Biological Activity of Peptide Fraction Derived from Hermetia illucens L. (Diptera: Stratiomyidae) Larvae Haemolymph on Gastric Cancer Cells
Abstract
1. Introduction
2. Results
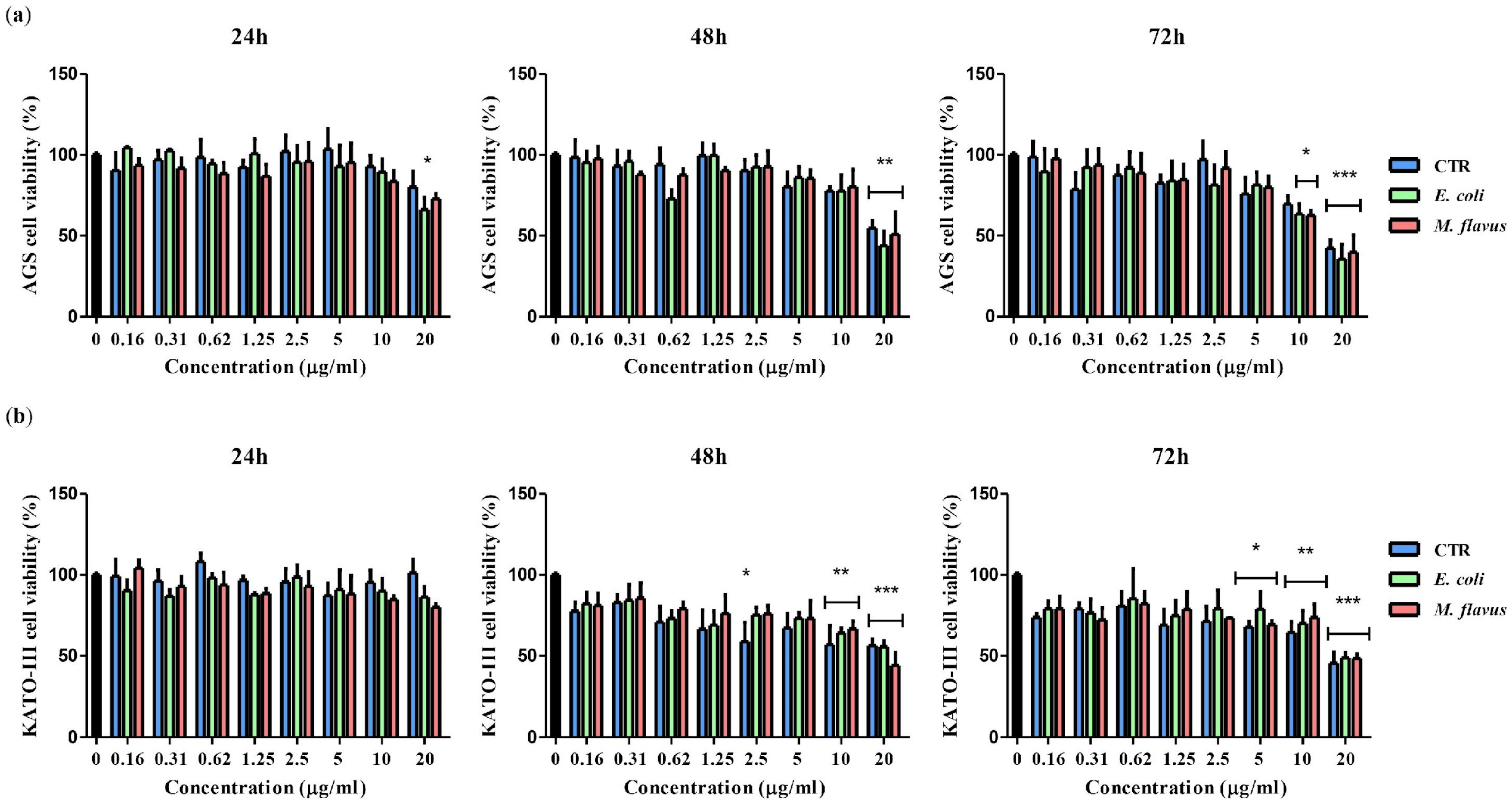
2.1. Reduction of Viability of KATO III and AGS Cell Lines by Peptide Fractions
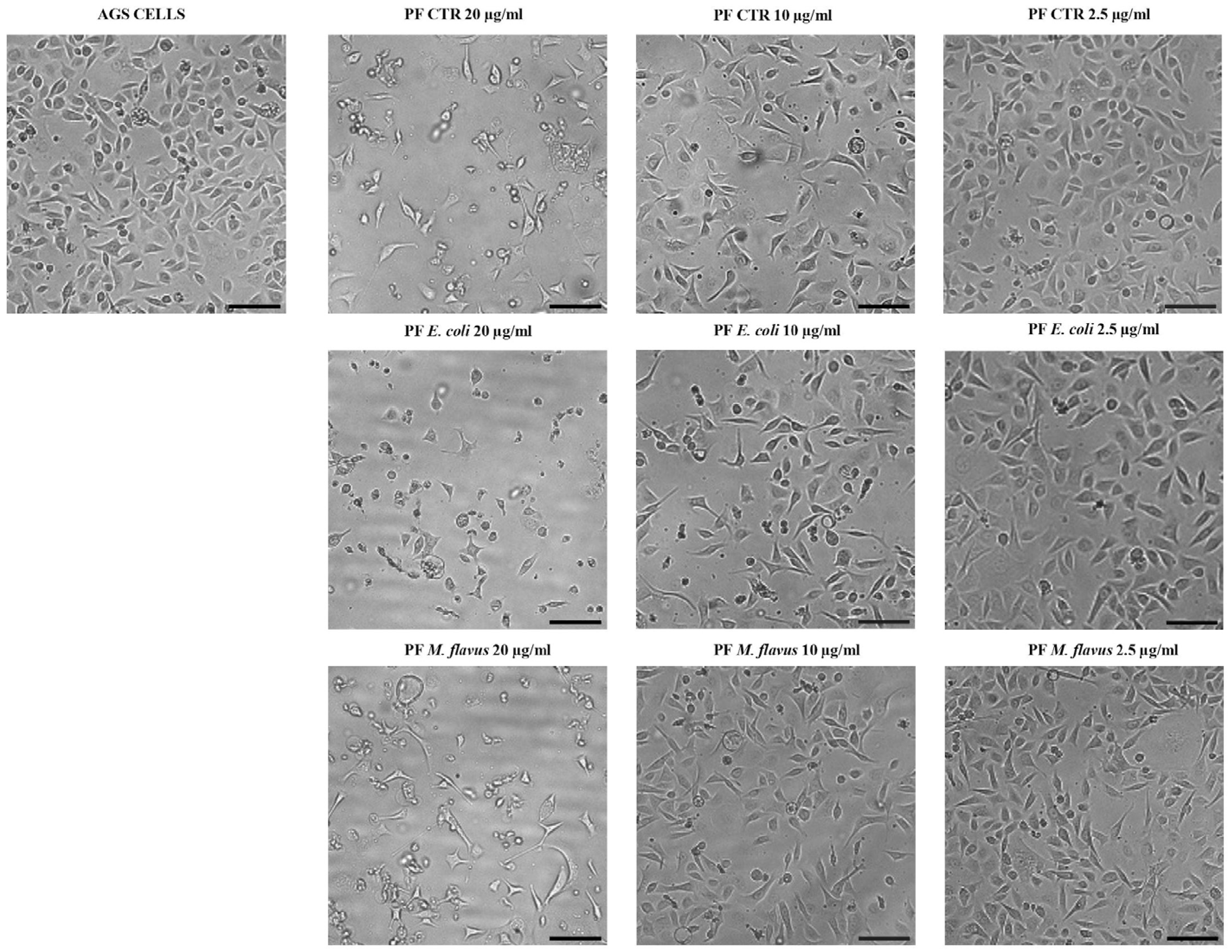
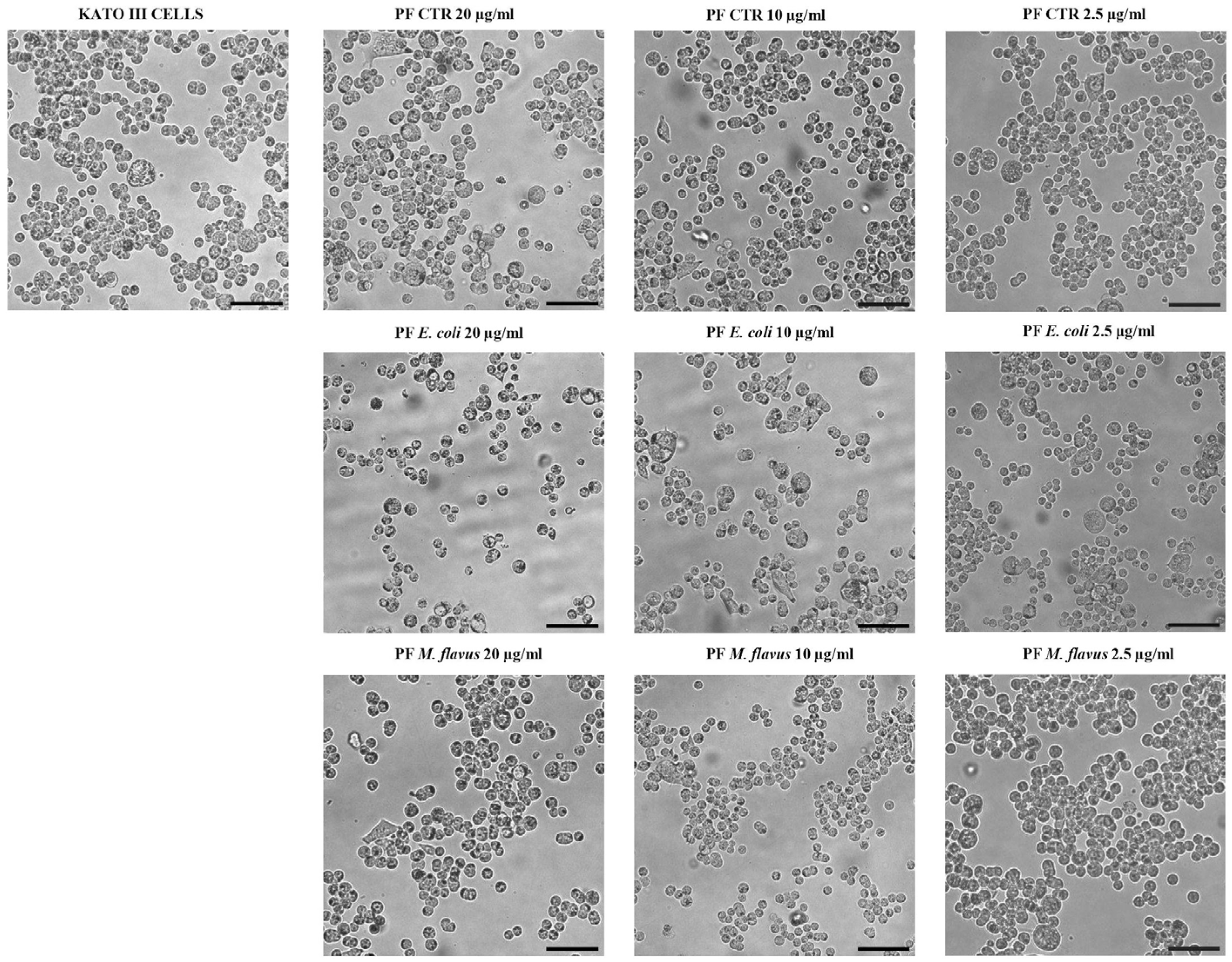
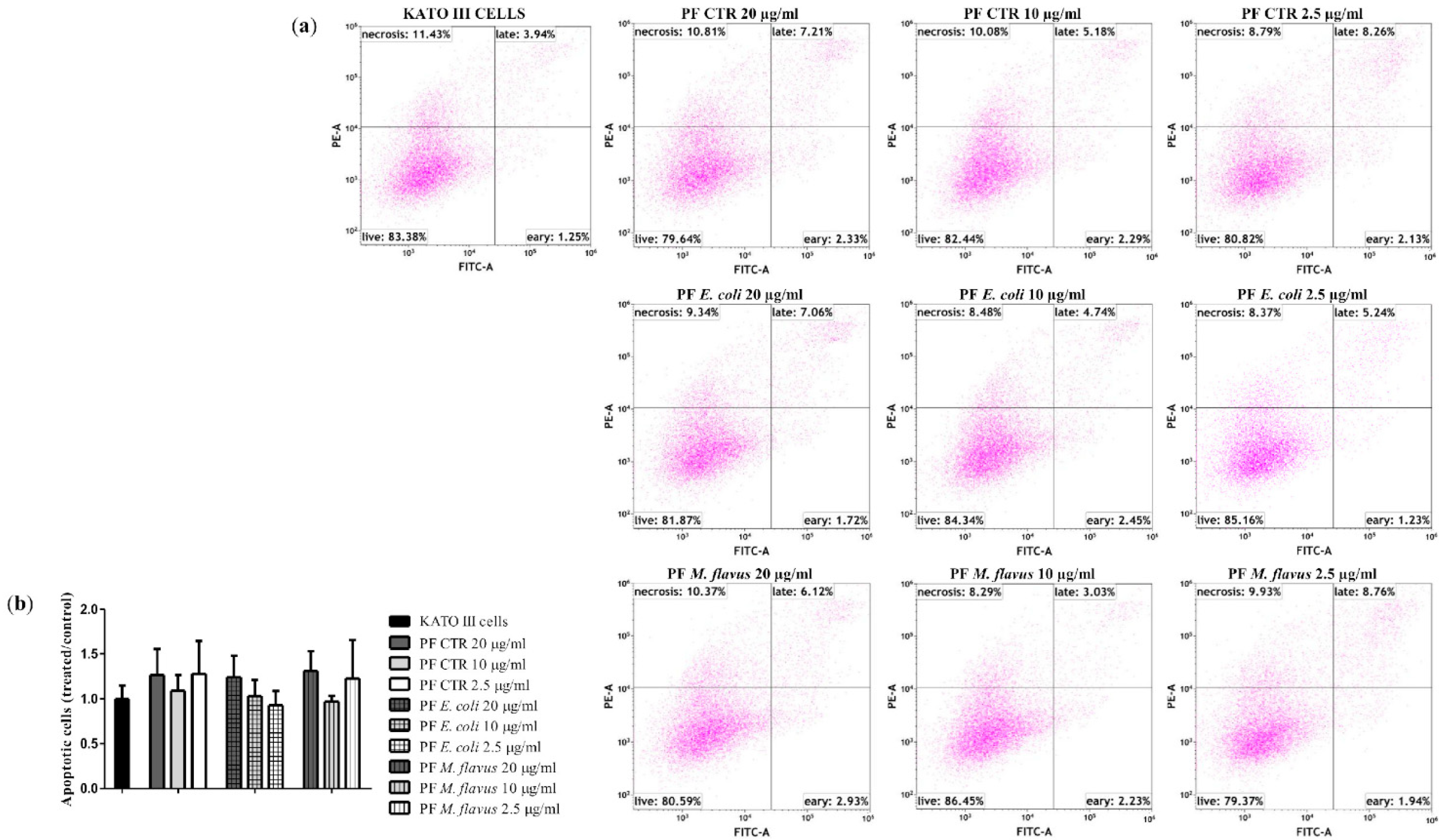
2.2. Cell Morphology Alteration of AGS and KATO III Cell Lines After Exposure to Peptide Fractions
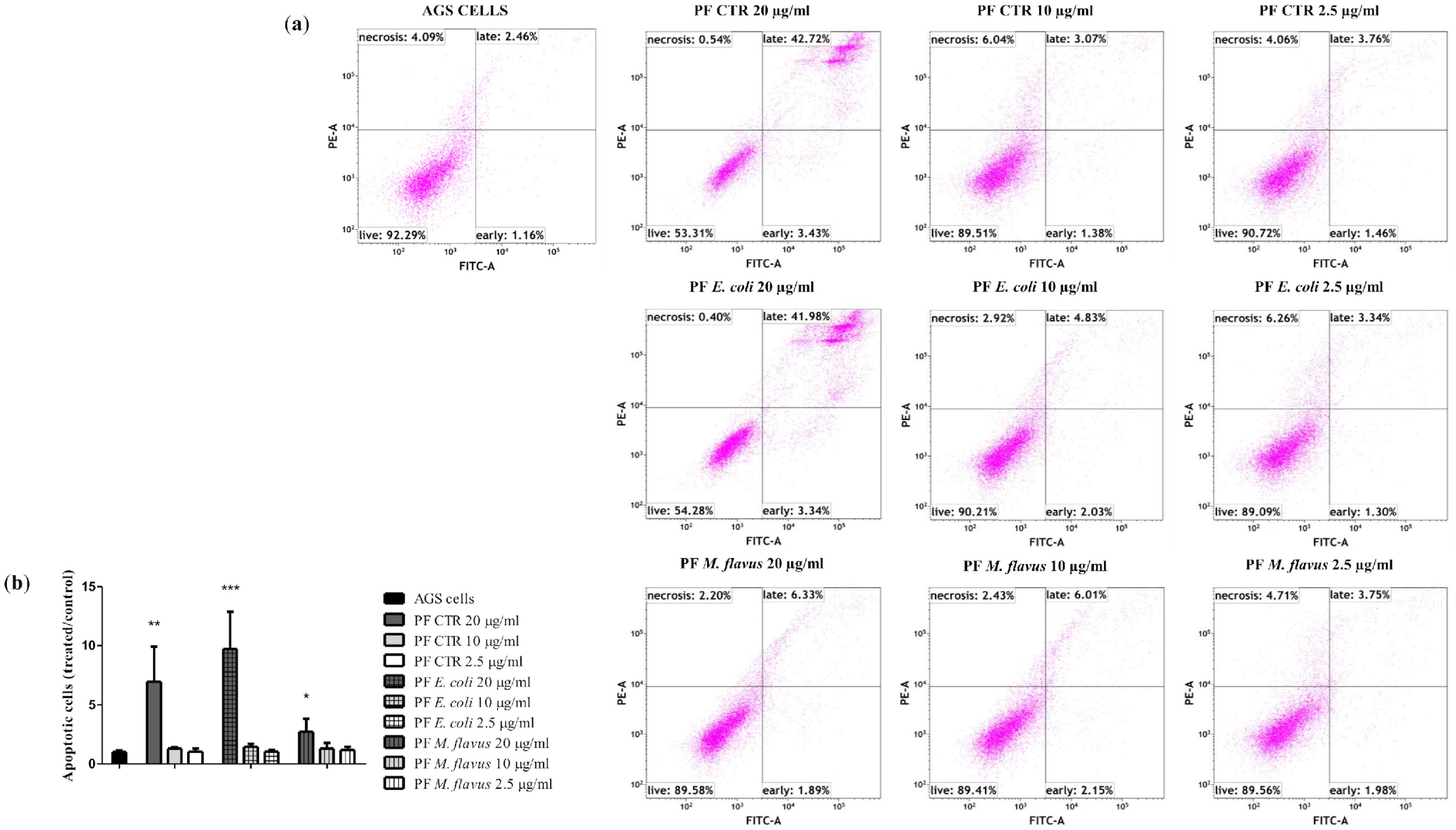
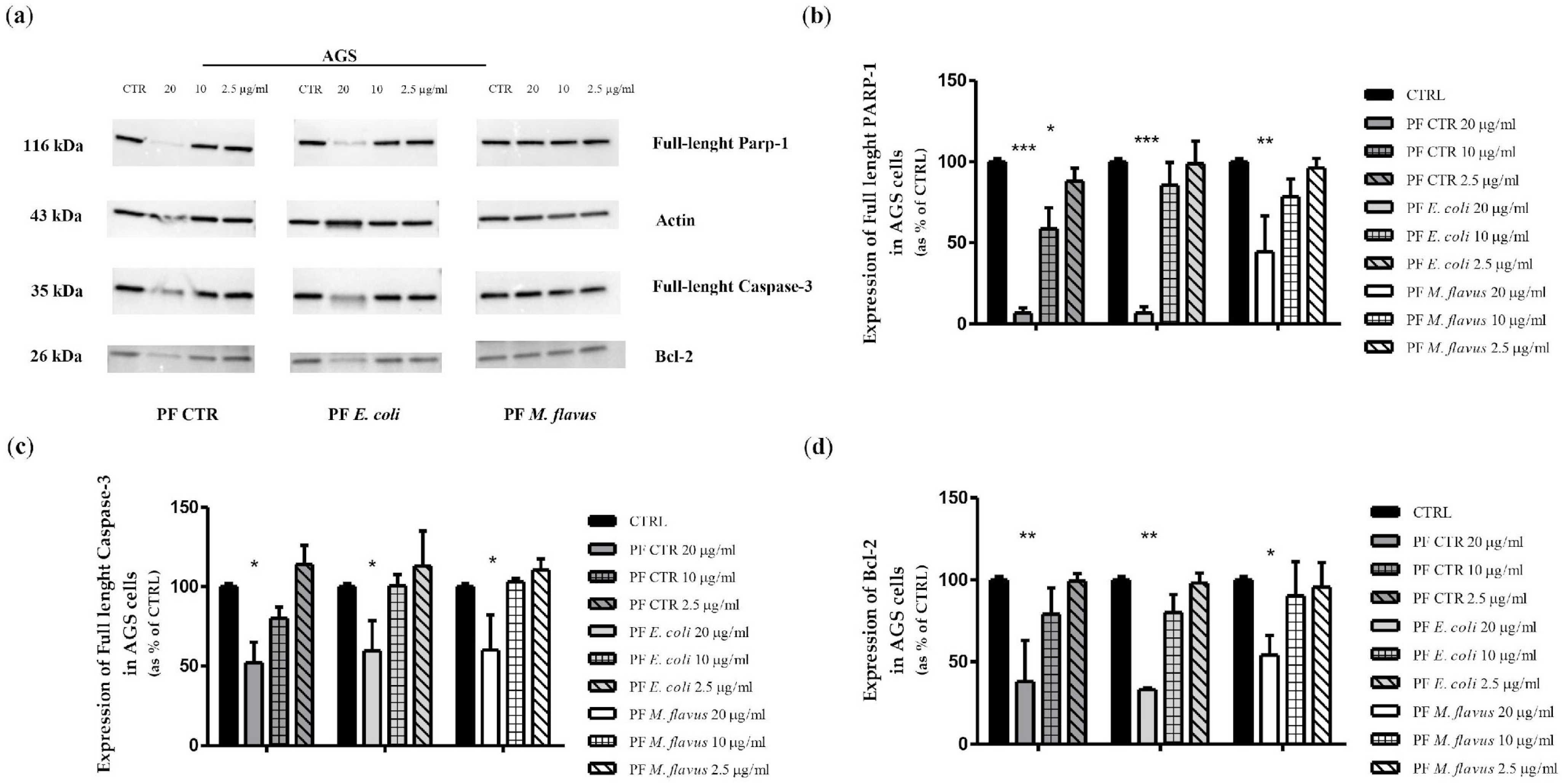
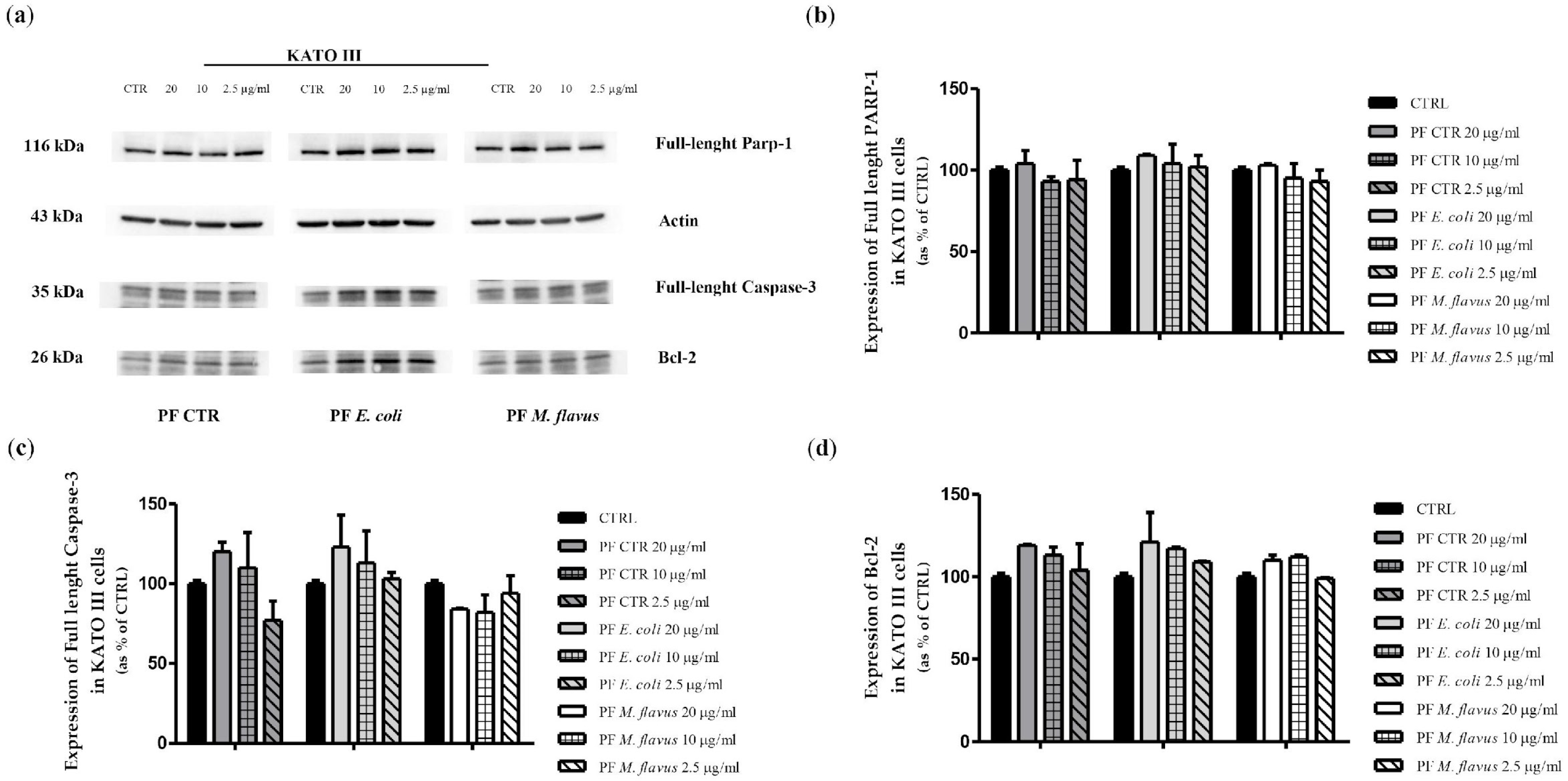
2.3. Apoptosis via Bcl-2/Caspase-3/PARP-1 Pathway in AGS Cell Line Induced by PFs
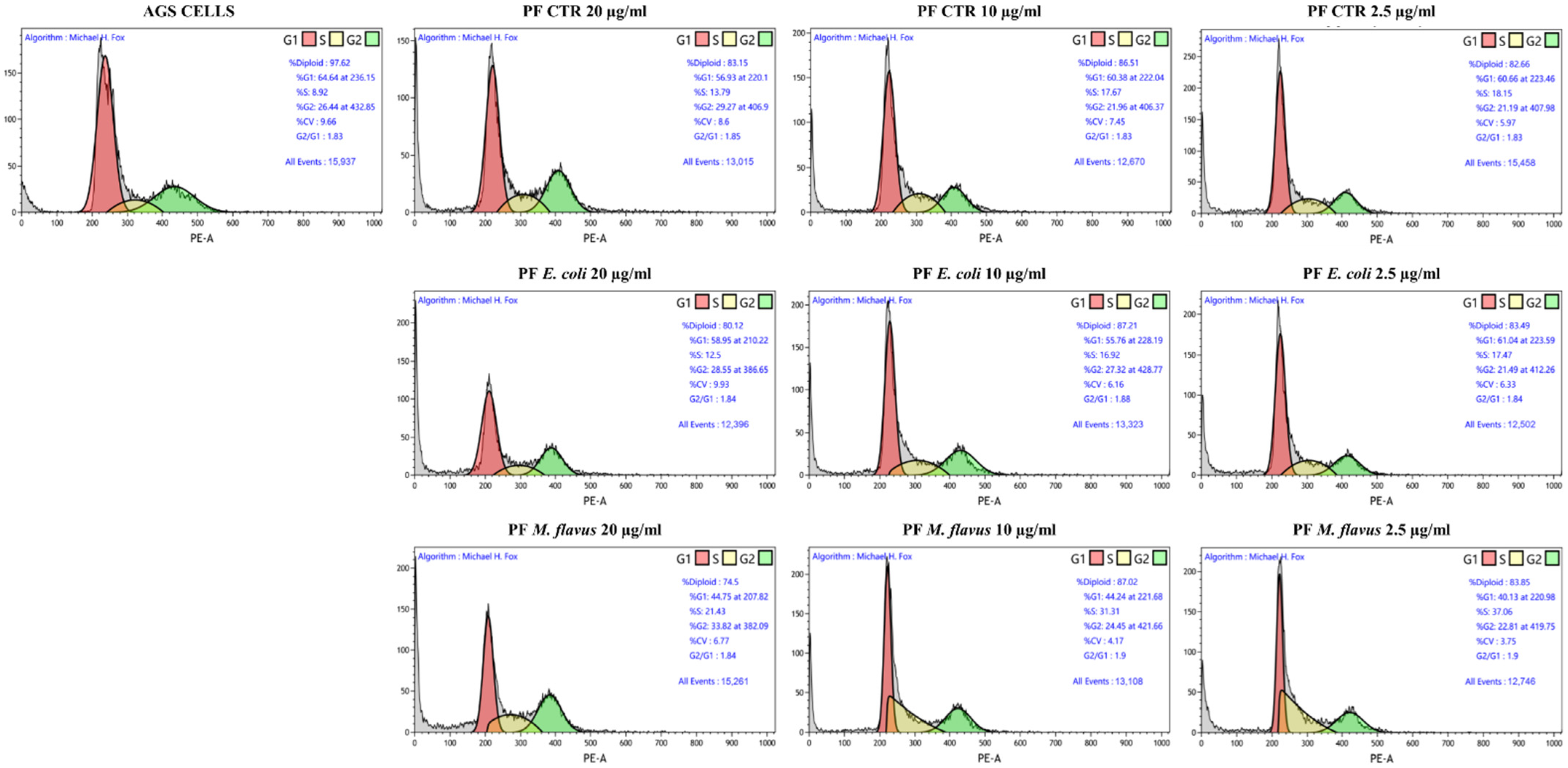
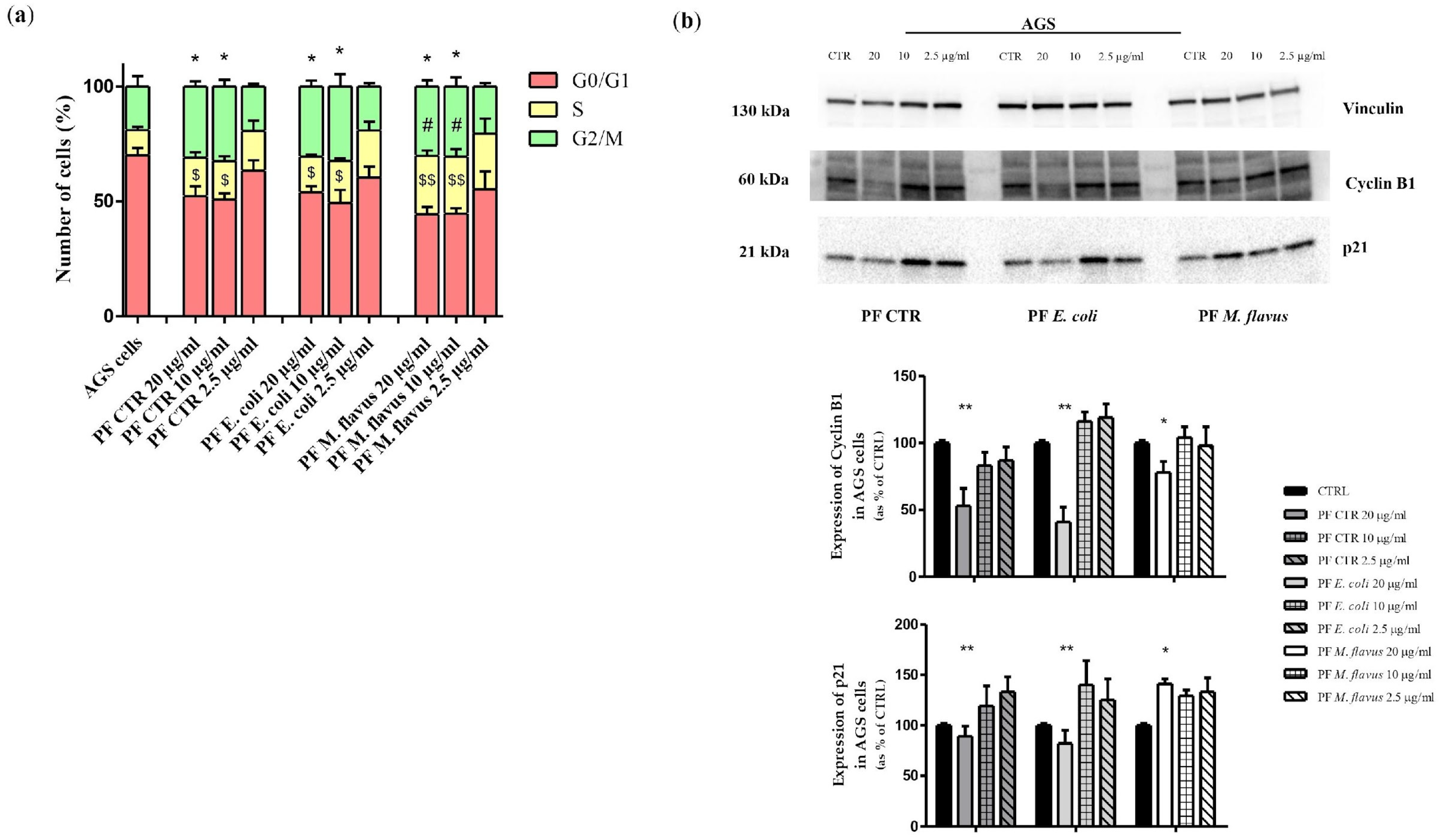
2.4. S Phase and G2/M Phase Arrest Induced by Peptide Fractions
3. Discussion
4. Materials and Methods
4.1. Hermetia Illucens Larvae Infection, Haemolymph Extraction and Peptide Fraction Isolation by Organic Solvents
4.2. Cell Lines and Culture Conditions
4.3. Measurement of Cell Viability
4.4. Observation of Morphological Changes
4.5. Cytofluorimetric Analysis for Apoptosis Assay
4.6. Cytofluorimetric Analysis for Cell Cycle Assay
4.7. Western Blot Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Cancer Institute. ‘Cancer Statistics’, National Institutes of Health; National Cancer Institute: Bethesda, MD, USA, 2024. [Google Scholar]
- Global Cancer Observatory. International Agency for Research on Cancer; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Global Health Observatory. Explore a World of Health Data; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- World Cancer Research Fund International. Cancer Trends—Worldwide Cancer Data; World Cancer Research Fund International: Sheffield, UK, 2022. [Google Scholar]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Kiri, S.; Ryba, T. Cancer, metastasis, and the epigenome. Mol. Cancer 2024, 23, 154. [Google Scholar] [CrossRef] [PubMed]
- Jokhadze, N.; Das, A.; Dizon, D.S. Global cancer statistics: A healthy population relies on population health. CA Cancer J. Clin. 2024, 74, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Mamun, T.I.; Younus, S.; Rahman, M.H. Gastric cancer-Epidemiology, modifiable and non-modifiable risk factors, challenges and opportunities: An updated review. Cancer Treat. Res. Commun. 2024, 41, 100845. [Google Scholar] [CrossRef] [PubMed]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Wang, Y.A. Gastric cancer immunosuppressive microenvironment heterogeneity: Implications for therapy development. Trends Cancer 2024, 10, 627–642. [Google Scholar] [CrossRef]
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef]
- Baylin, S.B.; Jones, P.A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019505. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Zahreddine, H.; Borden, K.L.B. Mechanisms and insights into drug resistance in cancer. Front. Pharmacol. 2013, 4, 28. [Google Scholar] [CrossRef]
- Joshi, S.S.; Badgwell, B.D. Current treatment and recent progress in gastric cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Emran, T.B.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef] [PubMed]
- Eslami, M.; Memarsadeghi, O.; Davarpanah, A.; Arti, A.; Nayernia, K.; Behnam, B. Overcoming Chemotherapy Resistance in Metastatic Cancer: A Comprehensive Review. Biomedicines 2024, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.E.; Hockings, H.; Hilton, D.M.; Kermorgant, S. The role of MET in chemotherapy resistance. Oncogene 2021, 40, 1927–1941. [Google Scholar] [CrossRef]
