Protocatechualdehyde Induced Breast Cancer Stem Cell Death via the Akt/Sox2 Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. Isolation of a CSC Inhibitor Derived from Artemisia Princeps Fermentation Using Lactobacillus rhamnosus
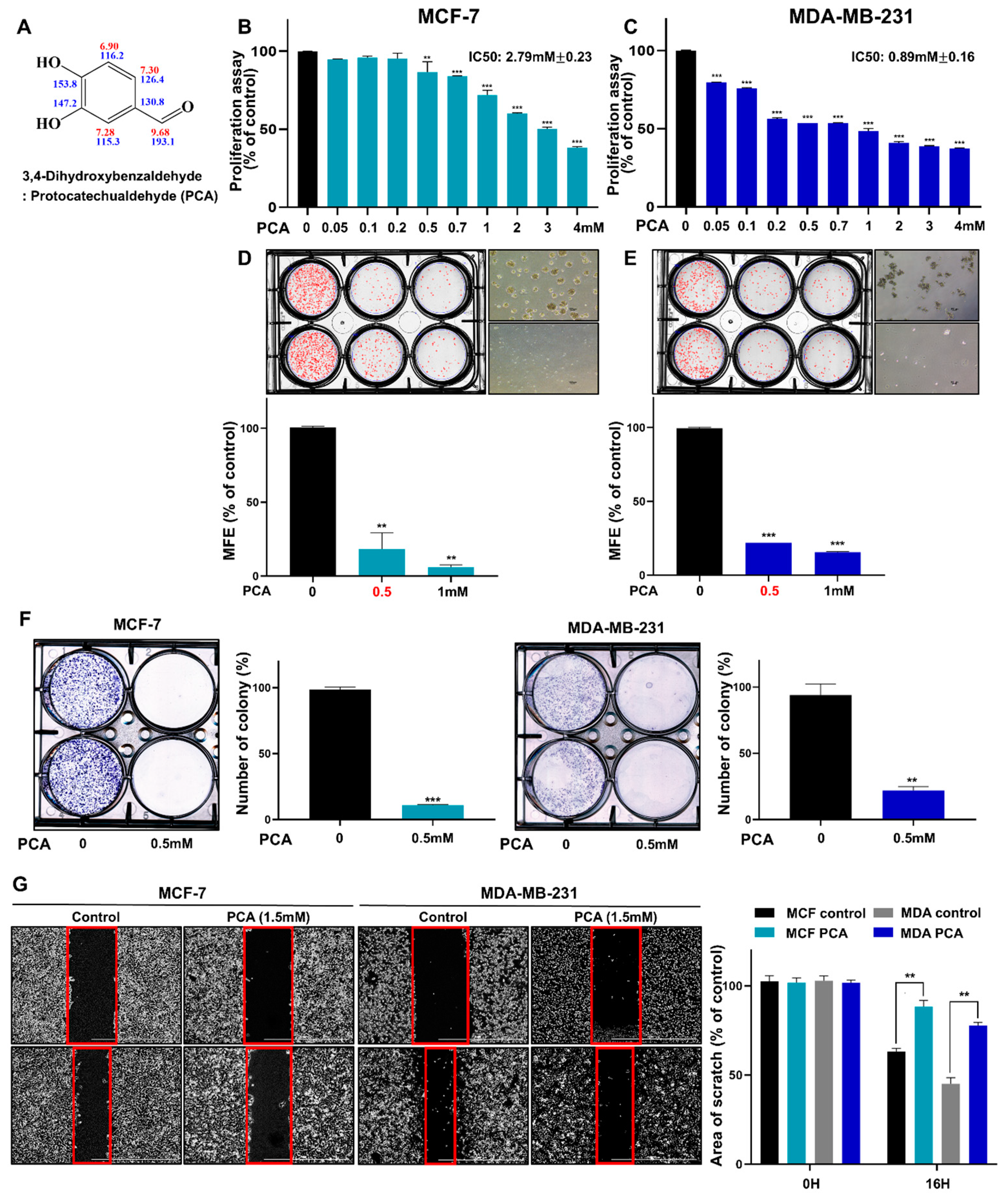
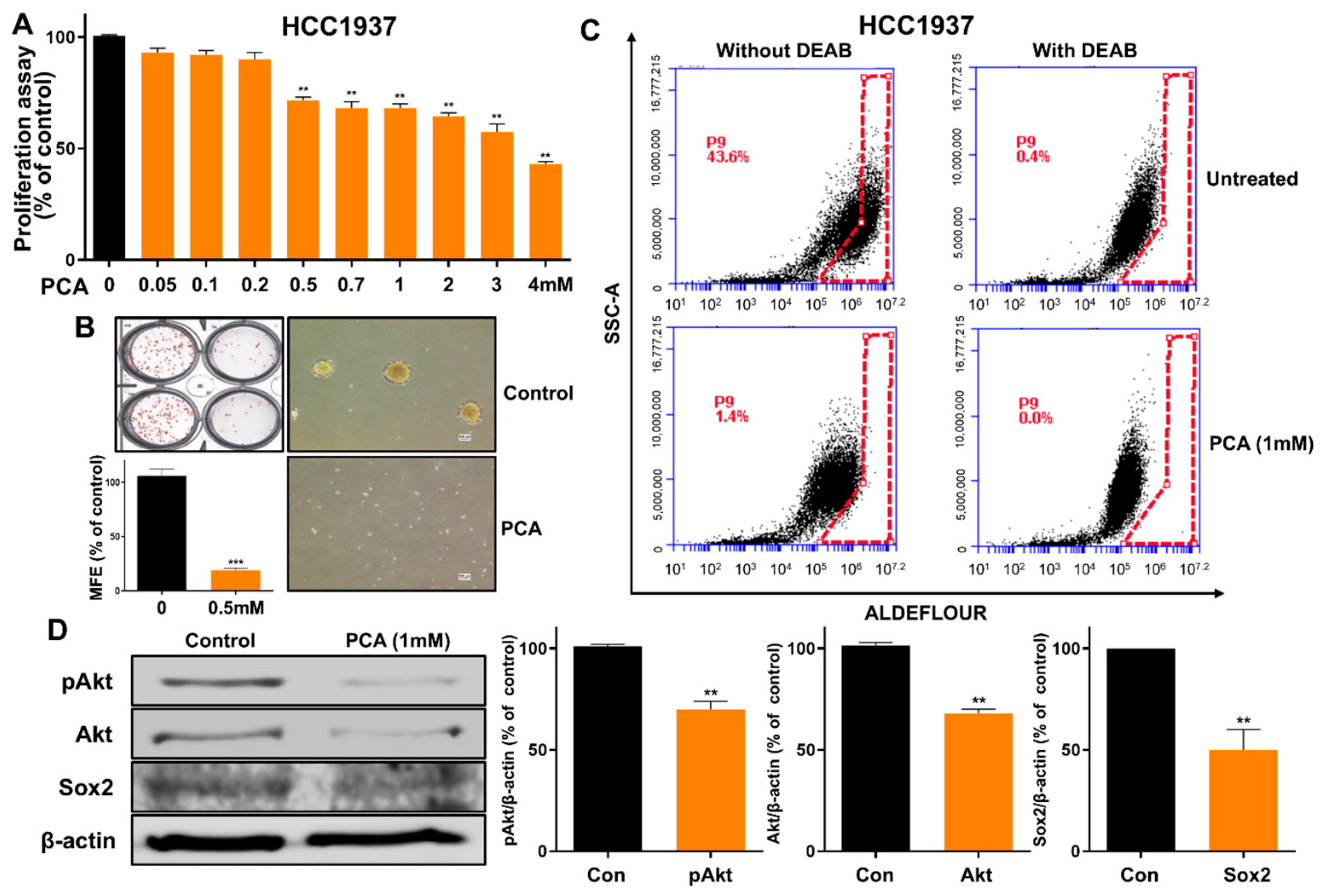
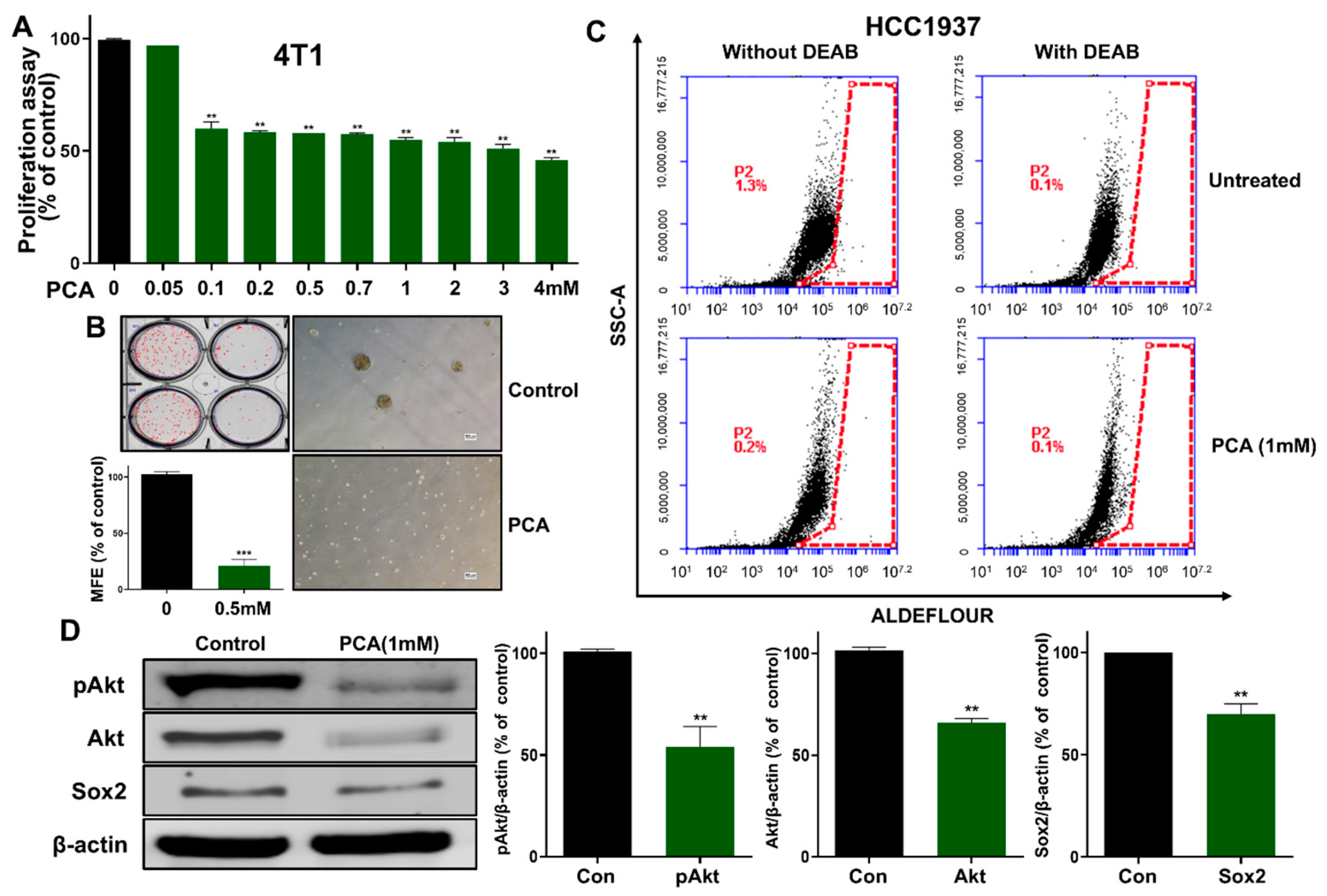
2.2. PCA Suppresses the Growth of BC Cells and the Formation of Mammosphere
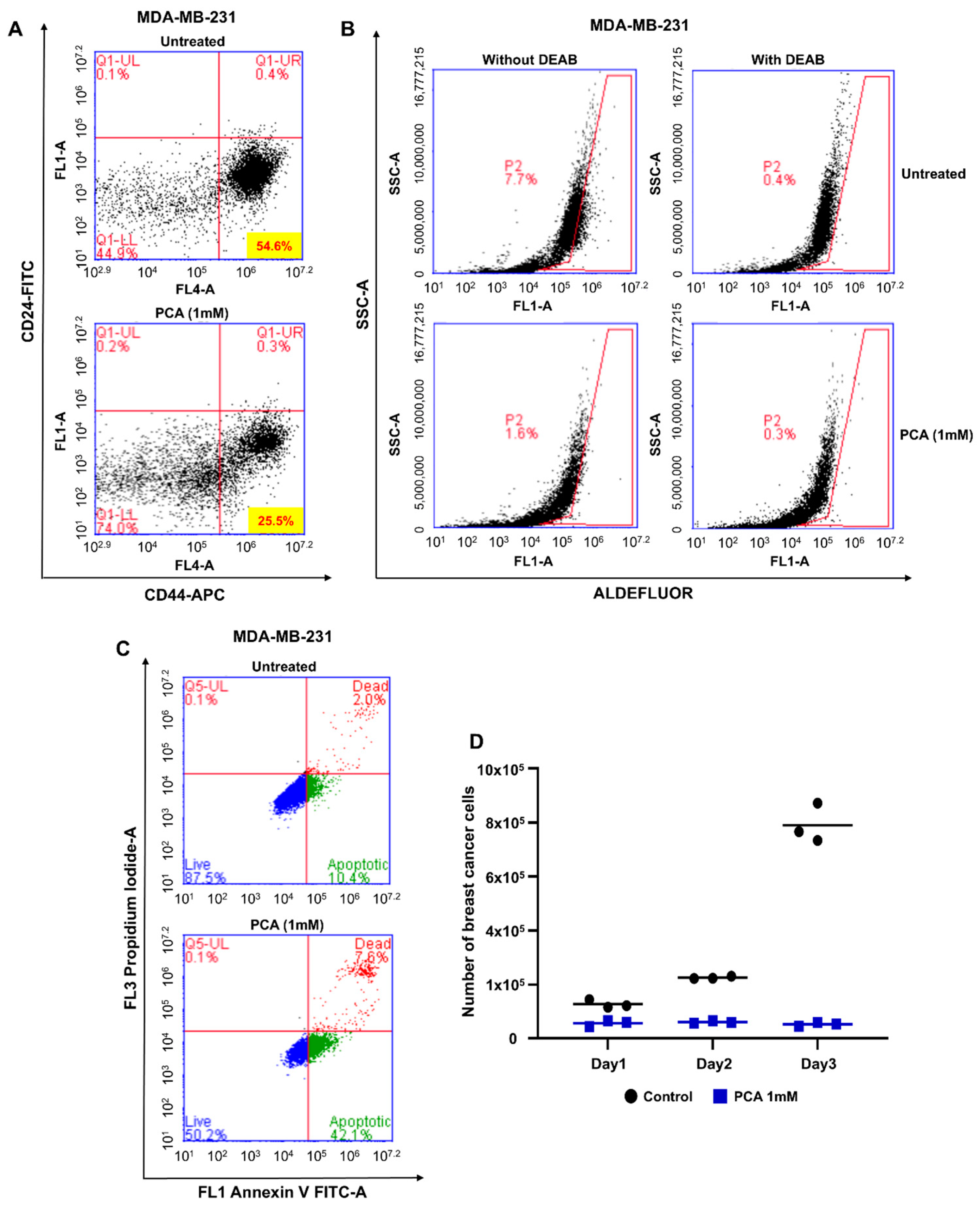
2.3. PCA Reduced CD44high/CD24low-Expressing and ALDH+ Cancer Cells
2.4. PCA Treatment Induces BCSC Apoptosis and Suppresses Mammosphere Proliferation
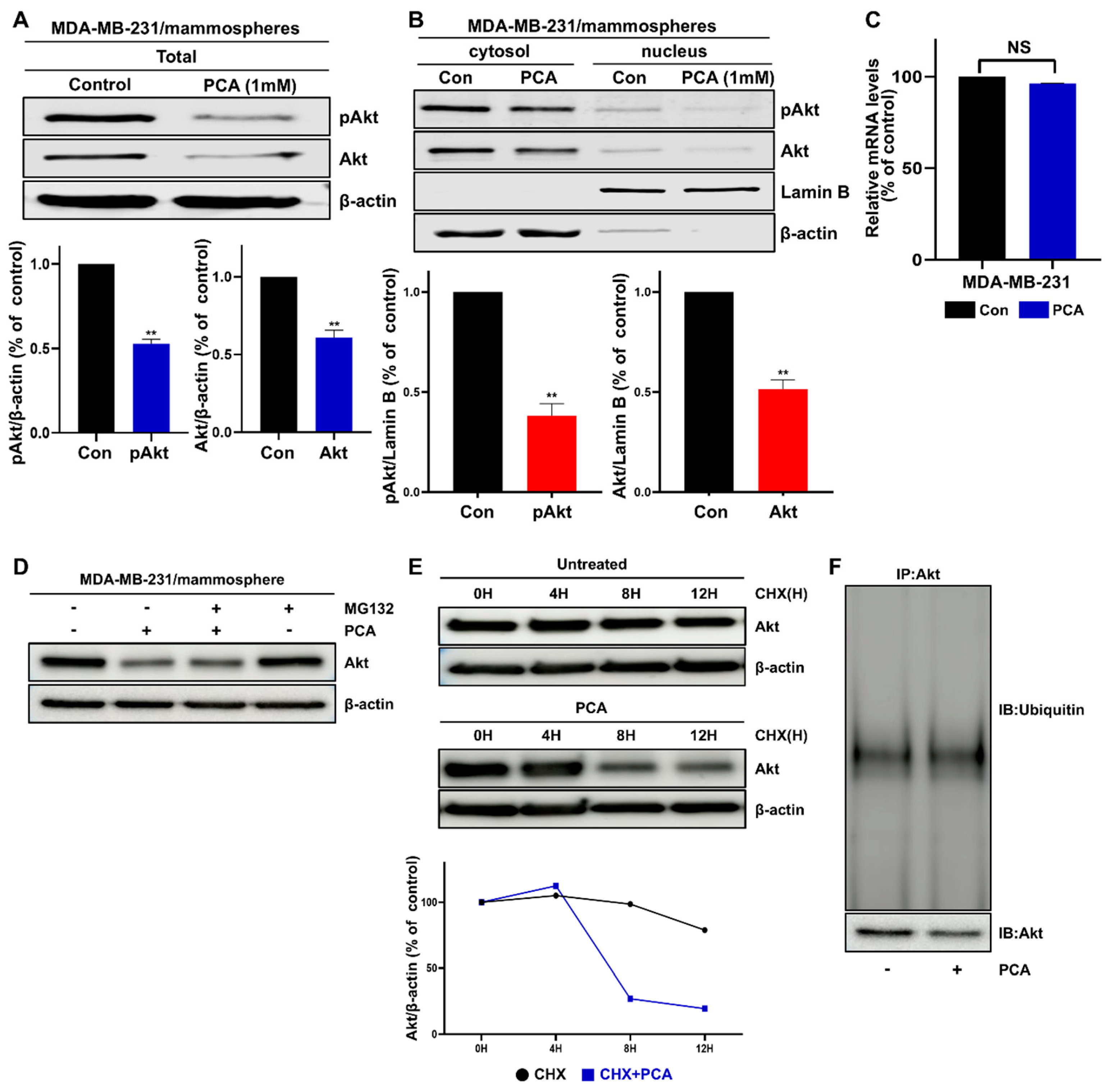
2.5. Effect of PCA on Akt and pAkt Protein Levels in Breast CSCs
2.6. PCA Mediates Akt Degradation in a Ubiquitin-Independent Manner
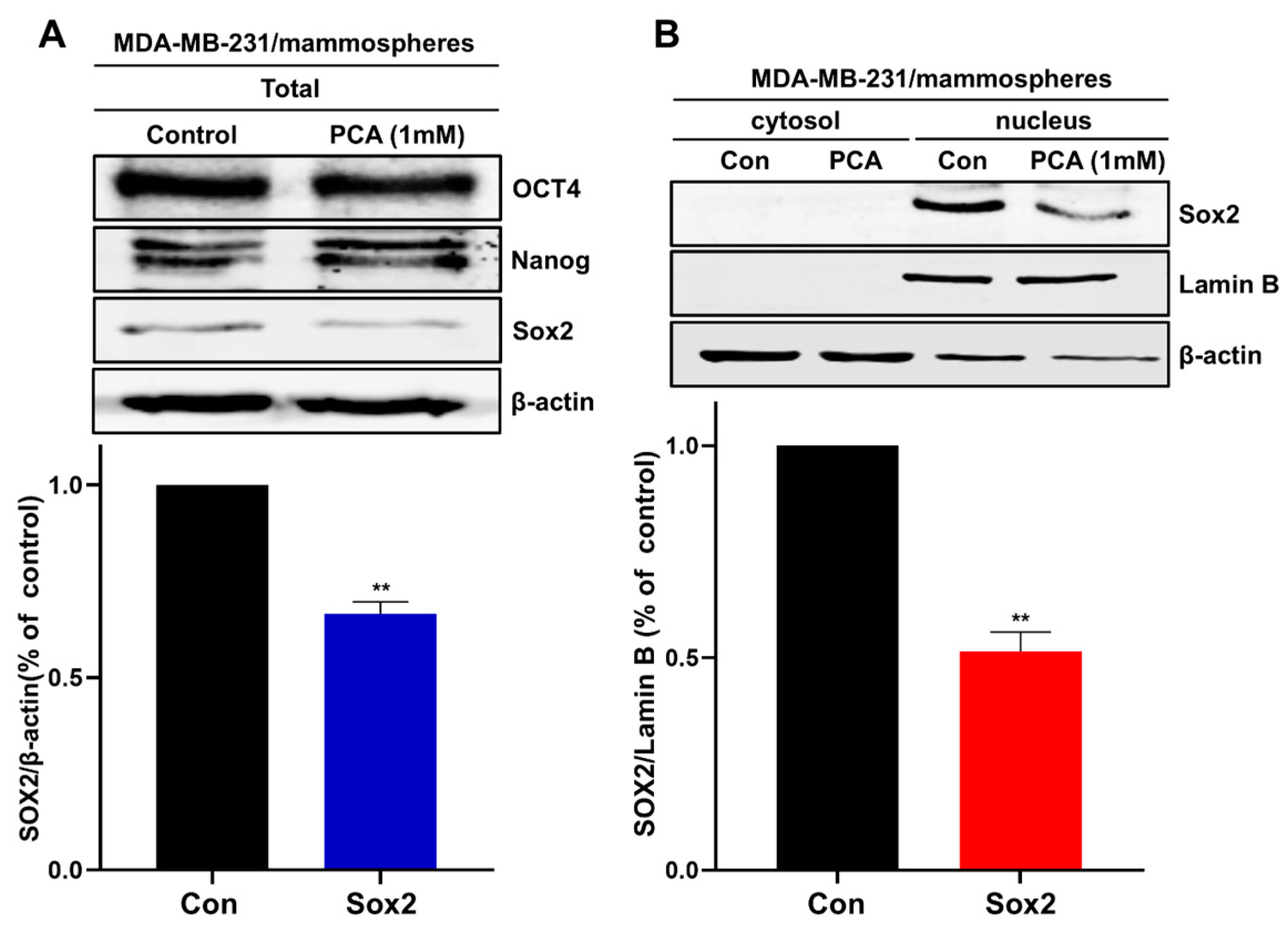
2.7. An Akt-Dependent Mechanism Mediates PCA Suppression of Sox2
2.8. Functional Assessment of PCA as an Anti-CSC Agent in Human HCC1937 Breast Cancer Cells
2.9. Effect of PCA on Anti-CSCs Using 4T1 Murine BC Cells
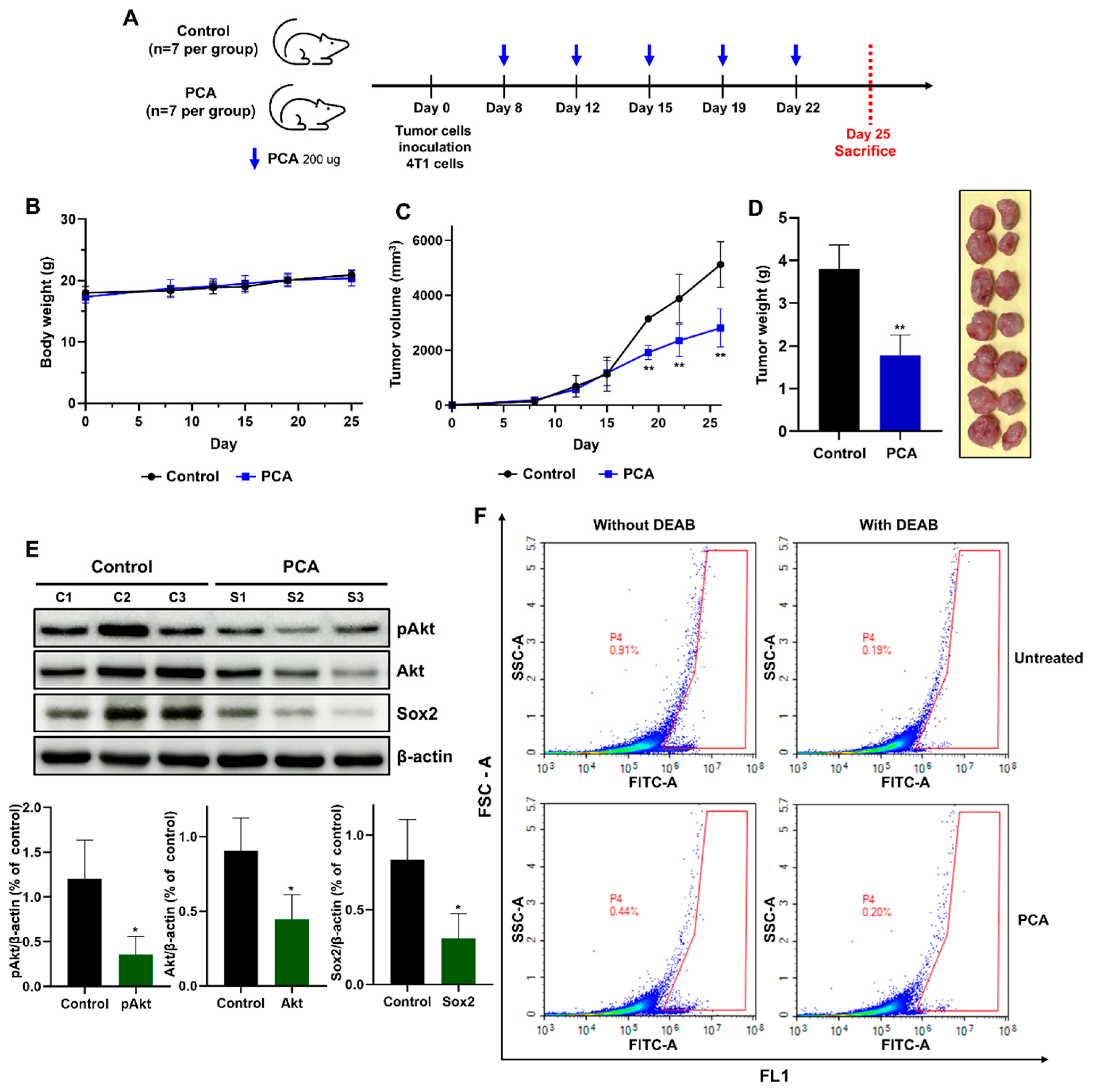
2.10. Anti-Tumor Effect of PCA in the 4T1 Mouse Model
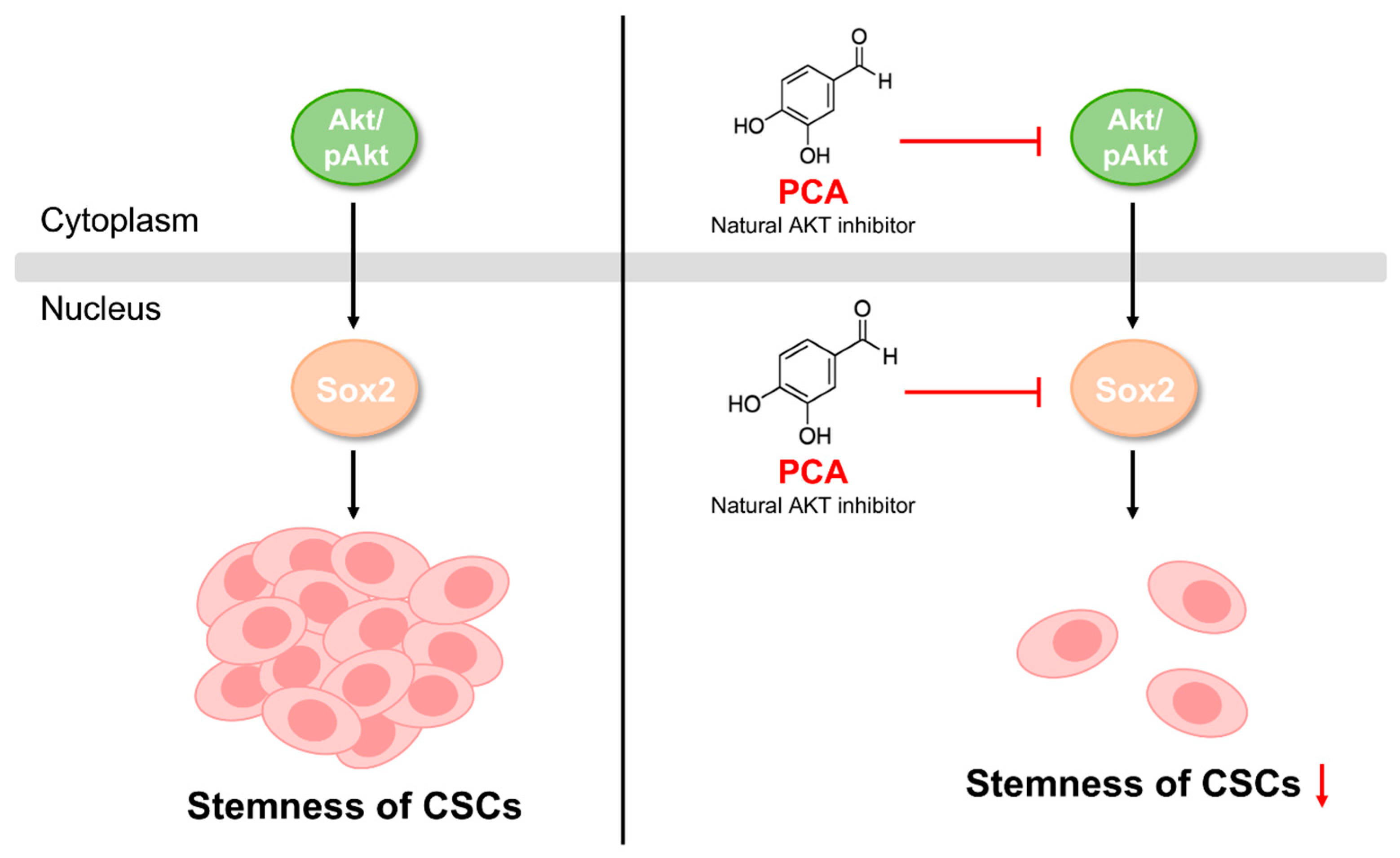
3. Discussion
4. Materials and Methods
4.1. Cell Cultivation and Reagents
4.2. Mammosphere Formation Assay
4.3. Cell Proliferation Assay
4.4. Colony Formation Assay
4.5. Scratch Migration Assay
4.6. Cancer Stem Cell Isolation and Detection
4.7. Apoptosis Assay
4.8. ALDEFLUOR Assay
4.9. Western Blotting and Immunoprecipitation
4.10. Mice
4.11. In Vivo Experiment
4.12. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | Breast cancer |
| CSC | Cancer stem cell |
| PCA | Protocatechualdehyde |
