No Benefit from Hydroxyurea Pre-Treatment in Frontline Chronic Myeloid Leukemia Therapy and Evidence of Quantitative Changes in the BCR::ABL1 Transcript Level
Abstract
1. Introduction
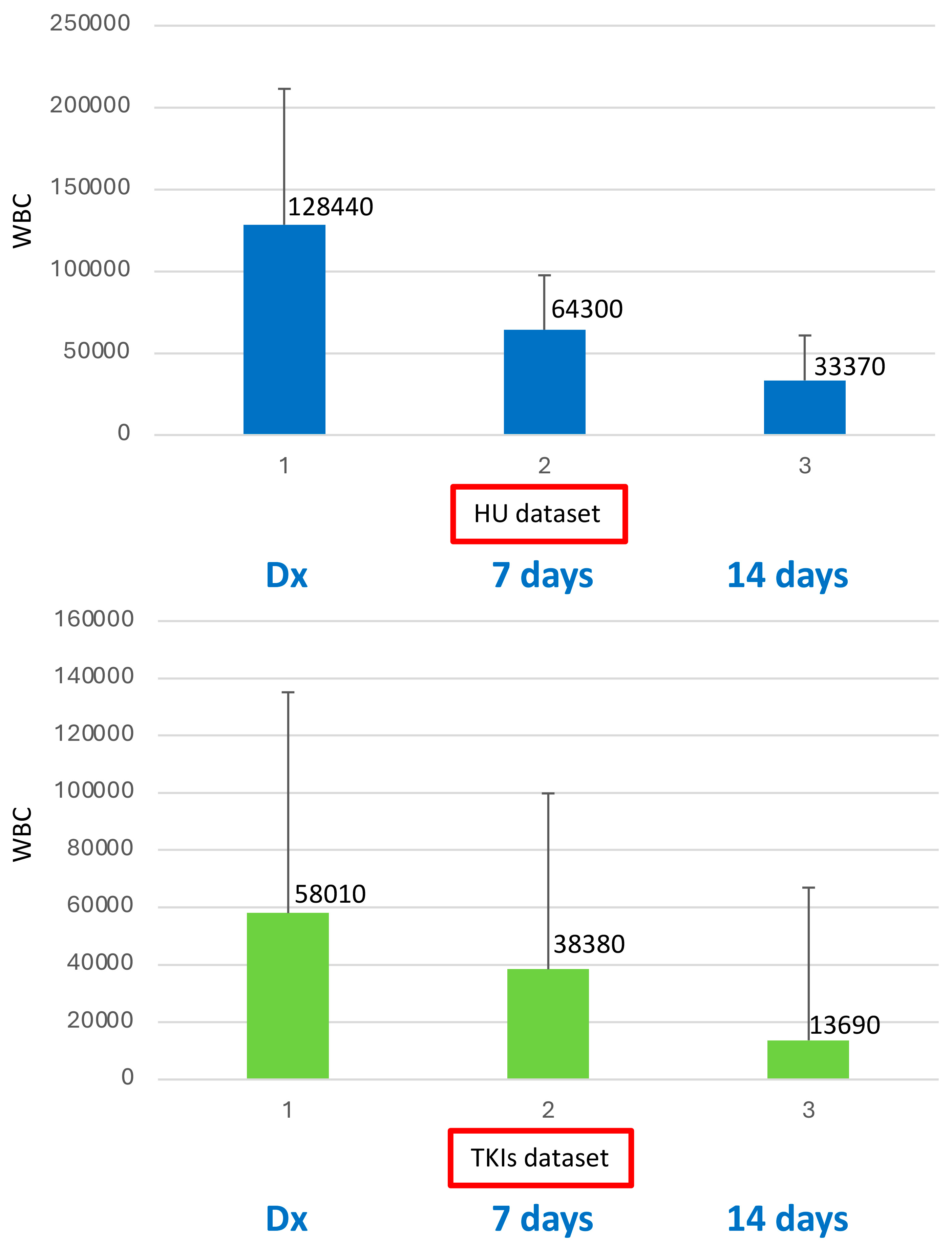
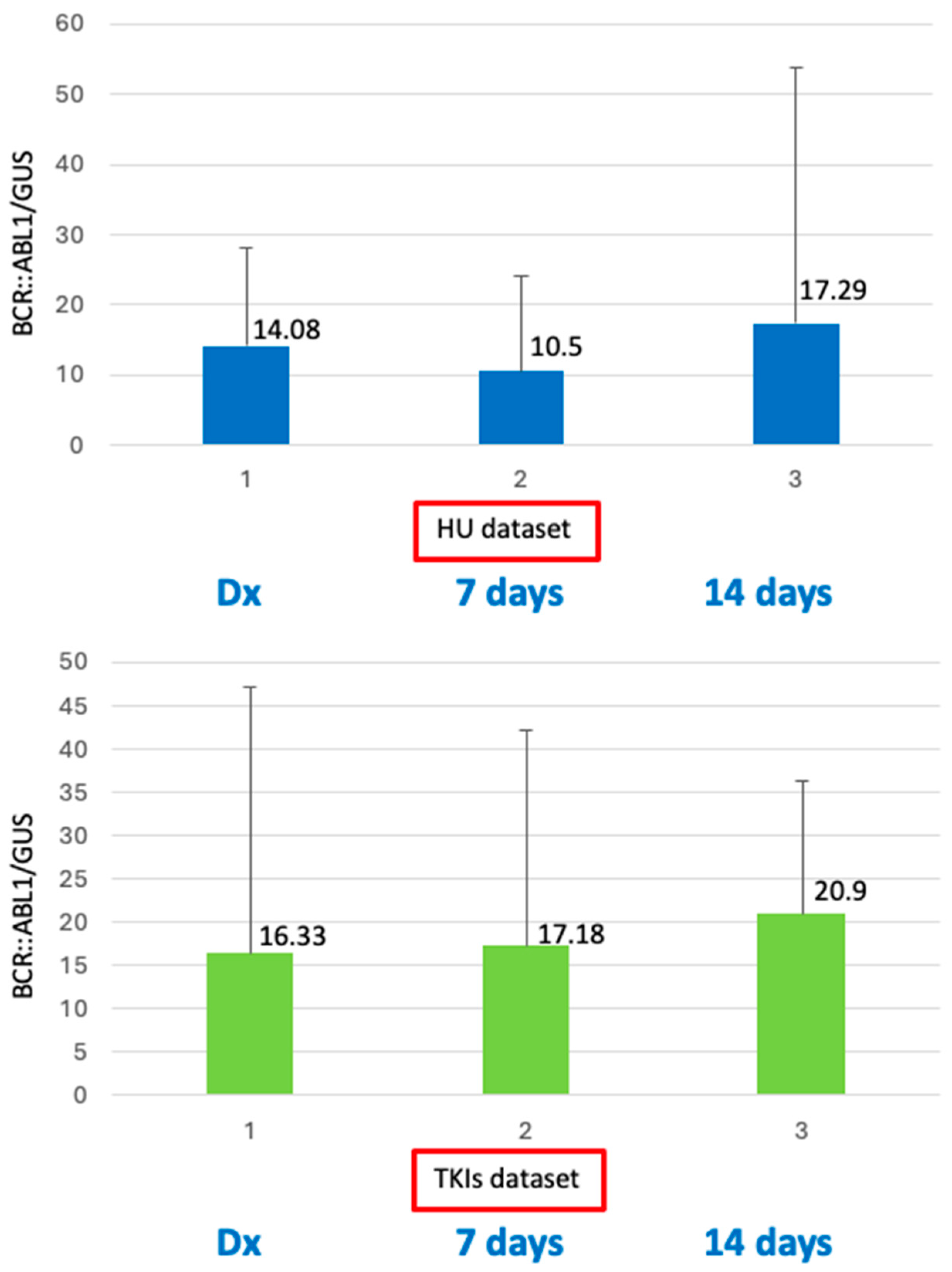
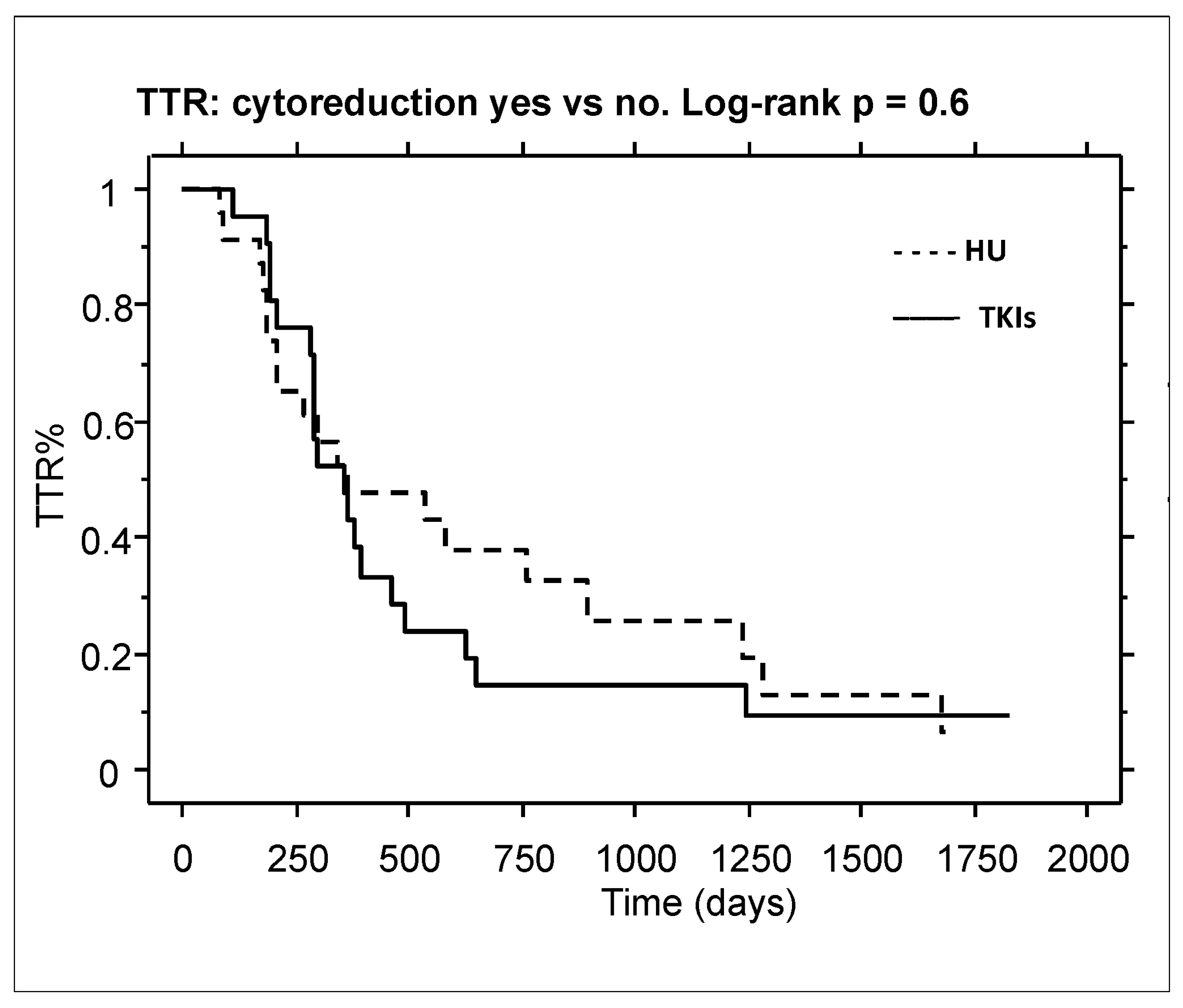
2. Results
3. Discussion
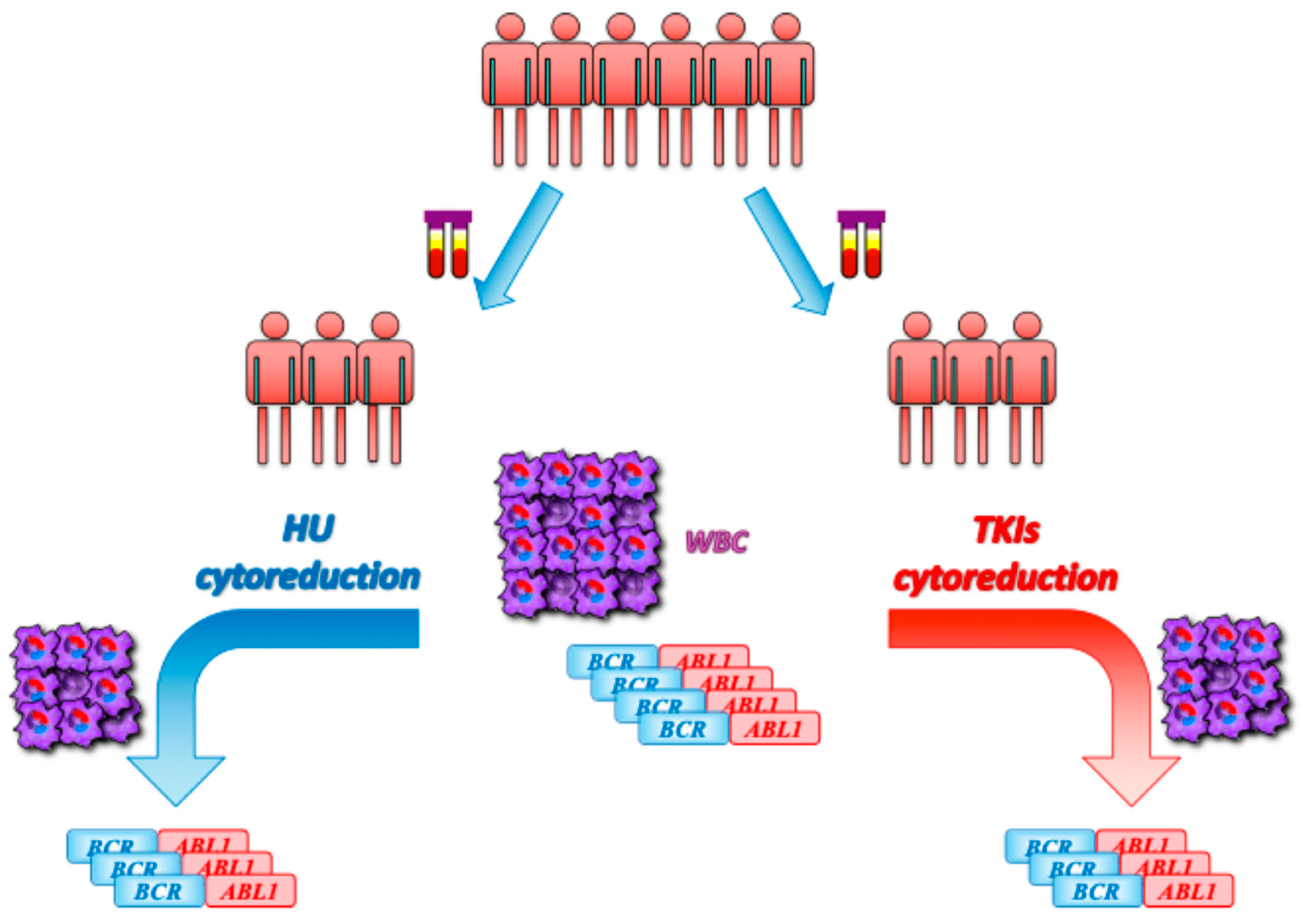
4. Patients and Methods
Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2025 update on diagnosis, therapy, and monitoring. Am. J. Hematol. 2024, 99, 2191–2212. [Google Scholar] [CrossRef] [PubMed]
- Baccarani, M.; Saglio, G.; Goldman, J.; Hochhaus, A.; Simonsson, B.; Appelbaum, F.; Apperley, J.; Cervantes, F.; Cortes, J.; Deininger, M.; et al. Evolving concepts in the management of chronic myeloid leukemia: Recommendations from an expert panel on behalf of the European Leukemia Net. Blood 2006, 108, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Baccarani, M.; Cortes, J.; Pane, F.; Niederwieser, D.; Saglio, G.; Apperley, J.; Cervantes, F.; Deininger, M.; Gratwohl, A.; Guilhot, F.; et al. Chronic myeloid leukemia: An update of concepts and management recommendations of European Leukemia Net. J. Clin. Oncol. 2009, 27, 6041–6051. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baccarani, M.; Deininger, M.W.; Rosti, G.; Hochhaus, A.; Soverini, S.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Guilhot, F.; et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood 2013, 122, 872–884. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stagno, F.; Stella, S.; Spitaleri, A.; Pennisi, M.S.; Di Raimondo, F.; Vigneri, P. Imatinib mesylate in chronic myeloid leukemia: Frontline treatment and long-term outcomes. Expert. Rev. Anticancer. Ther. 2016, 16, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Steegmann, J.L.; Baccarani, M.; Breccia, M.; Casado, L.F.; García-Gutiérrez, V.; Hochhaus, A.; Kim, D.W.; Kim, T.D.; Khoury, H.J.; Le Coutre, P.; et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia 2016, 30, 1648–1671. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hochhaus, A.; Baccarani, M.; Silver, R.T.; Schiffer, C.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Deininger, M.W.; Guilhot, F.; et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 2020, 34, 966–984. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hehlmann, R.; Berger, U.; Pfirrmann, M.; Hochhaus, A.; Metzgeroth, G.; Maywald, O.; Hasford, J.; Reiter, A.; Hossfeld, D.K.; Kolb, H.J.; et al. Randomized comparison of interferon alpha and hydroxyurea with hydroxyurea monotherapy in chronic myeloid leukemia (CML-study II): Prolongation of survival by the combination of interferon alpha and hydroxyurea. Leukemia 2003, 17, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Talpaz, M.; Mercer, J.; Hehlmann, R. The interferon-alpha revival in CML. Ann. Hematol. 2015, 94 (Suppl. 2), S195–S207. [Google Scholar] [CrossRef] [PubMed]