- Lim, Z.-F.; Ma, P.C. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J. Hematol. Oncol. 2019, 12, 134. [Google Scholar] [CrossRef]
- Ahronian, L.G.; Corcoran, R.B. Strategies for monitoring and combating resistance to combination kinase inhibitors for cancer therapy. Genome Med. 2017, 9, 37. [Google Scholar] [CrossRef]
- Bae, Y.H.; Park, K. Advanced drug delivery 2020 and beyond: Perspectives on the future. Adv. Drug Deliv. Rev. 2020, 158, 4–16. [Google Scholar] [CrossRef]
- Delou, J.M.A.; Souza, A.S.O.; Souza, L.C.M.; Borges, H.L. Highlights in Resistance Mechanism Pathways for Combination Therapy. Cells 2019, 8, 1013. [Google Scholar] [CrossRef]
- Gonzalez-Bosquet, J.; Gabrilovich, S.; McDonald, M.E.; Smith, B.J.; Leslie, K.K.; Bender, D.D.; Goodheart, M.J.; Devor, E. Integration of Genomic and Clinical Retrospective Data to Predict Endometrioid Endometrial Cancer Recurrence. Int. J. Mol. Sci. 2022, 23, 16014. [Google Scholar] [CrossRef]
- Sosinsky, A.; Ambrose, J.; Cross, W.; Turnbull, C.; Henderson, S.; Jones, L.; Hamblin, A.; Arumugam, P.; Chan, G.; Chubb, D.; et al. Insights for precision oncology from the integration of genomic and clinical data of 13,880 tumors from the 100,000 Genomes Cancer Programme. Nat. Med. 2024, 30, 279–289. [Google Scholar] [CrossRef]
- Guan, W.L.; He, Y.; Xu, R.H. Gastric cancer treatment: Recent progress and future perspectives. J. Hematol. Oncol. 2023, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.N.; Teng, Q.X.; Tian, Q.; Chen, W.; Xie, Y.; Wu, K.; Zeng, Q.; Zeng, L.; Pan, Y.; Chen, Z.-S.; et al. Signaling pathways and therapeutic interventions in gastric cancer. Signal Transduct. Target. Ther. 2022, 7, 358. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Shukla, P. Antimicrobial Peptides: Recent Insights on Biotechnological Interventions and Future Perspectives. Protein Pept. Lett. 2019, 26, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Azmiera, N.; Krasilnikova, A.; Sahudin, S.; Al-Talib, H.; Heo, C.C. Antimicrobial peptides isolated from insects and their potential applications. J. Asia-Pac. Entom. 2022, 25, 101892. [Google Scholar] [CrossRef]
- Wu, Q.; Patočka, J.; Kuča, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef]
- Bulet, P.; Stöcklin, R.; Menin, L. Anti-microbial peptides: From invertebrates to vertebrates. Immunol. Rev. 2004, 198, 169–184. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial Peptides as Anticancer Agents: Functional Properties and Biological Activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef]
- Fu, X.Y.; Yin, H.; Chen, X.T.; Yao, J.F.; Ma, Y.N.; Song, M.; Xu, H.; Yu, Q.-Y.; Du, S.-S.; Qi, Y.-K.; et al. Three rounds of stability-guided optimization and systematical evaluation of oncolytic peptide LTX-315. J. Med. Chem. 2024, 67, 3885–3908. [Google Scholar] [CrossRef]
- Yin, H.; Chen, X.T.; Chi, Q.N.; Ma, Y.N.; Fu, X.Y.; Du, S.S.; Qi, Y.-K.; Wang, K.W. The hybrid oncolytic peptide NTP-385 potently inhibits adherent cancer cells by targeting the nucleus. Acta Pharmacol. Sin. 2023, 44, 201–210. [Google Scholar] [CrossRef]
- Yin, H.; Fu, X.Y.; Gao, H.Y.; Ma, Y.N.; Yao, J.F.; Du, S.S.; Qi, Y.-N.; Wang, K.W. Design, synthesis and anticancer evaluation of novel oncolytic peptide-chlorambucil conjugates. Bioorg. Chem. 2023, 138, 106674. [Google Scholar] [CrossRef]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application (Review). Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Boparai, J.K.; Sharma, P.K. Mini Review on Antimicrobial Peptides, Sources, Mechanism and Recent Applications. Protein Pept. Lett. 2019, 27, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Papo, N.; Shai, Y. Host defense peptides as new weapons in cancer treatment. Cell Mol. Life Sci. 2005, 62, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Corradi, V.; Sejdiu, B.I.; Mesa-Galloso, H.; Abdizadeh, H.; Noskov, S.Y.; Marrink, S.J.; Tieleman, D.P. Emerging Diversity in Lipid-Protein Interactions. Chem. Rev. 2019, 119, 5775–5848. [Google Scholar] [CrossRef]