| TNBC | Triple negative breast cancer |
References
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes. Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef]
- Sopik, V.; Sun, P.; Narod, S.A. Impact of microinvasion on breast cancer mortality in women with ductal carcinoma in situ. Breast Cancer Res. Treat. 2018, 167, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.P.; Winer, E.P.; Foulkes, W.D.; Garber, J.; Perou, C.M.; Richardson, A.; Sledge, G.W.; Carey, L.A. Triple-negative breast cancer: Risk factors to potential targets. Clin. Cancer Res. 2008, 14, 8010–8018. [Google Scholar] [CrossRef] [PubMed]
- Stevens, K.N.; Vachon, C.M.; Couch, F.J. Genetic susceptibility to triple-negative breast cancer. Cancer Res. 2013, 73, 2025–2030. [Google Scholar] [CrossRef]
- Geng, S.Q.; Alexandrou, A.T.; Li, J.J. Breast cancer stem cells: Multiple capacities in tumor metastasis. Cancer Lett. 2014, 349, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.L.; Choi, H.S.; Kim, J.H.; Jeong, D.K.; Kim, K.S.; Lee, D.S. Dihydrotanshinone-Induced NOX5 Activation Inhibits Breast Cancer Stem Cell through the ROS/Stat3 Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 9296439. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Tanaka, K.; Tanaka, T.; Hara, A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget 2016, 7, 11018–11032. [Google Scholar] [CrossRef]
- Velasco-Velazquez, M.A.; Popov, V.M.; Lisanti, M.P.; Pestell, R.G. The role of breast cancer stem cells in metastasis and therapeutic implications. Am. J. Pathol. 2011, 179, 2–11. [Google Scholar] [CrossRef]