- Vigneri, P.; Stagno, F.; Stella, S.; Cupri, A.; Forte, S.; Massimino, M.; Antolino, A.; Siragusa, S.; Mannina, D.; Impera, S.S.; et al. High BCR-ABL/GUSIS Levels at Diagnosis of Chronic Phase CML Are Associated with Unfavorable Responses to Standard-Dose Imatinib. Clin. Cancer Res. 2017, 23, 7189–7198. [Google Scholar] [CrossRef] [PubMed]
- Hanfstein, B.; Müller, M.C.; Hehlmann, R.; Erben, P.; Lauseker, M.; Fabarius, A.; Schnittger, S.; Haferlach, C.; Göhring, G.; Proetel, U.; et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML). Leukemia 2012, 26, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Hehlmann, R.; Heimpel, H.; Hasford, J.; Kolb, H.J.; Pralle, H.; Hossfeld, D.K.; Queisser, W.; Löffler, H.; Heinze, B.; Georgii, A.; et al. Randomized comparison of busulfan and hydroxyurea in chronic myelogenous leukemia: Prolongation of survival by hydroxyurea. The German CML Study Group. Blood 1993, 82, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Lange, T.; Niederwieser, C.; Gil, A.; Krahl, R.; von Grünhagen, U.; Al-Ali, H.K.; Jentsch-Ullrich, K.; Spohn, C.; Lakner, V.; Assmann, M.; et al. No advantage of Imatinib in combination with hydroxyurea over Imatinib monotherapy: A study of the East German Study Group (OSHO) and the German CML study group. Leuk. Lymphoma 2020, 61, 2821–2830. [Google Scholar] [CrossRef] [PubMed]
