Cannabis sativa L. Leaf Oil Displays Cardiovascular Protective Effects in Hypertensive Rats
Abstract
1. Introduction
2. Results
2.1. Bioactive Compounds Found in HLO
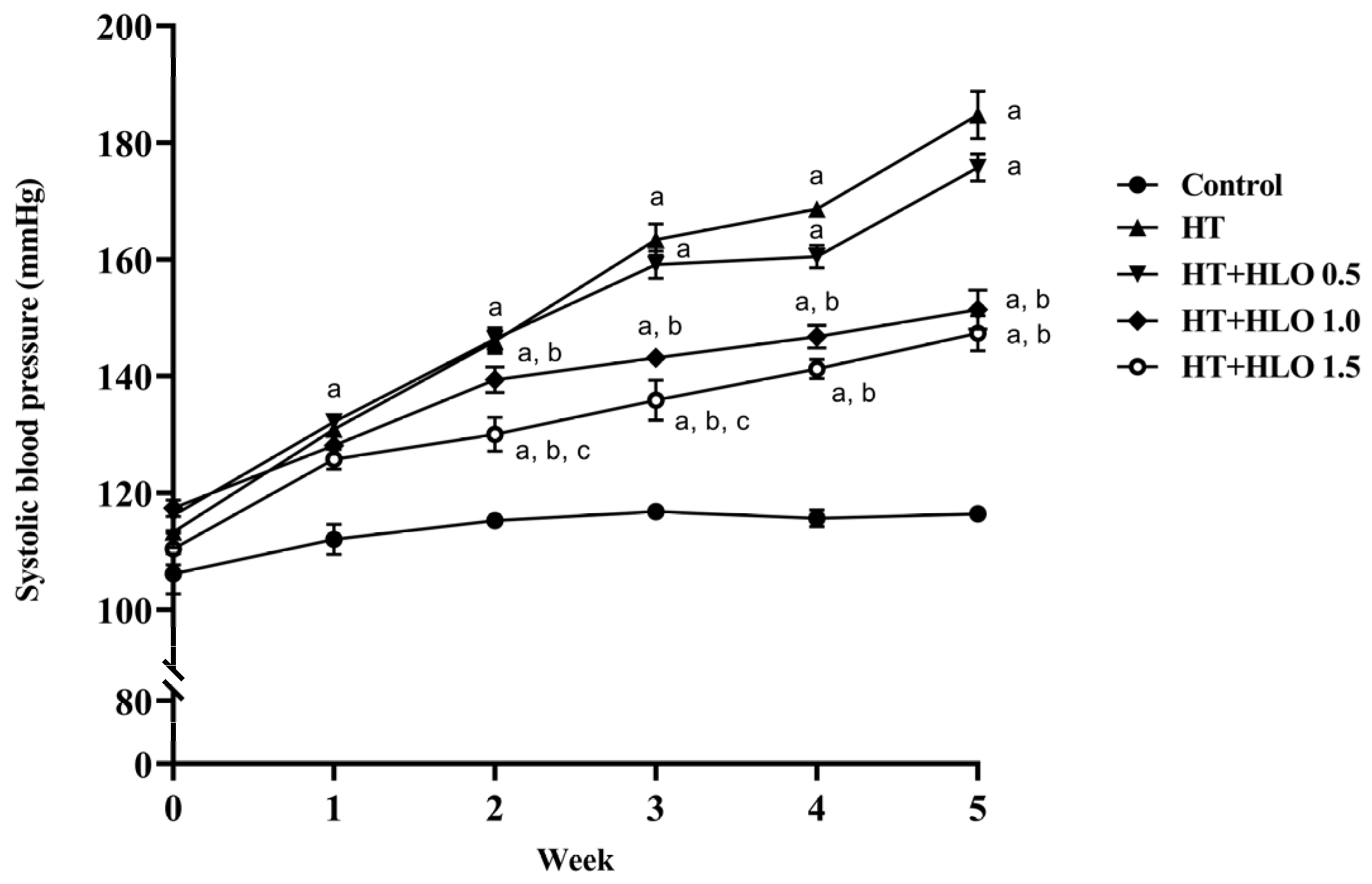
2.2. HLO Prevents the Gradual Increase in Systolic Blood Pressure (SBP) in Hypertensive Rats
2.3. HLO Decreases High Blood Pressure in Hypertensive Rats
2.4. Effects of HLO on Body Weight and Organ Weight in Hypertensive Rats
2.5. HLO Improves the Alteration of Cardiac Function Parameters in Hypertensive Rats
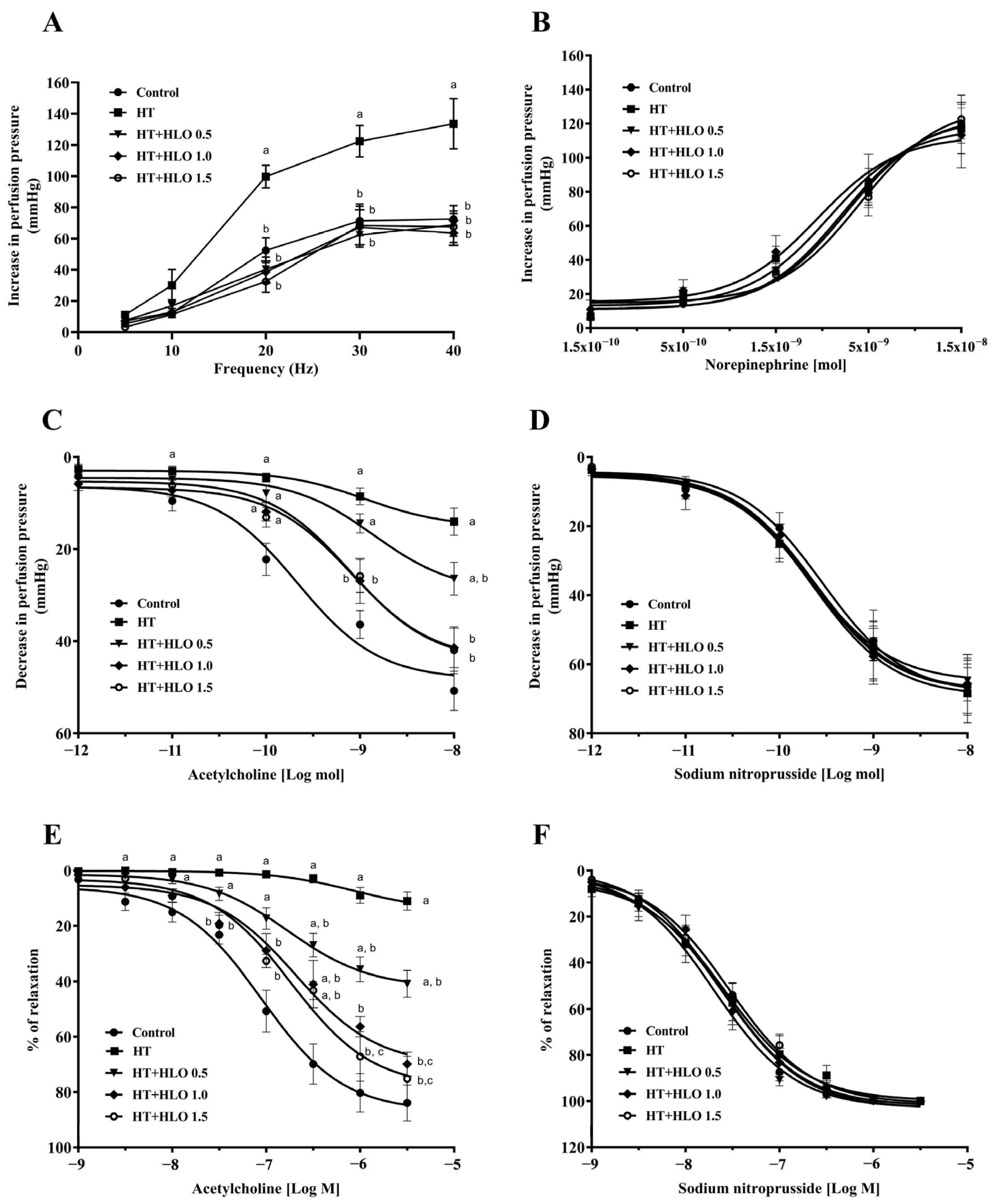
2.6. HLO Improves Vascular Responses to Sympathetic Nerve Stimulation and Endothelial Function in Hypertensive Rats
2.7. HLO Improves Cardiac Hypertrophy in Hypertensive Rats
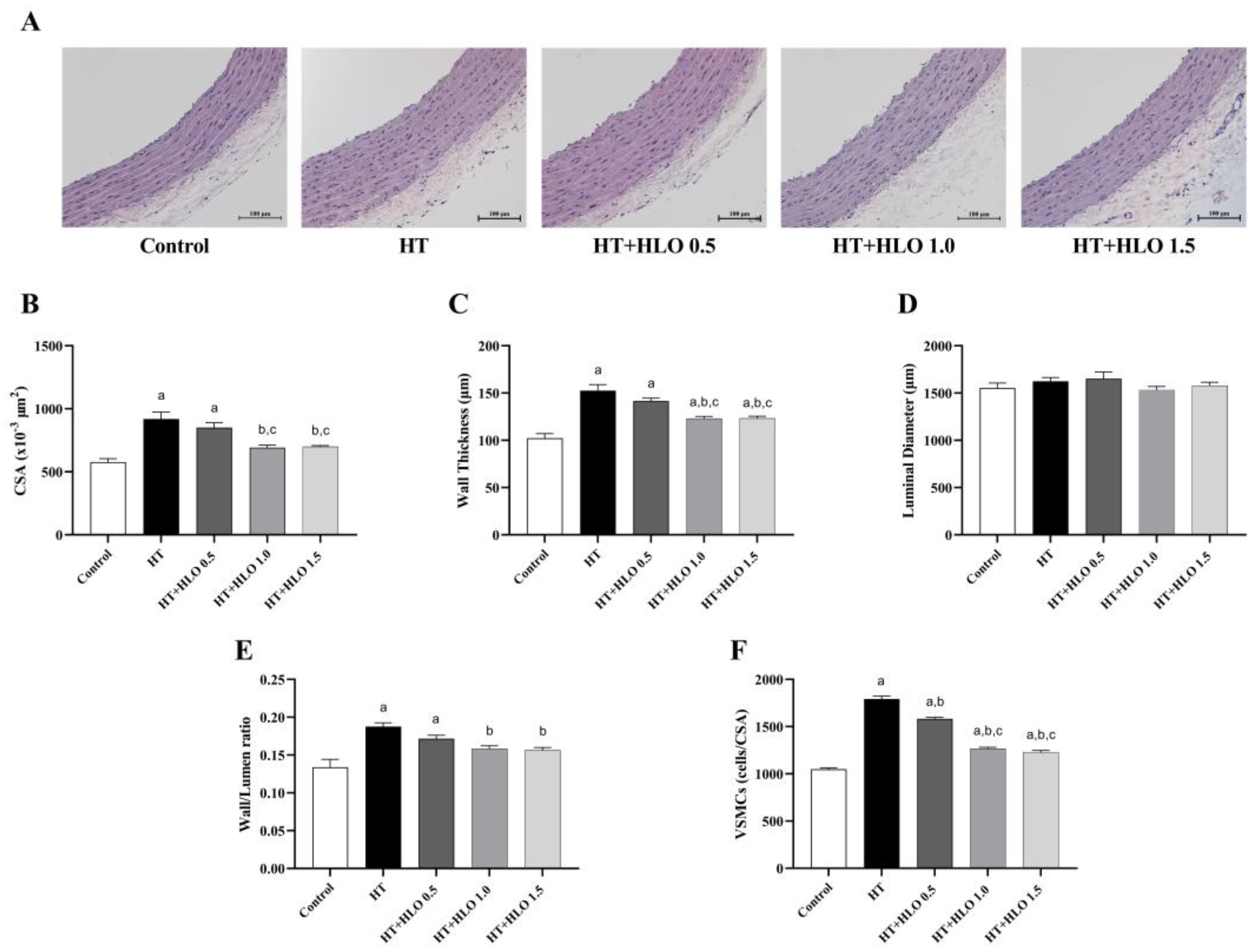
2.8. HLO Improves the Alteration of Vascular Morphology in Hypertensive Rats
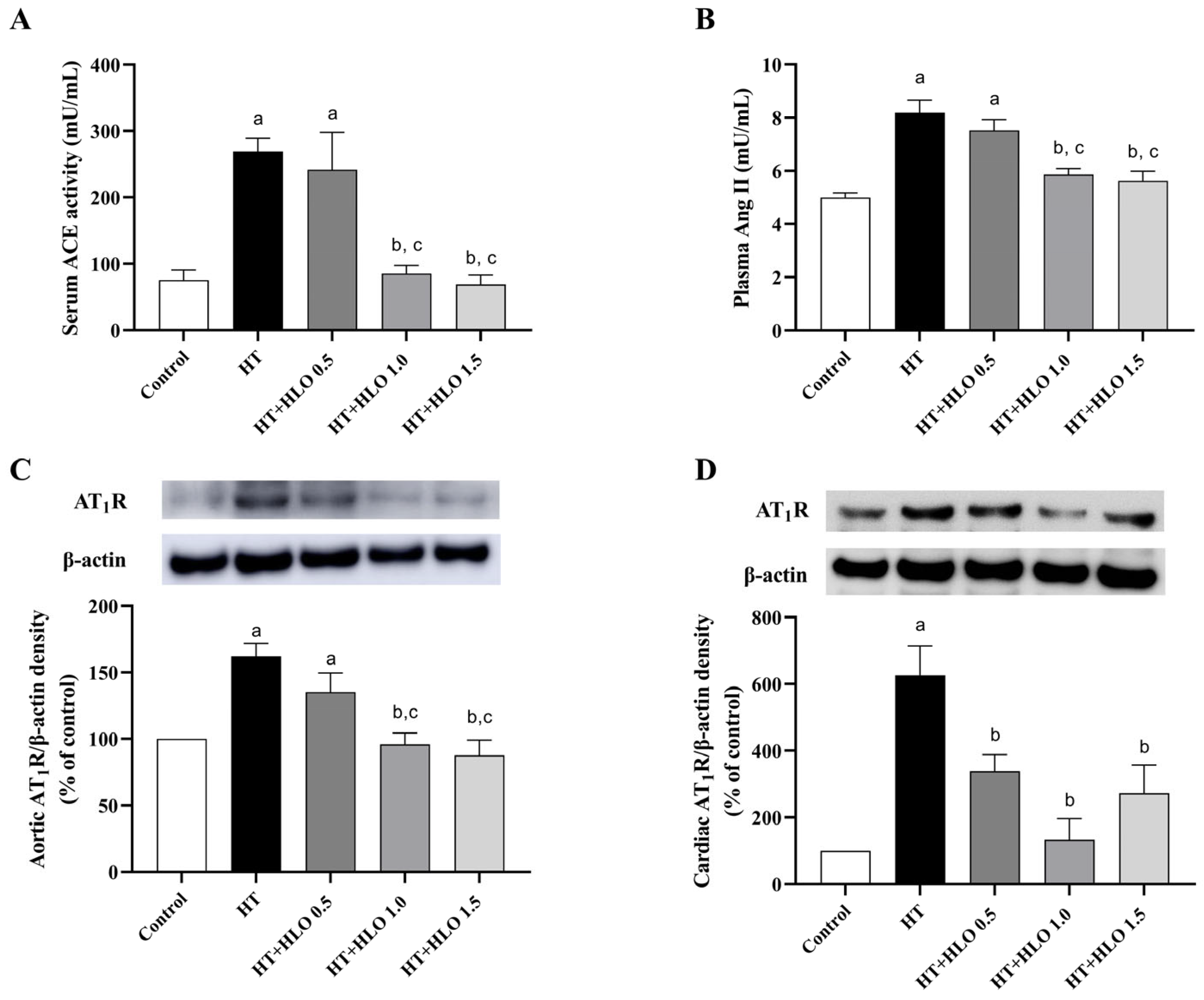
2.9. HLO Modulates RAS in Hypertensive Rats
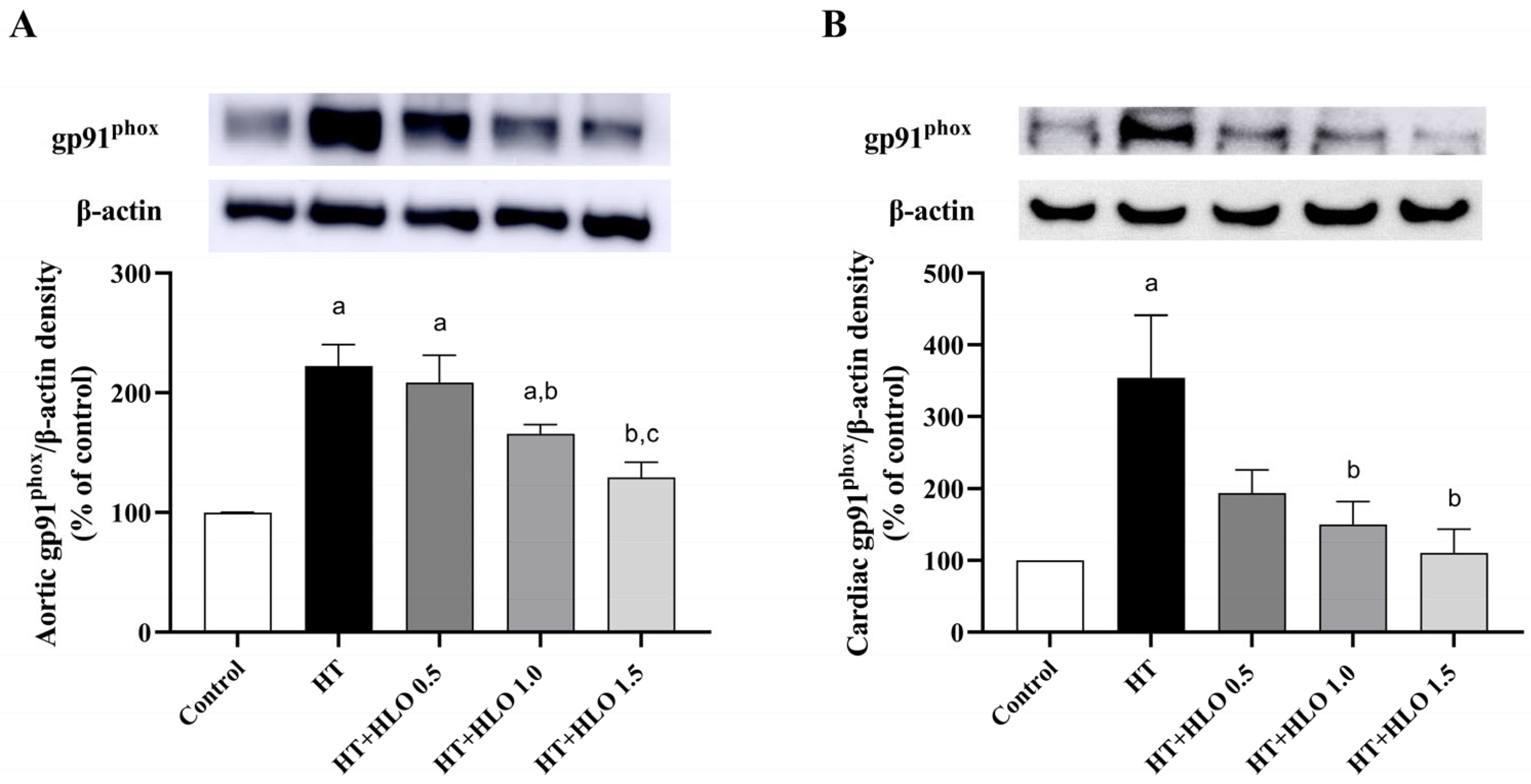
2.10. HLO Improves NADPH Oxidase Expression in Both Aortic and Cardiac Tissues of Hypertensive Rats
2.11. HLO Improves NO Level and Oxidative Status in Hypertensive Rats
3. Discussion
4. Materials and Methods
4.1. HLO Preparation
4.2. Animals and Experimental Protocols
4.3. Indirect Blood Pressure Measurement in Conscious Rats
4.4. Cardiac Function Assessment
4.5. Blood Pressure and Heart Rate Measurement in Unconscious Rats
4.6. Vascular Function Study
4.7. Histological Study of Aorta and Heart
4.8. RAS Measurement
4.8.1. Assay of Angiotensin-Converting Enzyme (ACE) Activity
4.8.2. Assay of Plasma Ang II Level
4.9. Protein Expression Measurement
4.10. Plasma Nitric Oxide Metabolites (NOx) Measurement
4.11. Oxidative Stress Marker Measurement
4.11.1. Superoxide Production Measurement
4.11.2. Malondialdehyde Level Measurement
4.11.3. Catalase Activity Measurement
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ross, S.; EISohly, M. Constituents of Cannabis sativa L. XXVIII A review of the natural constituents: 1980–1994. Zagazig J. Pharm. Sci. 1995, 4, 150–160. [Google Scholar] [CrossRef]
- Pojić, M.; Dapčević Hadnađev, T.; Hadnađev, M.; Rakita, S.; Brlek, T. Bread supplementation with hemp seed cake: A by-product of hemp oil processing. J. Food Qual. 2015, 38, 431–440. [Google Scholar] [CrossRef]
- Hrušková, M.; Švec, I. Cookie making potential of composite flour containing wheat, barley and hemp. Czech J. Food Sci. 2015, 33, 545–555. [Google Scholar] [CrossRef]
