Exploring the Potential of Coumarin Derivatives on Serotonin Receptors 5-HT1A and 5HT2A
Abstract
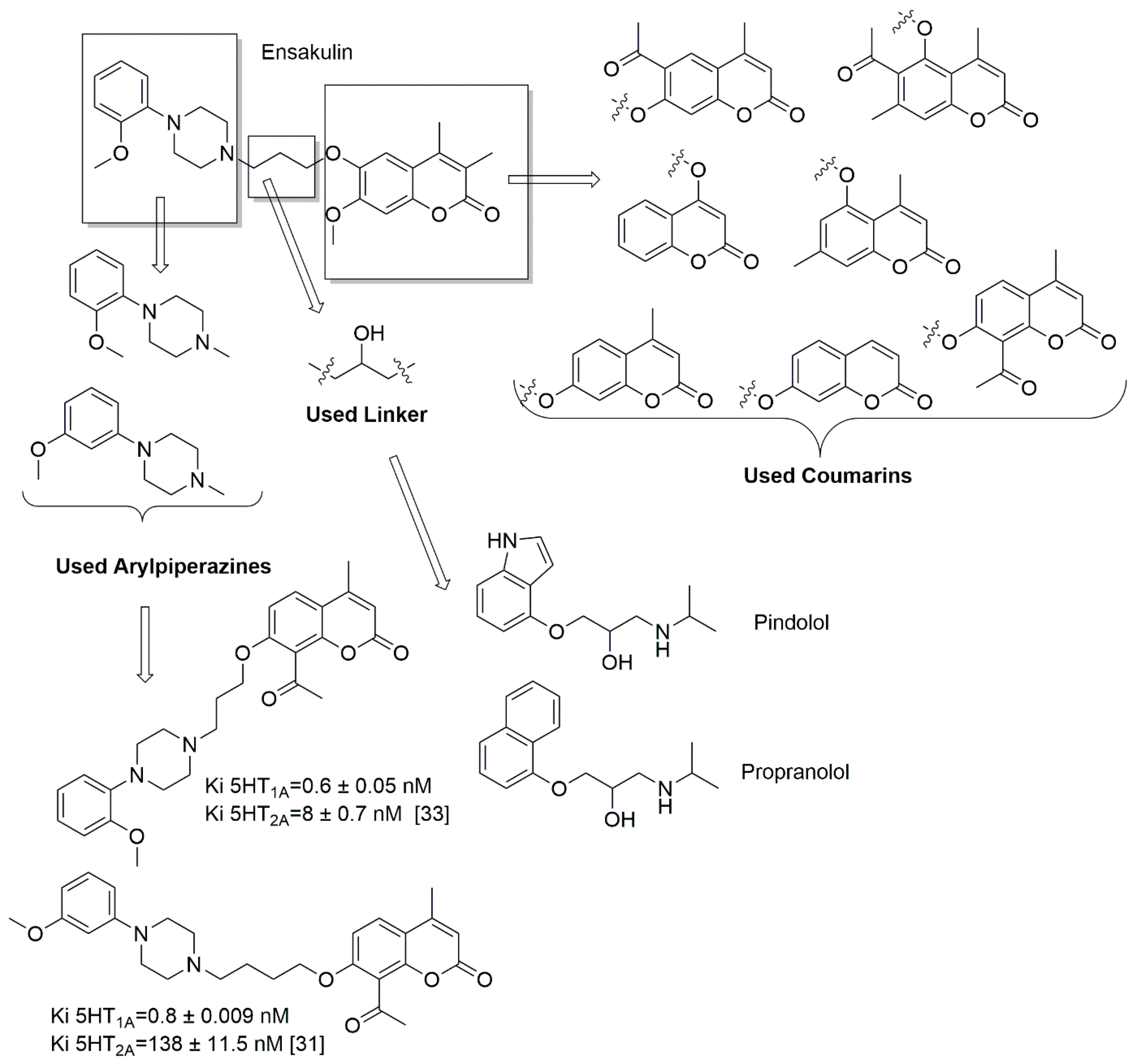
1. Introduction
2. Results and Discussion
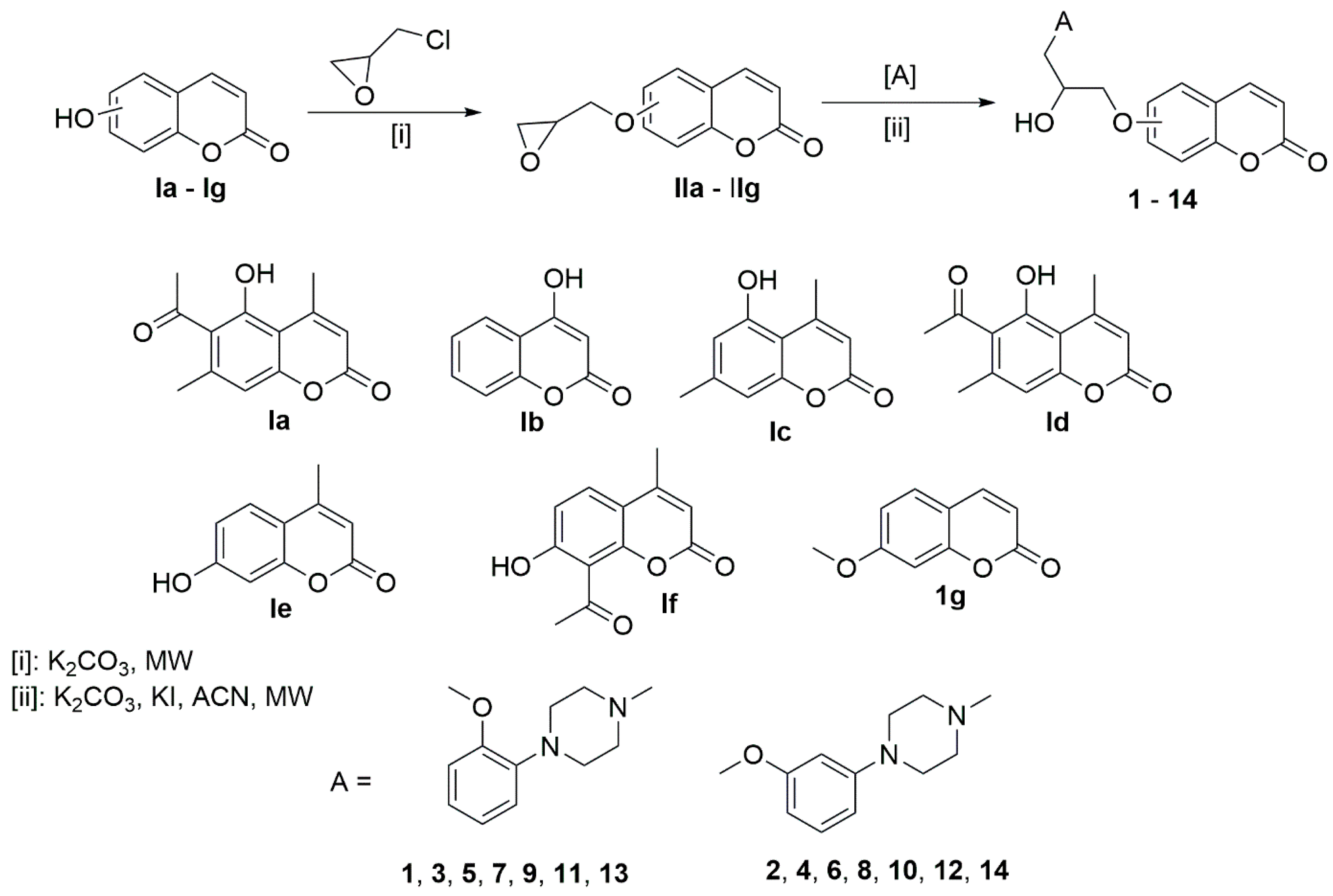
2.1. Chemistry
2.2. Pharmacology
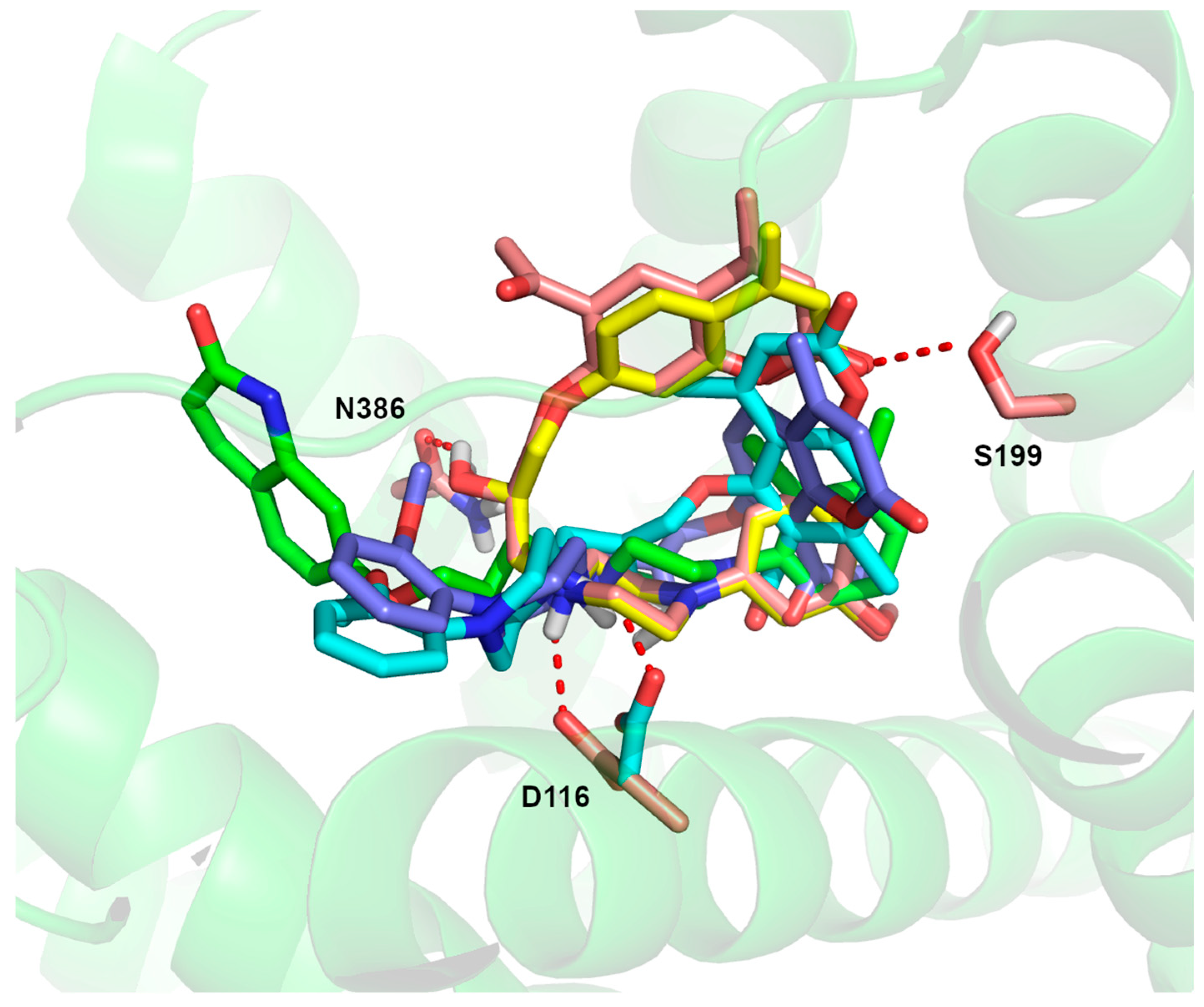
2.3. ADMET and Molecular Docking Studies
3. Materials and Methods
3.1. Experimental Section
3.1.1. Chemical Compounds
3.1.2. General Procedure for Preparing Compounds 1–14
General Procedure for Preparing Intermediate Derivatives IIa–IIg
General Procedure for Preparing Derivatives 1–14
6-Acetyl-7-(2-hydroxy-3-(4-(2-methoxyphenyl)piperazin-1-yl)propoxy)-4-methyl-2H-chromen-2-one (1)
6-Acetyl-7-(2-hydroxy-3-(4-(3-methoxyphenyl)piperazin-1-yl)propoxy)-4-methyl-2H-chromen-2-one (2)
4-(2-Hydroxy-3-(4-(2-methoxyphenyl)piperazin-1-yl)propoxy)-2H-chromen-2-one (3)
4-(2-Hydroxy-3-(4-(3-methoxyphenyl)piperazin-1-yl)propoxy)-2H-chromen-2-one (4)
5-(2-Hydroxy-3-(4-(2-methoxyphenyl)piperazin-1-yl)propoxy)-4,7-dimethyl-2H-chromen-2-one (5)
5-(2-Hydroxy-3-(4-(3-methoxyphenyl)piperazin-1-yl)propoxy)-4,7-dimethyl-2H-chromen-2-one (6)
6-Acetyl-5-(2-hydroxy-3-(4-(2-methoxyphenyl)piperazin-1-yl)propoxy)-4,7-dimethyl-2H-chromen-2-one (7)
6-Acetyl-5-(2-hydroxy-3-(4-(3-methoxyphenyl)piperazin-1-yl)propoxy)-4,7-dimethyl-2H-chromen-2-one (8)
7-(2-Hydroxy-3-(4-(2-methoxyphenyl)piperazin-1-yl)propoxy)-4-methyl-2H-chromen-2-one (9)
7-(2-Hydroxy-3-(4-(3-methoxyphenyl)piperazin-1-yl)propoxy)-4-methyl-2H-chromen-2-one (10)
8-Acetyl-7-(2-hydroxy-3-(4-(2-methoxyphenyl)piperazin-1-yl)propoxy)-4-methyl-2H-chromen-2-one (11)
8-Acetyl-7-(2-hydroxy-3-(4-(3-methoxyphenyl)piperazin-1-yl)propoxy)-4-methyl-2H-chromen-2-one (12)
7-(2-Hydroxy-3-(4-(2-methoxyphenyl)piperazin-1-yl)propoxy)-2H-chromen-2-one (13)
7-(2-Hydroxy-3-(4-(3-methoxyphenyl)piperazin-1-yl)propoxy)-2H-chromen-2-one (14)
3.1.3. Biological Assays
Membrane Preparation
Competitive Binding Assays
Antagonist Activity at 5-HT1A Receptors
3.1.4. Theoretical Methodology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meng, P.; Li, C.; Duan, S.; Ji, S.; Xu, Y.; Mao, Y.; Wang, H.; Tian, J. Epigenetic mechanism of 5-HT/NE/DA triple reuptake inhibi-tor on adult depression susceptibility in early stress mice. Front. Pharmacol. 2002, 13, 848251. [Google Scholar] [CrossRef]
