Prognostic Biomarkers in Breast Cancer via Multi-Omics Clustering Analysis
Abstract
1. Introduction
2. Results
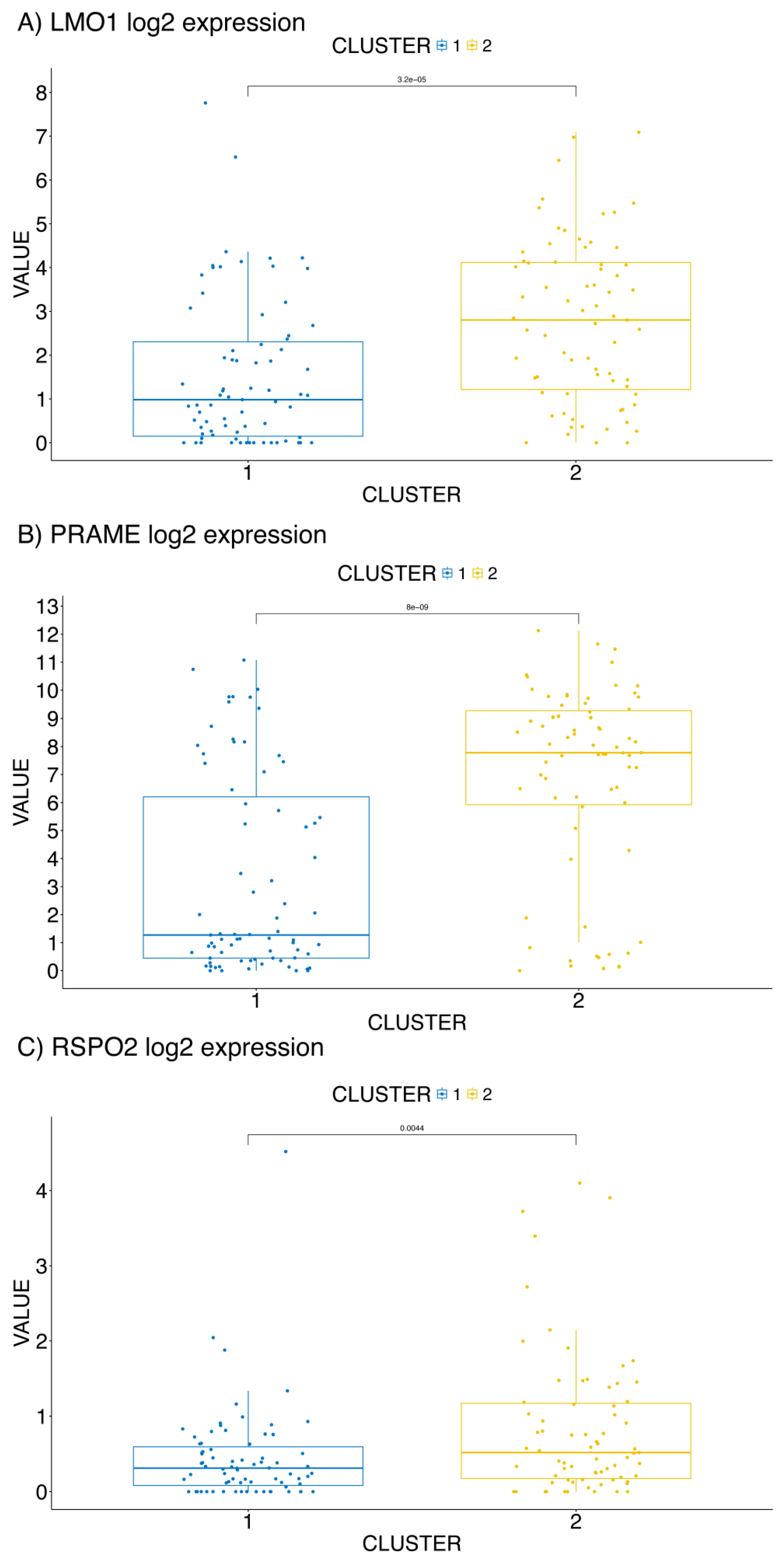
2.1. Biomarkers Identification
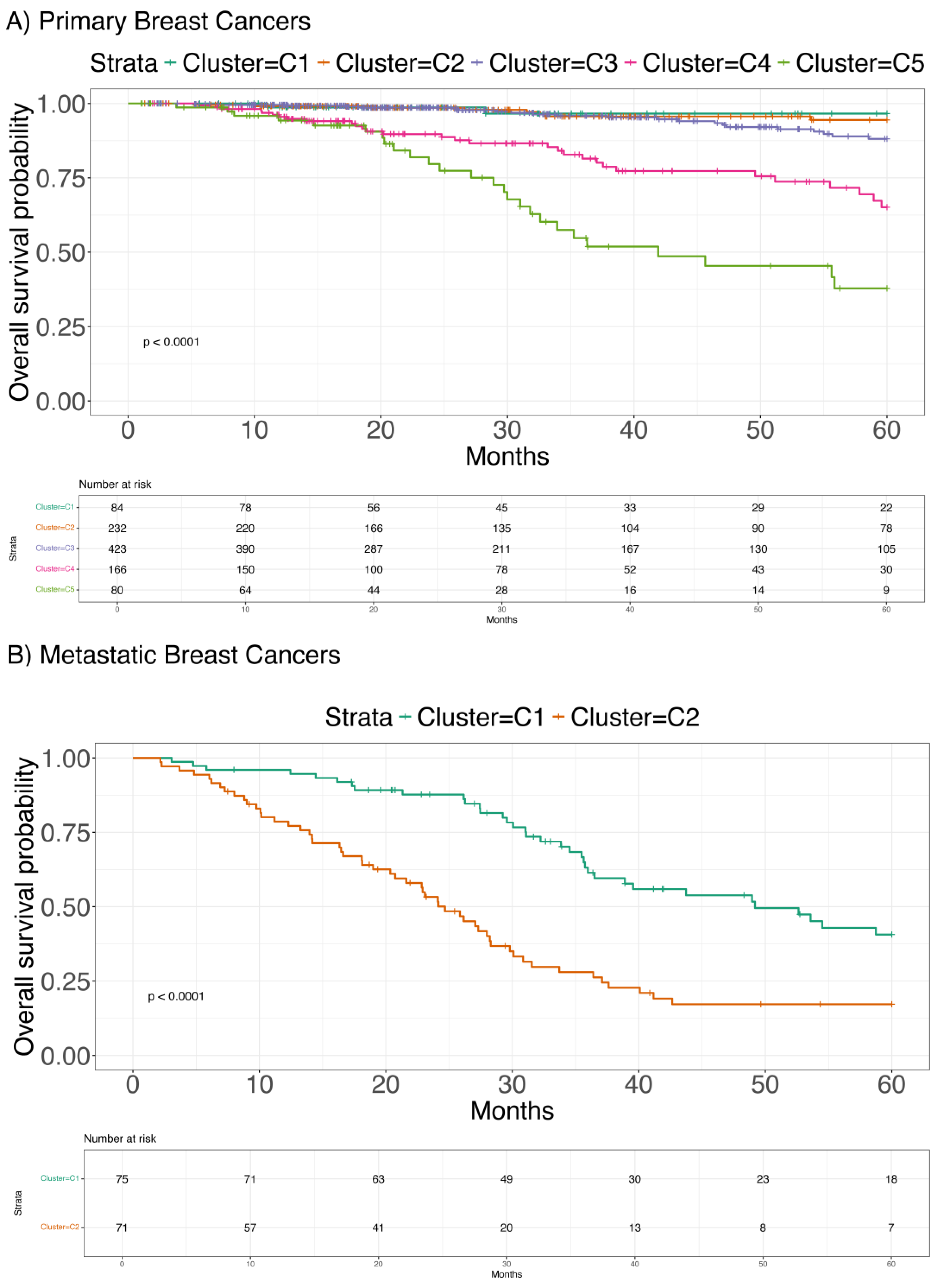
2.2. Clustering Analysis
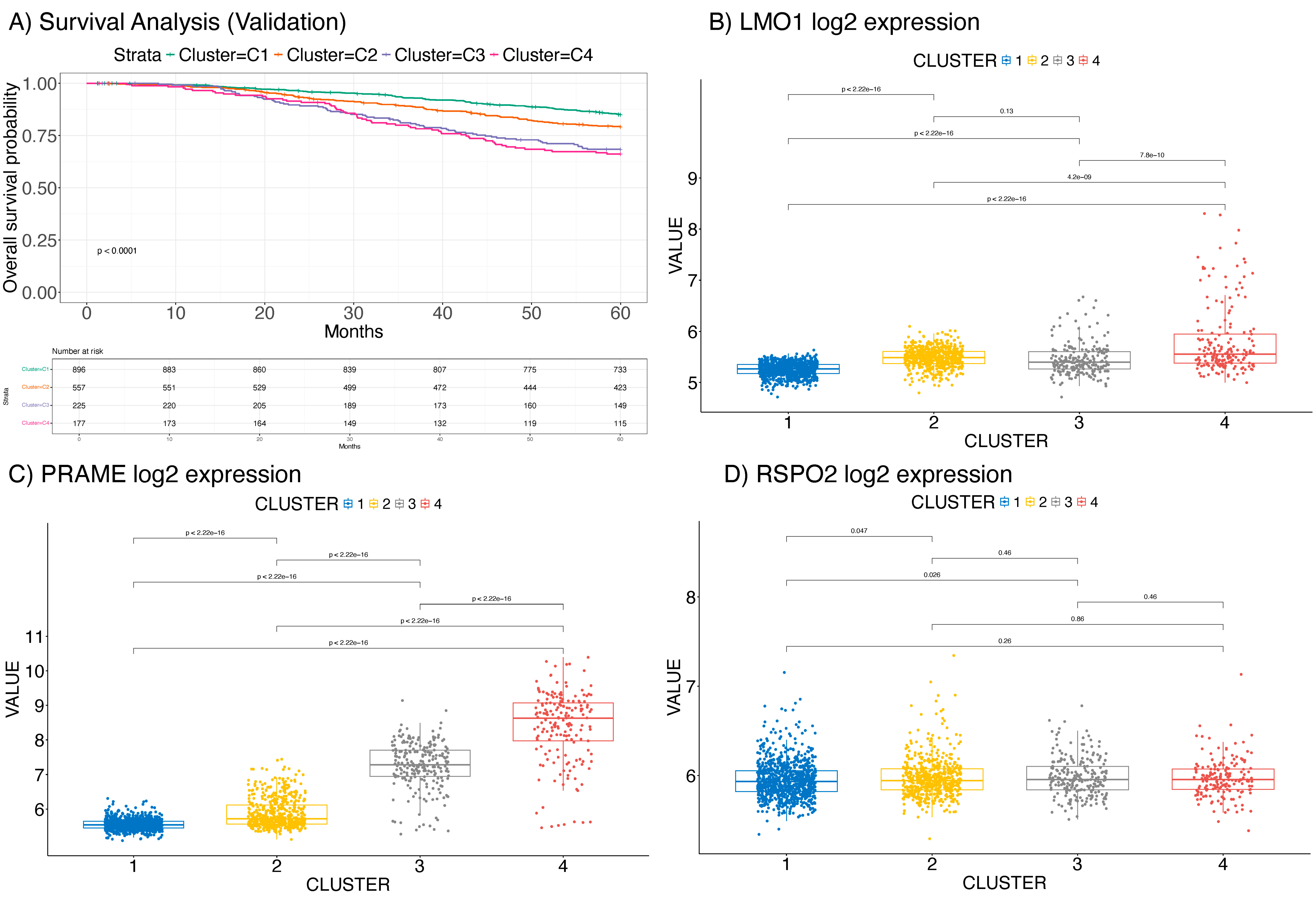
2.3. Validation on the METABRIC Dataset
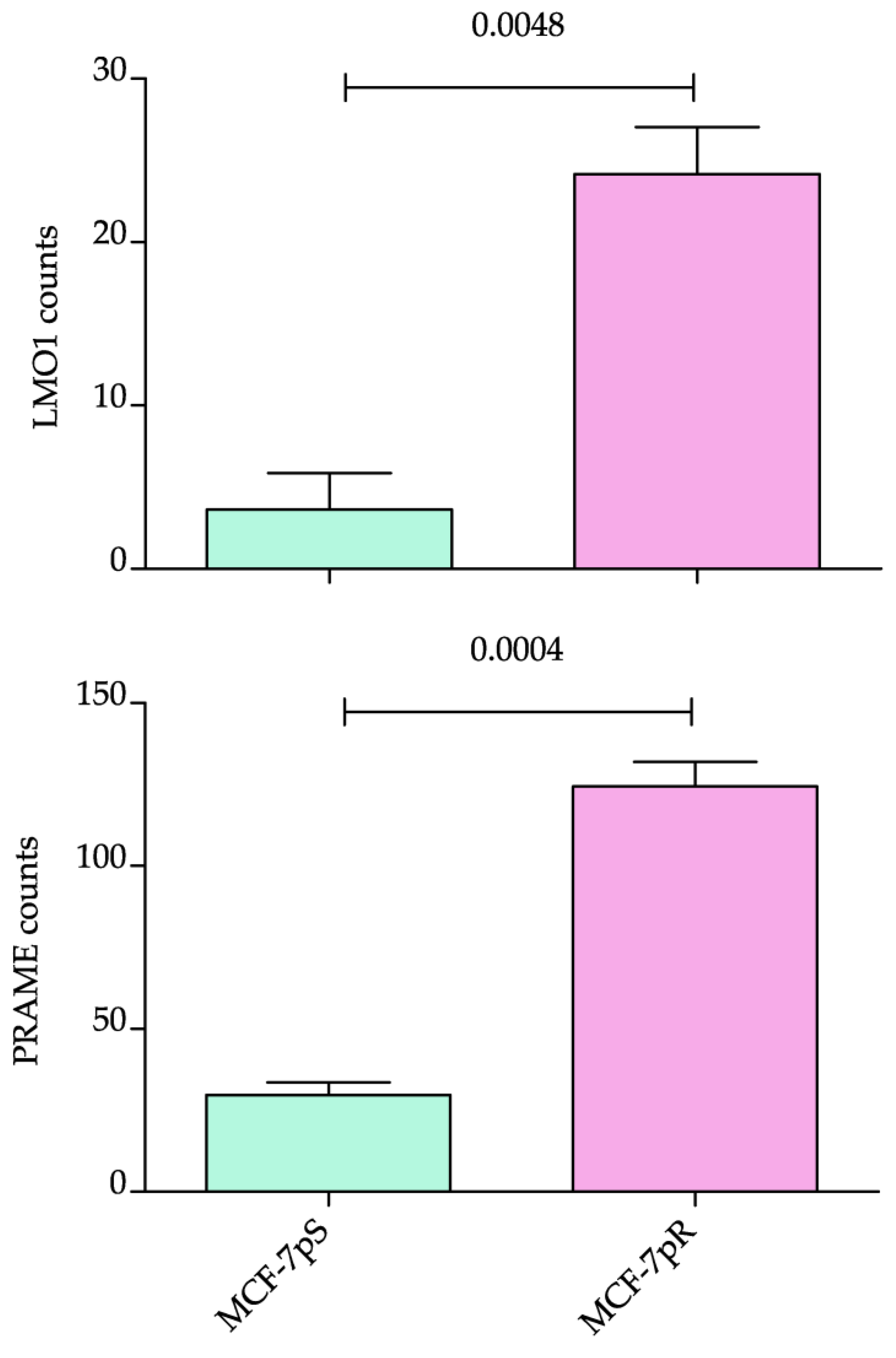
2.4. Expression of the Identified Biomarkers on Resistant Cell Lines and Metastatic BC Samples
3. Discussion
4. Materials and Methods
4.1. Data Preprocessing
4.2. Multi-Omics Integrative Clustering
4.3. Differential Analysis and Feature Selection
4.4. Survival Analysis
4.5. Regularized Cox Regression Analysis
4.6. RNA Extraction, Reverse Transcription and Real-Time Quantitative PCR
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Liu, S.; Tang, Y.; Li, J.; Zhao, W. Global, regional, and national trends in the burden of breast cancer among individuals aged 70 years and older from 1990 to 2021: An analysis based on the global burden of disease study 2021. Arch. Public Health 2024, 82, 170. [Google Scholar] [CrossRef] [PubMed]
- Ottaiano, A.; Ianniello, M.; Santorsola, M.; Ruggiero, R.; Sirica, R.; Sabbatino, F.; Perri, F.; Cascella, M.; Di Marzo, M.; Berretta, M.; et al. From Chaos to Opportunity: Decoding Cancer Heterogeneity for Enhanced Treatment Strategies. Biology 2023, 12, 1183. [Google Scholar] [CrossRef] [PubMed]