- Luo, M.; Clouthier, S.G.; Deol, Y.; Liu, S.; Nagrath, S.; Azizi, E.; Wicha, M.S. Breast cancer stem cells: Current advances and clinical implications. Methods Mol. Biol. 2015, 1293, 1–49. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.L.; Francescangeli, F.; Zeuner, A. Breast Cancer Stem Cells as Drivers of Tumor Chemoresistance, Dormancy and Relapse: New Challenges and Therapeutic Opportunities. Cancers 2019, 11, 1569. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Rathod, M.; De, A. Targeting stem cells in the realm of drug-resistant breast cancer. Breast Cancer 2019, 11, 115–135. [Google Scholar] [CrossRef] [PubMed]
- Palomeras, S.; Ruiz-Martinez, S.; Puig, T. Targeting Breast Cancer Stem Cells to Overcome Treatment Resistance. Molecules 2018, 23, 2193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, Y.; Zhang, X. Stemness-Related Markers in Cancer. Cancer Transl. Med. 2017, 3, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.W.; Zhou, Y.W.; Li, W.X.; Kang, B.; Zhang, X.Q.; Yang, Y.; Cheng, J.; Yin, S.Y.; Tong, Y.; He, J.Q.; et al. Akt-mediated phosphorylation of Oct4 is associated with the proliferation of stem-like cancer cells. Oncol. Rep. 2015, 33, 1621–1629. [Google Scholar] [CrossRef]
- Srinual, S.; Chanvorachote, P.; Pongrakhananon, V. Suppression of cancer stem-like phenotypes in NCI-H460 lung cancer cells by vanillin through an Akt-dependent pathway. Int. J. Oncol. 2017, 50, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, Y.; Li, W.; Chen, Q.; Li, J.; Pan, X.; Zhou, L.; Liu, C.; Chen, C.; He, J.; et al. Reciprocal regulation of Akt and Oct4 promotes the self-renewal and survival of embryonal carcinoma cells. Mol. Cell 2012, 48, 627–640. [Google Scholar] [CrossRef]
