Urinary Titin on the First Postoperative Day Predicts Long-Term Skeletal Muscle Loss in Patients with Gastroenterological Cancer
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
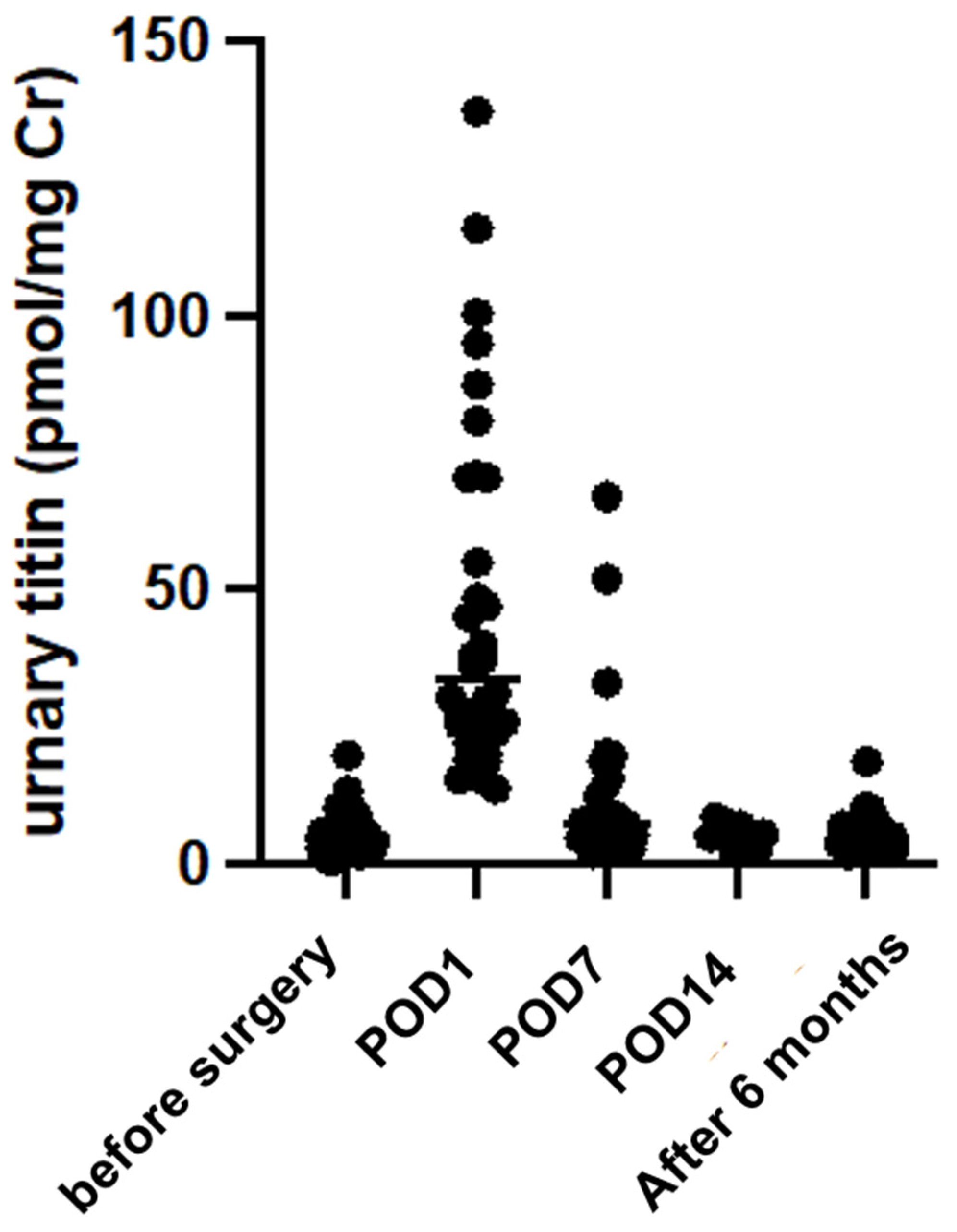
2.2. Changes in Urinary Titin Levels
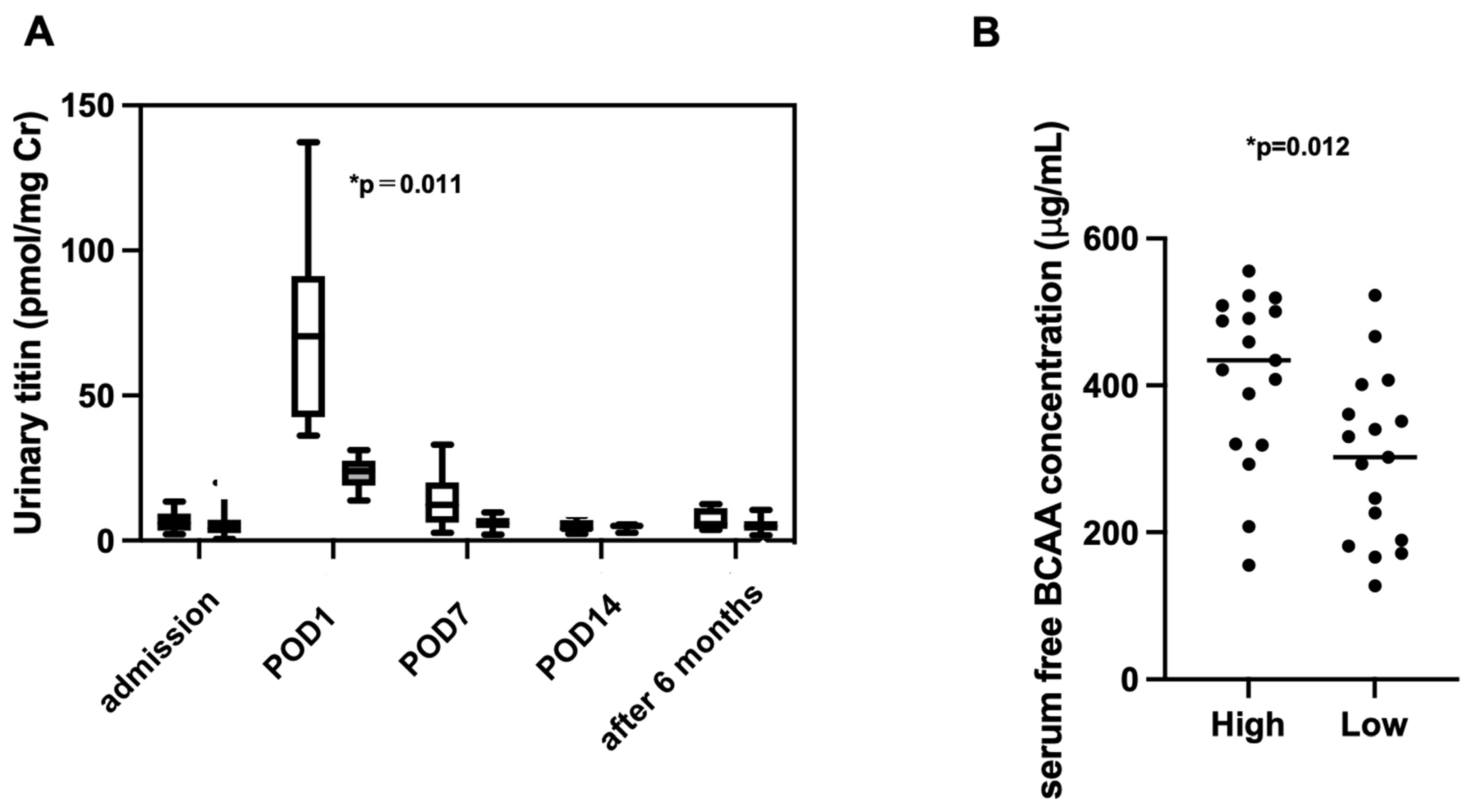
2.3. Association Between Urinary Titin on POD1 and Nutritional Status at 6 Months Postoperatively
2.4. Factors Associated with Postoperative Elevation of Urinary Titin
2.5. Factors Associated with Skeletal Muscle Loss 6 Months Postoperatively
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Patient Selection Criteria
4.3. Urinary Titin Measurement
4.4. Nutritional and Other Assessment
4.5. Measurement of Metabolites
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.S.; Wang, L. Preoperative malnutrition in elderly gastric cancer patients and adverse postoperative outcomes of radical gastrectomy. World J. Gastrointest. Surg. 2024, 16, 3618–3622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, S.; Gao, X.; Gao, T.; Huang, L.; Lian, B.; Gu, Y.; Chen, J.; Guo, D.; Jia, Z.; et al. Poor Pre-operative Nutritional Status Is a Risk Factor of Post-operative Infections in Patients with Gastrointestinal Cancer-A Multicenter Prospective Cohort Study. Front. Nutr. 2022, 9, 850063. [Google Scholar] [CrossRef] [PubMed]
- Hebuterne, X.; Lemarie, E.; Michallet, M.; de Montreuil, C.B.; Schneider, S.M.; Goldwasser, F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J. Parenter. Enteral Nutr. 2014, 38, 196–204. [Google Scholar] [CrossRef]
- Aoyama, T.; Kawabe, T.; Fujikawa, H.; Hayashi, T.; Yamada, T.; Tsuchida, K.; Yukawa, N.; Oshima, T.; Rino, Y.; Masuda, M.; et al. Loss of Lean Body Mass as an Independent Risk Factor for Continuation of S-1 Adjuvant Chemotherapy for Gastric Cancer. Ann. Surg. Oncol. 2015, 22, 2560–2566. [Google Scholar] [CrossRef] [PubMed]
- Kuwada, K.; Kikuchi, S.; Kuroda, S.; Yoshida, R.; Takagi, K.; Noma, K.; Nishizaki, M.; Kagawa, S.; Umeda, Y.; Fujiwara, T. Survival Impact of Postoperative Skeletal Muscle Loss in Gastric Cancer Patients Who Underwent Gastrectomy. Anticancer Res. 2023, 43, 223–230. [Google Scholar] [CrossRef]
- Nakashima, Y.; Saeki, H.; Hu, Q.; Tsuda, Y.; Zaitsu, Y.; Hisamatsu, Y.; Ando, K.; Kimura, Y.; Oki, E.; Mori, M. Skeletal Muscle Loss After Esophagectomy Is an Independent Risk Factor for Patients with Esophageal Cancer. Ann. Surg. Oncol. 2020, 27, 492–498. [Google Scholar] [CrossRef]
