Therapeutic Perspectives of Aminoflavonoids—A Review
Abstract
1. Introduction
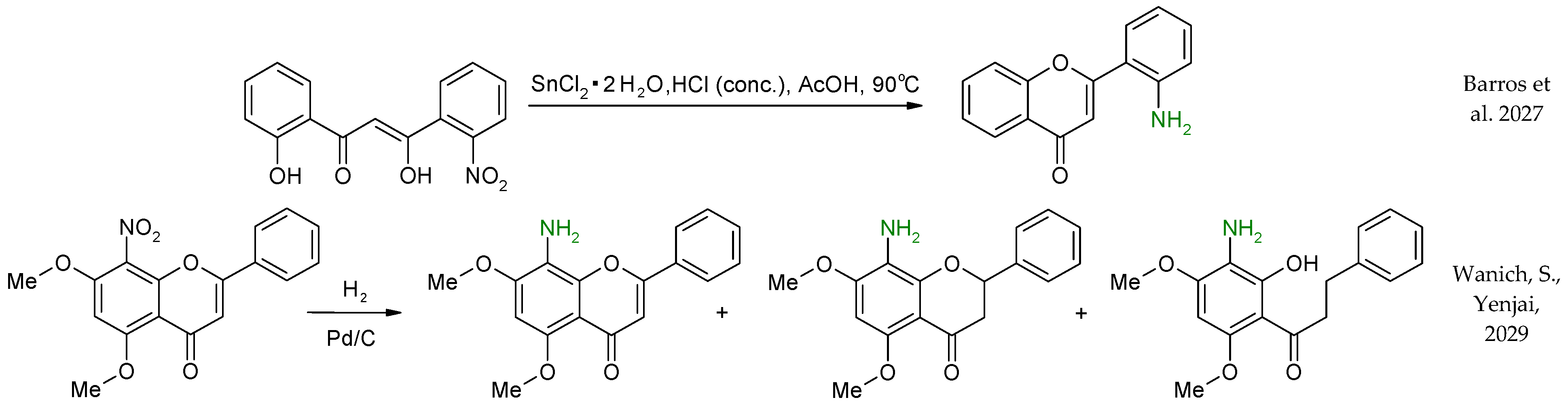
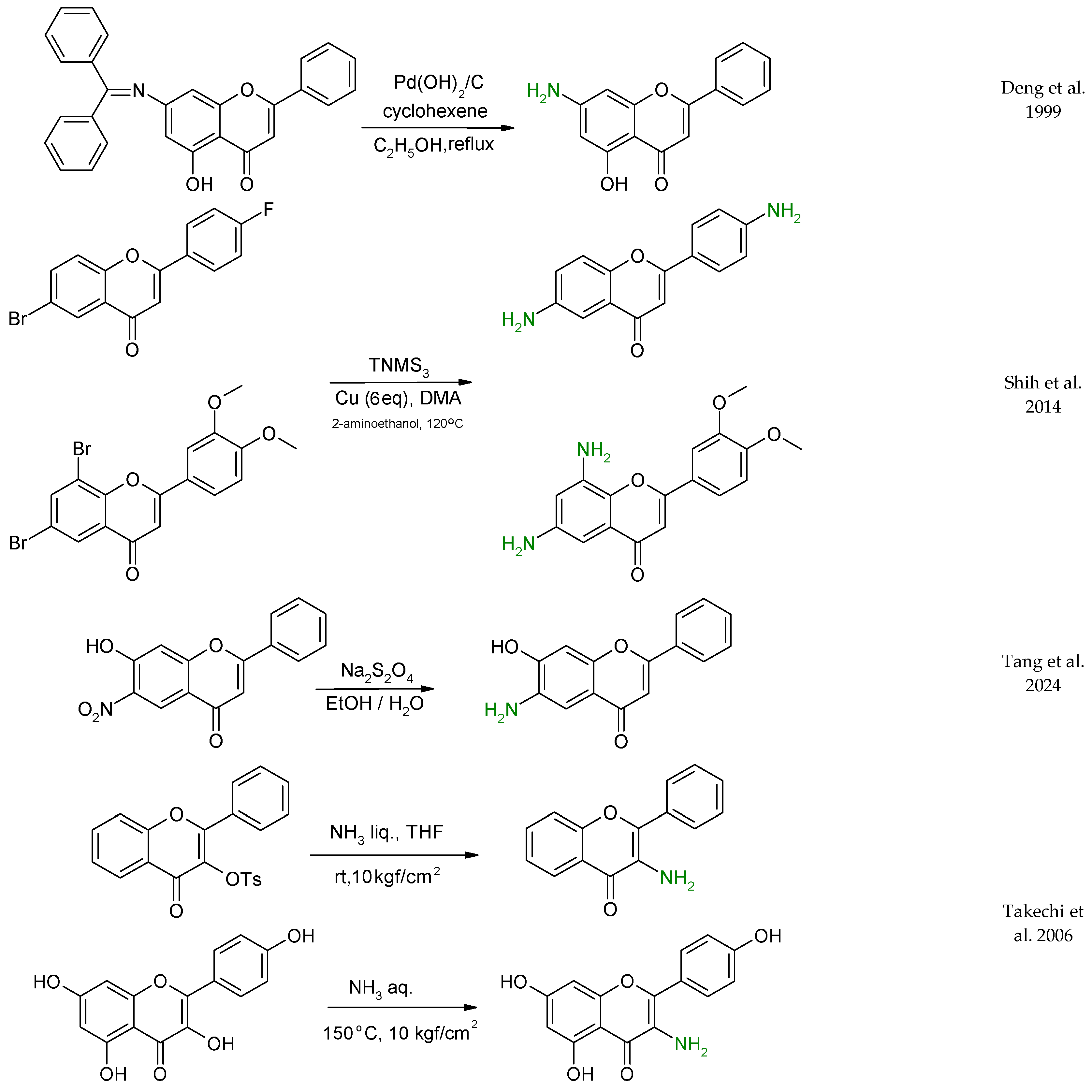
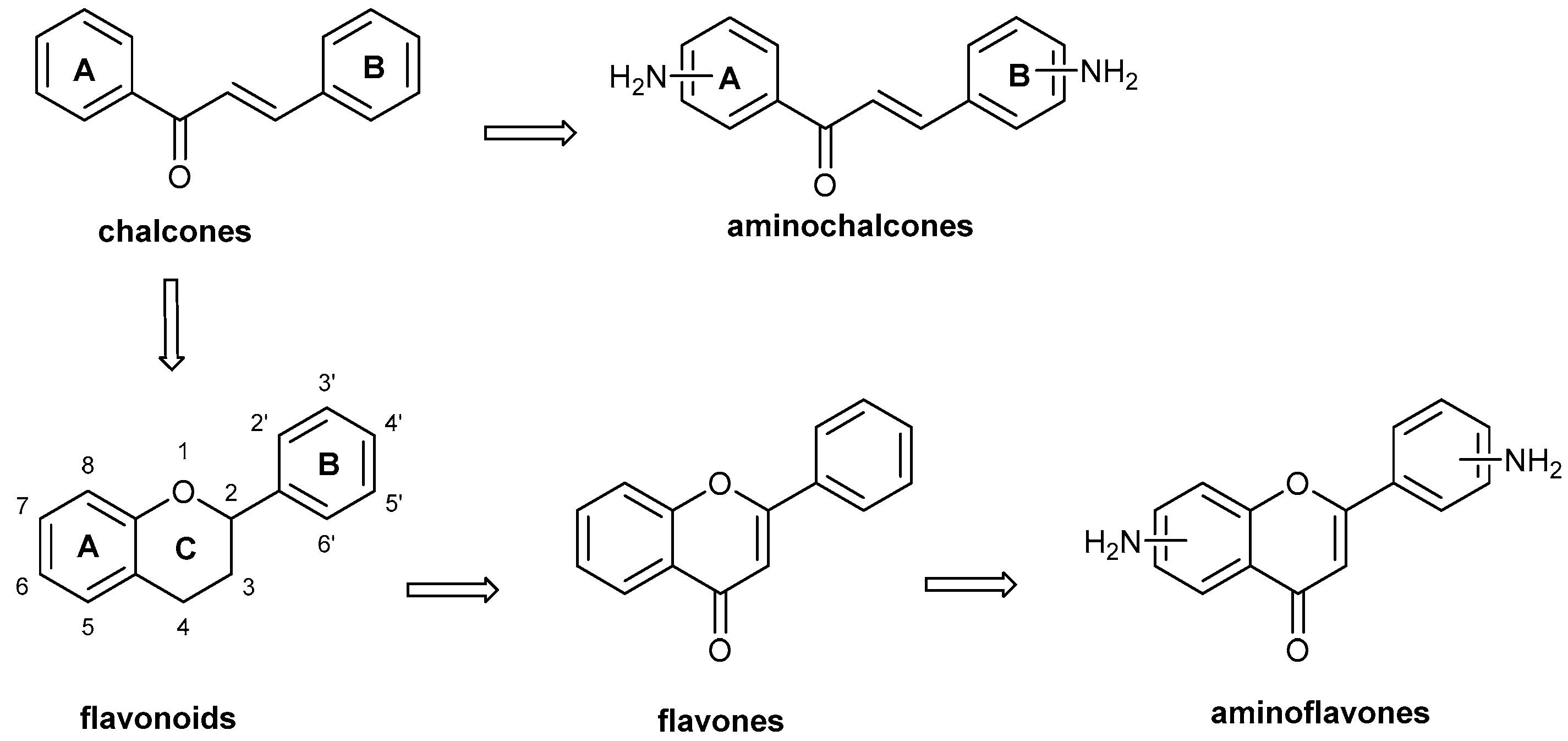
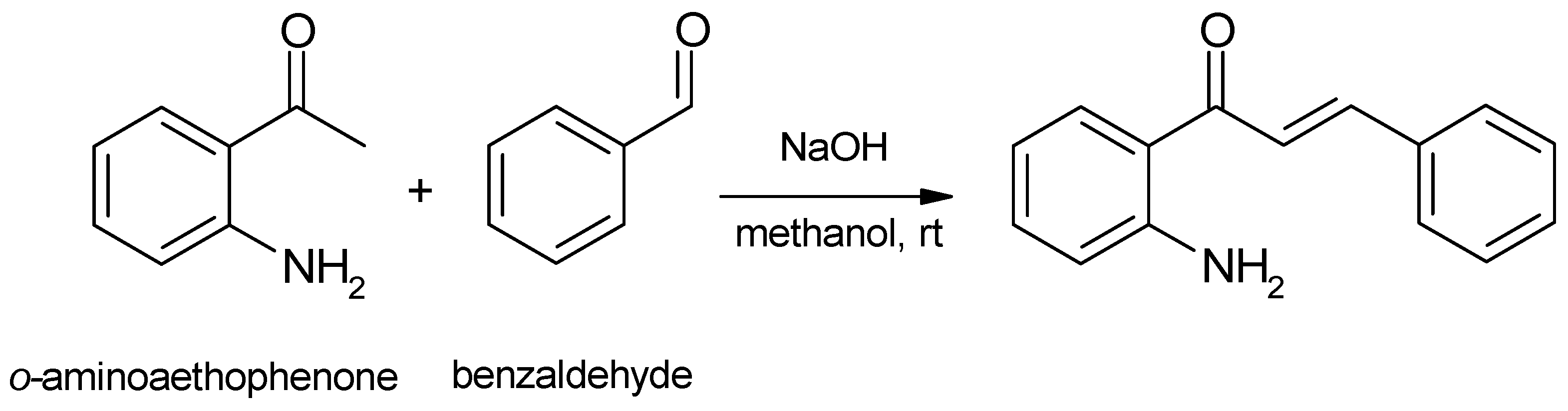
2. Preparation of Aminoflavone Derivatives
3. Biological Activities of Aminoflavones
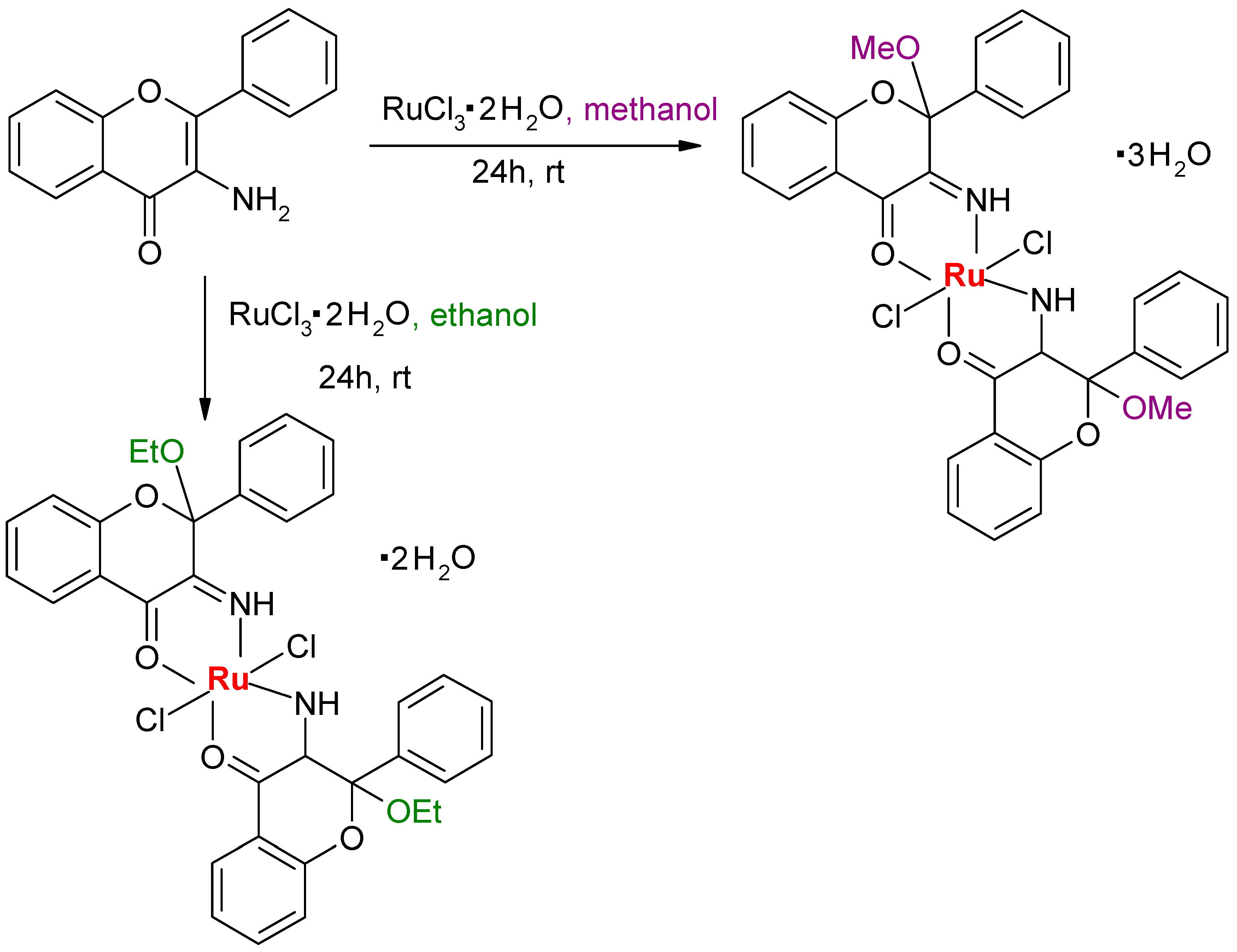
4. Aminoflavone Complexes
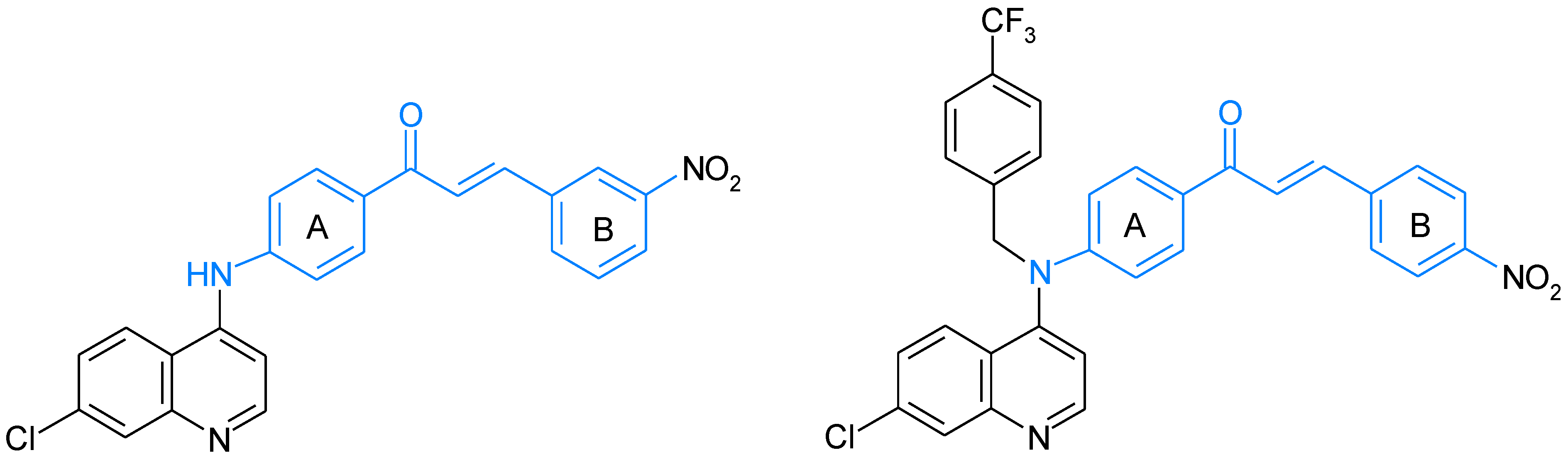
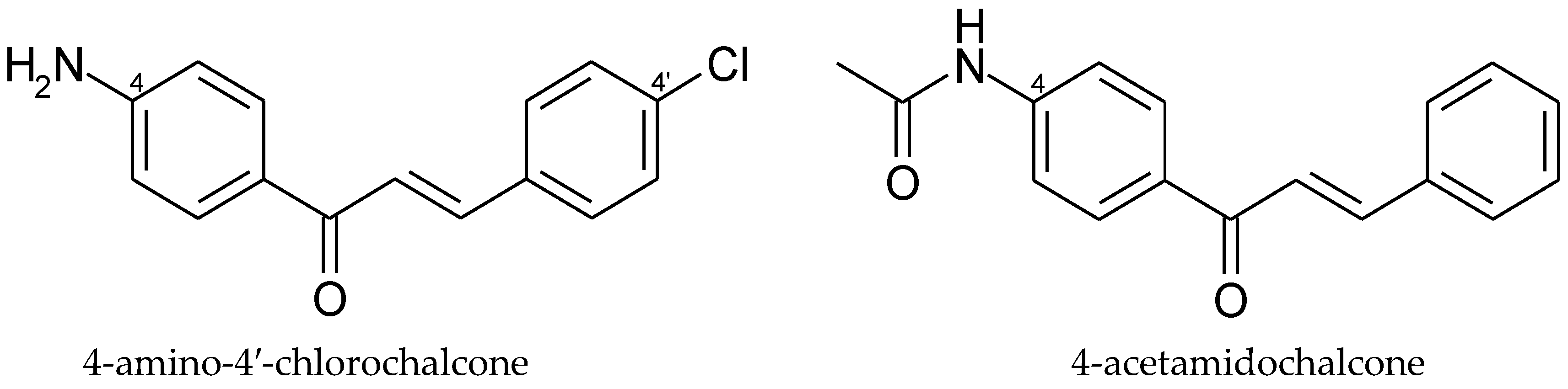
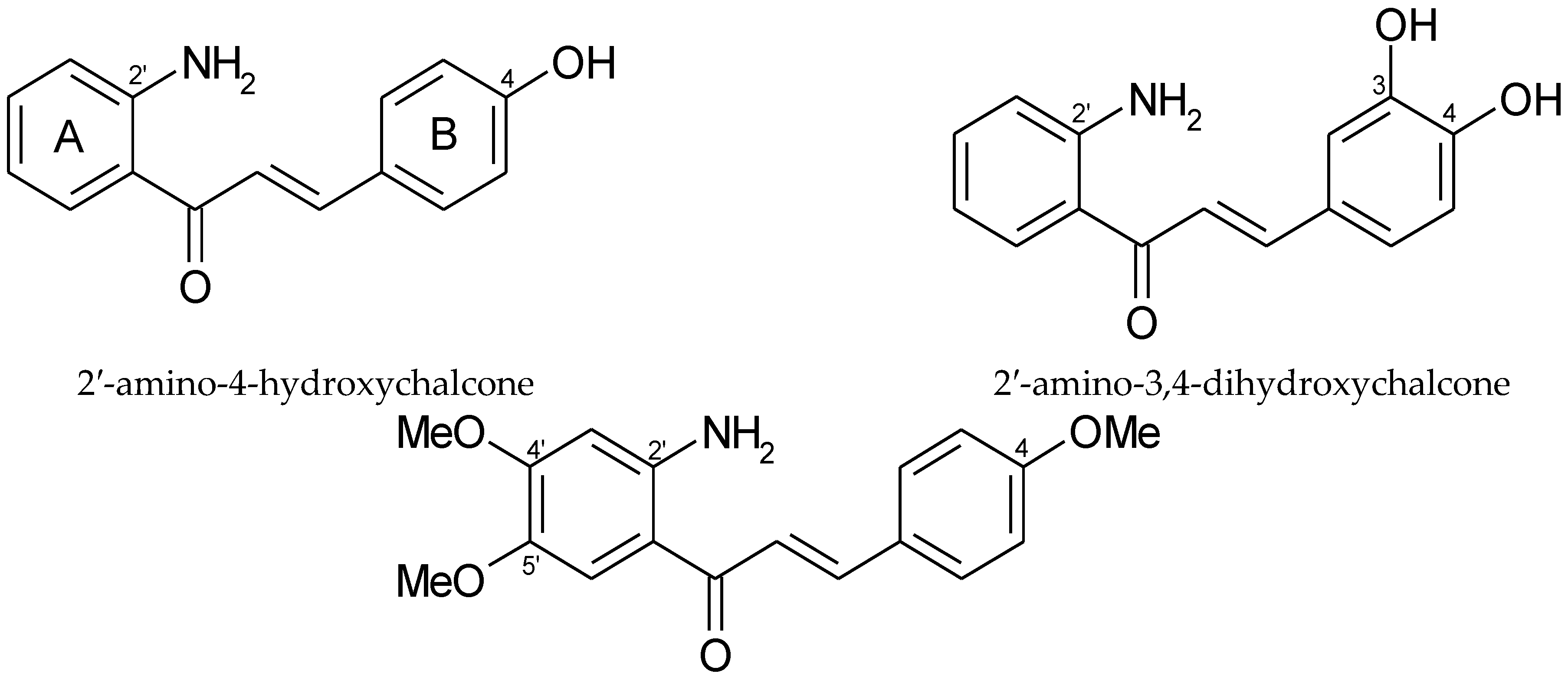
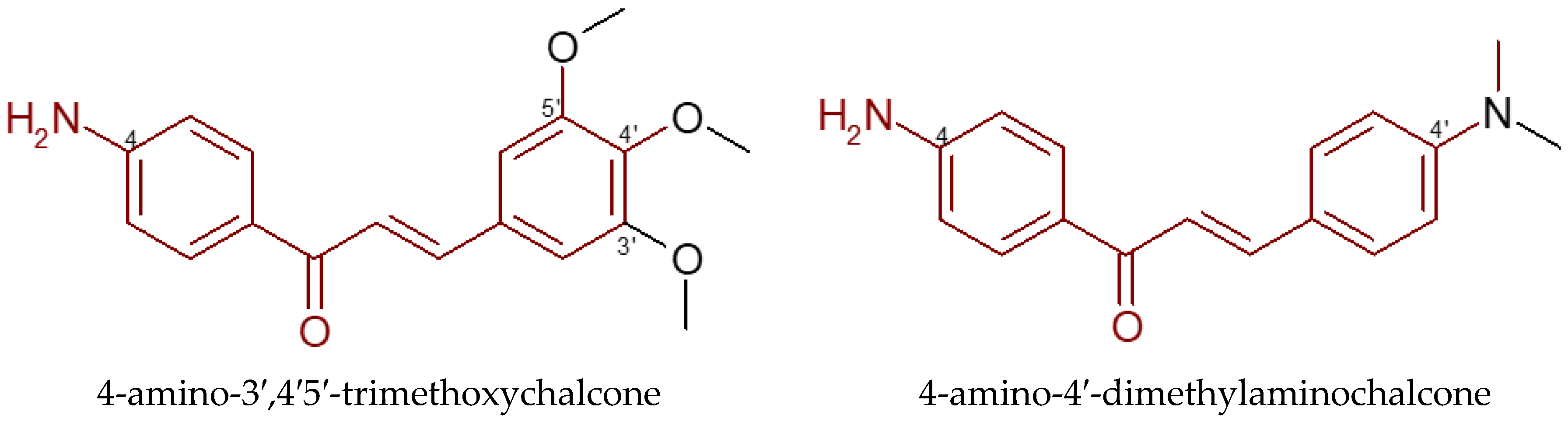
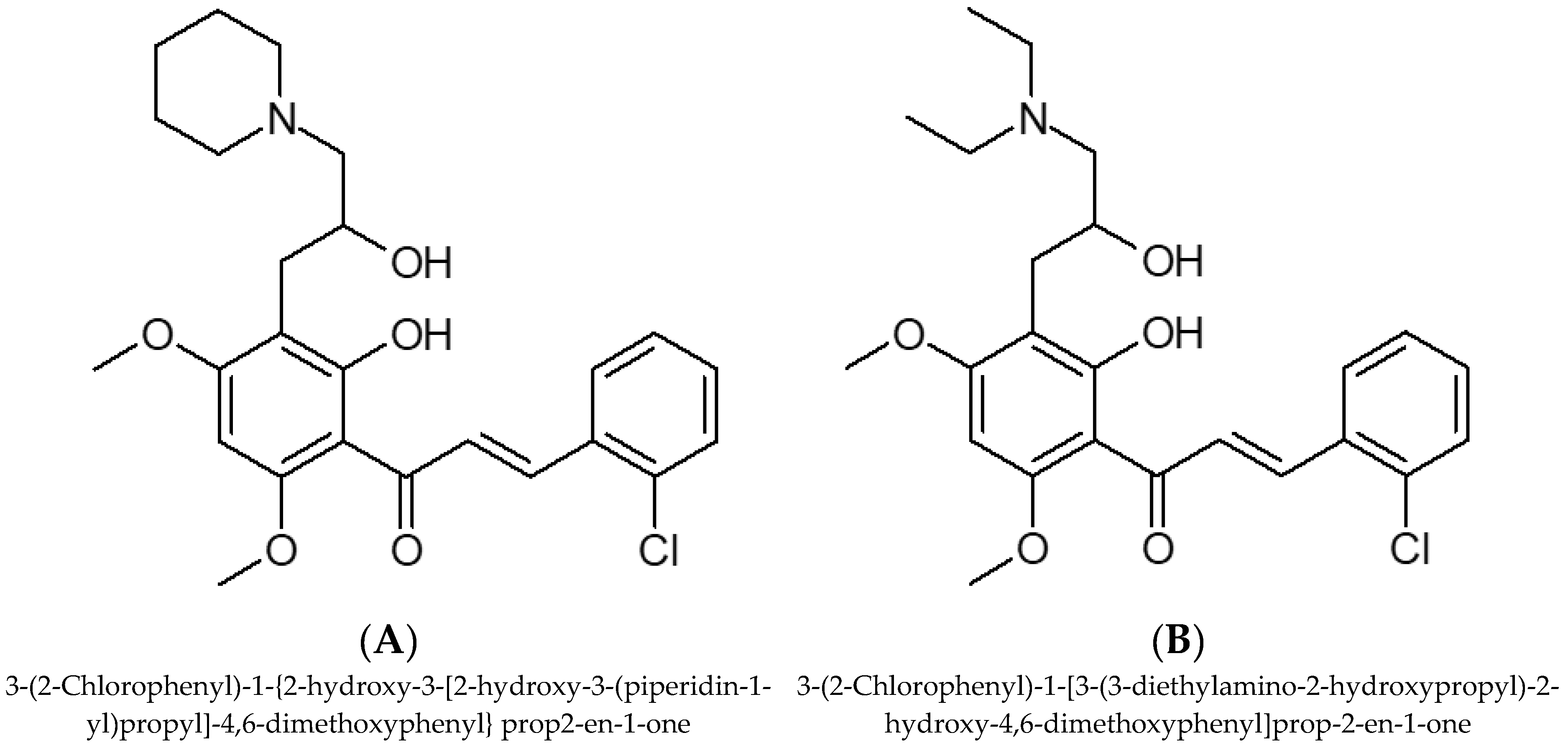
5. Aminochalcones
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tohge, T.; de Souza, L.P.; Fernie, A.R. Current understanding of the pathway of flavonoid biosynthesis in model and crop plants. J. Exp. Bot. 2017, 68, 4013–4028. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; Bijsman, M.N.; van Gameren, Y.; Cnossen, E.P.; de Vries, J.H.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Stompor, M.; Świtalska, M.; Wietrzyk, J. Synthesis and biological evaluation of acyl derivatives of hydroxyflavones as potent antiproliferative agents against resistance cell lines. Z. Naturforsch C J. Biosci. 2018, 73, 87–93. [Google Scholar] [CrossRef]
- Stompor-Gorący, M.; Bajek-Bil, A.; Machaczka, M. Chrysin: Perspectives on contemporary status and future possibilities as pro-health agent. Nutrients 2021, 13, 2038. [Google Scholar] [CrossRef]
- Stompor-Gorący, M.; Włoch, A.; Sengupta, P.; Nasulewicz-Goldeman, A.; Wietrzyk, J. Synergistic proliferation effects of xanthohumol and niflumic acid on Merkel and glioblastoma cancer cells: Role of cell membrane interactions. Int. J. Mol. Sci. 2024, 25, 11015. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Daiwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Rammohan, A.; Reddy, J.S.; Sravya, G.; Rao, C.N.; Zyrynov, G.V. Chalcone synthesis, properties and medicinal applications: A review. Environ. Chem. Lett. 2020, 18, 433–458. [Google Scholar] [CrossRef]
- Stompor, M.; Broda, D.; Bajek-Bil, A. Dihydrochalcones: Method of acquisition and pharmacological properties—A first systematic review. Molecules 2019, 24, 4468. [Google Scholar] [CrossRef]
- Stompor, M.; Świtalska, M.; Wietrzyk, J. The influence of a single and double biotinylation of xanthohumol on its anticancer activity. Acta Biochim. Pol. 2019, 66, 2876. [Google Scholar] [CrossRef] [PubMed]
- Stompor, M.; Żarowska, B. Antimicrobial activity of xanthohumol and its selected structural analogues. Molecules 2016, 21, 608. [Google Scholar] [CrossRef] [PubMed]