- Deslouches, B.; Di, Y.P. Antimicrobial peptides with selective antitumor mechanisms: Prospect for anticancer applications. Oncotarget 2017, 8, 46635–46651. [Google Scholar] [CrossRef]
- Leuschner, C.; Hansel, W. Membrane Disrupting Lytic Peptides for Cancer Treatments. Curr. Pharm. Des. 2004, 10, 2299–2310. [Google Scholar] [CrossRef]
- Szlasa, W.; Zendran, I.; Zalesińska, A.; Tarek, M.; Kulbacka, J. Lipid composition of the cancer cell membrane. J. Bioenerg. Biomembr. 2020, 52, 321–342. [Google Scholar] [CrossRef]
- Alves, A.C.; Ribeiro, D.; Nunes, C.; Reis, S. Biophysics in cancer: The relevance of drug-membrane interaction studies. Biochim. Biophys. Acta. 2016, 1858, 2231–2244. [Google Scholar] [CrossRef]
- Manrique-Moreno, M.; Santa-González, G.A.; Gallego, V. Bioactive cationic peptides as potential agents for breast cancer treatment. Biosci. Rep. 2021, 41, BSR20211218C. [Google Scholar] [CrossRef]
- Khan, T.; Waseem, R.; Zehra, Z.; Aiman, A.; Bhardwaj, P.; Ansari, J.; Hassan, M.I.; Islam, A. Mitochondrial Dysfunction: Pathophysiology and Mitochondria-Targeted Drug Delivery Approaches. Pharmaceutics 2022, 14, 2657. [Google Scholar] [CrossRef] [PubMed]
- Chernysh, S.; Kim, S.I.; Bekker, G.; Pleskach, V.A.; Filatova, N.A.; Anikin, V.B.; Platonov, V.G.; Bulet, P. Antiviral and antitumor peptides from insects. Proc. Natl. Acad. Sci. USA 2002, 99, 12628–12632. [Google Scholar] [CrossRef] [PubMed]
- Manniello, M.D.; Moretta, A.; Salvia, R.; Scieuzo, C.; Lucchetti, D.; Vogel, H.; Sgambato, A.; Falabella, P. Insect antimicrobial peptides: Potential weapons to counteract the antibiotic resistance. Cell Mol. Life Sci. 2021, 78, 4259–4282. [Google Scholar] [CrossRef]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell Infect. Microbiol. 2021, 11, 668632. [Google Scholar] [CrossRef]
- Paredes-Gamero, E.J.; Martins, M.N.C.; Cappabianco, F.A.M.; Ide, J.S.; Miranda, A. Characterization of dual effects induced by antimicrobial peptides: Regulated cell death or membrane disruption. Biochim. Biophys. Acta. 2012, 1820, 1062–1072. [Google Scholar] [CrossRef]
- Elena-Real, C.A.; Díaz-Quintana, A.; González-Arzola, K.; Velázquez-Campoy, A.; Orzáez, M.; López-Rivas, A.; Gil-Caballero, S.; De la Rosa, M.Á.; Díaz-Moreno, I. Cytochrome c speeds up caspase cascade activation by blocking 14-3-3ε-dependent Apaf-1 inhibition. Cell Death Dis. 2018, 9, 365. [Google Scholar] [CrossRef]
- Su, B.-C.; Chen, J.-Y. Antimicrobial Peptide Epinecidin-1 Modulates MyD88 Protein Levels via the Proteasome Degradation Pathway. Mar. Drugs. 2017, 15, 362. [Google Scholar] [CrossRef]
- Xie, M.; Liu, D.; Yang, Y. Anti-cancer peptides: Classification, mechanism of action, reconstruction and modification. Open Biol. 2020, 10, 200004. [Google Scholar] [CrossRef]
- Donehower, L.A.; Soussi, T.; Korkut, A.; Liu, Y.; Schultz, A.; Cardenas, M.; Li, X.; Babur, O.; Hsu, T.K.; Lichtarge, O.; et al. Integrated Analysis of TP53 Gene and Pathway Alterations in The Cancer Genome Atlas. Cell Rep. 2019, 28, 1370–1384.e5. [Google Scholar] [CrossRef]
