The Triad of Blood–Brain Barrier Integrity: Endothelial Cells, Astrocytes, and Pericytes in Perinatal Stroke Pathophysiology
Abstract
1. Introduction
2. Pediatric Stroke
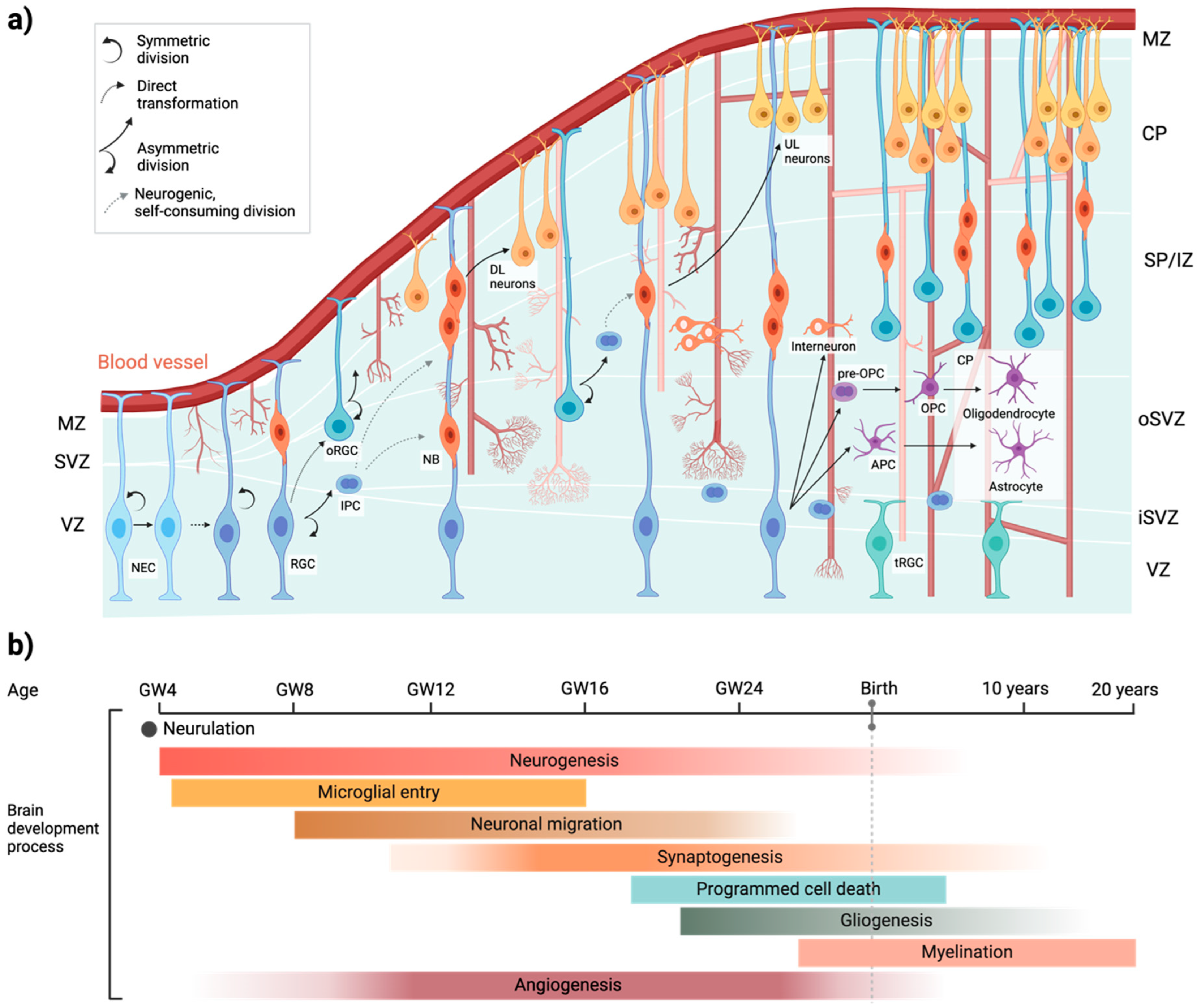
3. Human Brain Development
4. Human Vascular Development
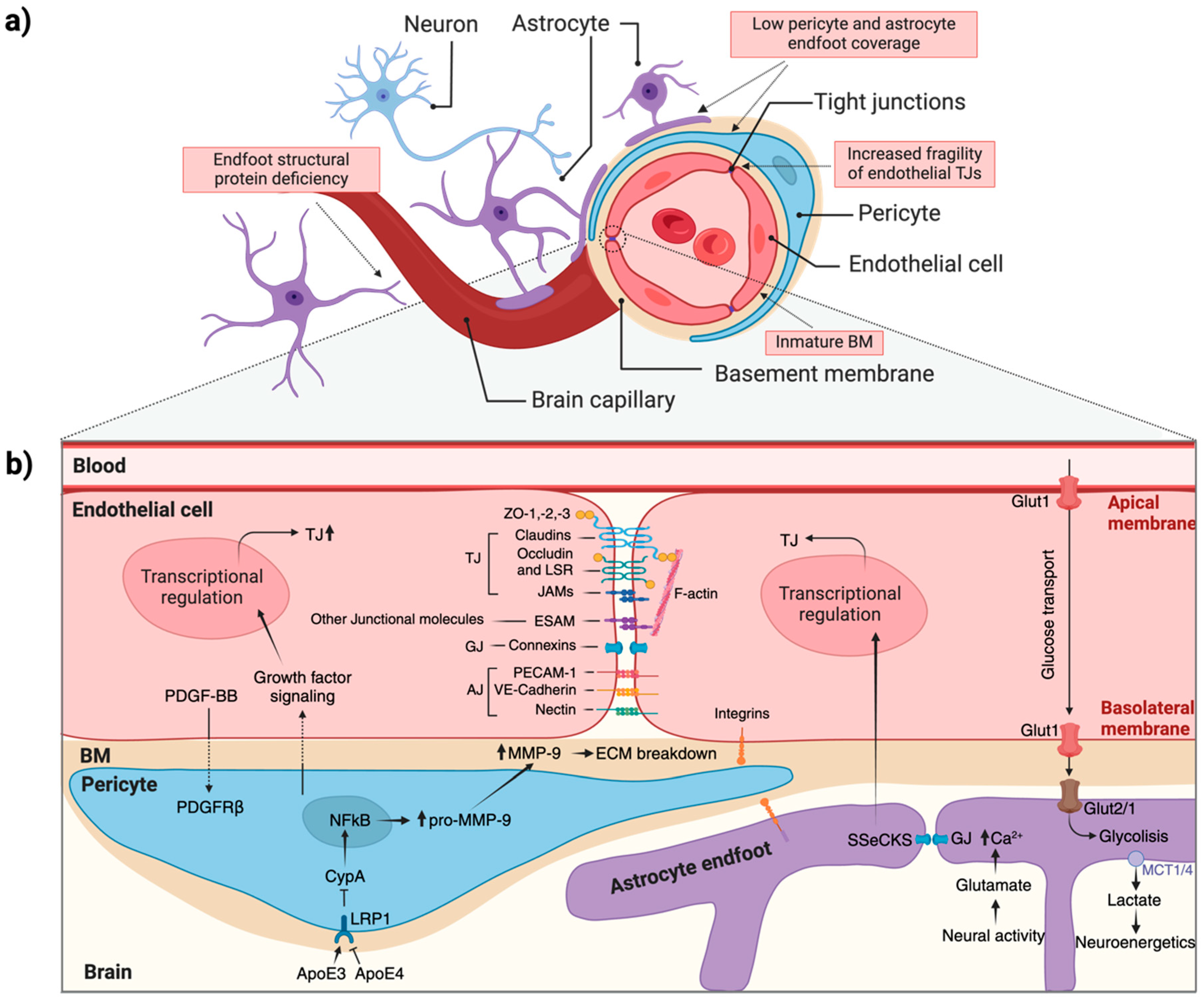
5. Neurovascular Unit Cell–Cell Interaction
6. Astrocyte, Pericytes, and ECs Interactions
7. Mechanisms of Brain Damage After a Perinatal Stroke
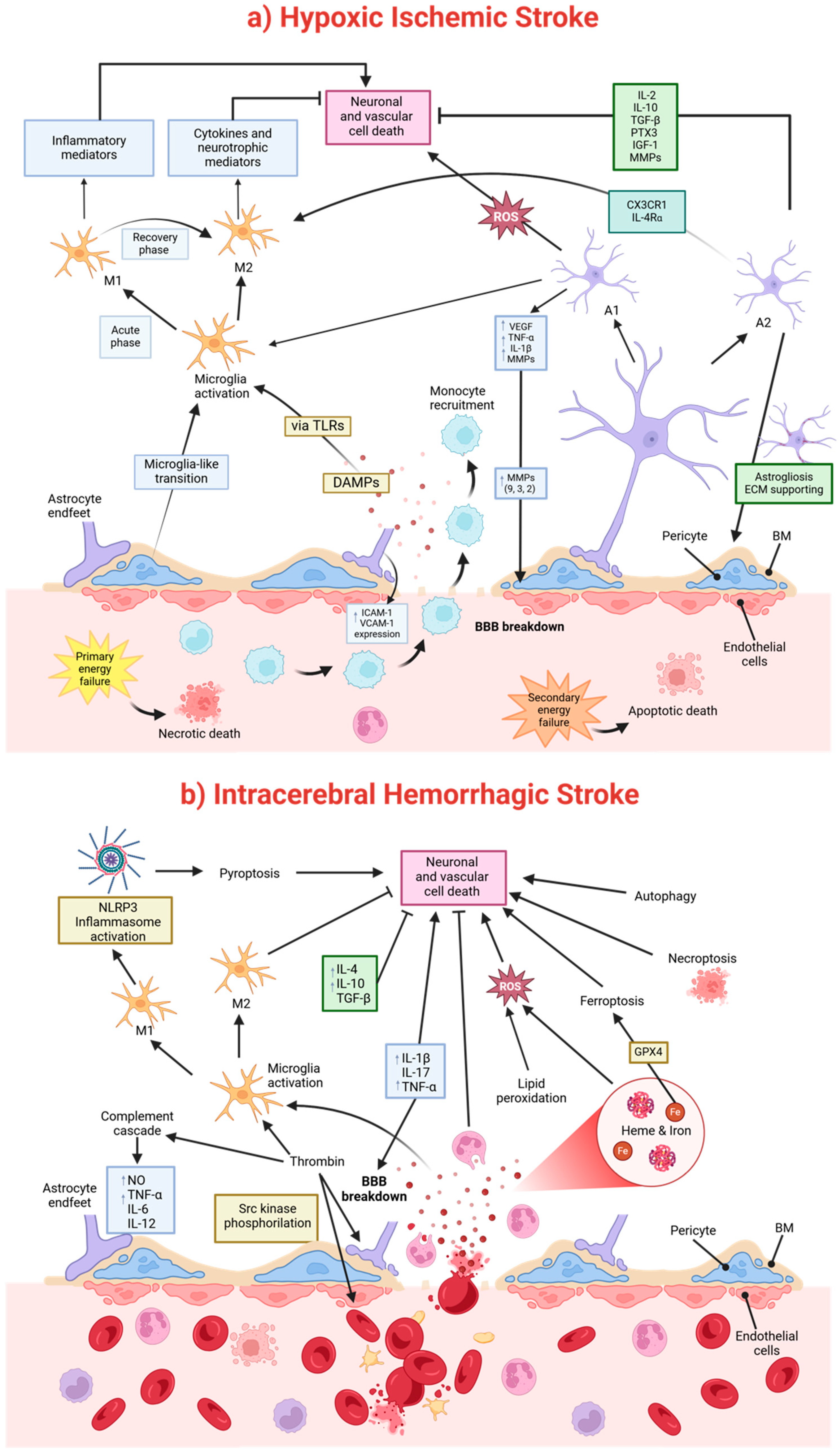
7.1. Pathophysiological Mechanisms of a Hypoxic–Ischemic Injury
7.2. Pathophysiological Mechanisms of Brain Damage After Intracerebral Hemorrhage (ICH)
8. Emerging Cell-Based Therapeutic Strategies for Pediatric Stroke
9. NVU and Monogenic Neurological Disorders
9.1. Pediatric Stroke Caused by Mutations in Genes Expressed in ECs
9.2. Pediatric Stroke Caused by Mutations in Genes Expressed in Vascular Mural Cells and Pericytes
10. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, Y.; Song, H.; Ming, G.L. Genetics of human brain development. Nat. Rev. Genet. 2024, 25, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Doi, M.; Usui, N.; Shimada, S. Prenatal Environment and Neurodevelopmental Disorders. Front. Endocrinol. 2022, 13, 860110. [Google Scholar] [CrossRef]
- Quinn, J.A.; Munoz, F.M.; Gonik, B.; Frau, L.; Cutland, C.; Mallett-Moore, T.; Kissou, A.; Wittke, F.; Das, M.; Nunes, T.; et al. Preterm birth: Case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine 2016, 34, 6047–6056. [Google Scholar] [CrossRef]
- Purisch, S.E.; Gyamfi-Bannerman, C. Epidemiology of preterm birth. Semin. Perinatol. 2017, 41, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, E.; Rayasam, A.; Vexler, Z.S. Brain Maturation as a Fundamental Factor in Immune-Neurovascular Interactions in Stroke. Transl. Stroke Res. 2024, 15, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.B. Perinatal ischemic stroke. Stroke 2007, 38, 742–745. [Google Scholar] [CrossRef]
- Hill, N.M.; Malone, L.A.; Sun, L.R. Stroke in the Developing Brain: Neurophysiologic Implications of Stroke Timing, Location, and Comorbid Factors. Pediatr. Neurol. 2023, 148, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ferriero, D.M.; Fullerton, H.J.; Bernard, T.J.; Billinghurst, L.; Daniels, S.R.; DeBaun, M.R.; deVeber, G.; Ichord, R.N.; Jordan, L.C.; Massicotte, P.; et al. Management of Stroke in Neonates and Children: A Scientific Statement From the American Heart Association/American Stroke Association. Stroke 2019, 50, e51–e96. [Google Scholar] [CrossRef]
- Jankovic, M.; Petrovic, B.; Novakovic, I.; Brankovic, S.; Radosavljevic, N.; Nikolic, D. The Genetic Basis of Strokes in Pediatric Populations and Insight into New Therapeutic Options. Int. J. Mol. Sci. 2022, 23, 1601. [Google Scholar] [CrossRef]
- Srivastava, R.; Mailo, J.; Dunbar, M. Perinatal Stroke in Fetuses, Preterm and Term Infants. Semin. Pediatr. Neurol. 2022, 43, 100988. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, M.; Kirton, A. Perinatal stroke: Mechanisms, management, and outcomes of early cerebrovascular brain injury. Lancet Child Adolesc. Health 2018, 2, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Lin, J.J.; Lin, K.L.; Lim, W.H.; Hsu, K.H.; Hsu, J.F.; Fu, R.H.; Chiang, M.C.; Chu, S.M.; Lien, R. Clinical Manifestations, Outcomes, and Etiologies of Perinatal Stroke in Taiwan: Comparisons between Ischemic, and Hemorrhagic Stroke Based on 10-year Experience in A Single Institute. Pediatr. Neonatol. 2017, 58, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Raju, T.N.; Nelson, K.B.; Ferriero, D.; Lynch, J.K. Ischemic perinatal stroke: Summary of a workshop sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke. Pediatrics 2007, 120, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Govaert, P.; Ramenghi, L.; Taal, R.; de Vries, L.; Deveber, G. Diagnosis of perinatal stroke I: Definitions, differential diagnosis and registration. Acta Paediatr. 2009, 98, 1556–1567. [Google Scholar] [CrossRef] [PubMed]