- Yoshida, S.; Nishigori, T.; Tsunoda, S.; Tanaka, E.; Okabe, H.; Kobayashi, A.; Nobori, Y.; Obama, K.; Hisamori, S.; Shide, K.; et al. Chronological Changes in Skeletal Muscle Mass Two Years after Minimally Invasive Esophagectomy: A Prospective Cohort Study. Surg. Endosc. 2022, 36, 1527–1535. [Google Scholar] [CrossRef]
- Mita, J.; Maeda, T.; Tsujita, E.; Hashimoto, N.; Fujikawa, R.; Ono, Y.; Sakai, A.; Tanaka, S.; Matono, R.; Ohmine, T.; et al. Skeletal Muscle Mass Index Reduction Rate as a Prognostic Indicator for Patients Undergoing Pancreatectomy for Pancreatic Cancer. Cancer Diagn. Progn. 2024, 4, 301–308. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Kobayashi, A.; Shirai, H.; Yao, S.; Yagi, S.; Kamo, N.; Seo, S.; Taura, K.; et al. Preoperative Visceral Adiposity and Muscularity Predict Poor Outcomes after Hepatectomy for Hepatocellular Carcinoma. Liver Cancer 2019, 8, 92–109. [Google Scholar] [CrossRef]
- de Luis Roman, D.; Lopez Gomez, J.J.; Munoz, M.; Primo, D.; Izaola, O.; Sanchez, I. Evaluation of Muscle Mass and Malnutrition in Patients with Colorectal Cancer Using the Global Leadership Initiative on Malnutrition Criteria and Comparing Bioelectrical Impedance Analysis and Computed Tomography Measurements. Nutrients 2024, 16, 3035. [Google Scholar] [CrossRef]
- Awano, H.; Matsumoto, M.; Nagai, M.; Shirakawa, T.; Maruyama, N.; Iijima, K.; Nabeshima, Y.I.; Matsuo, M. Diagnostic and clinical significance of the titin fragment in urine of Duchenne muscular dystrophy patients. Clin. Chim. Acta 2018, 476, 111–116. [Google Scholar] [CrossRef]
- Linke, W.A. Titin Gene and Protein Functions in Passive and Active Muscle. Annu. Rev. Physiol. 2018, 80, 389–411. [Google Scholar] [CrossRef] [PubMed]
- Freundt, J.K.; Linke, W.A. Titin as a force-generating muscle protein under regulatory control. J. Appl. Physiol. 2019, 126, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Awano, H.; Nishio, H. Can urinary titin be used for predicting Duchenne muscular dystrophy? Clin. Chim. Acta 2019, 490, 162. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, N.; Tsutsumi, R.; Hara, K.; Takashima, T.; Nakataki, E.; Itagaki, T.; Matsuo, M.; Oto, J.; Sakaue, H. Urinary Titin Is a Novel Biomarker for Muscle Atrophy in Nonsurgical Critically Ill Patients: A Two-Center, Prospective Observational Study. Crit. Care Med. 2020, 48, 1327–1333. [Google Scholar] [CrossRef]