- Di Sotto, A.; Gullì, M.; Acquaviva, A.; Tacchini, M.; Di Simone, S.C.; Chiavaroli, A.; Recinella, L.; Leone, S.; Brunetti, L.; Orlando, G.; et al. Phytochemical and pharmacological profiles of the essential oil from the inflorescences of the Cannabis sativa L. Ind. Crops Prod. 2022, 183, 114980. [Google Scholar] [CrossRef]
- Shin, J.; Choi, S.; Park, A.Y.; Ju, S.; Kweon, B.; Kim, D.-U.; Bae, G.-S.; Han, D.; Kwon, E.; Hong, J.; et al. In Vitro and In Vivo Anti-Inflammatory and Antidepressant-like Effects of Cannabis sativa L. Extracts. Plants 2024, 13, 1619. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Matsabisa, M.G.; Salau, V.F.; Islam, M.S. Tetrahydrocannabinol-Rich Extracts From Cannabis sativa L. Improve Glucose Consumption and Modulate Metabolic Complications Linked to Neurodegenerative Diseases in Isolated Rat Brains. Front. Pharmacol. 2020, 11, 592981. [Google Scholar] [CrossRef]
- Akarsu, G.D.; Akarsu, R.H. Therapeutic potential of cannabis for surgical wound healing in rats. Vet. Med. 2024, 69, 297–306. [Google Scholar] [CrossRef]
- Nagy, D.U.; Cianfaglione, K.; Maggi, F.; Sut, S.; Dall’Acqua, S. Chemical Characterization of Leaves, Male and Female Flowers from Spontaneous Cannabis (Cannabis sativa L.) Growing in Hungary. Chem. Biodivers. 2019, 16, e1800562. [Google Scholar] [CrossRef]
- Vega, G.A.; Dávila, J.A. Use of non-psychoactive residual biomass from Cannabis sativa L. for obtaining phenolic rich-extracts with antioxidant capacity. Nat. Prod. Res. 2022, 36, 4193–4199. [Google Scholar] [CrossRef]
- Bryan, N.S. Nitric oxide deficiency is a primary driver of hypertension. Biochem. Pharmacol. 2022, 206, 115325. [Google Scholar] [CrossRef]
- Ribeiro, M.O.; Antunes, E.; de Nucci, G.; Lovisolo, S.M.; Zatz, R. Chronic inhibition of nitric oxide synthesis. A new model of arterial hypertension. Hypertension 1992, 20, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.H.; Kim, H.Y.; Jang, Y.J.; Na, S.W.; Han, B.H.; Yoon, J.J.; Seo, C.S.; Lee, H.S.; Lee, Y.J.; Kang, D.G. New Therapeutic Insight into the Effect of Ma Huang Tang on Blood Pressure and Renal Dysfunction in the L-NAME-Induced Hypertension. Evid. Based Complement. Altern. Med. Ecam 2021, 2021, 9980429. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.Y.; Qian, L.B.; Zhu, L.G.; Liang, H.T.; Tan, Y.N.; Lu, H.T.; Lu, J.F.; Wang, H.P.; Xia, Q. Betulinic acid ameliorates endothelium-dependent relaxation in L-NAME-induced hypertensive rats by reducing oxidative stress. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2011, 44, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Belemnaba, L.; Nitiéma, M.; Ilboudo, S.; Ouédraogo, G.G.; Ouédraogo, N.; Belemlilga, M.B.; Compaoré, S.; Ouédraogo, S.; Ouédraogo, S. Preclinical Evaluation of the Antihypertensive Effect of an Aqueous Extract of Anogeissus leiocarpa (DC) Guill et Perr. Bark of Trunk in L-NAME-Induced Hypertensive Rat. J. Exp. Pharmacol. 2021, 13, 739–754. [Google Scholar] [CrossRef]
- García-Pedraza, J.; García-Domingo, M.; Gómez-Roso, M.; Ruiz-Remolina, L.; Rodríguez-Barbero, A.; Martín, M.L.; Morán, A. Hypertension exhibits 5-HT(4) receptor as a modulator of sympathetic neurotransmission in the rat mesenteric vasculature. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2019, 42, 618–627. [Google Scholar] [CrossRef]
- Potue, P.; Wunpathe, C.; Maneesai, P.; Kukongviriyapan, U.; Prachaney, P.; Pakdeechote, P. Nobiletin alleviates vascular alterations through modulation of Nrf-2/HO-1 and MMP pathways in l-NAME induced hypertensive rats. Food Funct. 2019, 10, 1880–1892. [Google Scholar] [CrossRef]
- Stanko, P.; Repova, K.; Baka, T.; Krajcirovicova, K.; Aziriova, S.; Barta, A.; Zorad, S.; Adamcova, M.; Simko, F. Sacubitril/Valsartan Alleviates Cardiac Remodeling and Dysfunction in L-NAME-Induced Hypertension and Hypertensive Heart Disease. Biomedicines 2024, 12, 733. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Z.; Zhang, Y.; Liu, Y.; Wang, S.; Sun, W.; Guo, J.; Yu, C.; Wang, Y.; Kong, W.; et al. Naringenin inhibits N(G)-nitro-L-arginine methyl ester-induced hypertensive left ventricular hypertrophy by decreasing angiotensin-converting enzyme 1 expression. Exp. Ther. Med. 2018, 16, 867–873. [Google Scholar] [CrossRef]
- Kanthlal, S.K.; Joseph, J.; Paul, B.; M, V.; P, U.D. Antioxidant and vasorelaxant effects of aqueous extract of large cardamom in L-NAME induced hypertensive rats. Clin. Exp. Hypertens. 2020, 42, 581–589. [Google Scholar] [CrossRef]
- Boe, A.E.; Eren, M.; Murphy, S.B.; Kamide, C.E.; Ichimura, A.; Terry, D.; McAnally, D.; Smith, L.H.; Miyata, T.; Vaughan, D.E. Plasminogen activator inhibitor-1 antagonist TM5441 attenuates Nω-nitro-L-arginine methyl ester-induced hypertension and vascular senescence. Circulation 2013, 128, 2318–2324. [Google Scholar] [CrossRef]