- Ecker, G.; Huber, D.; Schmid, D.; Chiba, P. The importance of a nitrogen atom in modulators of multidrug resistance. Mol. Pharmacol. 1999, 56, 791–796. [Google Scholar] [CrossRef]
- Casano, G.; Robin, M.; Barbier, P.; Peyrot, V.; Faure, R. Synthesis and complete assignment of the 1H and 13C NMR signals of new acetamido and aminoflavonoid derivatives. Magn. Reson. Chem. 2010, 48, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.I.R.N.A.; Dias, A.F.R.; Silva, A.M.S. Reductive coupling reactions of 2-nitrochalcones and their β-hydroxy- analogues: New synthesis of 2-arylquinoline and 2-aryl-4-hydroxyquinoline derivatives. Monatshefte Fur Chem. 2007, 138, 585–594. [Google Scholar] [CrossRef]
- Wanich, S.; Yenjai, C. Amino and nitro derivatives of 5,7-dimethoxyflavone from Kaempferia parviflora and cytotoxicity against KB cell line. Arch. Pharm. Res. 2009, 32, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Stompor, M.; Dancewicz, K.; Gabryś, B.; Anioł, M. Insect antifeedant potential of xanthohumol and isoxanthohumol and their derivatives. J. Agric. Food Chem. 2015, 63, 6749–6756. [Google Scholar] [CrossRef]
- Dymarska, M.; Janeczko, T.; Kostrzewa-Susłow, E. Biotransformations of flavones and an isoflavone (daidzein) in cultures of entomopathogenic filamentus fungi. Molecules 2018, 23, 1356. [Google Scholar] [CrossRef]
- Stompor, M.; Świtalska, M.; Bajek, A.; Wietrzyk, J. Influence of amide versus ester linkages on the anticancer properties of the new flavone-biotin conjugates. Z. Naturforsch C 2019, 74, 193–200. [Google Scholar] [CrossRef]
- Cushman, M.; Zhu, H.; Geahlen, R.L.; Kraker, A.J. Synthesis and biochemical evaluation of a series of aminoflavones as potential inhibitors of protein-tyrosine kinases p56lck, EGFr, and p60v-src. J. Med. Chem. 1994, 37, 3353–3362. [Google Scholar] [CrossRef]
- Deka, N.; Hadjeri, M.; Lawson, M.; Beney, C.; Boumendjel, A. Acetylated dimethoxyaniline as a key intermediate for the synthesis of aminoflavones and quinolones. Heterocycles 2002, 57, 123–128. [Google Scholar]
- Deng, B.; Lepoivre, A.; Lemière, G. Synthesis of 7-vinylflavone and 7-aminoflavone by palladium-catalyzed coupling reactions. Eur. J. Org. Chem. 1999, 10, 2683–2688. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, S.; Yang, J.; Gao, W.; Cui, J.; Zhuang, T. A novel approach to the synthesis of 6-amino-7-hydroxyflavone. Molecules 2004, 9, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Takechi, A.; Takikawa, H.; Miyake, H.; Sasaki, M. Synthesis of 3-aminoflavones from 3-hydroxyflavones via 3-tosyloxy or 3-mesyloxyflavones. Chem. Lett. 2006, 35, 128–129. [Google Scholar] [CrossRef]
