Impact of Titanium Plate Fixation on Diacylglycerol and Growth Factor Levels in the Periosteum of the Mandible and Maxilla in Patients with Dentofacial Deformities After Jaw Osteotomies
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of the Studied Groups
2.2. Diacylglycerols
2.3. Growth Factors
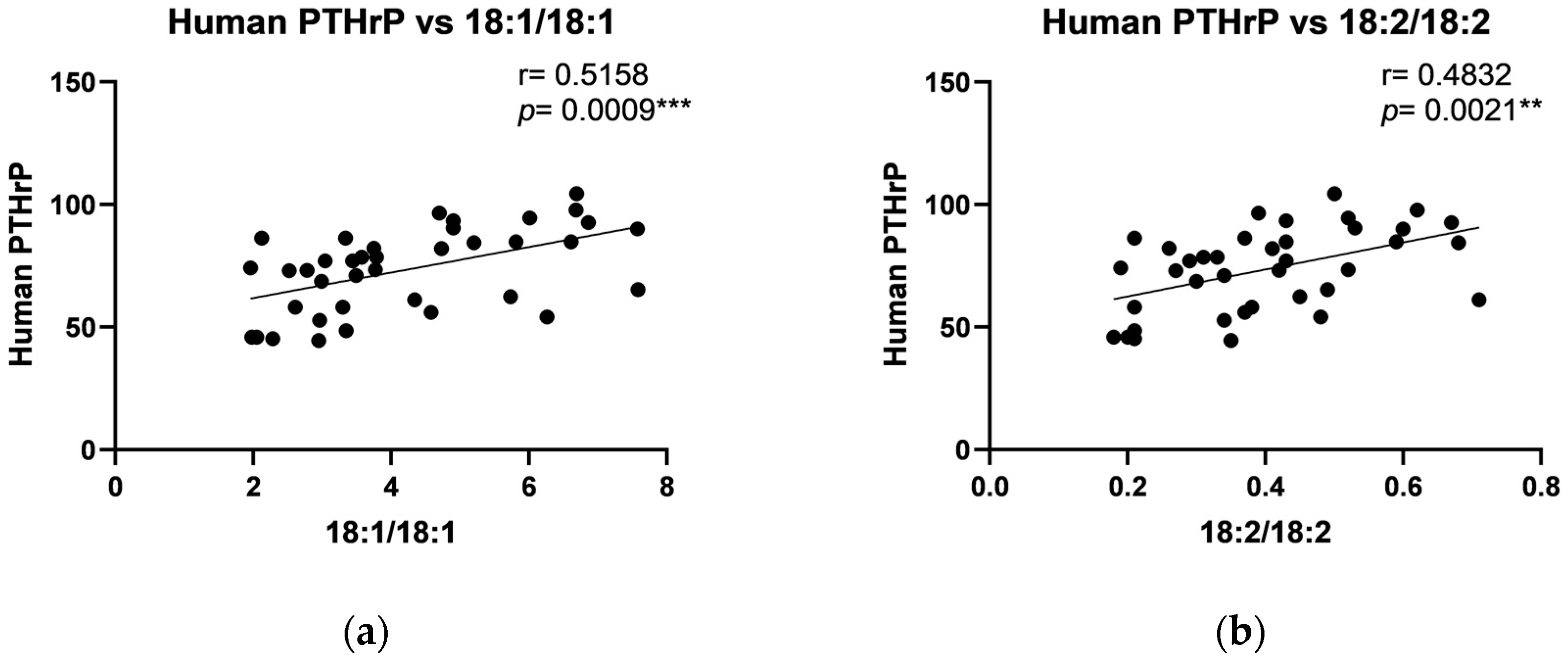
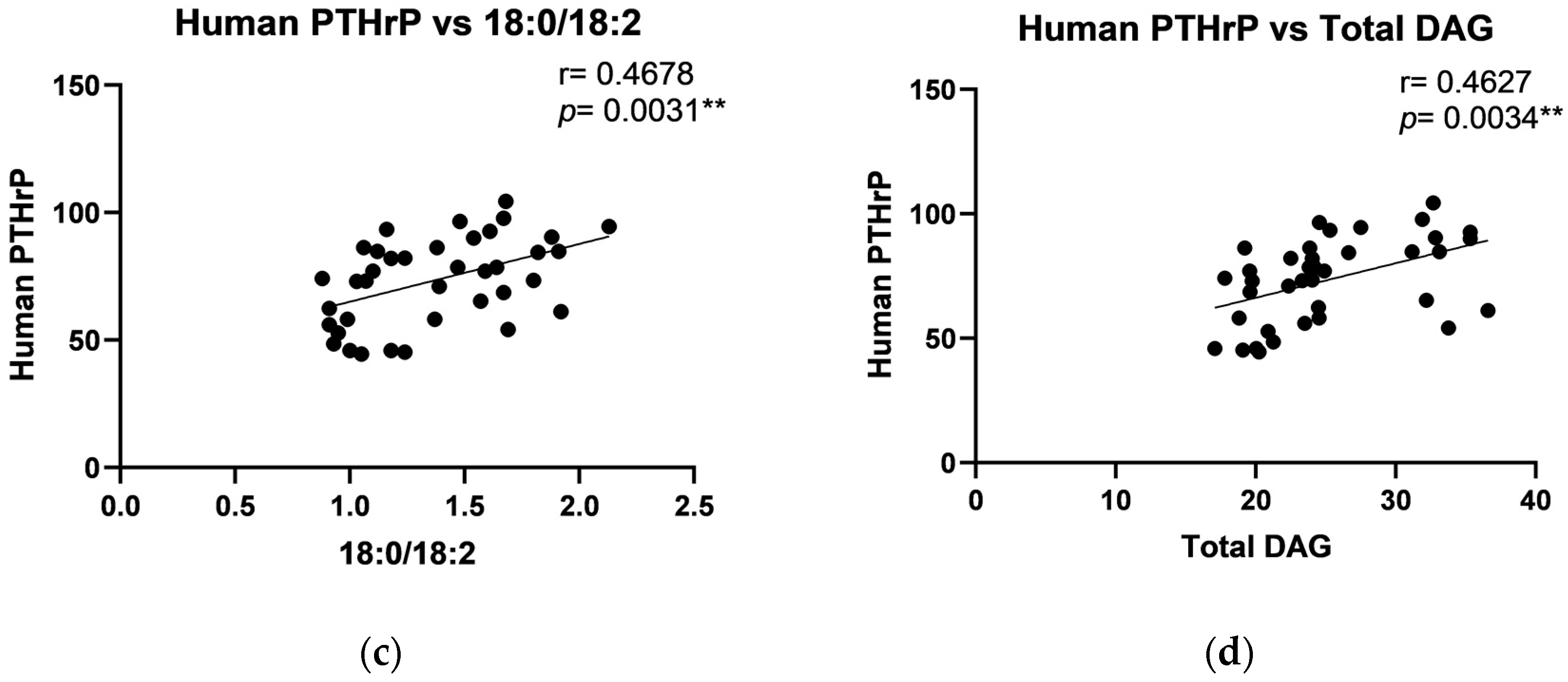
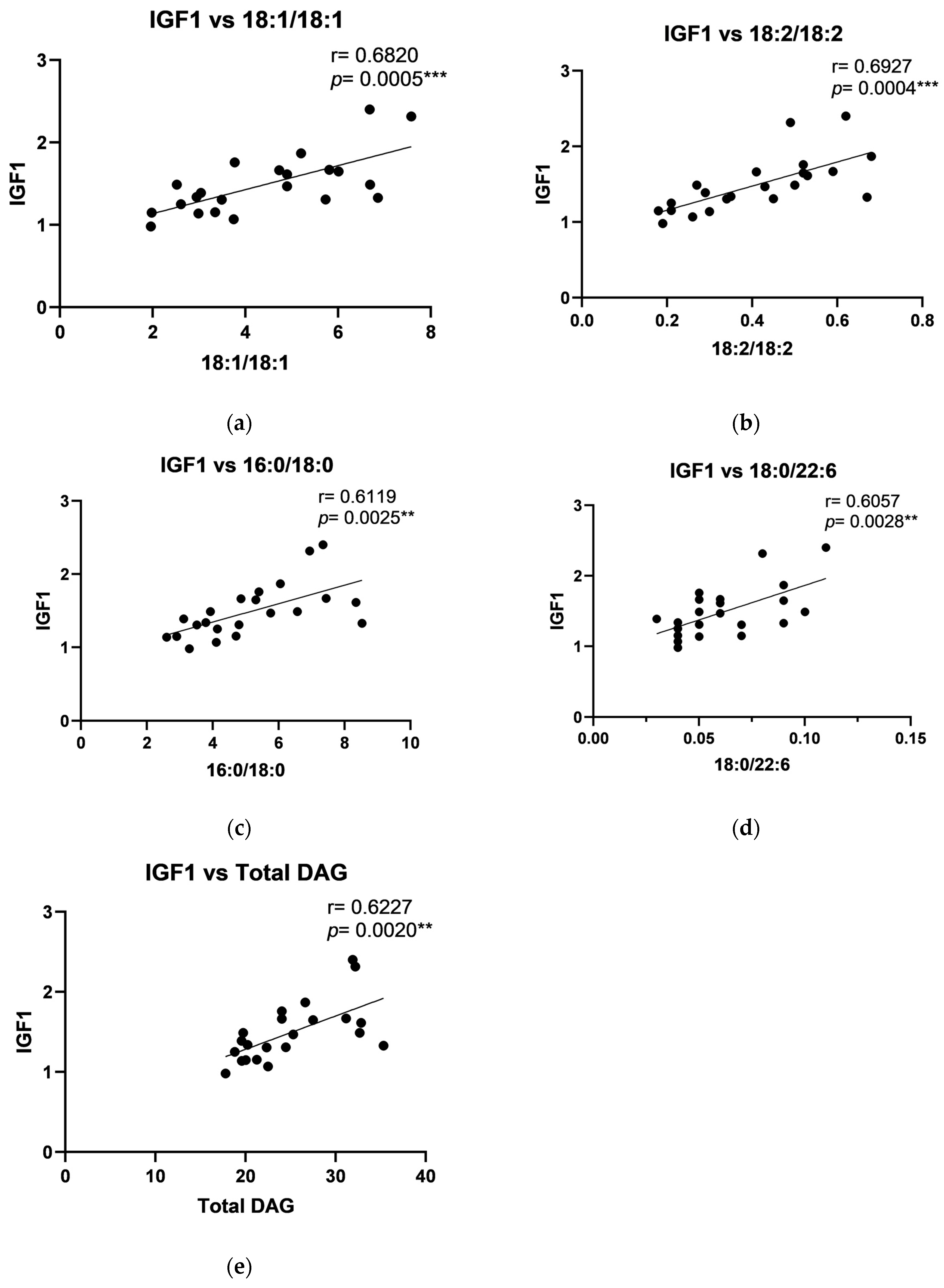
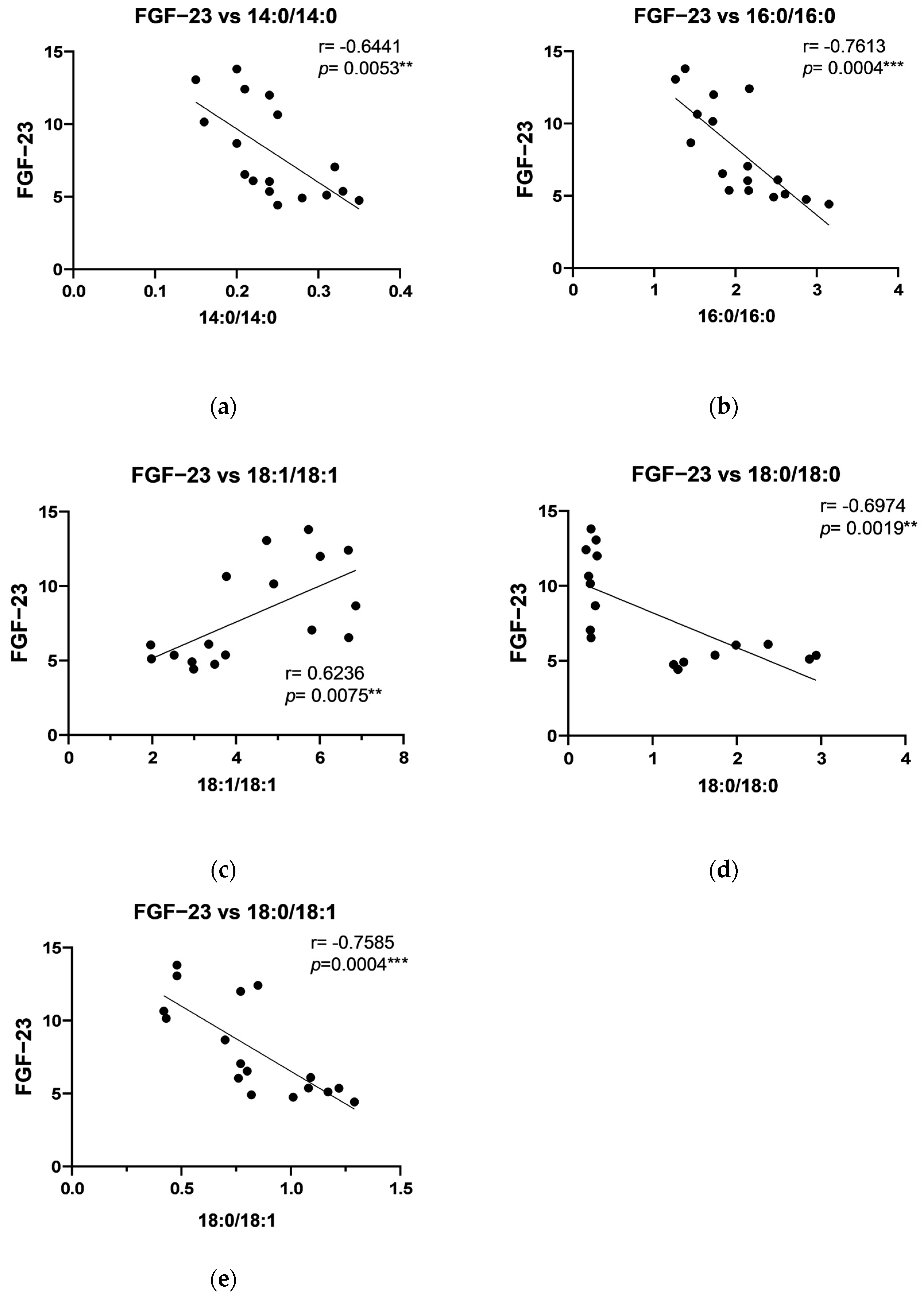
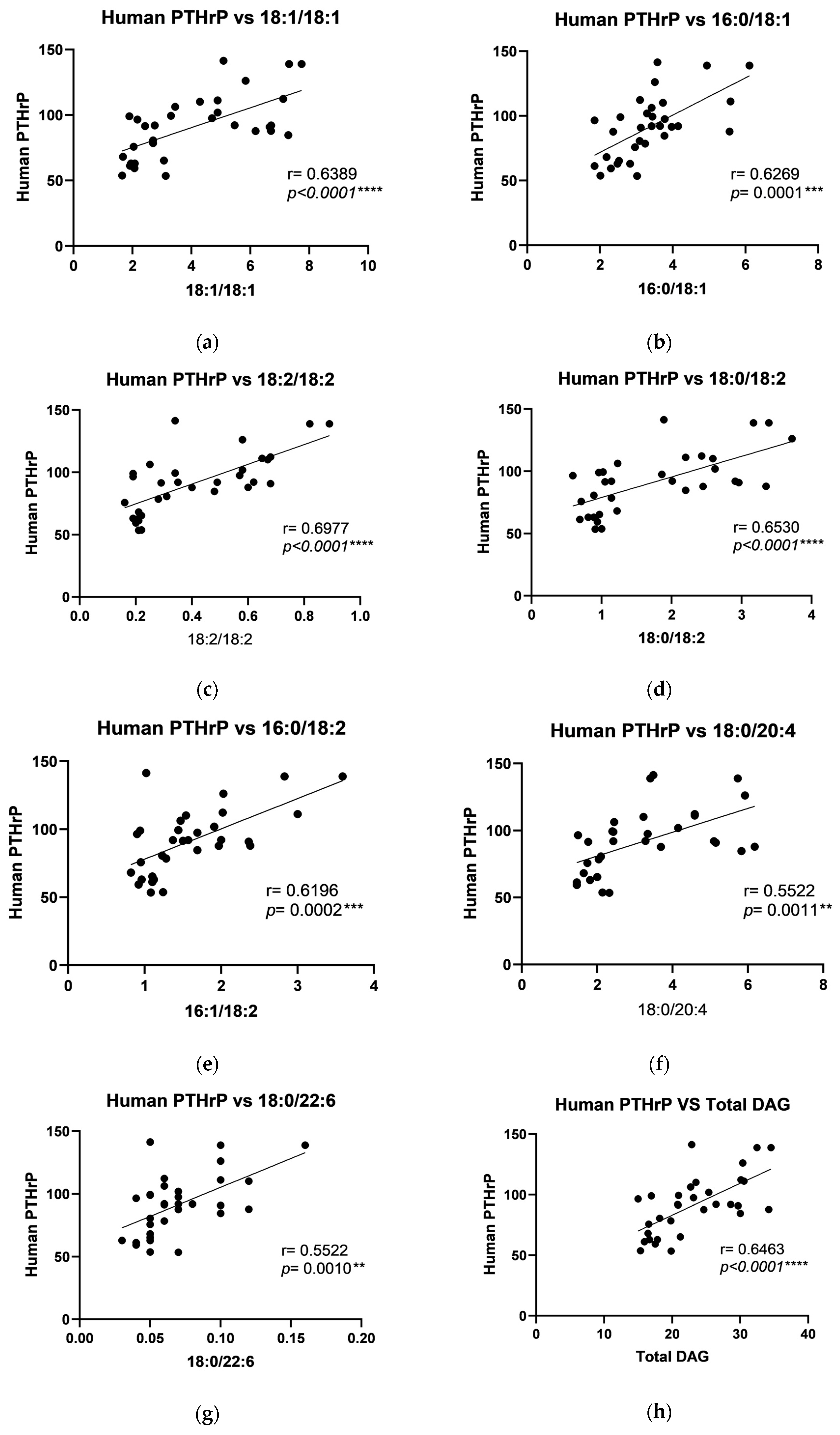
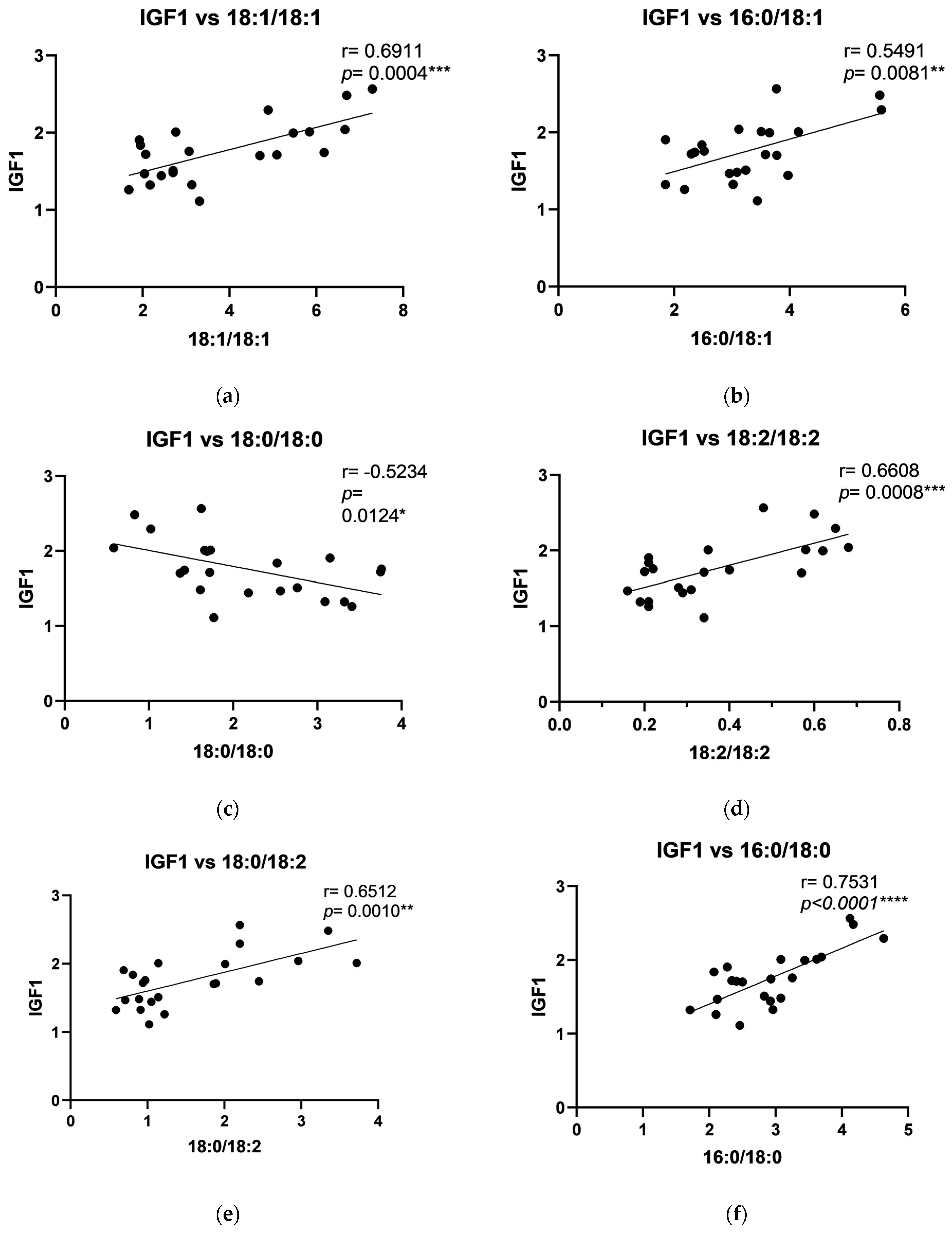
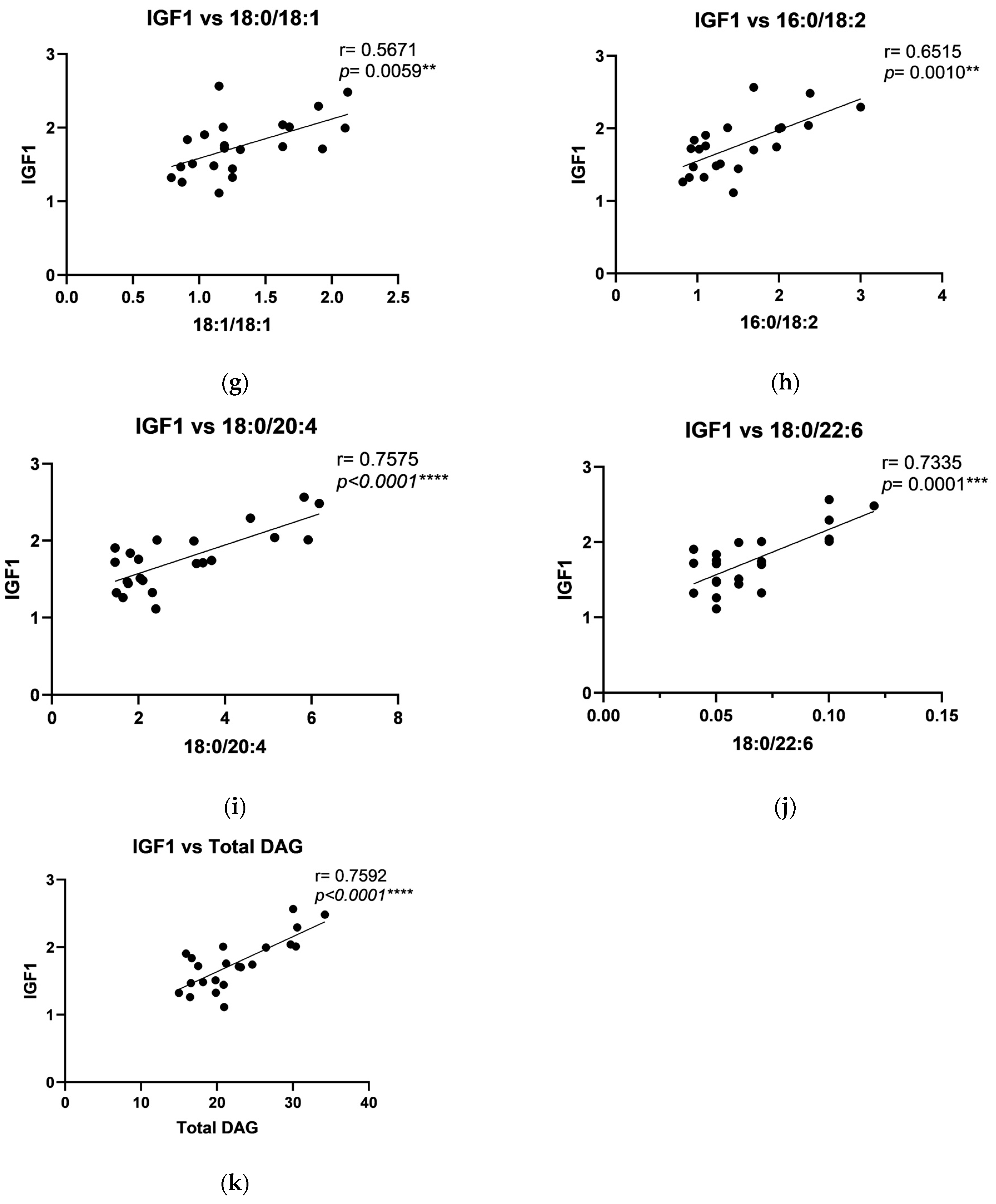
2.4. The Relationship Between DAG and Growth Factors
3. Discussion
4. Materials and Methods
4.1. Ethics Approval and Consent
4.2. Patient Selection
4.3. Sample Collection
4.4. Blood Analyses
4.5. Diacylglicerols Measurements
4.6. Growth Factors Measurements
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waiss, W.; Gosau, M.; Koyama, K.; Reichert, T. Frakturen von Maxilla und Mandibula. HNO 2011, 59, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Veras, S.R.D.; Bem, J.S.P.; Dealmeida, E.C.B.; Dos Santos, C.C.; Lins, A. Dental splints: Types and time of immobilization post tooth avulsion. Eur. Oral Res. 2017, 51, S69–S75. [Google Scholar] [CrossRef] [PubMed]
- Bouloux, G.F.; Chen, S.; Threadgill, J.M. Small and large titanium plates are equally effective for treating mandible fractures. J. Oral Maxillofac. Surg. 2012, 70, 1613–1621. [Google Scholar] [CrossRef]
- Borys, J.; Maciejczyk, M.; Krȩtowski, A.J.; Antonowicz, B.; Ratajczak-Wrona, W.; Jabłońska, E.; Załęski, P.; Waszkiel, D.; Ładny, J.R.; Żukowski, P. The redox balance in erythrocytes, plasma, and periosteum of patients with titanium fixation of the jaw. Front. Physiol. 2017, 8, 386. [Google Scholar] [CrossRef] [PubMed]