- Lindpaintner, K.; Ganten, D. The cardiac renin-angiotensin system. An appraisal of present experimental and clinical evidence. Circ. Res. 1991, 68, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Wilhelm, M.J.; Lang, R.E.; Unger, T.; Lindpaintner, K.; Ganten, D. Endogenous tissue renin-angiotensin systems: From molecular biology to therapy. Am. J. Med. 1988, 84, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.V.; Cicha, M.Z.; Nunez, S.; Meyerholz, D.K.; Chapleau, M.W.; Abboud, F.M. Angiotensin II-induced hypertension and cardiac hypertrophy are differentially mediated by TLR3-and TLR4-dependent pathways. Am. J. Physiol.-Heart Circ. Physiol. 2019, 316, H1027–H1038. [Google Scholar] [CrossRef]
- Das, D.K.; Maulik, N.; Engelman, R.M. Redox regulation of angiotensin II signaling in the heart. J. Cell. Mol. Med. 2004, 8, 144–152. [Google Scholar] [CrossRef]
- Rey, F.E.; Cifuentes, M.E.; Kiarash, A.; Quinn, M.T.; Pagano, P.J. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ. Res. 2001, 89, 408–414. [Google Scholar] [CrossRef]
- On-Nom, N.; Khaengamkham, K.; Kettawan, A.; Rungruang, T.; Suttisansanee, U.; Temviriyanukul, P.; Prangthip, P.; Chupeerach, C. Parboiled Germinated Brown Rice Improves Cardiac Structure and Gene Expression in Hypertensive Rats. Foods 2022, 12, 9. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, F.; Liu, Y.; Yin, S.; Pang, X.; Li, Z.; Wei, Z. Nebivolol alleviates aortic remodeling through eNOS upregulation and inhibition of oxidative stress in l-NAME-induced hypertensive rats. Clin. Exp. Hypertens. 2017, 39, 628–639. [Google Scholar] [CrossRef]
- Pechanova, O.; Matuskova, J.; Capikova, D.; Jendekova, L.; Paulis, L.; Simko, F. Effect of spironolactone and captopril on nitric oxide and S-nitrosothiol formation in kidney of L-NAME-treated rats. Kidney Int. 2006, 70, 170–176. [Google Scholar] [CrossRef]
- Holécyová, A.; Török, J.; Bernátová, I.; Pechánová, O. Restriction of nitric oxide rather than elevated blood pressure is responsible for alterations of vascular responses in nitric oxide-deficient hypertension. Physiol. Res. 1996, 45, 317–321. [Google Scholar]
- Moncada, S. Nitric oxide gas: Mediator, modulator, and pathophysiologic entity. J. Lab. Clin. Med. 1992, 120, 187–191. [Google Scholar]