- Bogousslavsky, J.; Regli, F.; Uské, A.; Maeder, P. Early spontaneous hematoma in cerebral infarct: Is primary cerebral hemorrhage overdiagnosed? Neurology 1991, 41, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.M.; Ly, J.V.; Srikanth, V.; Ma, H.; Chong, W.; Holt, M.; Phan, T.G. Differentiating between Hemorrhagic Infarct and Parenchymal Intracerebral Hemorrhage. Radiol. Res. Pract. 2012, 2012, 475497. [Google Scholar] [CrossRef]
- Fullerton, H.J.; Wu, Y.W.; Zhao, S.; Johnston, S.C. Risk of stroke in children: Ethnic and gender disparities. Neurology 2003, 61, 189–194. [Google Scholar] [CrossRef]
- Mallick, A.A.; Ganesan, V.; Kirkham, F.J.; Fallon, P.; Hedderly, T.; McShane, T.; Parker, A.P.; Wassmer, E.; Wraige, E.; Amin, S.; et al. Childhood arterial ischaemic stroke incidence, presenting features, and risk factors: A prospective population-based study. Lancet Neurol. 2014, 13, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Malone, L.A.; Felling, R.J. Pediatric Stroke: Unique Implications of the Immature Brain on Injury and Recovery. Pediatr. Neurol. 2020, 102, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.K.; Hirtz, D.G.; DeVeber, G.; Nelson, K.B. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics 2002, 109, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Budday, S.; Steinmann, P.; Kuhl, E. Physical biology of human brain development. Front. Cell. Neurosci. 2015, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Degl’Innocenti, E.; Dell’Anno, M.T. Human and mouse cortical astrocytes: A comparative view from development to morphological and functional characterization. Front. Neuroanat. 2023, 17, 1130729. [Google Scholar] [CrossRef] [PubMed]
- O’Rahilly, R.; Müller, F. The Embryonic Human Brain: An Atlas of Developmental Stages, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 1–358. [Google Scholar] [CrossRef]
- Stiles, J.; Jernigan, T.L. The basics of brain development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Baggiani, M.; Dell’Anno, M.T.; Pistello, M.; Conti, L.; Onorati, M. Human Neural Stem Cell Systems to Explore Pathogen-Related Neurodevelopmental and Neurodegenerative Disorders. Cells 2020, 9, 1893. [Google Scholar] [CrossRef]
- Gao, P.; Sultan, K.T.; Zhang, X.J.; Shi, S.H. Lineage-dependent circuit assembly in the neocortex. Development 2013, 140, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
- Tau, G.Z.; Peterson, B.S. Normal development of brain circuits. Neuropsychopharmacology 2010, 35, 147–168. [Google Scholar] [CrossRef]
- Lanzone, A.; Ferrazzani, S.; Botta, A. Delivery and late preterm birth. Ital. J. Pediatr. 2014, 40, A1. [Google Scholar] [CrossRef]
- Molliver, M.E.; Kostović, I.; van der Loos, H. The development of synapses in cerebral cortex of the human fetus. Brain Res. 1973, 50, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Riccomagno, M.M.; Kolodkin, A.L. Sculpting neural circuits by axon and dendrite pruning. Annu. Rev. Cell Dev. Biol. 2015, 31, 779–805. [Google Scholar] [CrossRef] [PubMed]
- Jithoo, A.; Penny, T.R.; Pham, Y.; Sutherland, A.E.; Smith, M.J.; Petraki, M.; Fahey, M.C.; Jenkin, G.; Malhotra, A.; Miller, S.L.; et al. The Temporal Relationship between Blood-Brain Barrier Integrity and Microglial Response following Neonatal Hypoxia Ischemia. Cells 2024, 13, 660. [Google Scholar] [CrossRef] [PubMed]
- Kierdorf, K.; Erny, D.; Goldmann, T.; Sander, V.; Schulz, C.; Perdiguero, E.G.; Wieghofer, P.; Heinrich, A.; Riemke, P.; Hölscher, C.; et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 2013, 16, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Roessmann, U.; Gambetti, P. Astrocytes in the developing human brain. An immunohistochemical study. Acta Neuropathol. 1986, 70, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.S.; Zdunek, S.; Bergmann, O.; Bernard, S.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Brundin, L.; et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 2014, 159, 766–774. [Google Scholar] [CrossRef]
- Raybaud, C. Normal and abnormal embryology and development of the intracranial vascular system. Neurosurg. Clin. N. Am. 2010, 21, 399–426. [Google Scholar] [CrossRef]
- Ballabh, P.; Hu, F.; Kumarasiri, M.; Braun, A.; Nedergaard, M. Development of tight junction molecules in blood vessels of germinal matrix, cerebral cortex, and white matter. Pediatr. Res. 2005, 58, 791–798. [Google Scholar] [CrossRef]