- Noh, K.H.; Kim, B.W.; Song, K.H.; Cho, H.; Lee, Y.H.; Kim, J.H.; Chung, J.Y.; Kim, J.H.; Hewitt, S.M.; Seong, S.Y.; et al. Nanog signaling in cancer promotes stem-like phenotype and immune evasion. J. Clin. Investig. 2012, 122, 4077–4093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sasaki, T.; Li, W.; Nagata, K.; Higai, K.; Feng, F.; Wang, J.; Cheng, M.; Koike, K. Identification of caffeoylquinic acid derivatives as natural protein tyrosine phosphatase 1B inhibitors from Artemisia princeps. Bioorganic Med. Chem. Lett. 2018, 28, 1194–1197. [Google Scholar] [CrossRef]
- Tan, R.X.; Zheng, W.F.; Tang, H.Q. Biologically active substances from the genus Artemisia. Planta Med. 1998, 64, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Lin, S.S.; Liao, P.H.; Young, S.C.; Yang, C.C. The immunopharmaceutical effects and mechanisms of herb medicine. Cell Mol. Immunol. 2008, 5, 23–31. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, G.H. Antioxidant and anticancer activity of Artemisia princeps var. orientalis extract in HepG2 and Hep3B hepatocellular carcinoma cells. Chin. J. Cancer Res. 2013, 25, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Son, H.U.; Lee, S.; Heo, J.C.; Lee, S.H. The solid-state fermentation of Artemisia capillaris leaves with Ganoderma lucidum enhances the anti-inflammatory effects in a model of atopic dermatitis. Int. J. Mol. Med. 2017, 39, 1233–1241. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yan, H.; Jung, K.H.; Kim, J.; Rumman, M.; Oh, M.S.; Hong, S.S. Artemisia capillaris extract AC68 induces apoptosis of hepatocellular carcinoma by blocking the PI3K/AKT pathway. Biomed. Pharmacother. 