- Gardiner, S.M.; Compton, A.M.; Bennett, T.; Palmer, R.M.; Moncada, S. Regional haemodynamic changes during oral ingestion of NG-monomethyl-L-arginine or NG-nitro-L-arginine methyl ester in conscious Brattleboro rats. Br. J. Pharmacol. 1990, 101, 10–12. [Google Scholar] [CrossRef]
- Rincón, J.; Correia, D.; Arcaya, J.L.; Finol, E.; Fernández, A.; Pérez, M.; Yaguas, K.; Talavera, E.; Chávez, M.; Summer, R.; et al. Role of Angiotensin II type 1 receptor on renal NAD(P)H oxidase, oxidative stress and inflammation in nitric oxide inhibition induced-hypertension. Life Sci. 2015, 124, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Mattson, D.L.; Meister, C.J. Renal cortical and medullary blood flow responses to L-NAME and ANG II in wild-type, nNOS null mutant, and eNOS null mutant mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R991–R997. [Google Scholar] [CrossRef]
- Girgih, A.T.; Alashi, A.; He, R.; Malomo, S.; Aluko, R.E. Preventive and treatment effects of a hemp seed (Cannabis sativa L.) meal protein hydrolysate against high blood pressure in spontaneously hypertensive rats. Eur. J. Nutr. 2014, 53, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Kubiliene, A.; Mickute, K.; Baranauskaite, J.; Marksa, M.; Liekis, A.; Sadauskiene, I. The Effects of Cannabis sativa L. Extract on Oxidative Stress Markers In Vivo. Life 2021, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Samak, M.; Fatullayev, J.; Sabashnikov, A.; Zeriouh, M.; Schmack, B.; Farag, M.; Popov, A.F.; Dohmen, P.M.; Choi, Y.H.; Wahlers, T.; et al. Cardiac Hypertrophy: An Introduction to Molecular and Cellular Basis. Med. Sci. Monit. Basic. Res. 2016, 22, 75–79. [Google Scholar] [CrossRef]
- Ma, E.; Wu, C.; Chen, J.; Wo, D.; Ren, D.N.; Yan, H.; Peng, L.; Zhu, W. Resveratrol prevents Ang II-induced cardiac hypertrophy by inhibition of NF-κB signaling. Biomed. Pharmacother. Biomed. Pharmacother. 2023, 165, 115275. [Google Scholar] [CrossRef]
- Zhang, Y.; Murugesan, P.; Huang, K.; Cai, H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2020, 17, 170–194. [Google Scholar] [CrossRef]
- Pakdeechote, P.; Rummery, N.M.; Ralevic, V.; Dunn, W.R. Raised tone reveals purinergic-mediated responses to sympathetic nerve stimulation in the rat perfused mesenteric vascular bed. Eur. J. Pharmacol. 2007, 563, 180–186. [Google Scholar] [CrossRef]
- Prasatthong, P.; Meephat, S.; Rattanakanokchai, S.; Bunbupha, S.; Prachaney, P.; Maneesai, P.; Pakdeechote, P. Hesperidin ameliorates signs of the metabolic syndrome and cardiac dysfunction via IRS/Akt/GLUT4 signaling pathway in a rat model of diet-induced metabolic syndrome. Eur. J. Nutr. 2021, 60, 833–848. [Google Scholar] [CrossRef]