- Liebner, S.; Czupalla, C.J.; Wolburg, H. Current concepts of blood-brain barrier development. Int. J. Dev. Biol. 2011, 55, 467–476. [Google Scholar] [CrossRef]
- Adams, R.H. Vascular patterning by Eph receptor tyrosine kinases and ephrins. Semin. Cell Dev. Biol. 2002, 13, 55–60. [Google Scholar] [CrossRef]
- Alvarez, J.I.; Dodelet-Devillers, A.; Kebir, H.; Ifergan, I.; Fabre, P.J.; Terouz, S.; Sabbagh, M.; Wosik, K.; Bourbonnière, L.; Bernard, M.; et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 2011, 334, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Agalliu, D.; Zhou, L.; Kuhnert, F.; Kuo, C.J.; Barres, B.A. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Liebner, S.; Corada, M.; Bangsow, T.; Babbage, J.; Taddei, A.; Czupalla, C.J.; Reis, M.; Felici, A.; Wolburg, H.; Fruttiger, M.; et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 2008, 183, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Shawber, C.J.; Kitajewski, J. Notch function in the vasculature: Insights from zebrafish, mouse and man. Bioessays 2004, 26, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.I.; Katayama, T.; Prat, A. Glial influence on the blood brain barrier. Glia 2013, 61, 1939–1958. [Google Scholar] [CrossRef]
- Lee, H.S.; Han, J.; Bai, H.J.; Kim, K.W. Brain angiogenesis in developmental and pathological processes: Regulation, molecular and cellular communication at the neurovascular interface. FEBS J. 2009, 276, 4622–4635. [Google Scholar] [CrossRef] [PubMed]
- Betz, C.; Lenard, A.; Belting, H.G.; Affolter, M. Cell behaviors and dynamics during angiogenesis. Development 2016, 143, 2249–2260. [Google Scholar] [CrossRef]
- Wacker, A.; Gerhardt, H. Endothelial development taking shape. Curr. Opin. Cell Biol. 2011, 23, 676–685. [Google Scholar] [CrossRef]
- Sauteur, L.; Affolter, M.; Belting, H.G. Distinct and redundant functions of Esama and VE-cadherin during vascular morphogenesis. Development 2017, 144, 1554–1565. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.H.; Miller, S.L.; Castillo-Melendez, M.; Malhotra, A. The Neurovascular Unit: Effects of Brain Insults During the Perinatal Period. Front. Neurosci. 2019, 13, 1452. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.A.; Chand, K.K.; Bell, A.; Miller, S.L.; Colditz, P.B.; Malhotra, A.; Wixey, J.A. Effects of fetal growth restriction on the perinatal neurovascular unit and possible treatment targets. Pediatr. Res. 2024, 95, 59–69. [Google Scholar] [CrossRef]
- Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004, 5, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, S.; Iadecola, C. Revisiting the neurovascular unit. Nat. Neurosci. 2021, 24, 1198–1209. [Google Scholar] [CrossRef]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Rizzuti, M.; Melzi, V.; Brambilla, L.; Quetti, L.; Sali, L.; Ottoboni, L.; Meneri, M.; Ratti, A.; Verde, F.; Ticozzi, N.; et al. Shaping the Neurovascular Unit Exploiting Human Brain Organoids. Mol. Neurobiol. 2024, 61, 6642–6657. [Google Scholar] [CrossRef] [PubMed]
- Manu, D.R.; Slevin, M.; Barcutean, L.; Forro, T.; Boghitoiu, T.; Balasa, R. Astrocyte Involvement in Blood-Brain Barrier Function: A Critical Update Highlighting Novel, Complex, Neurovascular Interactions. Int. J. Mol. Sci. 2023, 24, 17146. [Google Scholar] [CrossRef] [PubMed]
- Disdier, C.; Stonestreet, B.S. Hypoxic-ischemic-related cerebrovascular changes and potential therapeutic strategies in the neonatal brain. J. Neurosci. Res. 2020, 98, 1468–1484. [Google Scholar] [CrossRef]
- Langen, U.H.; Ayloo, S.; Gu, C. Development and Cell Biology of the Blood-Brain Barrier. Annu. Rev. Cell Dev. Biol. 2019, 35, 591–613. [Google Scholar] [CrossRef] [PubMed]
- Wolburg, H.; Lippoldt, A. Tight junctions of the blood–brain barrier: Development, composition and regulation. Vasc. Pharmacol. 2002, 38, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef]
- Nico, B.; Quondamatteo, F.; Herken, R.; Marzullo, A.; Corsi, P.; Bertossi, M.; Russo, G.; Ribatti, D.; Roncali, L. Developmental expression of ZO-1 antigen in the mouse blood-brain barrier. Dev. Brain Res. 1999, 114, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Zhou, L.; Kebede, A.A.; Barres, B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010, 468, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Shen, Q.; Goderie, S.K.; He, W.; Capela, A.; Davis, A.A.; Temple, S. Timing of CNS cell generation: A programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron 2000, 28, 69–80. [Google Scholar] [CrossRef]