2018, 98, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jiang, X.; Jeong, J.B.; Lee, S.H. Anticancer activity of protocatechualdehyde in human breast cancer cells. J. Med. Food 2014, 17, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.B.; Lee, S.H. Protocatechualdehyde possesses anti-cancer activity through downregulating cyclin D1 and HDAC2 in human colorectal cancer cells. Biochem. Biophys. Res. Commun. 2013, 430, 381–386. [Google Scholar] [CrossRef]
- Bae, S.; Kim, S.Y.; Jung, J.H.; Yoon, Y.; Cha, H.J.; Lee, H.; Kim, K.; Kim, J.; An, I.S.; Kim, J.; et al. Akt is negatively regulated by the MULAN E3 ligase. Cell Res. 2012, 22, 873–885. [Google Scholar] [CrossRef]
- Suizu, F.; Hiramuki, Y.; Okumura, F.; Matsuda, M.; Okumura, A.J.; Hirata, N.; Narita, M.; Kohno, T.; Yokota, J.; Bohgaki, M.; et al. The E3 ligase TTC3 facilitates ubiquitination and degradation of phosphorylated Akt. Dev. Cell 2009, 17, 800–810. [Google Scholar] [CrossRef]
- Lam, S.K.; Leung, L.L.; Li, Y.Y.; Zheng, C.Y.; Ho, J.C. Combination effects of arsenic trioxide and fibroblast growth factor receptor inhibitor in squamous cell lung carcinoma. Lung Cancer 2016, 101, 111–119. [Google Scholar] [CrossRef]
- Lu, X.; Mazur, S.J.; Lin, T.; Appella, E.; Xu, Y. The pluripotency factor nanog promotes breast cancer tumorigenesis and metastasis. Oncogene 2014, 33, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kang, L.; Zhang, H.; Huang, Y.; Fang, L.; Li, M.; Brown, P.J.; Arrowsmith, C.H.; Li, J.; Wong, J. AKT drives SOX2 overexpression and cancer cell stemness in esophageal cancer by protecting SOX2 from UBR5-mediated degradation. Oncogene 2019, 38, 5250–5264. [Google Scholar] [CrossRef] [PubMed]