- Gareb, B.; Van Bakelen, N.B.; Vissink, A.; Bos, R.R.M.; Van Minnen, B. Titanium or Biodegradable Osteosynthesis in Maxillofacial Surgery? In Vitro and In Vivo Performances. Polymers 2022, 14, 2782. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Lanzutti, A. Biomedical Applications of Titanium Alloys: A Comprehensive Review. Materials 2023, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.K.; Choi, Y.J.; Choi, W.C.; Song, I.S.; Lee, U.L. Reconstruction of maxillofacial bone defects using patient-specific long-lasting titanium implants. Sci. Rep. 2022, 12, 7538. [Google Scholar] [CrossRef]
- Yang, J.; Liu, C.; Sun, H.; Liu, Y.; Liu, Z.; Zhang, D.; Zhao, G.; Wang, Q.; Yang, D. The progress in titanium alloys used as biomedical implants: From the view of reactive oxygen species. Front. Bioeng. Biotechnol. 2022, 10, 1092916. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Partridge, N.C. Physiological bone remodeling: Systemic regulation and growth factor involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef]
- Toker, A. The biology and biochemistry of diacylglycerol signalling: Meeting on Molecular Advances in Diacylglycerol Signalling. EMBO Rep. 2005, 6, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Guasto, A.; Cormier-Daire, V. Signaling pathways in bone development and their related skeletal dysplasia. Int. J. Mol. Sci. 2021, 22, 4321. [Google Scholar] [CrossRef]
- Quarles, L.D. Skeletal secretion of FGF-23 regulates phosphate and vitamin D metabolism. Nat. Rev. Endocrinol. 2012, 8, 276–286. [Google Scholar] [CrossRef]
- Schliephake, H. Bone growth factors in maxillofacial skeletal reconstruction. Int. J. Oral Maxillofac. Surg. 2002, 31, 469–484. [Google Scholar] [CrossRef]