- Ishihara, M.; Nakanishi, N.; Tsutsumi, R.; Hara, K.; Machida, K.; Yamamoto, N.; Kanematsu, Y.; Sakaue, H.; Oto, J.; Takagi, Y. Elevated Urinary Titin and its Associated Clinical Outcomes after Acute Stroke. J. Stroke Cerebrovasc. Dis. 2021, 30, 105561. [Google Scholar] [CrossRef]
- Arase, M.; Nakanishi, N.; Tsutsumi, R.; Kawakami, A.; Arai, Y.; Sakaue, H.; Oto, J. The Utility of Urinary Titin to Diagnose and Predict the Prognosis of Acute Myocardial Infarction. Int. J. Mol. Sci. 2024, 25, 573. [Google Scholar] [CrossRef]
- Takiguchi, Y.; Tsutsumi, R.; Shimabukuro, M.; Tanabe, H.; Kawakami, A.; Hyodo, M.; Shiroma, K.; Saito, H.; Matsuo, M.; Sakaue, H. Urinary titin as a biomarker of sarcopenia in diabetes: A propensity score matching analysis. J. Endocrinol. Investig. 2024. [Google Scholar] [CrossRef]
- Tanihata, J.; Nishioka, N.; Inoue, T.; Bando, K.; Minamisawa, S. Urinary Titin Is Increased in Patients After Cardiac Surgery. Front. Cardiovasc. Med. 2019, 6, 7. [Google Scholar] [CrossRef]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef]
- Fanzani, A.; Conraads, V.M.; Penna, F.; Martinet, W. Molecular and cellular mechanisms of skeletal muscle atrophy: An update. J. Cachexia Sarcopenia Muscle 2012, 3, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Rennie, M.J. Anabolic resistance in critically ill patients. Crit. Care Med. 2009, 37, S398–S399. [Google Scholar] [CrossRef] [PubMed]
- Rennie, M.J. Anabolic resistance: The effects of aging, sexual dimorphism, and immobilization on human muscle protein turnover. Appl. Physiol. Nutr. Metab. 2009, 34, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, M.; Nomura, K.; Tsutsumi, R.; Izumi-Mishima, Y.; Kawaguchi, H.; Kawakami, A.; Hara, K.; Suzuki, Y.; Shirakawa, T.; Osawa, K.; et al. Urinary titin as an early biomarker of skeletal muscle proteolysis and atrophy in various catabolic conditions. Biochem. Biophys. Res. Commun. 2024, 737, 150918. [Google Scholar] [CrossRef]