- Gerhard, D.M.; Duman, R.S. Rapid-acting antidepressants: Mechanistic insights and future directions. Curr. Behav. Neurosci. Rep. 2018, 5, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.S.; Besckow, E.W.; da Silva Espindola, C.L.; Avila Nunes, G.D.; Zuge, N.P.; de Azeredo, M.P.; Rocha, M.J.D.; Carraro Junior, L.R.; Penteado, F.; Gomes, C.S. Antidepressant-like effect of aselenoindolizine in mice: In vivo and in silico evidence forthe involvement of the serotonergic 5-HT2A/C receptors. ACS Chem. Neurosci. 2022, 13, 1746–1755. [Google Scholar] [CrossRef]
- Cools, R.; Roberts, A.C.; Robbins, T.W. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn. Sci. 2008, 12, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Lesch, K.P.; Araragi, N.; Waider, J.; van den Hove, D.; Gutknecht, L. Targeting brain serotonin synthesis: Insights into neurodevelopmental disorders with long-term outcomes related to negative emotionality, aggression and antisocial behavior. Philos. Trans. R. Soc. Ser. B Biol. Sci. 2012, 367, 2426–2443. [Google Scholar] [CrossRef]
- Hoyer, D. Targeting the 5-HT system: Potential side effects. Neuropharmacology 2020, 179, 108233. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, D.; Hannon, J.P.; Martin, G.R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002, 71, 533–554. [Google Scholar] [CrossRef] [PubMed]
- Polter, A.M.; Li, X. 5-HT1A receptor-regulated signal transduction pathways in brain. Cell Signal 2010, 22, 1406–1412. [Google Scholar] [CrossRef]
- Dong, J.; de Montigny, C.; Blier, P. Effect of acute and repeated versus sustained administration of the 5-HT1A receptor agonist ipsapirone: Electrophysiological studies in the rat hippocampus and dorsal raphe. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1997, 356, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Sprouse, J.S.; Aghajanian, G.K. Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse 1987, 1, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Underwood, M.D.; Mann, J.J.; Arango, V. Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J. Psychiatr. Res. 2008, 42, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Celada, P.; Puig, M.; Amargós-Bosch, M.; Adell, A.; Artigas, F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J. Psychiatry Neurosci. 2004, 29, 252–265. [Google Scholar] [PubMed]