- Li, A.; Xia, X.; Yeh, J.; Kua, H.; Liu, H.; Mishina, Y.; Hao, A.; Li, B. PDGF-AA promotes osteogenic differentiation and migration of mesenchymal stem cell by down-regulating PDGFRα and derepressing BMP-Smad1/5/8 signaling. PLoS ONE 2014, 9, e113785. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, X.; Chen, X.; Wang, Z.; Zheng, S.; Cheng, Y.; Liu, S.; Hao, L. The role of insulin-like growth factor-1 in bone remodeling: A review. Int. J. Biol. Macromol. 2023, 238, 124125. [Google Scholar] [CrossRef] [PubMed]
- Wein, M.N.; Kronenberg, H.M. Regulation of bone remodeling by parathyroid hormone. Cold Spring Harb. Perspect. Med. 2018, 8, a031237. [Google Scholar] [CrossRef]
- Zamani, A.; Decker, C.; Cremasco, V.; Hughes, L.; Novack, D.V.; Faccio, R. Diacylglycerol kinase ζ (DGKζ) Is a critical regulator of bone homeostasis via modulation of c-Fos levels in osteoclasts. J. Bone Miner. Res. 2015, 30, 1852–1863. [Google Scholar] [CrossRef]
- Chen, L.; Tong, Z.; Luo, H.; Qu, Y.; Gu, X.; Si, M. Titanium particles in peri-implantitis: Distribution, pathogenesis and prospects. Int. J. Oral Sci. 2023, 15, 49. [Google Scholar] [CrossRef]
- Prestat, M.; Thierry, D. Corrosion of titanium under simulated inflammation conditions: Clinical context and in vitro investigations. Acta Biomater. 2021, 136, 72–87. [Google Scholar] [CrossRef]
- Zhou, Z.; Shi, Q.; Wang, J.; Chen, X.; Hao, Y.; Zhang, Y.; Wang, X. The unfavorable role of titanium particles released from dental implants. Nanotheranostics 2021, 5, 321. [Google Scholar] [CrossRef] [PubMed]