- Perrotti, D.; Jamieson, C.; Goldman, J.; Skorski, T. Chronic myeloid leukemia: Mechanisms of blastic transformation. J. Clin. Investig. 2010, 120, 2254–2264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hochhaus, A.; Wang, J.; Kim, D.-W.; Kim, D.D.H.; Mayer, J.; Goh, Y.-T.; le Coutre, P.; Takahashi, N.N.; Kim, I.; Etienne, G.; et al. Asciminib in newly diagnosed Chronic Myeloid Leukemia. N. Engl. J. Med. 2024, 391, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Yeung, D.T.; Shanmuganathan, N.; Reynolds, J.; Branford, S.; Walia, M.; Yong, A.S.M.; Shortt, J.; Chee, L.; Viiala, N.; Cunningham, I.; et al. Asciminib monotherapy as frontline treatment of chronic-phase chronic myeloid leukemia: Results from the ASCEND study. Blood 2024, 144, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Kockerols, C.C.B.; Geelen, I.; Levin, M.D.; Janssen, J.J.W.M.; Dinmohamed, A.G.; Hoogendoorn, M.; Cornelissen, J.J.; Westerweel, P.E. The use of hydroxyurea pretreatment in chronic myeloid leukemia in the current tyrosine kinase inhibitor era. Haematologica. 2022, 107, 1940–1943. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Komic, H.; Nilsson, M.S.; Wennström, L.; Bandaru, T.S.; Jaako, P.; Hellstrand, K.; Thorén, F.B.; Martner, A. Single-cell proteo-transcriptomic profiling reveals altered characteristics of stem and progenitor cells in patients receiving cytoreductive hydroxyurea in early-phase chronic myeloid leukemia. Haematologica 2024. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Massimino, M.; Stella, S.; Tirrò, E.; Pennisi, M.S.; Stagno, F.; Vitale, S.R.; Romano, C.; Tomarchio, C.; Parrinello, N.L.; Manzella, L.; et al. High BCR::ABL1 Expression Defines CD34+ Cells with Significant Alterations in Signal Transduction, Short-Proliferative Potential and Self-Renewal Ability. Onco Targets Ther. 2023, 16, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Chelysheva, E.; Apperley, J.; Turkina, A.; Yassin, M.A.; Rea, D.; Nicolini, F.E.; Barraco, D.; Kazakbaeva, K.; Saliev, S.; Abulafia, A.S.; et al. Chronic myeloid leukemia diagnosed in pregnancy: Management and outcome of 87 patients reported to the European LeukemiaNet international registry. Leukemia 2024, 38, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.C.; Erben, P.; Saglio, G.; Gottardi, E.; Nyvold, C.G.; Schenk, T.; Ernst, T.; Lauber, S.; Kruth, J.; Hehlmann, R.; et al. Harmonization of BCR-ABL mRNA quantification using a uniform multifunctional control plasmid in 37 international laboratories. Leukemia 2008, 22, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Vella, V.; Puppin, C.; Damante, G.; Vigneri, R.; Sanfilippo, M.; Vigneri, P.; Tell, G.; Frasca, F. DeltaNp73alpha inhibits PTEN expression in thyroid cancer cells. Int. J. Cancer. 2009, 124, 2539–2548. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.; Deininger, M.; Hochhaus, A.; Branford, S.; Radich, J.; Kaeda, J.; Baccarani, M.; Cortes, J.; Cross, N.C.; Druker, B.J.; et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: Review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood 2006, 108, 28–37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cross, N.C.; White, H.E.; Müller, M.C.; Saglio, G.; Hochhaus, A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia 2012, 26, 2172–2175. [Google Scholar] [CrossRef] [PubMed]