- Yamada, T.; Mehta, R.R.; Lekmine, F.; Christov, K.; King, M.L.; Majumdar, D.; Shilkaitis, A.; Green, A.; Bratescu, L.; Beattie, C.W.; et al. A peptide fragment of azurin induces a p53-mediated cell cycle arrest in human breast cancer cells. Mol. Cancer Ther. 2009, 8, 2947–2958. [Google Scholar] [CrossRef]
- Yi, H.Y.; Chowdhury, M.; Huang, Y.D.; Yu, X.Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Salvia, R.; Scieuzo, C.; Di Somma, A.; Vogel, H.; Pucci, P.; Sgambato, A.; Wolff, M.; Falabella, P. A bioinformatic study of antimicrobial peptides identified in the Black Soldier Fly (BSF) Hermetia illucens (Diptera: Stratiomyidae). Sci. Rep. 2020, 10, 16875. [Google Scholar] [CrossRef] [PubMed]
- Scieuzo, C.; Giglio, F.; Rinaldi, R.; Lekka, M.E.; Cozzolino, F.; Monaco, V.; Monti, M.; Salvia, R.; Falabella, P. In Vitro Evaluation of the Antibacterial Activity of the Peptide Fractions Extracted from the Hemolymph of Hermetia illucens (Diptera: Stratiomyidae). Insects 2023, 14, 464. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Scieuzo, C.; Salvia, R.; Mancini, I.M.; Canini, D.; Masi, S.; Falabella, P. Mobile black soldier fly farm for on-site disposal of animal dairy manure. Bull. Insectol. 2022, 75, 75–82. [Google Scholar]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Tomberlin, J.K.; Falabella, P. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci. Rep. 2020, 10, 19448. [Google Scholar] [CrossRef]
- Scieuzo, C.; Franco, A.; Salvia, R.; Triunfo, M.; Addeo, N.F.; Vozzo, S.; Piccolo, G.; Bovera, F.; Ritieni, A.; Francia, A.D.; et al. Enhancement of fruit byproducts through bioconversion by Hermetia illucens (Diptera: Stratiomyidae). Insect Sci. 2022, 30, 991–1010. [Google Scholar] [CrossRef]
- Di Somma, A.; Moretta, A.; Cané, C.; Scieuzo, C.; Salvia, R.; Falabella, P.; Duilio, A. Structural and functional characterization of a novel recombinant antimicrobial peptide from Hermetia illucens. Curr. Issues Mol. Biol. 2022, 44, 1–13. [Google Scholar] [CrossRef]
- Dho, M.; Candian, V.; Tedeschi, R. Insect Antimicrobial Peptides: Advancements, Enhancements and New Challenges. Antibiotics 2023, 12, 952. [Google Scholar] [CrossRef]
- Yeldag, G.; Rice, A.; Del Río Hernández, A. Chemoresistance and the Self-Maintaining Tumor Microenvironment. Cancers 2018, 10, 471. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A. Tumour Heterogeneity and Resistance to Cancer Therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Castells, M.; Thibault, B.; Delord, J.P.; Couderc, B. Implication of tumor microenvironment in chemoresistance: Tumor-associated stromal cells protect tumor cells from cell death. Int. J. Mol. Sci. 2012, 13, 9545–9571. [Google Scholar] [CrossRef] [PubMed]
- Puhr, H.C.; Karner, A.; Taghizadeh, H.; Jomrich, G.; Schoppmann, S.F.; Preusser, M.; Ilhan-Mutlu, A. Clinical characteristics and comparison of the outcome in young versus older patients with upper gastrointestinal carcinoma. J. Cancer Res. Clin. Oncol. 2020, 146, 3313–3322. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Punt, C.J.; Koopman, M.; Vermeulen, L. From tumour heterogeneity to advances in precision treatment of colorectal cancer. Nat. Rev. Clin. Oncol. 2017, 14, 235–246. [Google Scholar] [CrossRef]
- Wu, M.; Yuan, S.; Liu, K.; Wang, C.; Wen, F. Gastric Cancer Signaling Pathways and Therapeutic Applications. Technol. Cancer Res. Treat. 2024, 23, 15330338241271935. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Scieuzo, C.; Hahn, T.; Zibek, S.; Salvia, R.; Falabella, P. Insect Chitin-Based Nanomaterials for Innovative Cosmetics and Cosmeceuticals. Cosmetics 2021, 8, 40. [Google Scholar] [CrossRef]