- Lenz, G.; Onzi, G.R.; Lenz, L.S.; Buss, J.H.; Dos Santos, J.A.; Begnini, K.R. The Origins of Phenotypic Heterogeneity in Cancer. Cancer Res. 2022, 82, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, V.; Capici, S.; Pepe, F.F.; di Mauro, P.; Riva, F.; Cicchiello, F.; Maggioni, C.; Cordani, N.; Cerrito, M.G.; Cazzaniga, M.E. How to Treat HR+/HER2- Metastatic Breast Cancer Patients after CDK4/6 Inhibitors: An Unfinished Story. Life 2022, 12, 378. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef] [PubMed]
- Haffty, B.G.; Yang, Q.; Reiss, M.; Kearney, T.; Higgins, S.A.; Weidhaas, J.; Harris, L.; Hait, W.; Toppmeyer, D. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J. Clin. Oncol. 2006, 24, 5652–5657. [Google Scholar] [CrossRef] [PubMed]
- Schettini, F.; Braso-Maristany, F.; Kuderer, N.M.; Prat, A. A perspective on the development and lack of interchangeability of the breast cancer intrinsic subtypes. NPJ Breast Cancer 2022, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.S.; Mullins, M.; Cheang, M.C.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sharma, G.; Karmakar, S.; Banerjee, S. Multi-OMICS approaches in cancer biology: New era in cancer therapy. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167120. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Wang, P.; Zenklusen, J.C. SnapShot: TCGA-Analyzed Tumors. Cell 2018, 173, 530. [Google Scholar] [CrossRef]