- Poasakate, A.; Maneesai, P.; Potue, P.; Bunbupha, S.; Tong-Un, T.; Settheetham-Ishida, W.; Khamseekaew, J.; Pakdeechote, P. Genistein alleviates renin-angiotensin system mediated vascular and kidney alterations in renovascular hypertensive rats. Biomed. Pharmacother. 2022, 146, 112601. [Google Scholar] [CrossRef] [PubMed]
- Boonla, O.; Kukongviriyapan, U.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P.; Prachaney, P.; Greenwald, S.E. Curcumin improves endothelial dysfunction and vascular remodeling in 2K-1C hypertensive rats by raising nitric oxide availability and reducing oxidative stress. Nitric Oxide 2014, 42, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Bunbupha, S.; Pakdeechote, P.; Kukongviriyapan, U.; Prachaney, P.; Kukongviriyapan, V. Asiatic acid reduces blood pressure by enhancing nitric oxide bioavailability with modulation of eNOS and p47phox expression in L-NAME-induced hypertensive rats. Phytother. Res. 2014, 28, 1506–1512. [Google Scholar] [CrossRef]
- Lu, F.J.; Lin, J.T.; Wang, H.P.; Huang, W.C. A simple, sensitive, non-stimulated photon counting system for detection of superoxide anion in whole blood. Experientia 1996, 52, 141–144. [Google Scholar] [CrossRef]
- Nakmareong, S.; Kukongviriyapan, U.; Pakdeechote, P.; Donpunha, W.; Kukongviriyapan, V.; Kongyingyoes, B.; Sompamit, K.; Phisalaphong, C. Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with L-NAME-induced hypertension. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 519–529. [Google Scholar] [CrossRef]
- Poasakate, A.; Maneesai, P.; Rattanakanokchai, S.; Bunbupha, S.; Tong-Un, T.; Pakdeechote, P. Genistein Prevents Nitric Oxide Deficiency-Induced Cardiac Dysfunction and Remodeling in Rats. Antioxidants 2021, 10, 237. [Google Scholar] [CrossRef]
- Ming, M.; Guanhua, L.; Zhanhai, Y.; Guang, C.; Xuan, Z. Effect of the Lycium barbarum polysaccharides administration on blood lipid metabolism and oxidative stress of mice fed high-fat diet in vivo. Food Chem. 2009, 113, 872–877. [Google Scholar] [CrossRef]
- Ozmen, B.; Ozmen, D.; Erkin, E.; Güner, I.; Habif, S.; Bayindir, O. Lens superoxide dismutase and catalase activities in diabetic cataract. Clin. Biochem. 2002, 35, 69–72. [Google Scholar] [CrossRef]