- Loh, Y.H.; Wu, Q.; Chew, J.L.; Vega, V.B.; Zhang, W.; Chen, X.; Bourque, G.; George, J.; Leong, B.; Liu, J.; et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet. 2006, 38, 431–440. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Xiao, X.; Dong, Y.; Wu, J.; Yao, F.; Zhou, X.H. Effect of fermented wheat germ extract with lactobacillus plantarum dy-1 on HT-29 cell proliferation and apoptosis. J. Agric. Food Chem. 2015, 63, 2449–2457. [Google Scholar] [CrossRef] [PubMed]
- Yim, N.H.; Kim, A.; Jung, Y.P.; Kim, T.; Ma, C.J.; Ma, J.Y. Fermented So-Cheong-Ryong-Tang (FCY) induces apoptosis via the activation of caspases and the regulation of MAPK signaling pathways in cancer cells. BMC Complement. Altern. Med. 2015, 15, 336. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stanton, C.; Ross, R.P.; Fitzgerald, G.F.; Van Sinderen, D. Fermented functional foods based on probiotics and their biogenic metabolites. Curr. Opin. Biotechnol. 2005, 16, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Hwang, E.S. Quality Characteristics and Antioxidant Activity of Yogurt Supplemented with Aronia (Aronia melanocarpa) Juice. Prev. Nutr. Food Sci. 2016, 21, 330–337. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, J.H.; Kim, S.L.; Deng, H.Y.; Lee, D.; Kim, C.S.; Yun, B.S.; Lee, D.S. Catechol derived from aronia juice through lactic acid bacteria fermentation inhibits breast cancer stem cell formation via modulation Stat3/IL-6 signaling pathway. Mol. Carcinog. 2018, 57, 1467–1479. [Google Scholar] [CrossRef]
- Vaquero, I.; Marcobal, A.; Munoz, R. Tannase activity by lactic acid bacteria isolated from grape must and wine. Int. J. Food Microbiol. 2004, 96, 199–204. [Google Scholar] [CrossRef]