- Cancer Genome Atlas, N. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Pleasance, E.; Titmuss, E.; Williamson, L.; Kwan, H.; Culibrk, L.; Zhao, E.Y.; Dixon, K.; Fan, K.; Bowlby, R.; Jones, M.R.; et al. Pan-cancer analysis of advanced patient tumors reveals interactions between therapy and genomic landscapes. Nat. Cancer 2020, 1, 452–468. [Google Scholar] [CrossRef]
- Ramazzotti, D.; Lal, A.; Wang, B.; Batzoglou, S.; Sidow, A. Multi-omic tumor data reveal diversity of molecular mechanisms that correlate with survival. Nat. Commun. 2018, 9, 4453. [Google Scholar] [CrossRef] [PubMed]
- Cordani, N.; Mologni, L.; Piazza, R.; Tettamanti, P.; Cogliati, V.; Mauri, M.; Villa, M.; Malighetti, F.; Di Bella, C.; Jaconi, M.; et al. TWIST1 Upregulation Is a Potential Target for Reversing Resistance to the CDK4/6 Inhibitor in Metastatic Luminal Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 16294. [Google Scholar] [CrossRef] [PubMed]
- Valge-Archer, V.; Forster, A.; Rabbitts, T.H. The LMO1 and LDB1 proteins interact in human T cell acute leukaemia with the chromosomal translocation t(11;14)(p15;q11). Oncogene 1998, 17, 3199–3202. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, K.; Diskin, S.J.; Zhang, H.; Attiyeh, E.F.; Winter, C.; Hou, C.; Schnepp, R.W.; Diamond, M.; Bosse, K.; Mayes, P.A.; et al. Integrative genomics identifies LMO1 as a neuroblastoma oncogene. Nature 2011, 469, 216–220. [Google Scholar] [CrossRef]