- Babu, T.H.; Rao, V.R.S.; Tiwari, A.K.; Babu, K.S.; Srinivas, P.V.; Ali, A.Z.; Rao, J.M. Synthesis and biological evaluation of novel 8-aminomethylated oroxylin A analogues as α-glucosidase inhibitors. Bioorg Med. Chem. Lett. 2008, 18, 1659–1662. [Google Scholar] [CrossRef]
- Shih, T.L.; Chou, C.E.; Liao, W.Y.; Hsiao, C.A. Cooper-mediated trimethylsilyl azide in amination of bromoflavonoids to synthesize unique aminoflavonoids. Tetrahedron 2014, 70, 3657–3664. [Google Scholar] [CrossRef]
- Moorkoth, S.; Srinivasan, K.K.; Kutty, N.G.; Joseph, A.; Naseer, M. Synthesis and evaluation of a series of novel imidazolidinone analogues of 6-aminoflavone as anticancer and anti-inflammatory agents. Med. Chem. Res. 2013, 22, 5066–5075. [Google Scholar] [CrossRef]
- Stompor, M. 6-Acetamidoflavone obtained by microbiological and chemical methods and its antioxidant activity. J. Biotechnol. 2016, 237, 25–34. [Google Scholar] [CrossRef]
- Santos, M.B.; Pinhanelli, V.C.; Garcia, M.A.R.; Silva, G.; Baek, S.J.; França, S.C.; Fachin, A.L.; Marins, M.; Regasini, L.O. Antiproliferative and pro-apoptotic activities of 2′- and 4′-aminochalcones against tumor canine cells. Eur. J. Med. Chem. 2017, 138, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Luzzani, G.; Callero, M.A.; Kuruppu, A.I.; Trapani, V.; Flumian, C.; Todaro, L.; Bradshaw, T.D.; Perez, A.I.L. In vitro antitumor effect of AHR ligands aminoflavone (AFP 464) and benzothiazole (5F 203) in human renal carcinoma cells. J. Cell Biochem. 2017, 118, 4526–4535. [Google Scholar] [CrossRef]
- Callero, M.A.; Rodriguez, C.E.; Sόlimo, A.; Bal de Kier Joffé, E.; Loaiza Perez, A.l. The immune system as a new possible cell target for AFP 464 in a spontaneous mammary cancer mouse model. J. Cell Biochem. 2017, 118, 2841–2849. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, A.M.; Wu, J.; Ersland, K.; Xu, W. Estrogen receptor α and aryl hydrocarbon receptor independent growth inhibitory effects of aminoflavone in breast cancer cells. BMC Cancer 2014, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- Reinicke, K.E.; Kuffel, M.J.; Goetz, M.P.; Ames, M.M. Synergistic interactions between aminoflavone, paclitaxel and camptothecin in human breast cancer cells. Cancer Chemother. Pharmacol. 2010, 66, 575–578. [Google Scholar] [CrossRef]
- Dauzonne, D.; Folléas, B.; Martinez, L.; Chabot, G.G. Synthesis and in vitro cytotoxicity of a series of 3-aminoflavones. Eur. J. Med. Chem. 1997, 32, 71–82. [Google Scholar] [CrossRef]
- Akama, T.; Shida, Y.; Sugaya, T.; Ishida, H.; Gomi, K.; Kasai, M. Novel 5-aminoflavone derivatives as specific antitumor agents in breast cancer. J. Med. Chem. 1996, 39, 3461–3469. [Google Scholar] [CrossRef] [PubMed]