- Etoh, H.; Murakami, K.; Yogoh, T.; Ishikawa, H.; Fukuyama, Y.; Tanaka, H. Anti-oxidative compounds in barley tea. Biosci. Biotechnol. Biochem. 2004, 68, 2616–2618. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Miao, A.D.; Wang, S.Q. Protocatechuic aldehyde suppresses TNF-alpha-induced ICAM-1 and VCAM-1 expression in human umbilical vein endothelial cells. Eur. J. Pharmacol. 2005, 513, 1–8. [Google Scholar] [CrossRef]
- Deng, Y.; Guo, W.; Li, G.; Li, S.; Li, H.; Li, X.; Niu, B.; Song, M.; Zhang, Y.; Xu, Z.; et al. Protocatechuic Aldehyde Represses Proliferation and Migration of Breast Cancer Cells through Targeting C-terminal Binding Protein 1. J. Breast Cancer 2020, 23, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, L.; Zheng, Q.; Hao, H.; Ye, H.; Li, P.; Yang, H. Protocatechuic aldehyde protects cardiomycoytes against ischemic injury via regulation of nuclear pyruvate kinase M2. Acta Pharm. Sin. B 2021, 11, 3553–3566. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.C.; Lee, S.H. Protocatechuic Aldehyde Inhibits alpha-MSH-Induced Melanogenesis in B16F10 Melanoma Cells via PKA/CREB-Associated MITF Downregulation. Int. J. Mol. Sci. 2021, 22, 3861. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, E.; Sankari, L.S.; Malathi, L.; Krupaa, J.R. Naturally occurring products in cancer therapy. J. Pharm. Bioallied Sci. 2015, 7, S181–S183. [Google Scholar] [CrossRef] [PubMed]
- Walton, N.J.; Mayer, M.J.; Narbad, A. Vanillin. Phytochemistry 2003, 63, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Testa, J.R.; Tsichlis, P.N. AKT signaling in normal and malignant cells. Oncogene 2005, 24, 7391–7393. [Google Scholar] [CrossRef]