- Visvader, J.E.; Venter, D.; Hahm, K.; Santamaria, M.; Sum, E.Y.; O’Reilly, L.; White, D.; Williams, R.; Armes, J.; Lindeman, G.J. The LIM domain gene LMO4 inhibits differentiation of mammary epithelial cells in vitro and is overexpressed in breast cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 14452–14457. [Google Scholar] [CrossRef] [PubMed]
- Korša, L.; Abramović, M.; Kovačević, L.; Milošević, M.; Podolski, P.; Prutki, M.; Marušić, Z. PRAME expression and its prognostic significance in invasive breast carcinoma. Pathol.-Res. Pract. 2024, 254, 155096. [Google Scholar] [CrossRef] [PubMed]
- Epping, M.T.; Hart, A.A.; Glas, A.M.; Krijgsman, O.; Bernards, R. PRAME expression and clinical outcome of breast cancer. Br. J. Cancer 2008, 99, 398–403. [Google Scholar] [CrossRef]
- Ter Steege, E.J.; Bakker, E.R.M. The role of R-spondin proteins in cancer biology. Oncogene 2021, 40, 6469–6478. [Google Scholar] [CrossRef] [PubMed]
- Crippa, V.; Malighetti, F.; Villa, M.; Graudenzi, A.; Piazza, R.; Mologni, L.; Ramazzotti, D. Characterization of cancer subtypes associated with clinical outcomes by multi-omics integrative clustering. Comput. Biol. Med. 2023, 162, 107064. [Google Scholar] [CrossRef] [PubMed]