- Shaquiquzzaman, M.; Verma, G.; Marella, A.; Akhter, M.; Akhtar, W.; Khan, M.F.; Tasneem, S.M.; Alam, M.M. Piperazine scaffold: A remarkable tool in generation of diverse pharmacological agents. Eur. J. Med. Chem. 2015, 102, 487–529. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, N.; Vingtdeux, V.; Eddarkaoui, S.; Gay, M.; Evrard, C.; Le Fur, N.; Laurent, C.; Caillierez, R.; Obriot, H.; Larchanché, P.E.; et al. New piperazine multi-effect drugs prevent neurofibrillary degeneration and amyloid deposition and preserve memory in animal models of Alzheimer’s disease. Neurobiol. Dis. 2019, 129, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhang, Y.; Chen, Y.; Qiu, Y.; Yu, M.; Xu, X.; Liu, X.; Liu, B.F.; Zhang, L.; Zhang, G. Synthesis and biological evaluation of fused tricyclic heterocycle piperazine (piperi dine) derivatives as potential multireceptor atypical anti psychotics. J. Med. Chem. 2018, 61, 10017–10039. [Google Scholar] [CrossRef]
- Migliore, M.; Pontis, S.; Fuentes de Arriba, A.L.; Realini, N.; Torrente, E.; Armirotti, A.; Romeo, E.; Di Martino, S.; Russo, D.; Pizzirani, D.; et al. Second generation non-covalent NAAA inhibitors are protective in a model of multiple sclerosis. Angew. Chem. Int. Ed. Engl. 2016, 55, 11193–11197. [Google Scholar] [CrossRef] [PubMed]
- Moussa, I.A.; Banister, S.D.; Beinat, C.; Giboureau, N.; Reynolds, A.J.; Kassiou, M. Design, synthesis, and structure-affinity relationships of regioisomeric N-benzyl alkyl ether piperazine derivatives as sigma-1 receptor ligands. J. Med. Chem. 2010, 53, 6228–6239. [Google Scholar] [CrossRef] [PubMed]
- Foley, T.L.; Rai, G.; Yasgar, A.; Daniel, T.; Baker, H.L.; Attene-Ramos, M.; Kosa, N.M.; Leister, W.; Burkart, M.D.; Jadhav, A.; et al. 4-(3-Chloro-5-(trifluorome thyl)pyridin-2-yl)-N-(4-methoxypyridin-2-yl)piperazine-1-car bothioamide (ML267), a potent inhibitor of bacterial phosphopantetheinyl transferase that attenuates secondary metabolism and thwarts bacterial growth. J. Med. Chem. 2014, 57, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Deng, Q.; Li, B.; Shen, Y. Design, synthesis and biological evaluation of novel 5-(piperazin-1-yl)quinolin-2(1H)-one derivatives as potential chitin synthase inhibitors and antifungal agents. Eur. J. Med. Chem. 2019, 180, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, N.A.; Norman, M.H.; Bartberger, M.D.; Hong, F.T.; Bo, Y.; Liu, L.; Nishimura, N.; Yang, K.C.; Tadesse, S.; Fotsch, C.; et al. Small molecule disruptors of the glucokinase-glucokinase regulatory protein interaction: 5. A novel aryl sulfone series, optimiza tion through conformational analysis. J. Med. Chem. 2015, 58, 4462–4482. [Google Scholar] [CrossRef]
- Chen, F.H.; Zhang, L.B.; Qiang, L.; Yang, Z.; Wu, T.; Zou, M.J.; Tao, L.; You, Q.D.; Li, Z.Y.; Yang, Y.; et al. Reactive oxygen species-mitochondria pathway involved in LYG-202-induced apoptosis in human hepatocellular carcinoma HepG(2) cells. Cancer Lett. 2010, 296, 96–105. [Google Scholar] [CrossRef]
- He, Y.; Xie, F.; Ye, J.; Deuther-Conrad, W.; Cui, B.; Wang, L.; Lu, J.; Steinbach, J.; Brust, P.; Huang, Y.; et al. 1-(4-[F]Fluorobenzyl)-4-[(tetrahydro furan-2-yl)methyl]piperazine: A novel suitable radioligand with low lipophilicity for imaging r receptors in the brain. J. Med. Chem. 2017, 60, 4161–4172. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, K. Coumarin-Piperazine Derivatives as Biologically Active Compounds. Saudi Pharm. J. 2020, 28, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Hoerr, R.; Noeldner, M. Ensaculin (KA-672 HCl):A Multitransmitter Approach to Dementia Treatment. CNS Drug Rev. 2002, 8, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Hilgert, M.; Noldner, M.; Chatterjee, S.S.; Klein, J. KA-672 inhibits rat brain acetylcholinesterase in vitro but not in vivo. Neurosci. Lett. 1999, 263, 193–196. [Google Scholar] [CrossRef]
- Shen, Q.; Peng, Q.; Shao, J.; Liu, X.; Huang, Z.; Pu, X.; Ma, L.; Li, Y.M.; Chan, A.S.; Gu, L. Synthesis and biological evaluation of functionalized coumarins as acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2005, 40, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, X.B.; Wang, T.; Kong, L.Y. Design, synthesis, and acetylcholinesterase inhibitory activity of novel coumarin analogues. Bioorg Med. Chem. 2008, 16, 8011–8021. [Google Scholar] [CrossRef]
- Ostrowska, K.; Leśniak, A.; Czarnocka, Z.; Chmiel, J.; Bujalska-Zadrożny, M.; Trzaskowski, B. Design, Synthesis, and Biological Evaluation of a Series of 5- and 7-Hydroxycoumarin Derivatives as 5-HT1A Serotonin Receptor Antagonists. Pharmaceuticals 2021, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Kornischka, J.; Cordes, J.; Agelink, M.W. 40 Years Beta−Adrenoceptor Blockers in Psychiatry. Psychiatrie 2007, 75, 199–210. [Google Scholar] [CrossRef]