- Fiorin, L.G.; Matheus, H.R.; Ervolino, E.; Canciani, E.; Pellegrini, G.; Dellavia, C.; Maiorana, C.; de Almeida, J.M. Tamoxifen improves homeostasis in the peri-implant bone remodeling of osseointegrated titanium implants. J. Periodontal Res. 2022, 57, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Meyle, J.; Periodontology, G.D.o.t.E.W.o. Peri-implant diseases: Consensus report of the sixth European workshop on periodontology. J. Clin. Periodontol. 2008, 35, 282–285. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Maskomani, S.; Meenashisundaram, G.K.; Fuh, J.Y.H.; Dheen, S.T.; Anantharajan, S.K. A study of Titanium and Magnesium particle-induced oxidative stress and toxicity to human osteoblasts. Mater. Sci. Eng. C 2020, 117, 111285. [Google Scholar] [CrossRef]
- Antonowicz, B.; Maciejczyk, M.; Borys, J.; Łukaszuk, K.; Zięba, S.; Gołaś, E.; Żendzian-Piotrowska, M.; Zalewska, A. The Pattern of Cytokines, Chemokines, and Growth Factors of the Maxillary and Mandibular Periosteum After Exposure to Titanium Fixations—Ti6Al4V. J. Clin. Med. 2024, 13, 7064. [Google Scholar] [CrossRef]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Krętowski, A.; Waszkiel, D.; Bortnik, P.; Czarniecka-Bargłowska, K.; Kocisz, M.; Szulimowska, J.; Czajkowski, M. Exposure to Ti4Al4V titanium alloy leads to redox abnormalities, oxidative stress, and oxidative damage in patients treated for mandible fractures. Oxidative Med. Cell. Longev. 2018, 2018, 3714725. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.J.; Anderson, S.; Stafford, T.; Glant, T.; Jacobs, J.J. Lymphocyte responses in patients with total hip arthroplasty. J. Orthop. Res. 2005, 23, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Abdulhameed, E.A.; Al-Rawi, N.H.; Omar, M.; Khalifa, N.; Samsudin, A.R. Titanium dioxide dental implants surfaces related oxidative stress in bone remodeling: A systematic review. PeerJ 2022, 10, e12951. [Google Scholar] [CrossRef]