- Dore-Duffy, P.; Cleary, K. Morphology and properties of pericytes. Methods Mol. Biol. 2011, 686, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Dalkara, T.; Gursoy-Ozdemir, Y.; Yemisci, M. Brain microvascular pericytes in health and disease. Acta Neuropathol. 2011, 122, 1–9. [Google Scholar] [CrossRef]
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/pericyte interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.S.; Aguera, K.N.; Cha, B.; Davis, G.E. Defining Endothelial Cell-Derived Factors That Promote Pericyte Recruitment and Capillary Network Assembly. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2632–2648. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, W.J.; Choi, Y.K.; Song, H.S.; Son, M.J.; Gelman, I.H.; Kim, Y.J.; Kim, K.W. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat. Med. 2003, 9, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.D.; Winkler, E.A.; Singh, I.; Sagare, A.P.; Deane, R.; Wu, Z.; Holtzman, D.M.; Betsholtz, C.; Armulik, A.; Sallstrom, J.; et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012, 485, 512–516. [Google Scholar] [CrossRef]
- Paz, A.A.; González-Candia, A. Potential pharmacological target of tight junctions to improve the BBB permeability in neonatal Hypoxic-Ischemic encephalopathy Diseases. Biochem. Pharmacol. 2023, 207, 115356. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, Z.L.; Norris, E.H.; Strickland, S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat. Commun. 2014, 5, 3413. [Google Scholar] [CrossRef] [PubMed]
- Saunders, N.R.; Dziegielewska, K.M.; Mollgard, K.; Habgood, M.D. Physiology and molecular biology of barrier mechanisms in the fetal and neonatal brain. J. Physiol. 2018, 596, 5723–5756. [Google Scholar] [CrossRef]
- Martinez-Biarge, M.; Ferriero, D.M.; Cowan, F.M. Perinatal arterial ischemic stroke. Handb. Clin. Neurol. 2019, 162, 239–266. [Google Scholar] [CrossRef]
- Li, B.; Concepcion, K.; Meng, X.; Zhang, L. Brain-immune interactions in perinatal hypoxic-ischemic brain injury. Prog. Neurobiol. 2017, 159, 50–68. [Google Scholar] [CrossRef] [PubMed]
- Greco, P.; Nencini, G.; Piva, I.; Scioscia, M.; Volta, C.A.; Spadaro, S.; Neri, M.; Bonaccorsi, G.; Greco, F.; Cocco, I.; et al. Pathophysiology of hypoxic-ischemic encephalopathy: A review of the past and a view on the future. Acta Neurol. Belg. 2020, 120, 277–288. [Google Scholar] [CrossRef]
- Fernández-López, D.; Natarajan, N.; Ashwal, S.; Vexler, Z.S. Mechanisms of perinatal arterial ischemic stroke. J. Cereb. Blood Flow Metab. 2014, 34, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhao, A.; Zhang, X.; Zeng, M.; Wei, H.; Yan, X.; Wang, J.; Jiang, X.; Dai, Y. Glycolytic reprogramming in microglia: A potential therapeutic target for ischemic stroke. Cell. Signal. 2024, 124, 111466. [Google Scholar] [CrossRef]
- Lu, W.; Wen, J. Crosstalk Among Glial Cells in the Blood-Brain Barrier Injury After Ischemic Stroke. Mol. Neurobiol. 2024, 61, 6161–6174. [Google Scholar] [CrossRef] [PubMed]
- Gidday, J.M.; Gasche, Y.G.; Copin, J.C.; Shah, A.R.; Perez, R.S.; Shapiro, S.D.; Chan, P.H.; Park, T.S. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H558–H568. [Google Scholar] [CrossRef] [PubMed]
- McColl, B.W.; Rothwell, N.J.; Allan, S.M. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J. Neurosci. 2008, 28, 9451–9462. [Google Scholar] [CrossRef]
- Asahi, M.; Asahi, K.; Jung, J.C.; del Zoppo, G.J.; Fini, M.E.; Lo, E.H. Role for matrix metalloproteinase 9 after focal cerebral ischemia: Effects of gene knockout and enzyme inhibition with BB-94. J. Cereb. Blood Flow Metab. 2000, 20, 1681–1689. [Google Scholar] [CrossRef]
- Rosenberg, G.A.; Estrada, E.Y.; Dencoff, J.E. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke 1998, 29, 2189–2195. [Google Scholar] [CrossRef]
- Asahi, M.; Sumii, T.; Fini, M.E.; Itohara, S.; Lo, E.H. Matrix metalloproteinase 2 gene knockout has no effect on acute brain injury after focal ischemia. Neuroreport 2001, 12, 3003–3007. [Google Scholar] [CrossRef] [PubMed]