- Ostrowska, K.; Leśniak, A.; Karczyńska, U.; Jeleniewicz, P.; Głuch-Lutwin, M.; Mordyl, B.; Siwek, A.; Trzaskowski, B.; Sacharczuk, M.; Bujalska-Zadrożny, M. 6-Acetyl-5-hydroxy-4,7-dimethylcoumarin derivatives: Design, synthesis, modeling studies, 5-HT1A, 5-HT2A and D2 receptors affinity. Bioorg. Chem. 2020, 100, 103912. [Google Scholar] [CrossRef]
- Ostrowska, K.; Młodzikowska, K.; Głuch-Lutwin, M.; Gryboś, A.; Siwek, A. Synthesis of a new series of aryl/heteroarylpiperazinyl derivatives of 8-acetyl-7-hydroxy-4-methylcoumarin with low nanomolar 5-HT1A affinities. Eur. J. Med. Chem. 2017, 137, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, K.; Grzeszczuk, D.; Głuch-Lutwin, M.; Gryboś, A.; Siwek, A.; Dobrzycki, Ł.; Trzaskowski, B. Development of selective agents targeting serotonin 5HT1A receptors with subnanomolar activities based on a coumarin core. Med. Chem. Comm. 2017, 8, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, K.; Grzeszczuk, D.; Głuch-Lutwin, M.; Gryboś, A.; Siwek, A.; Leśniak, A.; Sacharczuk, M.; Trzaskowski, B. 5-HT1A and 5-HT2A receptors affinity, docking studies and pharmacological evaluation of a series of 8-acetyl-7-hydroxy-4-methylcoumarin derivatives. Bioorg Med. Chem. 2018, 26, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, K.; Leśniak, A.; Gryczka, W.; Dobrzycki, Ł.; Bujalska-Zadrożny, M.; Trzaskowski, B. New Piperazine Derivatives of 6-Acetyl-7-hydroxy-4-methylcoumarin as 5-HT1A Receptor Agents. Int. J. Mol. Sci. 2023, 24, 2779. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Yin, Y.; Wu, X.; Qiao, F.; Sha, S.; Lv, P.C.; Zhao, J.H.L. Synthesis, molecular docking and biological evaluation of coumarin derivatives containing piperazine skeleton as potential antibacterial agents. Bioorg. Med. Chem. 2014, 22, 5727–5737. [Google Scholar] [CrossRef] [PubMed]
- Farag, N.A.; Mohamed, S.R.; Soliman, G.A.H. Design, synthesis, and docking studies of novel benzopyrone derivatives as H(1)-antihistaminic agents. Bioorg Med. Chem. 2008, 16, 9009–9017. [Google Scholar] [CrossRef]