- Huhmann, M.B.; August, D.A. Nutrition support in surgical oncology. Nutr. Clin. Pract. 2009, 24, 520–526. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hubner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.G.; et al. ESPEN practical guideline: Clinical nutrition in surgery. Clin. Nutr. 2021, 40, 4745–4761. [Google Scholar] [CrossRef]
- Shitara, K.; Fleitas, T.; Kawakami, H.; Curigliano, G.; Narita, Y.; Wang, F.; Wardhani, S.O.; Basade, M.; Rha, S.Y.; Wan Zamaniah, W.I.; et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with gastric cancer. ESMO Open 2024, 9, 102226. [Google Scholar] [CrossRef]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-kinase- and exercise-related muscle damage implications for muscle performance and recovery. J. Nutr. Metab. 2012, 2012, 960363. [Google Scholar] [CrossRef]
- De Jonghe, B.; Sharshar, T.; Lefaucheur, J.P.; Authier, F.J.; Durand-Zaleski, I.; Boussarsar, M.; Cerf, C.; Renaud, E.; Mesrati, F.; Carlet, J.; et al. Paresis acquired in the intensive care unit: A prospective multicenter study. JAMA 2002, 288, 2859–2867. [Google Scholar] [CrossRef]
- Moghadam-Kia, S.; Oddis, C.V.; Aggarwal, R. Approach to asymptomatic creatine kinase elevation. Cleve Clin. J. Med. 2016, 83, 37–42. [Google Scholar] [CrossRef]
- Maruyama, N.; Asai, T.; Abe, C.; Inada, A.; Kawauchi, T.; Miyashita, K.; Maeda, M.; Matsuo, M.; Nabeshima, Y.I. Establishment of a highly sensitive sandwich ELISA for the N-terminal fragment of titin in urine. Sci. Rep. 2016, 6, 39375. [Google Scholar] [CrossRef]

| n = 34 | |
|---|---|
| Age (years) (mean ± SD) | 68.7 ± 8.5 |
| Male, n (%) | 16 (47.1) |
| BMI (kg/m2) | 22.3 (20.4–23.8) |
| Length of hospital stay (days) | 17.0 (13–23) |
| Malignancy rate, n (%) | 32 (94.2) |
| Esophageal cancer, n (%) | 6 (17.7) |
| Gastric cancer, n (%) | 18 (53.0) |
| Pancreatic cancer, n (%) | 7 (20.6) |
| Liver cancer, n (%) | 1 (2.9) |
| Gastrointestinal stromal tumor of the stomach, n (%) | 1 (2.9) |
| Mucinous cystic neoplasm of pancreas, n (%) | 1 (2.9) |
| Stage | |
| Stage 0, n (%) | 3 (9.7) |
| Stage I, n (%) | 17 (54.8) |
| Stage II, n (%) | 9 (29.0) |
| Stage III, n (%) | 2 (6.5) |
| Co-morbidity | |
| Hypertension, n (%) | 15 (44.1) |
| Diabetes, n (%) | 8 (23.5) |
| Dyslipidemia, n (%) | 8 (23.5) |
| Liver disease, n (%) | 8 (23.5) |
| Hyperuricemia, n (%) | 5 (14.7) |
| Renal failure, n (%) | 0 (0) |
| Resection site | |
| Esophagus, n (%) | 6 (17.7) |
| Stomach, n (%) | DG; 15 (44.2), PG; 3 (8.8), TG; 1 (2.9) |