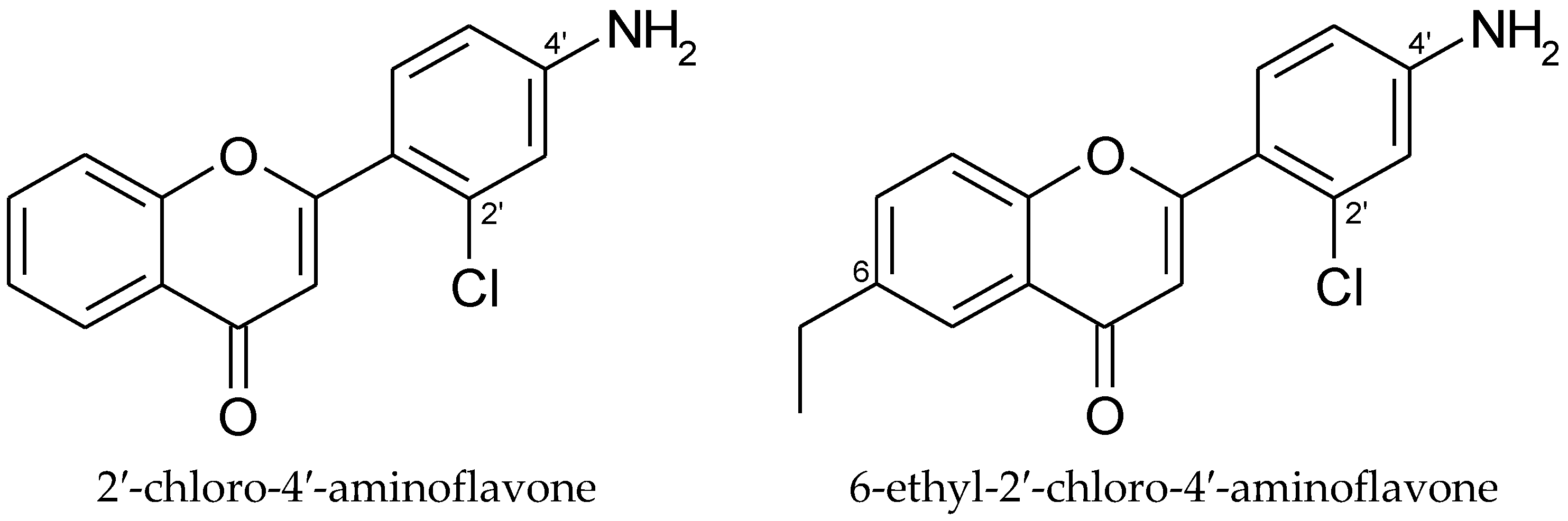
- Jin, F.; Zhang, N.; Tan, C.; Gao, D.; Zhang, C.; Liu, F.; Chen, Z.; Gao, C.; Liu, H.; Li, S.; et al. 2′-Chloro-4′-aminoflavone derivatives selectively targeting hepatocarcinoma cells: Convenient synthetic process G2/M cell cycle arrest and apoptosis triggers. Arch. Pharm. Chem. Life Sci. 2012, 345, 525–534. [Google Scholar] [CrossRef]
- Brantley, E.; Callero, M.A.; Berardi, D.E.; Campbell, P.; Rowland, L.; Zylstra, D.; Amis, L.; Yee, M.; Simian, M.; Todaro, L.; et al. AhR ligand aminoflavone inhibits α6-integrin expression and breast cancer sphere-initiating capacity. Cancer Lett. 2016, 376, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Terzuoli, E.; Puppo, M.; Rapisarda, A.; Uranchimeg, B.; Cao, L.; Burger, A.M.; Ziche, M.; Melillo, G. Aminoflavone, a ligand of the aryl hydrocarbon receptor, inhibits HIF-1α expression in an AhR-independent fashion. Cancer Res. 2010, 70, 6837–6848. [Google Scholar] [CrossRef]
- Król, W.; Czuba, Z.P.; Threadgill, M.D.; Cunningham, B.D.M.; Pietsz, G. Inhibition of nitric oxide (NO) production in murine macrophages by flavones. Biochem. Pharmacol. 1995, 50, 1031–1035. [Google Scholar] [CrossRef]
- Cushman, M.; Nagaratham, D.; Burg, D.L.; Geahlen, R.L. Synthesis and protein-tyrosine kinase inhibitory activities of flavonoid analogues. J. Med. Chem. 1991, 34, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Moorkoth, S. Synthesis and anticancer activity of novel thiazolidinone analogs of 6-aminoflavone. Chem. Pharm. Bull. 2015, 63, 974–985. [Google Scholar] [CrossRef]
- Mayer, S.; Keglevich, P.; Ábrànyi-Balogh, P.; Szigetvàri, Á.; Dèkàny, M.; Szàntay, C.; Hazai, L. Synthesis and in vitro anticancer evaluation of novel chrysin and 7-aminochrysin derivatives. Molecules 2020, 25, 888. [Google Scholar] [CrossRef] [PubMed]
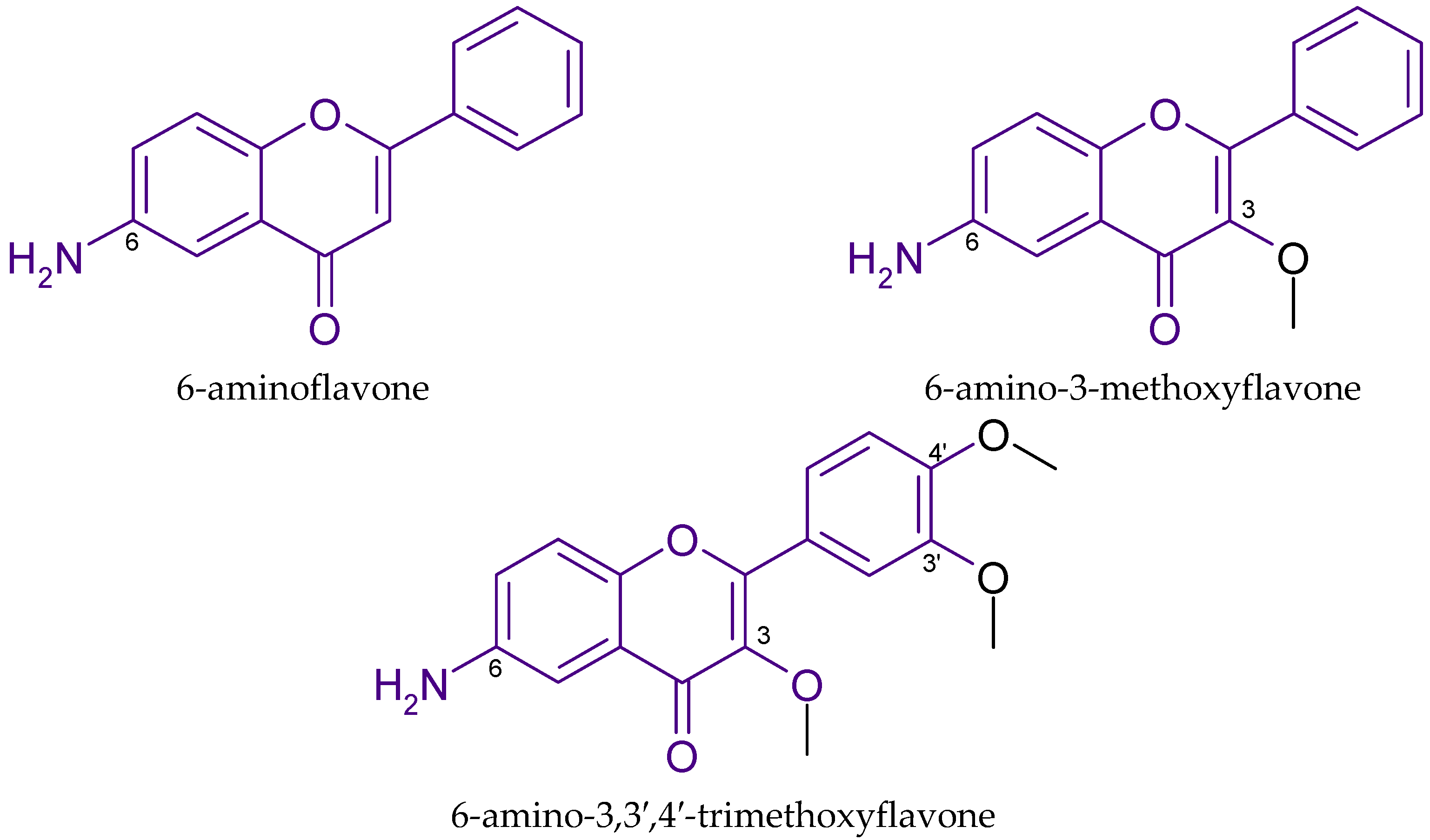
- Thorat, N.M.; Sarkate, A.P.; Lokwani, D.K.; Tiwari, S.V.; Azad, R.; Thopate, S.R. N-benzylation of 6-aminoflavone by reductive amination and efficient access to some novel anticancer agents via topoisomerase II inhibition. Mol. Diver 2021, 25, 937–948. [Google Scholar] [CrossRef]
- Shelke, R.N.; Pansare, D.N.; Sarkate, A.P.; Narula, I.K.; Lokwani, D.K.; Tiwari, S.V.; Azad, R.; Thopate, S.R. Synthesis and evaluation of novel sulfonamide analogues of 6/7-aminoflavones as anticancer agents via topoisomerase II inhibition. Bioorg. Med. Chem. Lett. 2020, 30, 127246. [Google Scholar] [CrossRef]