- Lo, E.H.; Dalkara, T.; Moskowitz, M.A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003, 4, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Zhang, L. Fetal hypoxia and programming of matrix metalloproteinases. Drug Discov. Today 2012, 17, 124–134. [Google Scholar] [CrossRef][Green Version]
- Borjini, N.; Sivilia, S.; Giuliani, A.; Fernandez, M.; Giardino, L.; Facchinetti, F.; Calzà, L. Potential biomarkers for neuroinflammation and neurodegeneration at short and long term after neonatal hypoxic-ischemic insult in rat. J. Neuroinflamm. 2019, 16, 194. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.Y.; Rocha-Ferreira, E.; Ek, C.J.; Wang, X.; Hagberg, H.; Mallard, C. Immune responses in perinatal brain injury. Brain Behav. Immun. 2017, 63, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Zonneveld, R.; Martinelli, R.; Shapiro, N.I.; Kuijpers, T.W.; Plötz, F.B.; Carman, C.V. Soluble adhesion molecules as markers for sepsis and the potential pathophysiological discrepancy in neonates, children and adults. Crit. Care 2014, 18, 204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.Q.; Wang, S.; Kim, H.Y.; Storrie, H.; Rosen, B.R.; Mooney, D.J.; Wang, X.; Lo, E.H. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat. Med. 2006, 12, 441–445. [Google Scholar] [CrossRef]
- Vaillant, C.; Didier-Bazès, M.; Hutter, A.; Belin, M.F.; Thomasset, N. Spatiotemporal expression patterns of metalloproteinases and their inhibitors in the postnatal developing rat cerebellum. J. Neurosci. 1999, 19, 4994–5004. [Google Scholar] [CrossRef] [PubMed]
- Norden, D.M.; Fenn, A.M.; Dugan, A.; Godbout, J.P. TGFbeta produced by IL-10 redirected astrocytes attenuates microglial activation. Glia 2014, 62, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Rakers, C.; Schleif, M.; Blank, N.; Matuskova, H.; Ulas, T.; Handler, K.; Torres, S.V.; Schumacher, T.; Tai, K.; Schultze, J.L.; et al. Stroke target identification guided by astrocyte transcriptome analysis. Glia 2019, 67, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Nakagomi, T.; Kubo, S.; Nakano-Doi, A.; Sakuma, R.; Lu, S.; Narita, A.; Kawahara, M.; Taguchi, A.; Matsuyama, T. Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells 2015, 33, 1962–1974. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, R.; Kawahara, M.; Nakano-Doi, A.; Takahashi, A.; Tanaka, Y.; Narita, A.; Kuwahara-Otani, S.; Hayakawa, T.; Yagi, H.; Matsuyama, T.; et al. Brain pericytes serve as microglia-generating multipotent vascular stem cells following ischemic stroke. J. Neuroinflamm. 2016, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Nirwane, A.; Yao, Y. SMA(low/undetectable) pericytes differentiate into microglia- and macrophage-like cells in ischemic brain. Cell. Mol. Life Sci. 2022, 79, 264. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Sheng, Z.; Liu, Y.; Zhang, R.; Yong, V.W.; Xue, M. Intracerebral haemorrhage: From clinical settings to animal models. Stroke Vasc. Neurol. 2020, 5, 388–395. [Google Scholar] [CrossRef]
- Xi, G.; Keep, R.F.; Hoff, J.T. Pathophysiology of brain edema formation. Neurosurg Clin N Am 2002, 13, 371–383. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, C.; Zhang, J.; Hu, Z. Mechanism and Therapy of Brain Edema after Intracerebral Hemorrhage. Cerebrovasc. Dis. 2016, 42, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.A.; Pandey, A.S.; Thompson, B.G.; Keep, R.F.; Hua, Y.; Xi, G. Injury mechanisms in acute intracerebral hemorrhage. Neuropharmacology 2018, 134, 240–248. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, Z.; Yu, J.; Yang, X.; He, F.; Liu, Z.; Che, F.; Chen, X.; Ren, H.; Hong, M.; et al. Role and mechanisms of cytokines in the secondary brain injury after intracerebral hemorrhage. Prog. Neurobiol. 2019, 178, 101610. [Google Scholar] [CrossRef]
- Guo, Y.; Dai, W.; Zheng, Y.; Qiao, W.; Chen, W.; Peng, L.; Zhou, H.; Zhao, T.; Liu, H.; Zheng, F.; et al. Mechanism and Regulation of Microglia Polarization in Intracerebral Hemorrhage. Molecules 2022, 27, 7080. [Google Scholar] [CrossRef]