| Pancreas, n (%) | DP; 3 (8.8), PD; 5 (14.7) |
| Liver, n (%) | 1 (2.9) |
| Surgery | |
| Laparotomy, n (%) | 5 (14.7) |
| Intraoperative hemorrhage (mL) | 25.0 (7–125) |
| Surgical time (h) | 6.8 (4.6–9.5) |
| POD1 High Titin Group (n = 17) | POD1 Low Titin Group (n = 17) | p-Value | |
|---|---|---|---|
| Age (years), (mean ± SD) | 68.8 (±10.3) | 68.6 (±6.9) | 0.973 |
| Male, n (%) | 6 (35.3) | 10 (58.8) | 0.303 |
| BMI (kg/m2) | 20.3 (18.2–22.5) | 22.8 (22.0–24.0) | 0.009 |
| Length of stay, (days) | 20 (13–25) | 17 (14–21) | 0.522 |
| Preoperative assessment | |||
| Serum albumin (g/dL) | 3.9 (3.7–4.1) | 3.8 (3.6–4.4) | 0.616 |
| Serum transthyretin (mg/dL) | 23.8 (21.0–26.3) | 25.5 (21.1–28.3) | 0.614 |
| Serum zinc (μg/dL) | 68 (66.8–79.5) | 73.0 (68.0–77.0) | 0.705 |
| Preoperative body composition | |||
| Skeletal muscle mass (kg) | 19.9 (17.9–25.7) | 25.4 (20.5–28.3) | 0.060 |
| Body cell mass (kg) | 24 (21.9–30.4) | 30.1 (24.7–33.3) | 0.058 |
| Grip strength (kg) | 19.6 (17.3–24.0) | 24.1 (20.6–29.3) | 0.081 |
| Preoperative nutritional screening | |||
| SGA A | 16 | 16 | 1.000 |
| SGA B | 0 | 1 | 1.000 |
| SGA C | 1 | 0 | 1.000 |
| Nutritional intake | |||
| Preoperative | |||
| Energy intake (kg/kcal) | 31.4 (26.3–37.8) | 29.7 (26.0–31.9) | 0.339 |
| Protein intake (g/kg) | 1.2 (1.0–1.5) | 1.2 (1.0–1.4) | 0.563 |
| POD 7 | |||
| Energy intake (kg/kcal) | 20.8 (16.2–28.6) | 21.0 (14.7–24.6) | 0.540 |
| Protein intake (g/kg) | 1.1 (0.7–1.2) | 0.9 (0.7–1.0) | 0.197 |
| Stage | 0.622 | ||
| Stage 0, n (%) | 2 (12.5) | 1 (6.7) | |
| Stage I, n (%) | 9 (56.3) | 8 (53.3) | |
| Stage II, n (%) | 5 (31.3) | 4 (26.7) | |
| Stage III, n (%) | 0 (0) | 2 (13.3) | |
| Malignant tumor, n (%) | 16 (94.1) | 16 (94.1) | 0.800 |
| Esophageal cancer, n (%) | 4 (23.5) | 2 (11.8) | |
| Gastric cancer, n (%) | 8 (47.1) | 10 (58.8) | |
| Pancreatic cancer, n (%) | 4 (23.5) | 3 (17.6) | |
| Liver cancer, n (%) | 0 (0) | 1 (5.9) | |
| Gastrointestinal stromal tumor of the stomach, n (%) | 1 (5.9) | 0 (0) | |
| Mucinous cystic neoplasm, n (%) | 0 (0) | 1 (5.9) | |
| Resection site | 0.264 | ||
| Esophagus, n (%) | 4 (23.5) | 2 (11.8) | |
| Stomach, n (%) | DG; 8 (47.1), PG; 1 (5.9), TG; 0 (0) | DG; 7 (41.1), PG; 2 (11.8), TG; 1(5.9) | |