- Flores-Flores, A.; Estrada-Soto, S.; Millán-Pacheco, C.; Bazán-Perkins, B.; Hernández-Pando, R.; Ibarra-Barajas, M.; Villalobos-Molina, R. Ex vivo and in silico approaches of tracheal relaxation through calcium channel blockade of 6-aminoflavone and its toxicological studies in murine models. Biomedicines 2023, 11, 1870–1881. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Shah, S.A.; Nishan, U.; Khan, N.; Almutairi, M.H.; Fozia, F.; Jamila, N.; Almutairi, B.B.; Ullah, Z. 6-Aminoflavone activates Nrf2 to inhibit the phosphor-JNK/TNF-α signaling pathway to reduce amyloid burden in an aging mouse model. ACS Omega 2023, 8, 26955–26964. [Google Scholar] [CrossRef] [PubMed]
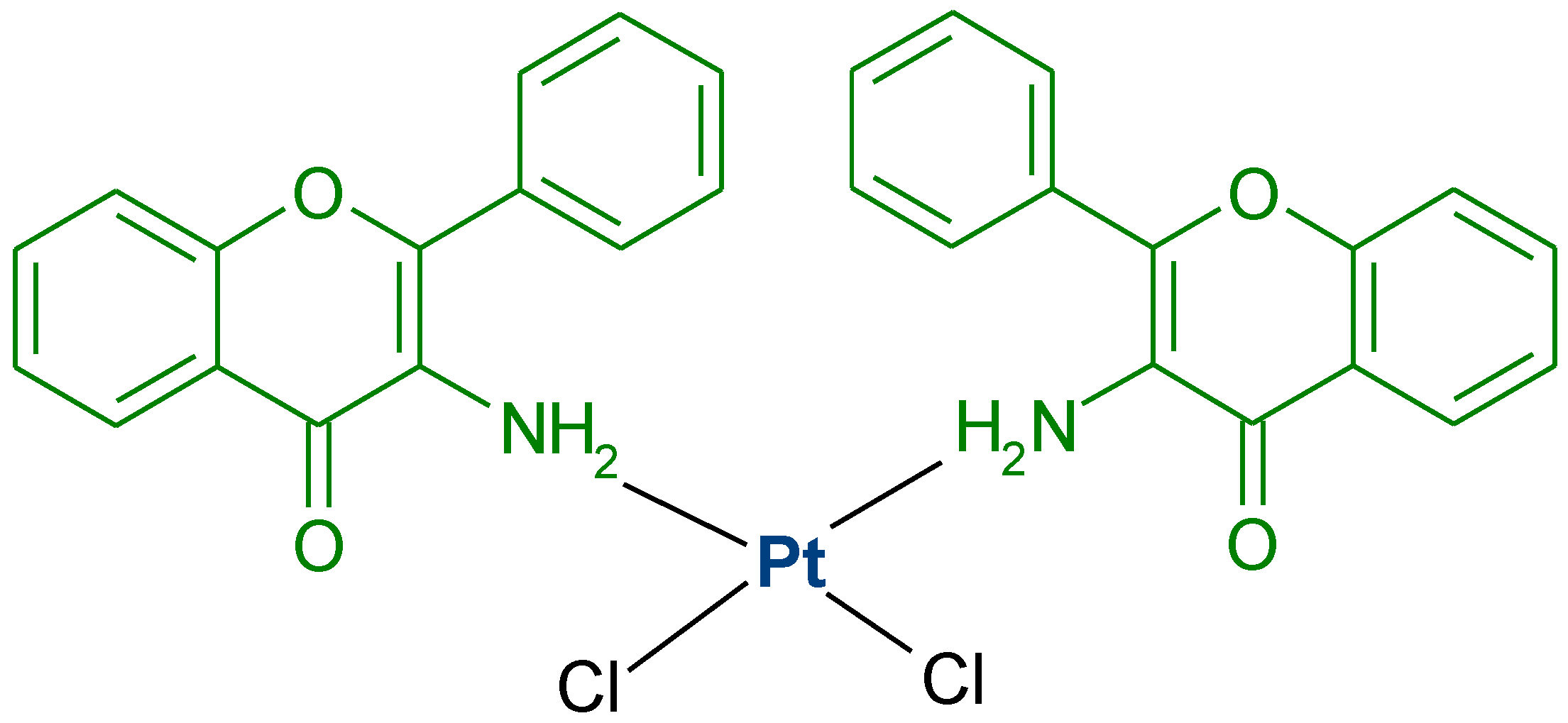
- Fabijańska, M.; Orzechowska, M.; Rybarczyk-Pirek, A.J.; Dominikowska, J.; Bieńkowska, A.; Małecki, M.; Ochocki, J. Simple trans-platinum complex bearing 3-aminoflavone ligand could be a useful drug: Structure—Activity relationship of platinum complex in comparison with cisplatin. Int. J. Mol. Sci. 2020, 21, 2116. [Google Scholar] [CrossRef]
- Mucha, P.; Hikisz, P.; Gwożdziński, K.; Krajewska, U.; Leniart, A.; Budzisz, E. Cytotoxic effect, generation of reactive oxygen/nitrogen species and electrochemical properties of Cu (II) complex in comparison to half-sandwich complexes of Ru(II) with aminochromone derivatives. RSC Adv. 2019, 9, 31943–31952. [Google Scholar] [CrossRef] [PubMed]
- Grazul, M.; Budzisz, E. Biological activity of metal ions complexes of chromones, coumarins and flavones. Coord. Chem. Rev. 2009, 253, 2588–2598. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Szmigiero, L.; Zyner, E.; Ochocki, J. Proapoptotic activity in vitro of two novel ruthenium(II) complexes with flavanone-based ligands that overcome cisplatin resistance in human bladder carcinoma cells. J. Inorg. Biochem. 2011, 105, 518–524. [Google Scholar] [CrossRef]
- Orzechowska, M.; Fabijanska, M.; Ochocki, J.; Małecki, M. Anticancer activity of trans-platinum (II) complex of 3-aminoflavone to ovarian cancer cells. Ginekol. Pol. 2017, 88, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Hartman, C.B.; Dube, P.S.; Legoabe, L.J.; Van Pelt, N.; Matheeussen, A.; Caljon, G.; Beteck, R.M. Novel quinoline derivatives with broad-spectrum antiprotozoal activities. Arch. Pharm. 2024, 357, 2300319. [Google Scholar] [CrossRef]
- Santos, M.B.D.; Carvalho Marques, B.; Miranda Ayusso, G.; Rocha Garcia, M.A.; Chiquetto Paracatu, L.; Pauli, I.; Silva Bolzani, V.; Defini Andricopulo, A.; Farias Ximenes, V.; Zeraik, M.L.; et al. Chalcones and their B-aryl analogues as myeloperoxidase inhibitors: In silico, in vitro and ex vivo investigations. Bioorg. Chem. 2021, 110, 104773. [Google Scholar] [CrossRef]