- Góth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta Int. J. Clin. Chem. 1991, 196, 143–151. [Google Scholar] [CrossRef]

| Parameters | Concentration |
|---|---|
| Total phenolic compounds (μg * QE/mL) | 26.02 ± 1.14 |
| Total flavonoid contents (μg * QE/mL) | 2.10 ± 0.48 |
| Quercetin (mg/100 mL) | 23.07 ± 0.45 |
| Parameter | Control | HT | HT + HLO 0.5 | HT + HLO 1.0 | HT + HLO 1.5 |
|---|---|---|---|---|---|
| SBP (mmHg) | 115.89 ± 2.50 | 181.89 ± 4.57 a | 158.02 ± 6.38 a,b | 137.20 ± 5.09 b,c | 134.18 ± 5.90 b,c |
| DBP (mmHg) | 70.58 ± 2.76 | 128.18 ± 2.37 a | 107.25 ± 7.23 a,b | 89.14 ± 8.24 a,b | 90.20 ± 5.44 b |
| MAP (mmHg) | 85.68 ± 2.37 | 146.08 ± 2.66 a | 124.17 ± 6.69 a,b | 105.16 ± 6.46 a,b | 104.86 ± 5.54 b,c |
| PP (mmHg) | 45.32 ± 2.62 | 53.71 ± 4.06 | 50.77 ± 4.08 | 48.07 ± 7.40 | 43.97 ± 1.73 |
| HR (beat/min) | 354.70 ± 11.88 | 373.65 ± 18.38 | 390.52 ± 8.89 | 379.40 ± 14.14 | 372.77 ± 12.33 |
| Parameter | Control | HT | HT + HLO 0.5 | HT + HLO 1.0 | HT + HLO 1.5 |
|---|---|---|---|---|---|
| BW (g) | 474.00 ± 11.19 | 445.12 ± 6.77 | 486.25 ± 11.15 | 486.5 ± 10.49 | 478.00 ± 6.49 |
| HW (g) | 1.38 ± 0.04 | 1.54 ± 0.04 | 1.59 ± 0.10 | 1.53 ± 0.07 | 1.62 ± 0.03 a |
| HW/BW (mg/g) | 2.92 ± 0.13 | 3.47 ± 0.11 a | 3.25 ± 0.15 | 3.15 ± 0.17 | 3.40 ± 0.03 |
| LVW (g) | 0.85 ± 0.02 | 1.02 ± 0.04 a | 0.95 ± 0.03 | 0.95 ± 0.03 | 0.95 ± 0.01 |
| LVW/BW (mg/g) | 1.80 ± 0.03 | 2.29 ± 0.10 a | 1.95 ± 0.03 b | 1.94 ± 0.05 b | 1.98 ± 0.03 b |
| Parameters | Control | HT | HT + HLO 0.5 | HT + HLO 1.0 | HT + HLO 1.5 |
|---|---|---|---|---|---|
| LVPWd (mm) | 1.79 ± 0.06 | 2.13 ± 0.13 a | 1.92 ± 0.07 | 1.75 ± 0.05 b | 1.76 ± 0.04 b |
| LVPWs (mm) | 2.66 ± 0.10 | 2.85 ± 0.14 | 2.73 ± 0.12 | 2.64 ± 0.10 | 2.74 ± 0.12 |
| LVIDd (mm) | 7.39 ± 0.13 | 5.96 ± 0.40 a | 6.73 ± 0.16 | 6.73 ± 0.16 | 6.83 ± 0.10 |
| LVIDs (mm) | 4.13 ± 0.16 | 3.94 ± 0.26 | 4.00 ± 0.19 | 3.64 ± 0.20 | 3.7 ± 0.21 |
| IVSd (mm) | 1.66 ± 0.04 | 1.93 ± 0.10 a | 1.90 ± 0.06 | 1.72 ± 0.04 | 1.76 ± 0.04 |
| IVSs (mm) | 2.55 ± 0.18 | 2.79 ± 0.09 | 2.74 ± 0.12 | 2.57 ± 0.12 | 2.84 ± 0.13 |
| EDV (mL) | 0.9 ± 0.04 | 0.53 ± 0.09 a | 0.70 ± 0.04 | 0.70 ± 0.05 | 0.73 ± 0.03 |
| ESV (mL) | 0.17 ± 0.02 | 0.16 ± 0.03 | 0.17 ± 0.02 | 0.13 ± 0.02 | 0.13 ± 0.02 |
| SV (mL) | 0.73 ± 0.04 | 0.36 ± 0.06 a | 0.53 ± 0.04 a | 0.58 ± 0.05 b | 0.59 ± 0.02 b |
| EF (%) | 80.74 ± 1.89 | 67.6 ± 3.14 a | 75.83 ± 2.98 | 81.5 ± 2.50 b | 81.67 ± 2.20 b |