- Rivas, S.; Gomez-Oro, C.; Anton, I.M.; Wandosell, F. Role of Akt Isoforms Controlling Cancer Stem Cell Survival, Phenotype and Self-Renewal. Biomedicines 2018, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.A.; Rudnicki, M.A. Oct4 interaction with Hmgb2 regulates Akt signaling and pluripotency. Stem Cells 2013, 31, 1107–1120. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Ohashi, A.; Huang, Y.; Pandita, T.K.; Ludwig, T.; Powell, S.N.; Yang, Q. Negative Regulation of AKT Activation by BRCA1. Cancer Res. 2008, 68, 10040–10044. [Google Scholar] [CrossRef] [PubMed]
- Erales, J.; Coffino, P. Ubiquitin-independent proteasomal degradation. Biochim. Biophys. Acta 2014, 1843, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.L.; Choi, H.S.; Lee, D.S. BRD4/nuclear PD-L1/RelB circuit is involved in the stemness of breast cancer cells. Cell Commun. Signal 2023, 21, 315. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, S.-Y.; Park, S.; Choi, Y.-H. Protocatechualdehyde Induced Breast Cancer Stem Cell Death via the Akt/Sox2 Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 1811. https://doi.org/10.3390/ijms26051811
Ko S-Y, Park S, Choi Y-H. Protocatechualdehyde Induced Breast Cancer Stem Cell Death via the Akt/Sox2 Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(5):1811. https://doi.org/10.3390/ijms26051811
Chicago/Turabian StyleKo, Seung-Yeon, Seonghee Park, and Youn-Hee Choi. 2025. "Protocatechualdehyde Induced Breast Cancer Stem Cell Death via the Akt/Sox2 Signaling Pathway" International Journal of Molecular Sciences 26, no. 5: 1811. https://doi.org/10.3390/ijms26051811
APA StyleKo, S.-Y., Park, S., & Choi, Y.-H. (2025). Protocatechualdehyde Induced Breast Cancer Stem Cell Death via the Akt/Sox2 Signaling Pathway. International Journal of Molecular Sciences, 26(5), 1811. https://doi.org/10.3390/ijms26051811