- Scieuzo, C.; Rinaldi, R.; Giglio, F.; Salvia, R.; Ali AlSaleh, M.; Jakše, J.; Pain, A.; Antony, B.; Falabella, P. Identification of Multifunctional Putative Bioactive Peptides in the Insect Model Red Palm Weevil (Rhynchophorus ferrugineus). Biomolecules 2024, 14, 1332. [Google Scholar] [CrossRef]
- Rahman, N.; Gope, A.; Khanrah, J.; Rawani, A. Drug Discovery Potential of Insect-Derived Compounds: A review. Curr. Drug Discov. Technol. 2024, 22, e300424229557. [Google Scholar] [CrossRef]
- Pascale, M.; Laurino, S.; Vogel, H.; Grimaldi, A.; Monné, M.; Riviello, L.; Tettamanti, G.; Falabella, P. The Lepidopteran endoribonuclease-U domain protein P102 displays dramatically reduced enzymatic activity and forms functional amyloids. Dev. Comp. Immunol. 2014, 47, 129–139. [Google Scholar] [CrossRef]
- Labella, C.; Kanawati, B.; Vogel, H.; Schmitt-Kopplin, P.; Laurino, S.; Bianco, G.; Falabella, P. Identification of two arginine kinase forms of endoparasitoid Leptomastix dactylopii venom by bottom up-sequence tag approach. J. Mass. Spectrom. 2015, 50, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; Falabella, P. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef] [PubMed]
- Adamski, Z.; Bufo, S.A.; Chowański, S.; Falabella, P.; Lubawy, J.; Marciniak, P.; Pacholska-Bogalska, J.; Salvia, R.; Scrano, L.; Słocińska, M.; et al. Beetles as Model Organisms in Physiological, Biomedical and Environmental Studies—A Review. Front. Physiol. 2019, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, E.; Podsiadlowski, L.; Muhammed, M.; Vilcinskas, A. Diversity, evolution and medical applications of insect antimicrobial peptides. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150290. [Google Scholar] [CrossRef]
- Nishide, Y.; Nagamine, K.; Kageyama, D.; Moriyama, M.; Futahashi, R.; Fukatsu, T. A new antimicrobial peptide, Pentatomicin, from the stinkbug Plautia stali. Sci. Rep. 2022, 12, 16503. [Google Scholar] [CrossRef]
- Huang, Y.B.; Wang, X.F.; Wang, H.Y.; Liu, Y.; Chen, Y. Studies on mechanism of action of anticancer peptides by modulation of hydrophobicity within a defined structural framework. Mol. Cancer Ther. 2011, 10, 416–426. [Google Scholar] [CrossRef]
- Chen, C.; Hu, J.; Zeng, P.; Pan, F.; Yaseen, M.; Xu, H.; Lu, J.R. Molecular mechanisms of anticancer action and cell selectivity of short α-helical peptides. Biomaterials 2014, 35, 1552–1561. [Google Scholar] [CrossRef]
- Gaspar, D.; Veiga, A.S.; Castanho, M.A. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013, 4, 294. [Google Scholar] [CrossRef]
- Sahalan, A.Z.; Omar, B.; Mohamed, A.Y.; Jeffery, J. Antibacterial activity of extracted hemolymph from larvae and pupae of local fly species, Musca domestica and Chrysomya megacephala. J. Sains Kesihat. Malays. 2007, 4, 1–11. [Google Scholar]
- Capparelli, R.; Romanelli, A.; Iannaccone, M.; Nocerino, N.; Ripa, R.; Pensato, S.; Pedone, C.; Iannelli, D. Synergistic antibacterial and anti-inflammatory activity of temporin A and modified temporin B in vivo. PLoS ONE 2009, 4, e7191. [Google Scholar] [CrossRef]
- Stączek, S.; Cytryńska, M.; Zdybicka-Barabas, A. Unraveling the role of antimicrobial peptides in insects. Int. J. Mol. Sci. 2023, 24, 5753. [Google Scholar] [CrossRef]
- Tonk, M.; Vilcinskas, A.; Rahnamaeian, M. Insect antimicrobial peptides: Potential tools for the prevention of skin cancer. Appl. Microbiol. Biotechnol. 2016, 100, 7397–7405. [Google Scholar] [CrossRef] [PubMed]
- Suttmann, H.; Retz, M.; Paulsen, F.; Harder, J.; Zwergel, U.; Kamradt, J.; Wullich, B.; Unteregger, G.; Stöckle, M.; Lehmann, J. Antimicrobial peptides of the Cecropin-family show potent antitumor activity against bladder cancer cells. BMC Urol. 2008, 8, 5. [Google Scholar] [CrossRef]