| FS (%) | 44.63 ± 1.92 | 33.37 ± 2.39 a | 40.32 ± 2.56 | 46.02 ± 2.96 b | 45.96 ± 2.58 b |
| Parameter | Control | HT | HT + HLO 0.5 | HT + HLO 1.0 | HT + HLO 1.5 |
|---|---|---|---|---|---|
| Plasma NOx (µM) | 92.96 ± 11.79 | 56.51 ± 8.27 a | 67.90 ± 7.70 | 89.92 ± 16.95 b | 87.73 ± 9.18 b |
| Superoxide production in aorta (Count/mg dry wt/min) | 76.08 ± 5.99 | 160.46 ± 26.03 a | 110.93 ± 20.41a | 80.76 ± 7.68 b | 60.14 ± 9.84 b |
| Superoxide production in cardiac tissues (Count/mg dry wt/min) | 60.69 ± 12.33 | 150.98 ± 41.01a | 118.78 ± 11.34 | 67.92 ± 15.92 b | 63.31 ± 12.93 b |
| Plasma MDA (µM) | 7.08 ± 0.69 | 25.65 ± 6.52 a | 20.36 ± 6.01 | 8.89 ± 1.59 b | 9.88 ± 1.52 b |
| Cardiac tissues MDA (µmol/g protein) | 3.98 ± 1.46 | 19.03 ± 2.58 a | 16.27 ± 2.55 a | 4.16 ± 1.15 b,c | 3.07 ± 0.87 b,c |
| Serum catalase (kU/L) | 270.92 ± 14.01 | 148.97 ± 20.82 a | 202.54 ± 11.02 a | 243.78 ± 7.78 b | 250.60 ± 20.39 b |
| Cardiac tissues catalase (U/g protein) | 15.88 ± 0.95 | 8.03 ± 1.46 a | 9.39 ± 0.77 | 16.73 ± 2.33 b,c | 17.61 ± 2.13 b,c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamseekaew, J.; Duangjinda, M.; Maneesai, P.; Labjit, C.; Rattanakanokchai, S.; Rongpan, S.; Pakdeechote, P.; Potue, P. Cannabis sativa L. Leaf Oil Displays Cardiovascular Protective Effects in Hypertensive Rats. Int. J. Mol. Sci. 2025, 26, 1897. https://doi.org/10.3390/ijms26051897
Khamseekaew J, Duangjinda M, Maneesai P, Labjit C, Rattanakanokchai S, Rongpan S, Pakdeechote P, Potue P. Cannabis sativa L. Leaf Oil Displays Cardiovascular Protective Effects in Hypertensive Rats. International Journal of Molecular Sciences. 2025; 26(5):1897. https://doi.org/10.3390/ijms26051897
Chicago/Turabian StyleKhamseekaew, Juthamas, Monchai Duangjinda, Putcharawipa Maneesai, Chanon Labjit, Siwayu Rattanakanokchai, Sudarat Rongpan, Poungrat Pakdeechote, and Prapassorn Potue. 2025. "Cannabis sativa L. Leaf Oil Displays Cardiovascular Protective Effects in Hypertensive Rats" International Journal of Molecular Sciences 26, no. 5: 1897. https://doi.org/10.3390/ijms26051897
APA StyleKhamseekaew, J., Duangjinda, M., Maneesai, P., Labjit, C., Rattanakanokchai, S., Rongpan, S., Pakdeechote, P., & Potue, P. (2025). Cannabis sativa L. Leaf Oil Displays Cardiovascular Protective Effects in Hypertensive Rats. International Journal of Molecular Sciences, 26(5), 1897. https://doi.org/10.3390/ijms26051897