- Shim, I.K.; Chung, H.J.; Jung, M.R.; Nam, S.Y.; Lee, S.Y.; Lee, H.; Heo, S.J.; Lee, S.J. Biofunctional porous anodized titanium implants for enhanced bone regeneration. J. Biomed. Mater. Res. Part A 2014, 102, 3639–3648. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, A.; Antonowicz, B.; Szulimowska, J.; Zieniewska-Siemieńczuk, I.; Leśniewska, B.; Borys, J.; Zięba, S.; Kostecka-Sochoń, P.; Żendzian-Piotrowska, M.; Lo Giudice, R. Mitochondrial Redox Balance of Fibroblasts Exposed to Ti-6Al-4V Microplates Subjected to Different Types of Anodizing. Int. J. Mol. Sci. 2023, 24, 12896. [Google Scholar] [CrossRef] [PubMed]
- Che, Z.; Sun, Q.; Zhao, Z.; Wu, Y.; Xing, H.; Song, K.; Chen, A.; Wang, B.; Cai, M. Growth factor-functionalized titanium implants for enhanced bone regeneration: A review. Int. J. Biol. Macromol. 2024, 274 Pt 2, 133153. [Google Scholar] [CrossRef] [PubMed]
- Maoqiang, L.; Zhenan, Z.; Fengxiang, L.; Gang, W.; Yuanqing, M.; Ming, L.; Xin, Z.; Tingting, T. Enhancement of osteoblast differentiation that is inhibited by titanium particles through inactivation of NFATc1 by VIVIT peptide. J. Biomed. Mater. Res. Part A 2010, 95, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Pioletti, D.P.; Takei, H.; Kwon, S.Y.; Wood, D.; Sung, K.L.P. The cytotoxic effect of titanium particles phagocytosed by osteoblasts. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 1999, 46, 399–407. [Google Scholar] [CrossRef]
- Blachnio-Zabielska, A.U.; Zabielski, P.; Jensen, M.D. Intramyocellular diacylglycerol concentrations and [U-¹³C]palmitate isotopic enrichment measured by LC/MS/MS. J. Lipid Res. 2013, 54, 1705–1711. [Google Scholar] [CrossRef]