- Ramos-Martín, F.; Herrera-León, C.; D’Amelio, N. Bombyx mori Cecropin D could trigger cancer cell apoptosis by interacting with mitochondrial cardiolipin. Biochim. Biophys. Acta 2022, 1864, 184003. [Google Scholar] [CrossRef] [PubMed]
- Parvy, J.P.; Yu, Y.; Dostalova, A.; Kondo, S.; Kurjan, A.; Bulet, P.; Lemaître, B.; Vidal, M.; Cordero, J.B. The antimicrobial peptide defensin cooperates with tumour necrosis factor to drive tumour cell death in Drosophila. Elife 2019, 8, e45061. [Google Scholar] [CrossRef] [PubMed]
- Rajasekhar, N.; Ramesh, N.; Prashantha, C.N. Isolation and Characterization of Hermetia Illucens Larval Protein for the Assessment of Inhibitory Activity against MCF7 and HeLa cell Lines. Intern. J. Innovat. Technol. Explor. Eng. 2020, 9, 3045–3050. [Google Scholar] [CrossRef]
- Zhou, L.; Meng, G.; Zhu, L.; Ma, L.; Chen, K. Insect Antimicrobial Peptides as Guardians of Immunity and Beyond: A Review. Int. J. Mol. Sci. 2024, 25, 3835. [Google Scholar] [CrossRef]
- Choi, W.; Yun, J.; Chu, J.; Chu, K. Antibacterial effect of extracts of Hermetia illucens (Diptera: Stratiomyidae) larvae against Gram-negative bacteria. Entomol. Res. 2012, 42, 219–226. [Google Scholar] [CrossRef]
- De Smet, J.; Wynants, E.; Cos, P.; Leen, C.; Campenhout, V. Microbial Community Dynamics during Rearing of Black Soldier Fly Larvae (Hermetia illucens) and Impact on Exploitation Potential. Appl. Environ. Microbiol. 2018, 84, e02722-17. [Google Scholar] [CrossRef]
- Elhag, O.; Zhou, D.; Song, Q.; Soomro, A.A.; Cai, M.; Zheng, L.; Yu, Z.; Zhang, J. Screening, expression, purification and functional characterization of novel antimicrobial peptide genes from Hermetia illucens (L.). PLoS ONE 2017, 12, e0169582. [Google Scholar] [CrossRef]
- Lee, K.S.; Yun, E.Y.; Goo, T.W. Antimicrobial activity of an extract of Hermetia illucens larvae immunized with lactobacillus casei against salmonella species. Insects 2020, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Chang, B.S.; Yoe, S.M. Detection of antimicrobial substances from larvae of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Entomol. Res. 2014, 44, 58–64. [Google Scholar] [CrossRef]
- Park, S.-I.; Kim, J.-W.; Yoe, S.M. Purification and characterization of a novel antibacterial peptide from black soldier fly (Hermetia illucens) larvae. Dev. Comp. Immunol. 2015, 52, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Ishibashi, J.; Tanaka, H.; Sato, M.; Asaoka, A.; Taylor, D.; Yamakawa, M. Selective cancer cell cytotoxicity of enantiomeric 9-mer peptides derived from beetle defensins depends on negatively charged phosphatidylserine on the cell surface. Peptides 2009, 30, 660–668. [Google Scholar] [CrossRef]
- Chowanski, S.; Adamski, Z.; Lubawy, J.; Marciniak, P.; Pacholska-Bogalska, J.; Slocinska, M.; Spochacz, M.; Szymczak, M.; Urbanski, A.; Walkowiak-Nowicka, K.; et al. Insect Peptides—Perspectives in Human Diseases Treatment. Curr. Med. Chem. 2017, 24, 3116–3152. [Google Scholar] [CrossRef]
- Koszałka, P.; Kamysz, E.; Wejda, M.; Kamysz, W.; Bigda, J. Antitumor activity of antimicrobial peptides against U937 histiocytic cell line. Acta Biochim. Pol. 2011, 58, 111–117. [Google Scholar] [CrossRef]
- Zhao, S.; Tan, J.; Yu, H.M.; Tian, Y.; Wu, Y.F.; Luo, R.; Guo, J.J. In vivo and in vitro antiproliferative and antimetastatic effects of hemolymph of Aspongopus chinensis Dallas on breast cancer cell. J. Tradit. Chin. Med. 2021, 41, 523–529. [Google Scholar]