- Sakata, R.P.; Antoniolli, G.; Lancellotti, M.; Kawano, D.F.; Guimarāes Barbosa, E.; Almeida, W.P. Synthesis and biological evaluation of 2′-aminochalcone: A multi-target approach to find drug candidates to treat Alzheimer’s disease. Bioorg. Chem. 2020, 103, 104201. [Google Scholar] [CrossRef]
- Apiraksattayakul, S.; Pingaew, R.; Leechaisit, R.; Prachayasittikul, V.; Ruankham, W.; Songtawee, N.; Tantimongcolwat, T.; Ruchirawat, S.; Prachayasittikul, V.; Prachayasittikul, S.; et al. Aminochalcones attenuate neuronal cell death under oxidative damage via sirtuin 1 activity. ACS Omega 2024, 8, 33367–33379. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, J.N.; de Andtade Ferreira Barreto, J.; de Alcântara Oliveira, F.A.; Haydée Lima Ferreira, J.; Dias Rufino Arcanjo, D.; Emidio Sampaio Nogueira, C.; Machado Marinho, M.; dos Santos, H.S.; Maria Lins Rolim, H.; de Siqueira-Júnior, J.P.; et al. ADMET study and inhibition of Staphylococcus aureus efflux pumps by a synthetic p-aminochalcone. Results Chem. 2024, 7, 101449. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S.; Marti, L. Process intensification with bifunctional heterogeneous catalysts: Selective one-pot synthesis of 2′-aminochalcones. ACS Catal. 2015, 5, 157–166. [Google Scholar] [CrossRef]
- Tristão, T.C.; Campos-Buzzi, F.; Corrêa, R.; Cruz, R.C.B.; CechinelFelho, V.; Bella Cruz, A. Antimicrobial and cytotoxicity potential of acetamido, amino and nitrochalcones. Arzneim. Forsch. Drug Res. 2012, 62, 590–594. [Google Scholar] [CrossRef]
- Sulpizio, C.; Roller, A.; Giester, G.; Rompel, A. Synthesis, structure, and antioxidant activity of methoxy- and hydroxyl-substituted 2′-aminochalcones. Monatsh Chem. 2016, 147, 1747–1757. [Google Scholar] [CrossRef]
- Prasad, Y.R.; Rani, V.J.; Rao, A.S. In vitro antioxidant activity and scavenging effects of some synthesized 4′-aminochalcones. Asian J. Chem. 2013, 25, 52–58. [Google Scholar] [CrossRef]
- Liu, G.; Ge, Z.; Zhao, M.; Zhou, Y. Design, synthesis and cytotoxic activities of novel aliphatic amino-substituted flavonoids. Molecules 2013, 18, 14070–14084. [Google Scholar] [CrossRef] [PubMed]
- Zeraik, M.L.; Ximenes, V.F.; Regasini, L.O.; Dutra, L.A.; Silva, D.H.S.; Fonseca, L.M.; Coelho, D.; Machado, S.A.S.; Bolzani, V.S. 4′-Aminochalcones as a novel inhibitors of the chlorinating activity of myeloperoxidase. Curr. Med. Chem. 2012, 19, 5405–5413. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.; Prabhakar, V.; Sangith, A.; Chandrika, B.; Balasubramanian, R. Synthesis, anti-inflammatory and antioxidant activity of ring-A-monosubstituted chalcone derivatives. Med. Chem. Res. 2014, 23, 4383–4394. [Google Scholar] [CrossRef]