| Pancreas, n (%) | DP; 0 (0), PD; 4 (23.5) | DP; 3 (17.6), PD; 1 (5.9) | |
| Liver, n (%) | 0 (0) | 1 (5.9) | |
| Surgery | |||
| Laparotomy, n (%) | 4 (23.5) | 1 (5.9) | 1.000 |
| Intraoperative hemorrhage (mL) | 25.0 (15–125) | 25.0 (5.8–99.5) | 0.829 |
| Surgical time (h) | 8.0 (4.0–10.2) | 6.2 (5.1–8.7) | 0.760 |
| Univariate Analysis | |||||
|---|---|---|---|---|---|
| Variables | Partial Regression Coefficient | 95% CI | Standard Error | t-Statistic | p-Value |
| Serum albumin (g/dL) | −3.020 | (−28.665–22.626) | 12.590 | −0.240 | 0.812 |
| Serum c-reactive protein (mg/dL) | −35.461 | (−110.463–39.542) | 36.821 | −0.963 | 0.343 |
| Serum creatine kinase (IU/L) | 0.064 | (−0.243–0.3704) | 0.150 | 0.425 | 0.673 |
| Serum lactate dehydrogenase (U/L) | 0.574 | (0.267–0.882) | 0.151 | 3.801 | <0.001 |
| Serum cholinesterase (IU/L) | 0.011 | (−0.142–0.164) | 0.075 | 0.150 | 0.882 |
| Hemoglobin (g/dL) | −4.005 | (−9.587–1.576) | 2.740 | −1.462 | 0.154 |
| Serum transthyretin (mg/dL) | 1.424 | (0.023–2.826) | 0.687 | 2.073 | 0.047 |
| Serum transferrin (mg/dL) | 0.124 | (−0.125–0.373) | 0.122 | 1.013 | 0.319 |
| Serum retinol-binding protein (mg/dL) | 9.251 | (−0.655–19.158) | 4.857 | 1.905 | 0.066 |
| Serum zinc (μg/dL) | 0.378 | (−0.516–1.272) | 0.438 | 0.863 | 0.395 |
| Urinary titin at the time of admission (pmol/mg Cr) | 0.757 | (−2.053–3.568) | 1.380 | 0.549 | 0.587 |
| BMI (kg/m2) | −4.470 | (−8.428–0.512) | 1.943 | −2.301 | 0.028 |
| Grip strength (kg) | −0.986 | (−2.630–0.657) | 0.806 | −1.224 | 0.230 |
| Skeletal muscle mass (kg) | −1.826 | (−4.121–0.469) | 1.127 | −1.621 | 0.115 |
| Body cell mass (kg) | −1.673 | (−3.764–0.417) | 1.026 | −1.631 | 0.113 |
| Body fat percentage (%) | 0.127 | (−1.495–1.749) | 0.797 | 0.159 | 0.874 |
| ECW/TBW | 405.922 | (−547.519–1359.37) | 468.077 | 0.867 | 0.392 |
| Phase Angle | −11.745 | (−25.8019–2.3113) | 6.901 | −1.702 | 0.098 |
| Length of stay (days) | 1.282 | (0.447–2.118) | 0.410 | 3.125 | 0.004 |
| Age (years) | −1.139 | (−2.418–0.139) | 0.628 | −1.816 | 0.079 |
| Complications | 21.401 | (−6.956–49.758) | 13.921 | 1.537 | 0.134 |
| Surgical time (h) | 4.521 | (1.417–7.626) | 1.524 | 2.967 | 0.006 |
| Intraoperative blood loss (mL) | 0.052 | (0.001–0.103) | 0.025 | 2.097 | 0.044 |
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Length of stay (days) | 1.00 (0.88–1.13) | 0.971 | 1.05 (0.89–1.22) | 0.563 | 1.05 (0.96–1.15) | 0.263 |