- Conboy, C.B.; Velez-Reyes, G.L.; Rathe, S.K.; Abrahante, J.E.; Temiz, N.A.; Burns, M.B.; Harris, R.S.; Starr, T.K.; Largaespada, D.A. R-Spondins 2 and 3 Are Overexpressed in a Subset of Human Colon and Breast Cancers. DNA Cell Biol. 2021, 40, 70–79. [Google Scholar] [CrossRef]
- Curtis, C.; Shah, S.P.; Chin, S.F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.; Chin, S.F.; Rueda, O.M.; Vollan, H.K.; Provenzano, E.; Bardwell, H.A.; Pugh, M.; Jones, L.; Russell, R.; Sammut, S.J.; et al. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat. Commun. 2016, 7, 11479. [Google Scholar] [CrossRef] [PubMed]
- Rueda, O.M.; Sammut, S.J.; Seoane, J.A.; Chin, S.F.; Caswell-Jin, J.L.; Callari, M.; Batra, R.; Pereira, B.; Bruna, A.; Ali, H.R.; et al. Dynamics of breast-cancer relapse reveal late-recurring ER-positive genomic subgroups. Nature 2019, 567, 399–404. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, l1. [Google Scholar] [CrossRef] [PubMed]
- Simon, N.; Friedman, J.; Hastie, T.; Tibshirani, R. Regularization Paths for Cox’s Proportional Hazards Model via Coordinate Descent. J. Stat. Softw. 2011, 39, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tibshirani, R.; Bien, J.; Friedman, J.; Hastie, T.; Simon, N.; Taylor, J.; Tibshirani, R.J. Strong rules for discarding predictors in lasso-type problems. J. R. Stat. Soc. Ser. B Stat. Methodol. 2012, 74, 245–266. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malighetti, F.; Villa, M.; Villa, A.M.; Pelucchi, S.; Aroldi, A.; Cortinovis, D.L.; Canova, S.; Capici, S.; Cazzaniga, M.E.; Mologni, L.; et al. Prognostic Biomarkers in Breast Cancer via Multi-Omics Clustering Analysis. Int. J. Mol. Sci. 2025, 26, 1943. https://doi.org/10.3390/ijms26051943
Malighetti F, Villa M, Villa AM, Pelucchi S, Aroldi A, Cortinovis DL, Canova S, Capici S, Cazzaniga ME, Mologni L, et al. Prognostic Biomarkers in Breast Cancer via Multi-Omics Clustering Analysis. International Journal of Molecular Sciences. 2025; 26(5):1943. https://doi.org/10.3390/ijms26051943
Chicago/Turabian StyleMalighetti, Federica, Matteo Villa, Alberto Maria Villa, Sara Pelucchi, Andrea Aroldi, Diego Luigi Cortinovis, Stefania Canova, Serena Capici, Marina Elena Cazzaniga, Luca Mologni, and et al. 2025. "Prognostic Biomarkers in Breast Cancer via Multi-Omics Clustering Analysis" International Journal of Molecular Sciences 26, no. 5: 1943. https://doi.org/10.3390/ijms26051943
APA StyleMalighetti, F., Villa, M., Villa, A. M., Pelucchi, S., Aroldi, A., Cortinovis, D. L., Canova, S., Capici, S., Cazzaniga, M. E., Mologni, L., Ramazzotti, D., & Cordani, N. (2025). Prognostic Biomarkers in Breast Cancer via Multi-Omics Clustering Analysis. International Journal of Molecular Sciences, 26(5), 1943. https://doi.org/10.3390/ijms26051943