- Cross, N.C.P.; Ernst, T.; Branford, S.; Cayuela, J.M.; Deininger, M.; Fabarius, A.; Kim, D.D.H.; Machova Polakova, K.; Radich, J.P.; Hehlmann, R.; et al. European LeukemiaNet laboratory recommendations for the diagnosis and management of chronic myeloid leukemia. Leukemia 2023, 37, 2150–2167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| HU | TKIs | |
|---|---|---|
| Patients no. | 21 | 24 |
| Age, median years (range) | 58 (18–79) | 64.5 (17–83) |
| Sex (M/F) | 15/6 | 15/9 |
| WBC × 109/L, median (range) | 128.44 (26.5–197) | 58.01 (24.46–368.38) |
| Sokal risk | ||
| - Low | 4 | 9 |
| - Intermediate | 11 | 13 |
| - High | 5 | 2 |
| - ND | 1 | 0 |
| BCR::ABL1 transcript | ||
| - e13a2 | 6 | 14 |
| - e14a2 | 14 | 9 |
| - e13a2-e14a2 | 1 | 1 |
| BCR::ABL1/GUSIS, median % @ diagnosis | 14.08 | 16.33 |
| BCR::ABL1/ABLIS < 10% @ 3 Months | HU Group | TKI Group | p |
|---|---|---|---|
| Optimal response | 16 | 22 | 0.2 |
| Non optimal response | 5 | 2 | |
| ELN responders (EMR) | 76.1% | 91.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stagno, F.; Stella, S.; Leotta, S.; Caserta, S.; Vitale, S.R.; Markovic, U.; Tomarchio, C.; Mirabile, G.; Russo, S.; Massimino, M.; et al. No Benefit from Hydroxyurea Pre-Treatment in Frontline Chronic Myeloid Leukemia Therapy and Evidence of Quantitative Changes in the BCR::ABL1 Transcript Level. Int. J. Mol. Sci. 2025, 26, 1840. https://doi.org/10.3390/ijms26051840