- Trein, M.R.; Rodrigues e Oliveira, L.; Rigo, G.V.; Garcia, M.A.R.; Petro-Silveira, B.; da Silva Trentin, D.; Macedo, A.J.; Regasini, L.O.; Tasca, T. Anti-Trichomonas vaginalis activity of chalcone and amino-analogues. Parasitol. Res. 2019, 118, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.Y.; Li, Y.; Qiang, X.M.; Xu, R.; Zheng, Y.X.Z.; Cao, Z.C.; Luo, L.; Yang, X.; Sang, Z.P.; Su, F.; et al. Design, synthesis and biological evaluation of 4′-aminochalcone-rivastigmine hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2017, 25, 1030–1041. [Google Scholar] [CrossRef]
- Seba, V.; Silva, G.; Dos Santos, M.B.; Baek, S.J.; França, S.C.; Fachin, A.L.; Regasini, L.O.; Marins, M. Chalcone derivatives 4′-amino-1-naphthyl-chalcone (D14) and 4′-amino-4-methyl-1-naphthyl-chalcone (D15) suppress migration and invasion of osteosarcoma cells mediated by p53 regulating EMT-related genes. Int. J. Mol. Sci. 2018, 19, 2838. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, J.W.; Maia, M.M.G.; da Silva, F.R.C.; Ali, A.; Marinho, M.M.; Marinho, E.S.; dos Santos, H.S.; Martins, A.M.C.; de Menezes, R.R.P.P.B. Trypanocidal potential of synthetic p-aminochalcones: In silico and in vitro evaluation. Bioorg. Chem. 2023, 141, 106931–106943. [Google Scholar]
- Prasad, Y.R.; Rao, A.S.; Rambabu, R. Synthesis of some 4′-amino chalcones and their anti-inflammatory and antimicrobial activity. Asian J. Chem. 2009, 21, 907–914. [Google Scholar]
- Mariño, P.A.; Pereira, D.B.; Santi, G.; de Souza, R.O.; Faoro, D.; de Oliveira, L.F.S.; Machado, M.M.; Paula, F.R. In vitro and in silico toxicity evaluation of bioactive 4′-aminochalcone derivatives. Drug Chem. Toxicol. 2016, 39, 147–152. [Google Scholar] [CrossRef]
- Patel, J.R.; Dholakiya, B.Z. Synthesis of 1-(4-((E)-3-arylacryloyl) phenyl)-3,4-dibromo-1H-pyrrole-2,5-diones and screening for anti-candida and antituberculosis activity. Med. Chem. Res. 2012, 21, 1977–1983. [Google Scholar] [CrossRef]
- Goetz, M.P.; Reid, J.M.; Qi, Y.; Chen, A.; McGovern, R.M.; Kuffel, M.J.; Scanlon, P.D.; Erlichman, C.; Armes, M.M. A phase I study of once-weekly aminoflavone prodrug (AFP464) in solid tumorpatients. J. Clin. Oncol. 2011, 29, 2546. [Google Scholar] [CrossRef]
- Xu, K.; Ren, X.; Wang, J.; Zhang, Q.; Fu, X.; Zhang, P.-C. Clinical development and informatics analysis of natural and semi-synthetic flavonoid drugs: A critical review. J. Adv. Res. 2024, 63, 269–284. [Google Scholar] [CrossRef] [PubMed]
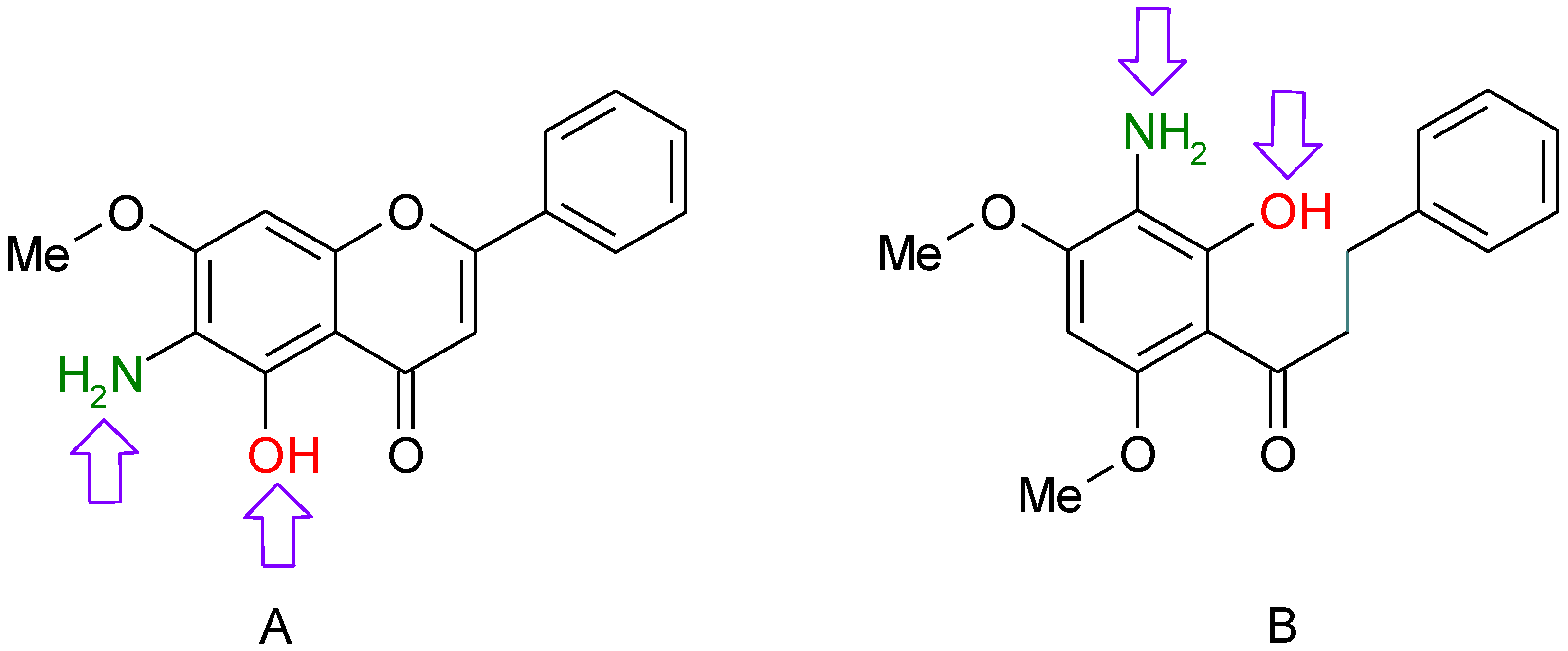