| Intraoperative blood loss (mL) | 1.00 (0.99–1.00) | 0.566 | 1.00 (0.99–1.00) | 0.621 | 0.10 (0.99–1.00) | 0.680 |
| Surgical time (h) | 1.41 (0.85–2.35) | 0.184 | 1.10 (0.71–1.70) | 0.671 | 1.06 (0.73–1.53) | 0.761 |
| BMI at admission (kg/m2) | 0.53 (0.31–0.91) | 0.022 | ||||
| Serum lactate dehydrogenase at admission (U/L) | 1.05 (1.01–1.09) | 0.015 | ||||
| Serum transthyretin at admission (mg/dL) | 0.10 (0.89–1.12) | 0.942 | ||||
| OR (95% CI) | p-Value | |
|---|---|---|
| BMI at admission (kg/m2) | 1.07 (0.76–1.5) | 0.711 |
| Serum lactate dehydrogenase at admission (U/L) | 0.96 (0.92–1.0) | 0.054 |
| Urinary titin on postoperative day 1 (pmol/mg Cr) | 1.05 (1.01–1.1) | 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyomen, M.; Tatsumi, A.; Tsutsumi, R.; Izumi-Mishima, Y.; Hyodo, M.; Tanaka, E.; Iguchi, K.; Taura, K.; Terajima, H.; Honjo, S.; et al. Urinary Titin on the First Postoperative Day Predicts Long-Term Skeletal Muscle Loss in Patients with Gastroenterological Cancer. Int. J. Mol. Sci. 2025, 26, 2026. https://doi.org/10.3390/ijms26052026
Kyomen M, Tatsumi A, Tsutsumi R, Izumi-Mishima Y, Hyodo M, Tanaka E, Iguchi K, Taura K, Terajima H, Honjo S, et al. Urinary Titin on the First Postoperative Day Predicts Long-Term Skeletal Muscle Loss in Patients with Gastroenterological Cancer. International Journal of Molecular Sciences. 2025; 26(5):2026. https://doi.org/10.3390/ijms26052026
Chicago/Turabian StyleKyomen, Momoko, Ayako Tatsumi, Rie Tsutsumi, Yuna Izumi-Mishima, Mizusa Hyodo, Eiji Tanaka, Kohta Iguchi, Kojiro Taura, Hiroaki Terajima, Sachiko Honjo, and et al. 2025. "Urinary Titin on the First Postoperative Day Predicts Long-Term Skeletal Muscle Loss in Patients with Gastroenterological Cancer" International Journal of Molecular Sciences 26, no. 5: 2026. https://doi.org/10.3390/ijms26052026
APA StyleKyomen, M., Tatsumi, A., Tsutsumi, R., Izumi-Mishima, Y., Hyodo, M., Tanaka, E., Iguchi, K., Taura, K., Terajima, H., Honjo, S., Hamasaki, A., Nomura, K., & Sakaue, H. (2025). Urinary Titin on the First Postoperative Day Predicts Long-Term Skeletal Muscle Loss in Patients with Gastroenterological Cancer. International Journal of Molecular Sciences, 26(5), 2026. https://doi.org/10.3390/ijms26052026