- Simard, J.M.; Geng, Z.; Woo, S.K.; Ivanova, S.; Tosun, C.; Melnichenko, L.; Gerzanich, V. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2009, 29, 317–330. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Cui, G.Z.; Yan, X.L.; Wang, X.; Qu, Y.; Guo, Z.N.; Jin, H. Mechanism of Ferroptosis and Its Relationships with Other Types of Programmed Cell Death: Insights for Potential Interventions After Intracerebral Hemorrhage. Front. Neurosci. 2020, 14, 589042. [Google Scholar] [CrossRef]
- Buccilli, B. Exploring new horizons: Emerging therapeutic strategies for pediatric stroke. Exp. Neurol. 2024, 374, 114701. [Google Scholar] [CrossRef] [PubMed]
- Bruschettini, M.; Badura, A.; Romantsik, O. Stem cell-based interventions for the treatment of stroke in newborn infants. Cochrane Database Syst. Rev. 2023, 11, CD015582. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Rojas, M. Anti-inflammatory effects of mesenchymal stem cells: Novel concept for future therapies. Expert Opin. Biol. Ther. 2008, 8, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Sung, S.I.; Yoo, H.S.; Lee, J.H.; Oh, W.I.; Park, W.S. Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke 2013, 44, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Lehnerer, V.; Roidl, A.; Romantsik, O.; Guzman, R.; Wellmann, S.; Bruschettini, M. Mesenchymal stem cell therapy in perinatal arterial ischemic stroke: Systematic review of preclinical studies. Pediatr. Res. 2024, 95, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Taguchi, A.; Ohshima, M.; Kasahara, Y.; Sato, Y.; Tsuda, H.; Otani, K.; Yamahara, K.; Ihara, M.; Harada-Shiba, M.; et al. Effects of intravenous administration of umbilical cord blood CD34(+) cells in a mouse model of neonatal stroke. Neuroscience 2014, 263, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Rocha-Ferreira, E.; Fleiss, B.; Nijboer, C.H.; Gressens, P.; Mallard, C.; Hagberg, H. Neuroprotection offered by mesenchymal stem cells in perinatal brain injury: Role of mitochondria, inflammation, and reactive oxygen species. J. Neurochem. 2021, 158, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Ren, L.N.; Guo, G.; Cannella, L.A.; Chernaya, V.; Samuel, S.; Liu, S.X.; Wang, H.; Yang, X.F. Endothelial progenitor cells in ischemic stroke: An exploration from hypothesis to therapy. J. Hematol. Oncol. 2015, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Jeong, Y.W.; Choi, W.; Noh, J.E.; Lee, S.; Kim, H.S.; Song, J. Multimodal Therapeutic Effects of Neural Precursor Cells Derived from Human-Induced Pluripotent Stem Cells through Episomal Plasmid-Based Reprogramming in a Rodent Model of Ischemic Stroke. Stem Cells Int. 2020, 2020, 4061516. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Ling, X.; Hu, G.; Zhu, Q.; Zhang, J.; Li, Q.; Zhao, B.; Wang, Y.; Deng, Z. Small extracellular vesicles secreted by human iPSC-derived MSC enhance angiogenesis through inhibiting STAT3-dependent autophagy in ischemic stroke. Stem Cell Res. Ther. 2020, 11, 313. [Google Scholar] [CrossRef] [PubMed]
- Oki, K.; Tatarishvili, J.; Wood, J.; Koch, P.; Wattananit, S.; Mine, Y.; Monni, E.; Tornero, D.; Ahlenius, H.; Ladewig, J.; et al. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells 2012, 30, 1120–1133. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, D.; Faustino, J.; Daneman, R.; Zhou, L.; Lee, S.Y.; Derugin, N.; Wendland, M.F.; Vexler, Z.S. Blood-brain barrier permeability is increased after acute adult stroke but not neonatal stroke in the rat. J. Neurosci. 2012, 32, 9588–9600. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.S.; Abdel-Salam, G.M.H.; Issa, M.Y.; Emam, B.A.; Zaki, M.S. Band-like calcification with simplified gyration and polymicrogyria: Report of 10 new families and identification of five novel OCLN mutations. J. Hum. Genet. 2017, 62, 553–559. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, M.C.; Daly, S.B.; Urquhart, J.E.; Black, G.C.; Pilz, D.T.; Brockmann, K.; McEntagart, M.; Abdel-Salam, G.; Zaki, M.; Wolf, N.I.; et al. Recessive mutations in the gene encoding the tight junction protein occludin cause band-like calcification with simplified gyration and polymicrogyria. Am. J. Hum. Genet. 2010, 87, 354–364. [Google Scholar] [CrossRef]