- Orozco-Flores, A.A.; Valadez-Lira, J.A.; Covarrubias-Cárdenas, K.E.; Pérez-Trujillo, J.J.; Gomez-Flores, R.; Caballero-Hernández, D.; Tamez-Guerra, R.; Rodríguez-Padilla, C.; Tamez-Guerra, P. In vitro antitumor, pro-inflammatory, and pro-coagulant activities of Megalopyge opercularis J.E. Smith hemolymph and spine venom. Sci. Rep. 2020, 10, 18395. [Google Scholar] [CrossRef]
- Mahmoud, S.; Abou El-Khashab, L.; Moselhy, W.; Zayed, A.; Salama, M. In vitro anti-cancer activity of larval hemolymph and fat body of flesh fly Sarcophaga argyrostoma (Diptera: Sarcophagidae). Adv. Entomol. 2020, 8, 93. [Google Scholar] [CrossRef]
- Januszanis, B.; Staczek, S.; Zdybicka, A.B.; Badziul, D.; Jakubowicz-Gil, J.; Langner, E.; Rzeski, W.; Cytrynska, M. The effect of Galleria mellonella polypeptides on human brain glioblastoma multiforme cell line-a preliminary study. Ann. Univ. Mariae Curie-Sklodowska 2012, 67, 53. [Google Scholar]
- Qu, B.; Yuan, J.; Liu, X.; Zhang, S.; Ma, X.; Lu, L. Anticancer activities of natural antimicrobial peptides from animals. Front. Microbiol. 2024, 14, 1321386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.T.; Liu, Z.D.; Wang, Z.; Wang, T.; Wang, N.; Wang, N.; Zhang, B.; Zhao, Y.F. Recent advances in small peptides of marine origin in cancer therapy. Mar. Drugs 2021, 19, 115. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri, N.; Javidan, M.; Sheikhi, S.; Taştan, Ö.; Parodi, A.; Liao, Z.; Azar, M.T.; Ganjalıkhani-Hakemi, M. Bioactive peptides: An alternative therapeutic approach for cancer management. Front. Immunol. 2024, 15, 1310443. [Google Scholar] [CrossRef]
- Bakare, O.O.; Gokul, A.; Wu, R.; Niekerk, L.A.; Klein, A.; Keyster, M. Biomedical relevance of novel anticancer peptides in the sensitive treatment of cancer. Biomolecules 2021, 11, 1120. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- Gortany, N.K.; Panahi, G.; Ghafari, H.; Shekari, M.; Ghazi-Khansari, M. Foretinib induces G2/M cell cycle arrest, apoptosis, and invasion in human glioblastoma cells through c-MET inhibition. Cancer Chemother. Pharmacol. 2021, 87, 827–842. [Google Scholar] [CrossRef]
- Xu, P.; Cai, X.; Zhang, W.; Li, Y.; Qiu, P.; Lu, D.; He, X. Flavonoids of Rosa roxburghii Tratt exhibit radioprotection and anti-apoptosis properties via the Bcl-2(Ca2+)/Caspase-3/PARP-1 pathway. Apoptosis 2016, 21, 1125–1143. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Park, J.H.; Kim, M.J.; Kim, W.J.; Ha, K.T.; Choi, B.T.; Lee, S.Y.; Shin, H.K. Isolinderalactone regulates the BCL-2/caspase-3/PARP pathway and suppresses tumor growth in a human glioblastoma multiforme xenograft mouse model. Cancer Lett. 2019, 443, 25–33. [Google Scholar] [CrossRef]
- Wei, J.; Huang, Y.; Wu, N.; Yu, L.; Liu, B. KRAS mutation and protein levels in gastric cancer patients and response to MEK inhibitors. Ann. Oncol. 2016, 27, vi214. [Google Scholar] [CrossRef]
- Ke, X.; Qin, Q.; Deng, T.; Liao, Y.; Gao, S.J. Heterogeneous Responses of Gastric Cancer Cell Lines to Tenovin-6 and Synergistic Effect with Chloroquine. Cancers 2020, 12, 365. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, X.; Yu, P.; Chen, X.; Lu, P.; Li, M.; Liu, X.; Li, Z.; Wei, F.; Wang, K.; et al. Cinobufagin Induces Cell Cycle Arrest at the G2/M Phase and Promotes Apoptosis in Malignant Melanoma Cells. Front. Oncol. 2019, 9, 853. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, S.; Kwon, Y.S.; Lee, M.G.; Lee, K.S.; Nam, K.S. Cell cycle arrest-mediated cell death by morin in MDA-MB-231 triple-negative breast cancer cells. Pharmacol. Rep. 2021, 73, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Cariou, S.; Donovan, J.C.; Flanagan, W.M.; Milic, A.; Bhattacharya, N.; Slingerland, J.M. Down-regulation of p21WAF1/CIP1 or p27Kip1 abrogates antiestrogen-mediated cell cycle arrest in human breast cancer cells. Proc. Natl. Acad. Sci. USA 2000, 97, 9042–9046. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, M.T.; Crispi, S. The dual role played by p21 may influence the apoptotic or anti-apoptotic fate in cancer. J. Cancer Res. Updates 2012, 1, 189–202. [Google Scholar] [CrossRef]