| Control Me (Q1–Q3) | Study Me (Q1–Q3) | |
|---|---|---|
| WBC (×103/µL) | 6.5 (5.4–7.5) | 5.9 (5.2–6.5) ns |
| RBC (×106/µL) | 4.5 (4.2–5.0) | 4.8 (4.5–5.2) ns |
| HGB (g/dL) | 15.7 (14.8–16.2) | 14.8 (14.5–15.7) ns |
| HCT (%) | 47.4 (43.8–50.0) | 45.1 (41.6–48.8) ns |
| MCV (fL) | 85.4 (82.3–89.9) | 83.1 (81.3–88.6) ns |
| MCHC (g/dL) | 35.0 (34.0–36.1) | 34.4 (32.7–35.2) ns |
| PLT (×103/µL) | 272 (233–307) | 239 (194–259) ns |
| PT (s) | 13 (12–14) | 14 (12–14) ns |
| APTT (s) | 27.2 (26.3–29.6) | 28.2 (26.6–30.4) ns |
| INR (-) | 1.00 (0.91–1.08) | 0.98 (0.90–1.06) ns |
| Na+ (mmol/L) | 139 (136–142) | 138 (136–140) ns |
| K+ (mmol/L) | 4.2 (3.7–4.8) | 4.8 (3.7–4.9) ns |
| Creatinine (mg/dL) | 1.05 (0.89–1.12) | 0.87 (0.78–1.04) ** |
| Glucose (mg/dL) | 90.1 (87.9–95.8) | 86.5 (84.1–91.1) ns |
| Urea (mg/dL) | 28.33 (24.61–30.20) | 25.74 (18.23–28.29) ns |
| AST (U/I) | 28 (25–32) | 29 (26–32) ns |
| ALT (U/I) | 33 (29–37) | 28 (22–37) ns |
| CRP (mg/L) | 1.4 (1.0–2.2) | 2.8 (1.8–3.7) **** |
| Mandible | Maxilla | |||
|---|---|---|---|---|
| Control Me (Q1–Q3) (pmol/mg Tissue) | Control Me (Q1–Q3) (pmol/mg Tissue) | Control Me (Q1–Q3) (pmol/mg Tissue) | Study Me (Q1–Q3) (pmol/mg Tissue) | |
| 14:0/14:0 | 0.280 (0.230–0.320) | 0.300 (0.250–0.365) ns | 0.225 (0.198–0.265) | 0.285 (0.240–0.345) ** |
| 16:0/16:0 | 1.690 (1.370–1.850) | 2.070 (1.865–2.650) *** | 1.725 (1.373–2.030) | 2.480 (2.113–2.825) **** |
| 18:1/18:1 | 6.180 (4.890–7.120) | 2.170 (1.935–2.915) **** | 5.770 (4.723–6.683) | 2.975 (2.340–3.418) **** |
| 16:0/18:1 | 3.650 (3.290–4.940) | 2.830 (2.240–3.330) *** | 6.540 (5.670–8.438) | 3.125 (2.640–3.768) **** |