- Cen, Z.; Chen, Y.; Chen, S.; Wang, H.; Yang, D.; Zhang, H.; Wu, H.; Wang, L.; Tang, S.; Ye, J.; et al. Biallelic loss-of-function mutations in JAM2 cause primary familial brain calcification. Brain 2020, 143, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Schottlaender, L.V.; Abeti, R.; Jaunmuktane, Z.; Macmillan, C.; Chelban, V.; O’Callaghan, B.; McKinley, J.; Maroofian, R.; Efthymiou, S.; Athanasiou-Fragkouli, A.; et al. Bi-allelic JAM2 Variants Lead to Early-Onset Recessive Primary Familial Brain Calcification. Am. J. Hum. Genet. 2020, 106, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Lamagna, C.; Meda, P.; Mandicourt, G.; Brown, J.; Gilbert, R.J.; Jones, E.Y.; Kiefer, F.; Ruga, P.; Imhof, B.A.; Aurrand-Lions, M. Dual interaction of JAM-C with JAM-B and alpha(M)beta2 integrin: Function in junctional complexes and leukocyte adhesion. Mol. Biol. Cell 2005, 16, 4992–5003. [Google Scholar] [CrossRef] [PubMed]
- Stamatovic, S.M.; Johnson, A.M.; Keep, R.F.; Andjelkovic, A.V. Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers 2016, 4, e1154641. [Google Scholar] [CrossRef] [PubMed]
- Lecca, M.; Pehlivan, D.; Suner, D.H.; Weiss, K.; Coste, T.; Zweier, M.; Oktay, Y.; Danial-Farran, N.; Rosti, V.; Bonasoni, M.P.; et al. Bi-allelic variants in the ESAM tight-junction gene cause a neurodevelopmental disorder associated with fetal intracranial hemorrhage. Am. J. Hum. Genet. 2023, 110, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Gould, D.B.; Phalan, F.C.; Breedveld, G.J.; van Mil, S.E.; Smith, R.S.; Schimenti, J.C.; Aguglia, U.; van der Knaap, M.S.; Heutink, P.; John, S.W. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science 2005, 308, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Durrani-Kolarik, S.; Manickam, K.; Chen, B. COL4A1 Mutation in a Neonate with Intrauterine Stroke and Anterior Segment Dysgenesis. Pediatr. Neurol. 2017, 66, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Garvey, A.A.; Walsh, B.H.; Inder, T.E. Pathogenesis and prevention of intraventricular hemorrhage. Semin. Perinatol. 2022, 46, 151592. [Google Scholar] [CrossRef]
- Abdel-Salam, G.M.H.; Esmail, A.; Nagy, D.; Abdel-Ghafar, S.F.; Abdel-Hamid, M.S. Novel homozygous ESAM variants in two families with perinatal strokes showing variable neuroradiologic and clinical findings. J. Hum. Genet. 2024, 70, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Mochida, G.H.; Ganesh, V.S.; Felie, J.M.; Gleason, D.; Hill, R.S.; Clapham, K.R.; Rakiec, D.; Tan, W.H.; Akawi, N.; Al-Saffar, M.; et al. A homozygous mutation in the tight-junction protein JAM3 causes hemorrhagic destruction of the brain, subependymal calcification, and congenital cataracts. Am. J. Hum. Genet. 2010, 87, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Duong, C.N.; Nottebaum, A.F.; Butz, S.; Volkery, S.; Zeuschner, D.; Stehling, M.; Vestweber, D. Interference with ESAM (Endothelial Cell-Selective Adhesion Molecule) Plus Vascular Endothelial-Cadherin Causes Immediate Lethality and Lung-Specific Blood Coagulation. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 378–393. [Google Scholar] [CrossRef]
- Stahl, S.; Gaetzner, S.; Voss, K.; Brackertz, B.; Schleider, E.; Surucu, O.; Kunze, E.; Netzer, C.; Korenke, C.; Finckh, U.; et al. Novel CCM1, CCM2, and CCM3 mutations in patients with cerebral cavernous malformations: In-frame deletion in CCM2 prevents formation of a CCM1/CCM2/CCM3 protein complex. Hum. Mutat. 2008, 29, 709–717. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.; Wooderchak-Donahue, W.; VanSant Webb, C.; Whitehead, K.; Stevenson, D.A.; Bayrak-Toydemir, P. Hereditary hemorrhagic telangiectasia: Genetics and molecular diagnostics in a new era. Front. Genet. 2015, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Wooderchak-Donahue, W.L.; Johnson, P.; McDonald, J.; Blei, F.; Berenstein, A.; Sorscher, M.; Mayer, J.; Scheuerle, A.E.; Lewis, T.; Grimmer, J.F.; et al. Expanding the clinical and molecular findings in RASA1 capillary malformation-arteriovenous malformation. Eur. J. Hum. Genet. 2018, 26, 1521–1536. [Google Scholar] [CrossRef]