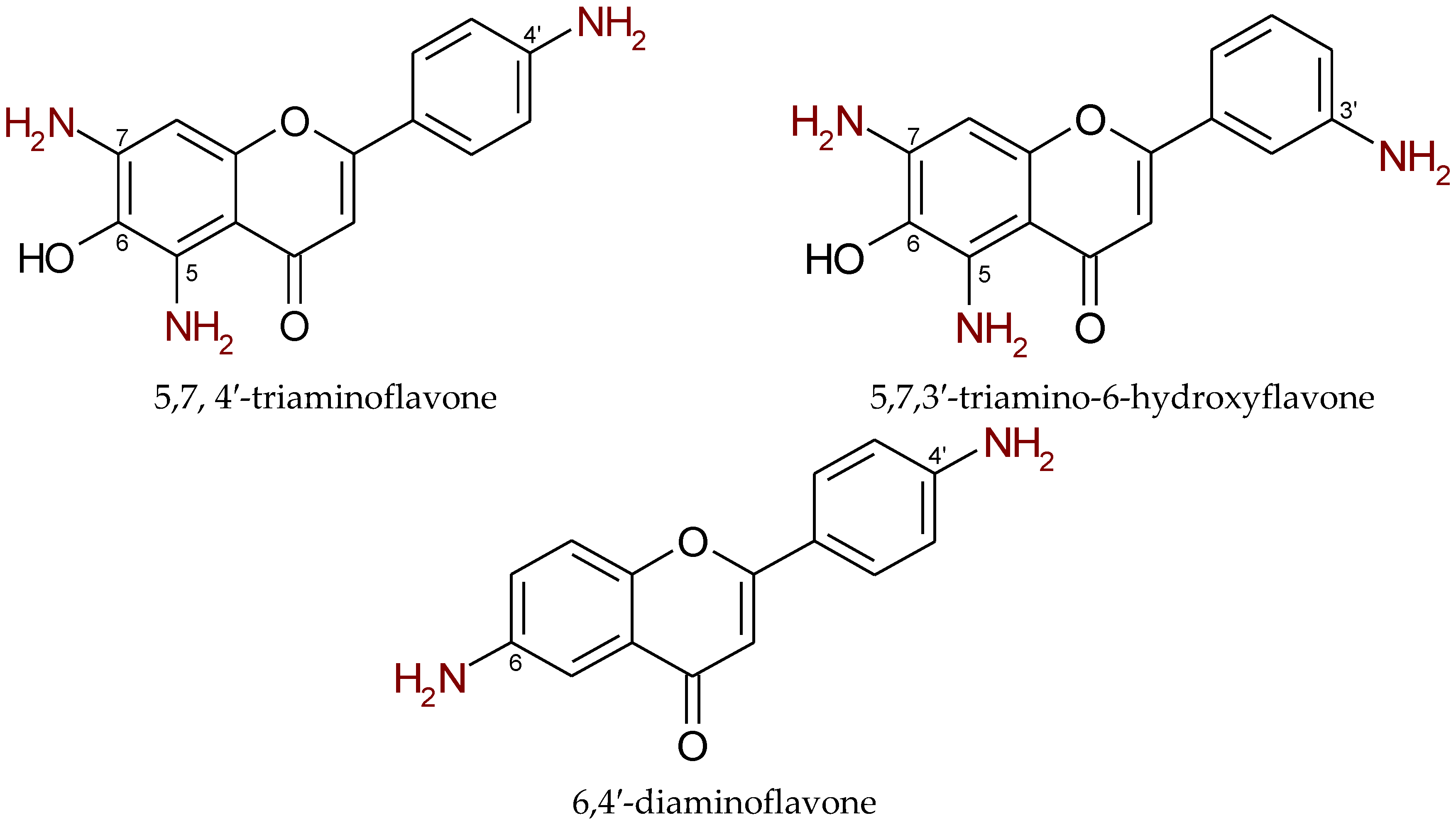

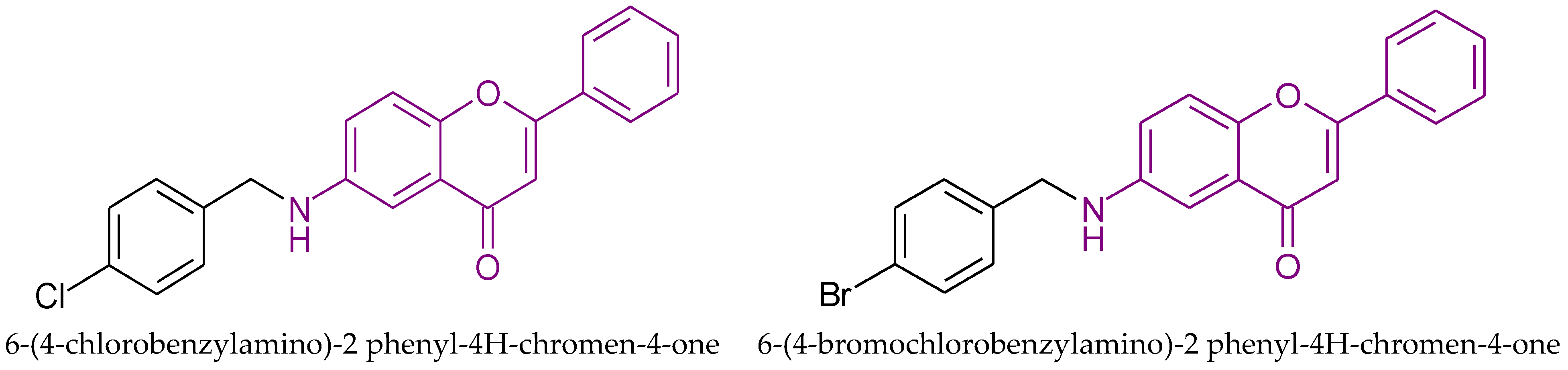


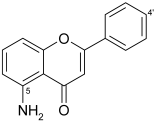 5-aminoflavone (5-AF) | ||
|---|---|---|
| IC50 [µM] | ||
| Estradiol (10−4 µM) | ||
| − | + | |
| 5-AF | >100 | 13 |
| 4′-OH | >100 | 1.5 |
| 4′-OMe | >100 | 12 |
| 4′-Br | 9.5 | 1.0 |
| 4′-CN | 94 | >100 |
| 4′-COOH | >10 | >10 |
| 4′-NH2 | 0.0098 | 0.0072 |
| 4′-NHAc | 18 | 13 |
| 4′-NHMe | 0.0054 | 0.0023 |
| 4′-NHEt | 0.0017 | 0.0013 |
| 4′-NHCH2Ph | 4.3 | 4.1 |
| 4′-NMe2 | 0.0050 | 0.0040 |
| 4′-NEt2 | 0.010 | 0.0020 |
| Aminoflavone Derivatives with Anticancer Effects | |||
|---|---|---|---|
| Chemical Structure | Cell Line | IC50 (μM) | Reference |
 | Human renal cancer cells | Luzzani et al. [29] Brantley et al. [36] | |
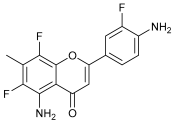 | Human breast cancer cell lines | Brinkman et al. [31] | |
 | Murine L1210 leukemia cells | 22 | Dauzonne et al. [33] |
 | Murine L1210 leukemia cells | 10 | Dauzonne et al. [33] |
 | Hepatocarcinoma cells (HepG2), breast adenocarcinoma cells (MCF-7), and human chronic myelogenous leukemia cells (K562) | 1.8 | Jin et al. [35] |
 | Inhibitors of protein tyrosine kinase | Król et al. [38] | |
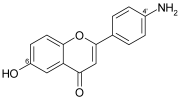 | Inhibitors of protein tyrosine kinase | 1.2 | Cushman et al. [39] |
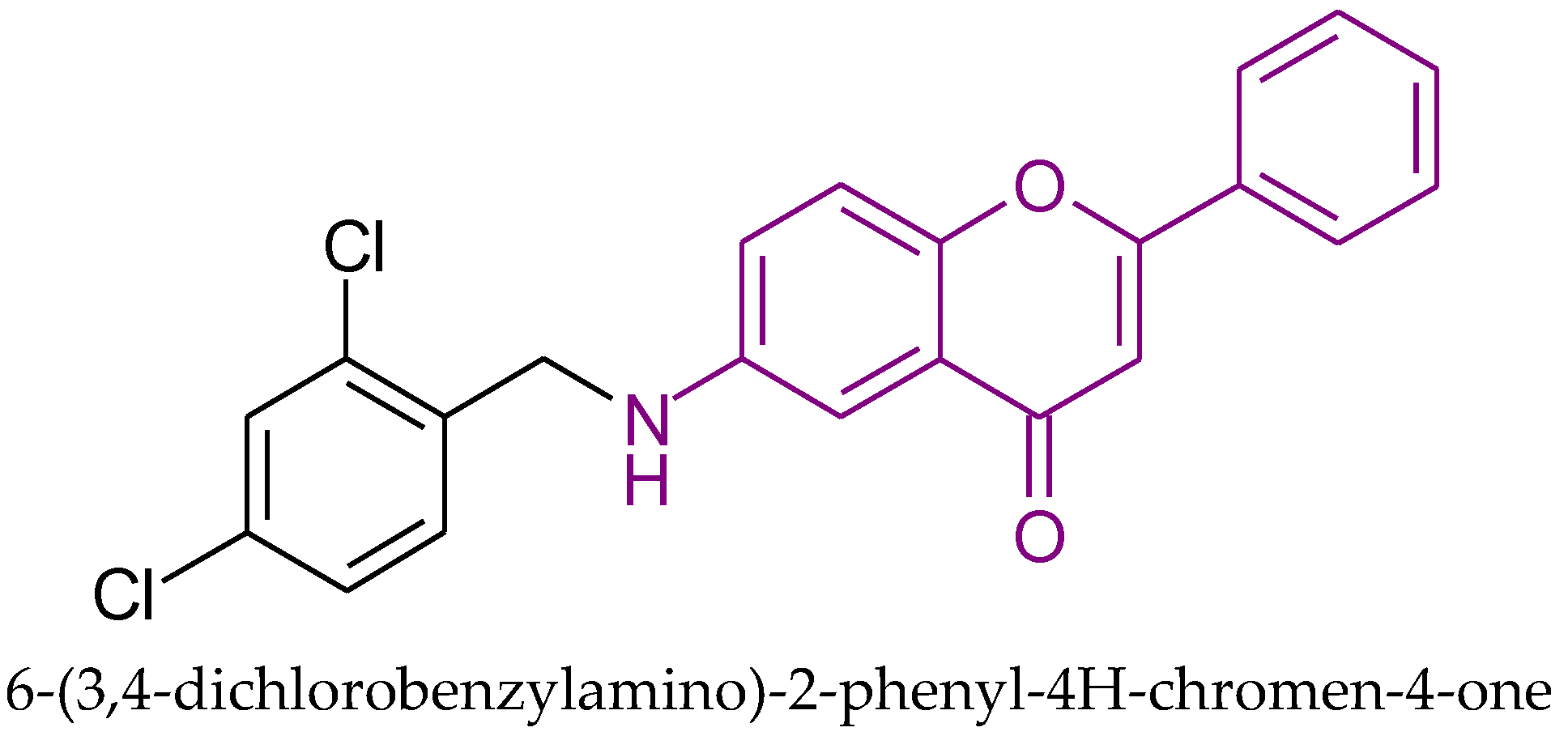
 | Anticancer activity to MCF-7 cells | 9.35 | Thorat et al. [41] |
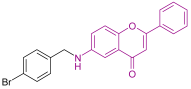 | Anticancer activity to MCF-7 cells | 9.58 | Thorat et al. [41] |
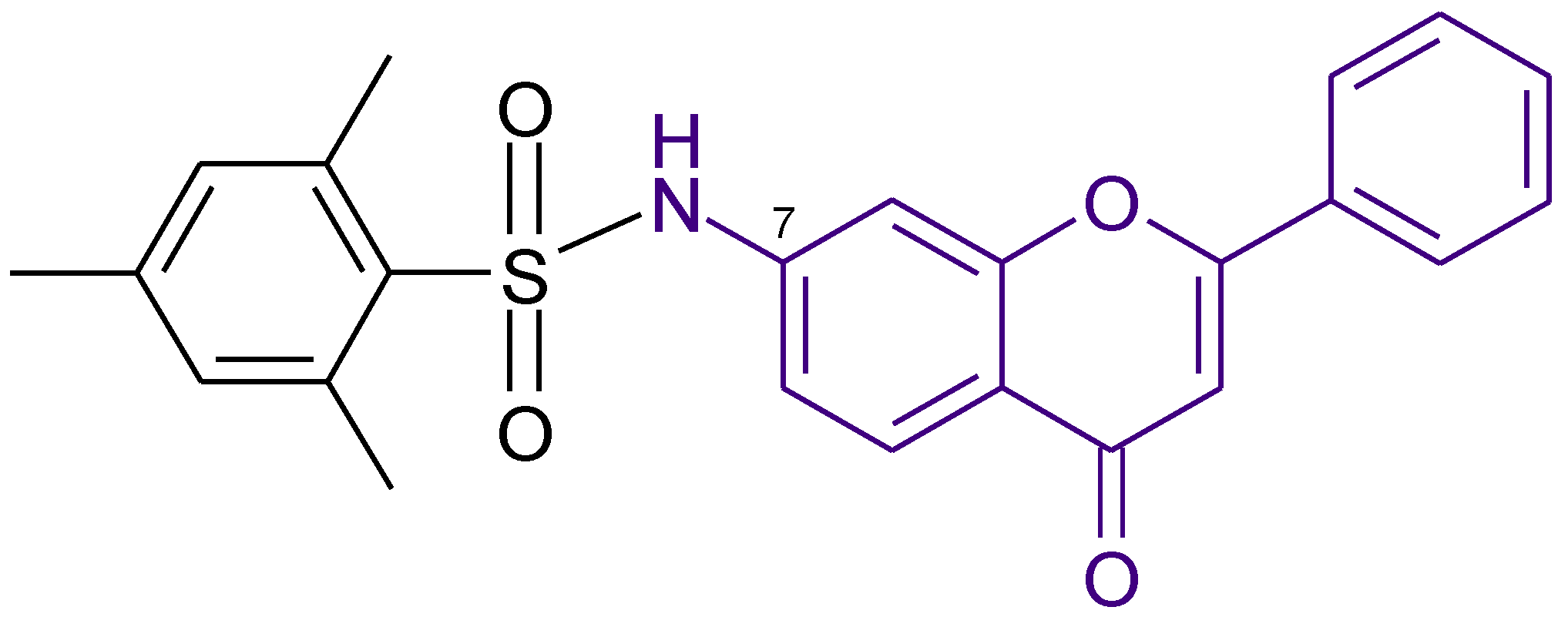
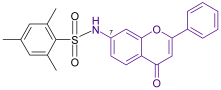 | Anticancer to HepG-2, A-549, and Caco-2cell lines | 0.98 | Shelke et al. [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stompor-Gorący, M.; Bajek-Bil, A.; Potocka, N.; Zawlik, I. Therapeutic Perspectives of Aminoflavonoids—A Review. Int. J. Mol. Sci. 2025, 26, 2014. https://doi.org/10.3390/ijms26052014
Stompor-Gorący M, Bajek-Bil A, Potocka N, Zawlik I. Therapeutic Perspectives of Aminoflavonoids—A Review. International Journal of Molecular Sciences. 2025; 26(5):2014. https://doi.org/10.3390/ijms26052014
Chicago/Turabian StyleStompor-Gorący, Monika, Agata Bajek-Bil, Natalia Potocka, and Izabela Zawlik. 2025. "Therapeutic Perspectives of Aminoflavonoids—A Review" International Journal of Molecular Sciences 26, no. 5: 2014. https://doi.org/10.3390/ijms26052014
APA StyleStompor-Gorący, M., Bajek-Bil, A., Potocka, N., & Zawlik, I. (2025). Therapeutic Perspectives of Aminoflavonoids—A Review. International Journal of Molecular Sciences, 26(5), 2014. https://doi.org/10.3390/ijms26052014