- Lacivita, E.; Niso, M.; Stama, M.L.; Arzuaga, A.; Altamura, C.; Costa, L.; Desaphy, J.F.; Ragozzino, M.L.; Ciranna, L.; Leopoldo, M. Privileged scaffold-based design to identify a novel drug-like 5-HT7 receptor-preferring agonist to target Fragile X syndrome. Eur. J. Med. Chem. 2022, 199, 112395. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, K.; Ciesielski, A.; Sadocha, A.; Prokopiuk, D.; Trzaskowski, B. A detailed structural analysis of selected (oxiran-2-yl)methoxy- and 3-chloro-2-hydroxypropoxycoumarin. J. Mol. Struct. 2025, 1321, 140269. [Google Scholar] [CrossRef]
- González-Gómez, J.C.; Santana, L.; Uriarte, E.; Brea, J.; Villazón, M.; Loza, M.I.; De Luca, M.; Rivas, M.E.; Montenegro, G.Y.; Fontenla, J.A. New arylpiperazine derivatives with high affinity for α1A, D2 and 5-HT2A receptors. Bioorg. Med. Chem. Lett. 2003, 13, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lan, Y.; Wang, S.; Zhang, H.; Xu, X.; Liu, X.; Yu, M.; Liu, B.F.; Zhang, G. Synthesis and evaluation of new coumarin derivatives as potential atypical antipsychotics. Eur. J. Med. Chem. 2014, 74, 427–439. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development. Adv. Drug Deliver Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Shelley, J.C.; Cholleti, A.; Frye, L.L.; Greenwood, J.R.; Timlin, M.R.; Uchimaya, M. Epik: A software program for pKa prediction and protonation state generation for drug-like molecules. J. Comput. Aided Mol. Des. 2007, 21, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Yu, J.; Wang, H.; Luo, Z.; Liu, X.; He, L.; Qi, J.; Fan, L.; Tang, L.; Chen, Z.; et al. Structure-based discovery of nonhallucinogenic psychedelic analogs. Science 2022, 375, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fan, L.; Wang, H.; Yu, J.; Lu, D.; Qi, J.; Nie, F.; Luo, Z.; Liu, Z.; Cheng, J.; et al. Structure-based design of a novel third-generation antipsychotic drug lead with potential antidepressant properties. Nat. Neurosci. 2022, 25, 39–49. [Google Scholar] [CrossRef]
- Xu, P.; Huang, S.; Zhang, H.; Mao, C.; Zhou, X.E.; Cheng, X.; Simon, I.A.; Shen, D.-D.; Yen, H.-Y.; Robinson, C.V.; et al. Structural insights into the lipid and ligand regulation of serotonin receptors. Nature 2021, 592, 469–473. [Google Scholar] [CrossRef]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2024, 5, 513–520. [Google Scholar] [CrossRef]
| Compound | 5-HT1A Ki (nM, 95% CI) | 5-HT1A IC50 (µM, 95% CI) | 5-HT2A Ki (nM, 95% CI) |