- Amyere, M.; Revencu, N.; Helaers, R.; Pairet, E.; Baselga, E.; Cordisco, M.; Chung, W.; Dubois, J.; Lacour, J.P.; Martorell, L.; et al. Germline Loss-of-Function Mutations in EPHB4 Cause a Second Form of Capillary Malformation-Arteriovenous Malformation (CM-AVM2) Deregulating RAS-MAPK Signaling. Circulation 2017, 136, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M.D.; Tang, H.; Gallione, C.J.; Baugher, J.D.; Frelin, L.P.; Cohen, B.; North, P.E.; Marchuk, D.A.; Comi, A.M.; Pevsner, J. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N. Engl. J. Med. 2013, 368, 1971–1979. [Google Scholar] [CrossRef]
- Nasim, S.; Bichsel, C.; Pinto, A.; Alexandrescu, S.; Kozakewich, H.; Bischoff, J. Similarities and differences between brain and skin GNAQ p.R183Q driven capillary malformations. Angiogenesis 2024, 27, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, I.; Bianchi, S.; De Stefano, N.; Dichgans, M.; Dotti, M.T.; Duering, M.; Jouvent, E.; Korczyn, A.D.; Lesnik-Oberstein, S.A.; Malandrini, A.; et al. Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) as a model of small vessel disease: Update on clinical, diagnostic, and management aspects. BMC Med. 2017, 15, 41. [Google Scholar] [CrossRef]
- Henshall, T.L.; Keller, A.; He, L.; Johansson, B.R.; Wallgard, E.; Raschperger, E.; Mae, M.A.; Jin, S.; Betsholtz, C.; Lendahl, U. Notch3 is necessary for blood vessel integrity in the central nervous system. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, L.; Moens, C.B.; Appel, B. Notch3 establishes brain vascular integrity by regulating pericyte number. Development 2014, 141, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Hansson, E.M.; Tikka, S.; Lanner, F.; Sahlgren, C.; Farnebo, F.; Baumann, M.; Kalimo, H.; Lendahl, U. Notch signaling regulates platelet-derived growth factor receptor-beta expression in vascular smooth muscle cells. Circ. Res. 2008, 102, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, T.; Bogue, W.; Bigit, B.; Cuervo, H. Deficiency of Notch signaling in pericytes results in arteriovenous malformations. JCI Insight 2020, 5, e125940. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, P.; Johansson, B.R.; Leveen, P.; Betsholtz, C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 1997, 277, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Tallquist, M.D.; French, W.J.; Soriano, P. Additive effects of PDGF receptor beta signaling pathways in vascular smooth muscle cell development. PLoS Biol. 2003, 1, E52. [Google Scholar] [CrossRef]
- Kundishora, A.J.; Peters, S.T.; Pinard, A.; Duran, D.; Panchagnula, S.; Barak, T.; Miyagishima, D.F.; Dong, W.; Smith, H.; Ocken, J.; et al. DIAPH1 Variants in Non-East Asian Patients with Sporadic Moyamoya Disease. JAMA Neurol. 2021, 78, 993–1003. [Google Scholar] [CrossRef]
- Guo, D.C.; Papke, C.L.; Tran-Fadulu, V.; Regalado, E.S.; Avidan, N.; Johnson, R.J.; Kim, D.H.; Pannu, H.; Willing, M.C.; Sparks, E.; et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. Am. J. Hum. Genet. 2009, 84, 617–627. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Martínez, T.; Gornatti, D.G.; Ortiz, M.; Cañellas, G.; Heine-Suñer, D.; Vives-Bauzà, C. The Triad of Blood–Brain Barrier Integrity: Endothelial Cells, Astrocytes, and Pericytes in Perinatal Stroke Pathophysiology. Int. J. Mol. Sci. 2025, 26, 1886. https://doi.org/10.3390/ijms26051886
Garcia-Martínez T, Gornatti DG, Ortiz M, Cañellas G, Heine-Suñer D, Vives-Bauzà C. The Triad of Blood–Brain Barrier Integrity: Endothelial Cells, Astrocytes, and Pericytes in Perinatal Stroke Pathophysiology. International Journal of Molecular Sciences. 2025; 26(5):1886. https://doi.org/10.3390/ijms26051886
Chicago/Turabian StyleGarcia-Martínez, Tania, Denise G. Gornatti, Marina Ortiz, Guillem Cañellas, Damià Heine-Suñer, and Cristòfol Vives-Bauzà. 2025. "The Triad of Blood–Brain Barrier Integrity: Endothelial Cells, Astrocytes, and Pericytes in Perinatal Stroke Pathophysiology" International Journal of Molecular Sciences 26, no. 5: 1886. https://doi.org/10.3390/ijms26051886
APA StyleGarcia-Martínez, T., Gornatti, D. G., Ortiz, M., Cañellas, G., Heine-Suñer, D., & Vives-Bauzà, C. (2025). The Triad of Blood–Brain Barrier Integrity: Endothelial Cells, Astrocytes, and Pericytes in Perinatal Stroke Pathophysiology. International Journal of Molecular Sciences, 26(5), 1886. https://doi.org/10.3390/ijms26051886