- Cazzalini, O.; Scovassi, A.I.; Savio, M.; Stivala, L.A.; Prosperi, E. Multiple roles of the cell cycle inhibitor p21(CDKN1A) in the DNA damage response. Mutat. Res. 2010, 704, 12–20. [Google Scholar] [CrossRef]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 2016, 42, 63–71. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Fagundes, R.; Teixeira, L.K. Cyclin E/CDK2: DNA replication, replication stress and genomic instability. Front. Cell Dev. Biol. 2021, 9, 774845. [Google Scholar] [CrossRef]
- Lu, X.; Liu, J.; Legerski, R.J. Cyclin E is stabilized in response to replication fork barriers leading to prolonged S phase arrest. J. Biol. Chem. 2009, 284, 35325–35337. [Google Scholar] [CrossRef]
- Razavipour, S.F.; Harikumar, K.B.; Slingerland, J.M. p27 as a transcriptional regulator: New roles in development and cancer. Cancer Res. 2020, 80, 3451–3458. [Google Scholar] [CrossRef]
- Durand, B.; Gao, F.B.; Raff, M. Accumulation of the cyclin-dependent kinase inhibitor p27/Kip1 and the timing of oligodendrocyte differentiation. EMBO J. 1997, 16, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Drexler, H.; Pebler, S. Inducible p27Kip1 expression inhibits proliferation of K562 cells and protects against apoptosis induction by proteasome inhibitors. Cell Death Differ. 2003, 10, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.A.; Reichhart, J.M. Drosophila innate immunity: An evolutionary perspective. Nat. Immunol. 2002, 3, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wuang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, R.; Laurino, S.; Salvia, R.; Russi, S.; De Stefano, F.; Galasso, R.; Sgambato, A.; Scieuzo, C.; Falco, G.; Falabella, P. Biological Activity of Peptide Fraction Derived from Hermetia illucens L. (Diptera: Stratiomyidae) Larvae Haemolymph on Gastric Cancer Cells. Int. J. Mol. Sci. 2025, 26, 1885. https://doi.org/10.3390/ijms26051885
Rinaldi R, Laurino S, Salvia R, Russi S, De Stefano F, Galasso R, Sgambato A, Scieuzo C, Falco G, Falabella P. Biological Activity of Peptide Fraction Derived from Hermetia illucens L. (Diptera: Stratiomyidae) Larvae Haemolymph on Gastric Cancer Cells. International Journal of Molecular Sciences. 2025; 26(5):1885. https://doi.org/10.3390/ijms26051885
Chicago/Turabian StyleRinaldi, Roberta, Simona Laurino, Rosanna Salvia, Sabino Russi, Federica De Stefano, Rocco Galasso, Alessandro Sgambato, Carmen Scieuzo, Geppino Falco, and Patrizia Falabella. 2025. "Biological Activity of Peptide Fraction Derived from Hermetia illucens L. (Diptera: Stratiomyidae) Larvae Haemolymph on Gastric Cancer Cells" International Journal of Molecular Sciences 26, no. 5: 1885. https://doi.org/10.3390/ijms26051885
APA StyleRinaldi, R., Laurino, S., Salvia, R., Russi, S., De Stefano, F., Galasso, R., Sgambato, A., Scieuzo, C., Falco, G., & Falabella, P. (2025). Biological Activity of Peptide Fraction Derived from Hermetia illucens L. (Diptera: Stratiomyidae) Larvae Haemolymph on Gastric Cancer Cells. International Journal of Molecular Sciences, 26(5), 1885. https://doi.org/10.3390/ijms26051885