|---|---|---|---|
| 1 | 243 (136–437) | 331 (55–1985) | 1846 (1126–10,440) |
| 2 | 196 (134–288) | 59 (18–160) | 83 (30–229) |
| 3 | 243 (159–372) | 68 (37–129) | 4006 (1832–8761) |
| 4 | 190 (118–307) | 10 (0.49–23) | 315 (131–760) |
| 5 | 90 (54–150) | 14.7 (7.1–30) | 1182 (516–2806) |
| 6 | 176 (97–319) | 6.5 (2.5–17.3) | 662 (320–1373) |
| 7 | 90 (56–147) | 2.4 (0.58–9.8) | 4150 (2276–7568) |
| 8 | 1776 (354–8916) | 16.2 (6.2–42.7) | 115 (36–367) |
| 9 | 245 (143–419) | 2.4 (0.7–7.9) | 291 (134–629) |
| 10 | 87 (53–142) | 89.5 (30.2–265) | 260 (111–607) |
| 11 | 96 (50–183) | 0.043 (0.014–0.137) | 6157 (3184–11,910) |
| 12 | 264 (162–432) | 6.1 (1.76–21.5) | 67 (26–169) |
| 13 | 222 (124–396) | 1.43 (0.63–32.6) | 18 (6–61) |
| 14 | 135 (85–216) | 112 (48.9–256.3) | 68 (41–113) |
| 8-OH-DPAT | 0.68 (0.45–1.02) | - | - |
| Ketanserin | - | - | 0.56 (0.23–1.36) |
| WAY-100635 | 0.0043 (0.00186–0.0075) |
| Compound | MW a | Dipole b | vol c | SASA d | dHB e | aHB f | logP g | metab h | Ro3 i | Ro5 j | pKa k | LD50 l |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 466.5 | 7.1 | 777.6 | 1426.2 | 1 | 10.7 | 2.36 | 6 | 0 | 0 | 8.97 | 500 |
| 2 | 466.5 | 3.7 | 764.7 | 1418.1 | 1 | 10.7 | 2.30 | 5 | 0 | 0 | 8.97 | 550 |
| 3 | 410.5 | 3.5 | 705.7 | 1270.5 | 1 | 8.7 | 2.77 | 5 | 0 | 0 | 9.05 | 1100 |
| 4 | 410.5 | 4.5 | 705.1 | 1273.8 | 1 | 8.7 | 2.78 | 5 | 0 | 0 | 9.05 | 550 |
| 5 | 438.5 | 6.7 | 753.1 | 1367.3 | 1 | 8.7 | 3.38 | 7 | 0 | 1 | 8.99 | 1100 |
| 6 | 438.5 | 7.6 | 761.9 | 1387.3 | 1 | 8.7 | 3.37 | 6 | 0 | 0 | 9.00 | 676 |
| 7 | 480.6 | 6.1 | 774.1 | 1433.4 | 1 | 10.7 | 2.76 | 7 | 0 | 1 | 8.99 | 500 |
| 8 | 480.6 | 8.7 | 763.9 | 1425.7 | 1 | 10.7 | 2.70 | 7 | 0 | 1 | 8.99 | 550 |
| 9 | 424.5 | 9.0 | 758.5 | 1356.8 | 1 | 8.7 | 3.17 | 6 | 0 | 0 | 8.97 | 1100 |
| 10 | 424.5 | 6.5 | 745.6 | 1348.7 | 1 | 8.7 | 3.11 | 5 | 0 | 0 | 8.97 | 1100 |
| 11 | 466.5 | 12.7 | 775.3 | 1420.3 | 1 | 10.7 | 2.52 | 6 | 0 | 0 | 8.97 | 550 |
| 12 | 466.5 | 10.0 | 762.4 | 1412.2 | 1 | 10.7 | 2.46 | 5 | 0 | 0 | 8.97 | 1000 |
| 13 | 410.5 | 8.7 | 733.7 | 1305.2 | 1 | 8.7 | 2.91 | 5 | 0 | 0 | 8.97 | 1100 |
| 14 | 410.5 | 6.1 | 720.7 | 1297.1 | 1 | 8.7 | 2.85 | 4 | 0 | 0 | 8.97 | 550 |
| aripiprazole | 448.4 | 7.8 | 709 | 1318.3 | 1 | 6.25 | 4.43 | 5 | 0 | 0 | 7.39 | 800 |