Stagno F, Stella S, Leotta S, Caserta S, Vitale SR, Markovic U, Tomarchio C, Mirabile G, Russo S, Massimino M, et al. No Benefit from Hydroxyurea Pre-Treatment in Frontline Chronic Myeloid Leukemia Therapy and Evidence of Quantitative Changes in the BCR::ABL1 Transcript Level. International Journal of Molecular Sciences. 2025; 26(5):1840. https://doi.org/10.3390/ijms26051840
Chicago/Turabian StyleStagno, Fabio, Stefania Stella, Salvatore Leotta, Santino Caserta, Silvia Rita Vitale, Uros Markovic, Cristina Tomarchio, Giuseppe Mirabile, Sabina Russo, Michele Massimino, and et al. 2025. "No Benefit from Hydroxyurea Pre-Treatment in Frontline Chronic Myeloid Leukemia Therapy and Evidence of Quantitative Changes in the BCR::ABL1 Transcript Level" International Journal of Molecular Sciences 26, no. 5: 1840. https://doi.org/10.3390/ijms26051840
APA StyleStagno, F., Stella, S., Leotta, S., Caserta, S., Vitale, S. R., Markovic, U., Tomarchio, C., Mirabile, G., Russo, S., Massimino, M., Vigneri, P., Manzella, L., Di Raimondo, F., & Allegra, A. (2025). No Benefit from Hydroxyurea Pre-Treatment in Frontline Chronic Myeloid Leukemia Therapy and Evidence of Quantitative Changes in the BCR::ABL1 Transcript Level. International Journal of Molecular Sciences, 26(5), 1840. https://doi.org/10.3390/ijms26051840