| 18:0/18:0 | 1.420 (1.020–1.690) | 2.570 (2.030–3.250) **** | 0.265 (0.210–0.330) | 1.975 (1.383–2.430) **** |
| 18:2/18:2 | 0.600 (0.490–0.680) | 0.2100 (0.195–0.285) **** | 0.510 (0.430–0.605) | 0.295 (0.210–0.348) **** |
| 18:0/18:2 | 2.590 (2.200–3.170) | 0.960 (0.850–1.095) **** | 1.640 (1.175–1.835) | 1.140 (1.008–1.388) ** |
| 16:0/18:0 | 3.560 (2.930–4.120) | 2.460 (2.110–3.020) *** | 6.755 (5.378–7.593) | 4.020 (3.163–4.458) **** |
| 18:0/18:1 | 1.650 (1.340–1.930) | 1.110 (0.890–1.200) **** | 0.645 (0.480–0.763) | 1.105 (0.935–1.305) **** |
| 16:0/18:2 | 2.000 (1.690–2.380) | 1.100 (0.9450–1.325) **** | 2.595 (2.243–3.238) | 1.380 (1.203–1.773) **** |
| 18:0/20:4 | 4.590 (3.410–5.730) | 2.000 (1.690–2.360) **** | 2.720 (2.133–3.003) | 2.285 (2.065–2.498) ns |
| 18:0/22:6 | 0.100 (0.070–0.100) | 0.0500 (0.0450–0.060) **** | 0.075 (0.058–0.093) | 0.055 (0.0400–0.070) ** |
| Total | 29.69 (24.65–30.56) | 17.83 (16.51–20.85) **** | 31.54 (24.53–33.28) | 20.56 (19.31–23.70) **** |
| Control Me (Q1–Q3) | Study Me (Q1–Q3) | |
|---|---|---|
| 14:0/14:0 | 20.64 (18.08–23.57) | 28.54 (20.46–31.96) ** |
| 16:0/16:0 | 372.0 (336.9–444.5) | 388.8 (341.4–443.2) ns |
| 18:1/18:1 | 2155 (1915–2580) | 3105 (2616–3291) **** |
| 16:0/18:1 | 1825 (1458–2253) | 2667 (2087–3003) **** |
| 18:0/18:0 | 52.67 (39.60–58.43) | 38.96 (33.48–42.69) *** |
| 18:2/18:2 | 1079 (893.3–1364) | 1103 (939.6–1343) ns |
| 18:0/18:2 | 117.4 (101.5–138.4) | 177.3 (158.9–189.6) **** |
| 16:0/18:0 | 1750 (1359–2063) | 2322 (1945–2774) *** |
| 18:0/18:1 | 156.7 (129.5–174.0) | 126.2 (108.6–145.7)* |
| 16:0/18:2 | 892.7 (728.2–1016) | 1688 (1415–1861) **** |
| 18:0/20:4 | 21.69 (18.87–24.42) | 48.26 (38.43–58.64) **** |
