.; Osorio, J.E. Lovastatin Delays Infection and Increases Survival Rates in AG129 Mice Infected with Dengue Virus Serotype 2. PLoS ONE 2014, 9, e87412. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Wilcox, R. One-Way and Higher Designs for Independent Groups. In Introduction to Robust Estimation and Hypothesis Testing; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 9780123869838. [Google Scholar]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]


| GSE Accession | Treatment | Group (Treatment/Control) | Exposure Times | Number of Transcriptomes | Array Platform |
|---|---|---|---|---|---|
| GSE77597 | Untreated | Control | 0 h | 2 | Illumina HiSeq 2000 (Homo sapiens) |
| ANG2 Activating Antibody | Treatment | 4 h | 2 | Illumina HiSeq 2000 (Homo sapiens) | |
| GSE2639 | TNF | Treatment | 5 h | 4 | Affymetrix Human Genome U133A Array |
| Untreated | Control | 0 h | 4 | Affymetrix Human Genome U133A Array | |
| GSE9055 | Untreated | Control | 0 h | 1 | Affymetrix Human Genome U133 Plus 2.0 Array |
| TNF | Treatment | From 1 to 8 h | 21 | Affymetrix Human Genome U133 Plus 2.0 Array | |
| GSE15464 | VEGF | Treatment | From 0.5 to 2.5 h | 3 | Affymetrix Human Genome U133 Plus 2.0 Array |
| Untreated | Control | From 0.5 to 2.5 h | 1 | Affymetrix Human Genome U133 Plus 2.0 Array | |
| GSE10778 | VEGF | Treatment | From 0.5 to 6 h | 4 | Affymetrix Human Genome U133A Array |
| Gene | Drug | Interaction Type | PMIDs |
|---|---|---|---|
| CD69 | TOCILIZUMAB | 27339827 | |
| CDKN2B | PALBOCICLIB | 23898052 | |
| CXCL10 | METHYLPREDNISOLONE | 17220550 | |
| CXCL8 | DACARBAZINE | ||
| HDAC9 | VORINOSTAT | inhibitor | 19344175 |
| IFNGR1 | INTERFERON GAMMA-1B | Binder—agonist | 17618444 |
| IGF1R | PAZOPANIB | ||
| IL1R1 | ANAKINRA | Inhibitor—antagonist | 17083033 |
| NT5C2 | MERCAPTOPURINE | 15990089 | |
| PDGFD | SUNITINIB | inhibitor | |
| PIK3CD | IDELALISIB | inhibitor | |
| PMAIP1 | BORTEZOMIB | 16024631 | |
| PTK2 | PAZOPANIB | ||
| SMAD2 | BLEOMYCIN | 17274978 | |
| SOD2 | PACLITAXEL | 25495407 | |
| TNF | THALIDOMIDE | inhibitor | 8755512 |
| TNFSF13B | BELIMUMAB | Antibody—inhibitor—modulator | |
| VCAM1 | DEXAMETHASONE | 7694584 | |
| GAS6 | DOCETAXEL | 27153245 | |
| BCL2L11 | IMATINIB | 24223824 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfaro-García, J.P.; Orozco-Castaño, C.A.; Sánchez-Rendón, J.A.; Casanova-Yépes, H.F.; Vicente-Manzanares, M.; Gallego-Gómez, J.C. Characterization of the Temporal Dynamics of the Endothelial–Mesenchymal-like Transition Induced by Soluble Factors from Dengue Virus Infection in Microvascular Endothelial Cells. Int. J. Mol. Sci. 2025, 26, 2139. https://doi.org/10.3390/ijms26052139
Alfaro-García JP, Orozco-Castaño CA, Sánchez-Rendón JA, Casanova-Yépes HF, Vicente-Manzanares M, Gallego-Gómez JC. Characterization of the Temporal Dynamics of the Endothelial–Mesenchymal-like Transition Induced by Soluble Factors from Dengue Virus Infection in Microvascular Endothelial Cells. International Journal of Molecular Sciences. 2025; 26(5):2139. https://doi.org/10.3390/ijms26052139
Chicago/Turabian StyleAlfaro-García, Jenny Paola, Carlos Alberto Orozco-Castaño, Julián Andrés Sánchez-Rendón, Herley Fernando Casanova-Yépes, Miguel Vicente-Manzanares, and Juan Carlos Gallego-Gómez. 2025. "Characterization of the Temporal Dynamics of the Endothelial–Mesenchymal-like Transition Induced by Soluble Factors from Dengue Virus Infection in Microvascular Endothelial Cells" International Journal of Molecular Sciences 26, no. 5: 2139. https://doi.org/10.3390/ijms26052139
APA StyleAlfaro-García, J. P., Orozco-Castaño, C. A., Sánchez-Rendón, J. A., Casanova-Yépes, H. F., Vicente-Manzanares, M., & Gallego-Gómez, J. C. (2025). Characterization of the Temporal Dynamics of the Endothelial–Mesenchymal-like Transition Induced by Soluble Factors from Dengue Virus Infection in Microvascular Endothelial Cells. International Journal of Molecular Sciences, 26(5), 2139. https://doi.org/10.3390/ijms26052139