| ketanserin | 395.4 | 7.8 | 699.4 | 1235.5 | 1 | 7.5 | 2.93 | 3 | 0 | 0 | 6.45 | 790 |
| 8-OH-DPAT | 247.4 | 1 | 556.1 | 959.5 | 1 | 2.75 | 3.46 | 4 | 0 | 0 | 9.4 | 150 |
| WAY100635 | 422.6 | 3.9 | 761.8 | 1413.2 | 0 | 8.25 | 3.95 | 6 | 0 | 0 | 6.34 | 874 |
| 5HT1A | 5HT2A | |||
|---|---|---|---|---|
| Compound | ΔG [kcal/mol] | Ki [nM] | ΔG [kcal/mol] | Ki [nM] |
| 1 | <−9.0 | >250 | −10.8 | 12.1 |
| 2 | −9.8 | 65.5 | −10.3 | 28.2 |
| 3 | <−9.0 | >250 | −11.0 | 8.6 |
| 4 | −9.6 | 91.9 | −10.7 | 14.3 |
| 5 | −9.4 | 128.7 | −10.7 | 14.3 |
| 6 | <−9.0 | >250 | −10.5 | 20.1 |
| 7 | −10.0 | 46.8 | −11.3 | 5.2 |
| 8 | <−9.0 | >250 | −10.9 | 10.2 |
| 9 | <−9.0 | >250 | −9.8 | 65.5 |
| 10 | −9.2 | 180.4 | −10.0 | 46.8 |
| 11 | −9.4 | 128.7 | −10.9 | 10.2 |
| 12 | −9.2 | 180.4 | −10.3 | 28.2 |
| 13 | <−9.0 | >250 | −9.7 | 77.6 |
| 14 | −9.5 | 108.8 | −9.8 | 65.5 |
| aripiprazole | −9.5 | 108.8 | −9.9 | 55.4 |
| ketanserin | −9.6 | - | −11.7 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostrowska, K.; Horosz, G.; Kruk, K.; Sieroń, B.; Leśniak, A.; Czartoryska, Z.; Bujalska-Zadrożny, M.; Milenkovic, D.; Trzaskowski, B. Exploring the Potential of Coumarin Derivatives on Serotonin Receptors 5-HT1A and 5HT2A. Int. J. Mol. Sci. 2025, 26, 1946. https://doi.org/10.3390/ijms26051946
Ostrowska K, Horosz G, Kruk K, Sieroń B, Leśniak A, Czartoryska Z, Bujalska-Zadrożny M, Milenkovic D, Trzaskowski B. Exploring the Potential of Coumarin Derivatives on Serotonin Receptors 5-HT1A and 5HT2A. International Journal of Molecular Sciences. 2025; 26(5):1946. https://doi.org/10.3390/ijms26051946
Chicago/Turabian StyleOstrowska, Kinga, Gabriela Horosz, Karolina Kruk, Bartłomiej Sieroń, Anna Leśniak, Zofia Czartoryska, Magdalena Bujalska-Zadrożny, Dejan Milenkovic, and Bartosz Trzaskowski. 2025. "Exploring the Potential of Coumarin Derivatives on Serotonin Receptors 5-HT1A and 5HT2A" International Journal of Molecular Sciences 26, no. 5: 1946. https://doi.org/10.3390/ijms26051946
APA StyleOstrowska, K., Horosz, G., Kruk, K., Sieroń, B., Leśniak, A., Czartoryska, Z., Bujalska-Zadrożny, M., Milenkovic, D., & Trzaskowski, B. (2025). Exploring the Potential of Coumarin Derivatives on Serotonin Receptors 5-HT1A and 5HT2A. International Journal of Molecular Sciences, 26(5), 1946. https://doi.org/10.3390/ijms26051946