| 18:0/22:6 | 2.135 (1.748–3.083) | 4.000 (3.488–4.435) **** |
| Total | 8426 (7224–9695) | 11922 (10036–13372) **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonowicz, B.; Borys, J.; Roszczyc-Owsiejczuk, K.; Łukaszuk, K.; Zalewska, A.; Błachnio-Zabielska, A.U. Impact of Titanium Plate Fixation on Diacylglycerol and Growth Factor Levels in the Periosteum of the Mandible and Maxilla in Patients with Dentofacial Deformities After Jaw Osteotomies. Int. J. Mol. Sci. 2025, 26, 2020. https://doi.org/10.3390/ijms26052020
Antonowicz B, Borys J, Roszczyc-Owsiejczuk K, Łukaszuk K, Zalewska A, Błachnio-Zabielska AU. Impact of Titanium Plate Fixation on Diacylglycerol and Growth Factor Levels in the Periosteum of the Mandible and Maxilla in Patients with Dentofacial Deformities After Jaw Osteotomies. International Journal of Molecular Sciences. 2025; 26(5):2020. https://doi.org/10.3390/ijms26052020
Chicago/Turabian StyleAntonowicz, Bożena, Jan Borys, Kamila Roszczyc-Owsiejczuk, Kamila Łukaszuk, Anna Zalewska, and Agnieszka U. Błachnio-Zabielska. 2025. "Impact of Titanium Plate Fixation on Diacylglycerol and Growth Factor Levels in the Periosteum of the Mandible and Maxilla in Patients with Dentofacial Deformities After Jaw Osteotomies" International Journal of Molecular Sciences 26, no. 5: 2020. https://doi.org/10.3390/ijms26052020
APA StyleAntonowicz, B., Borys, J., Roszczyc-Owsiejczuk, K., Łukaszuk, K., Zalewska, A., & Błachnio-Zabielska, A. U. (2025). Impact of Titanium Plate Fixation on Diacylglycerol and Growth Factor Levels in the Periosteum of the Mandible and Maxilla in Patients with Dentofacial Deformities After Jaw Osteotomies. International Journal of Molecular Sciences, 26(5), 2020. https://doi.org/10.3390/ijms26052020





