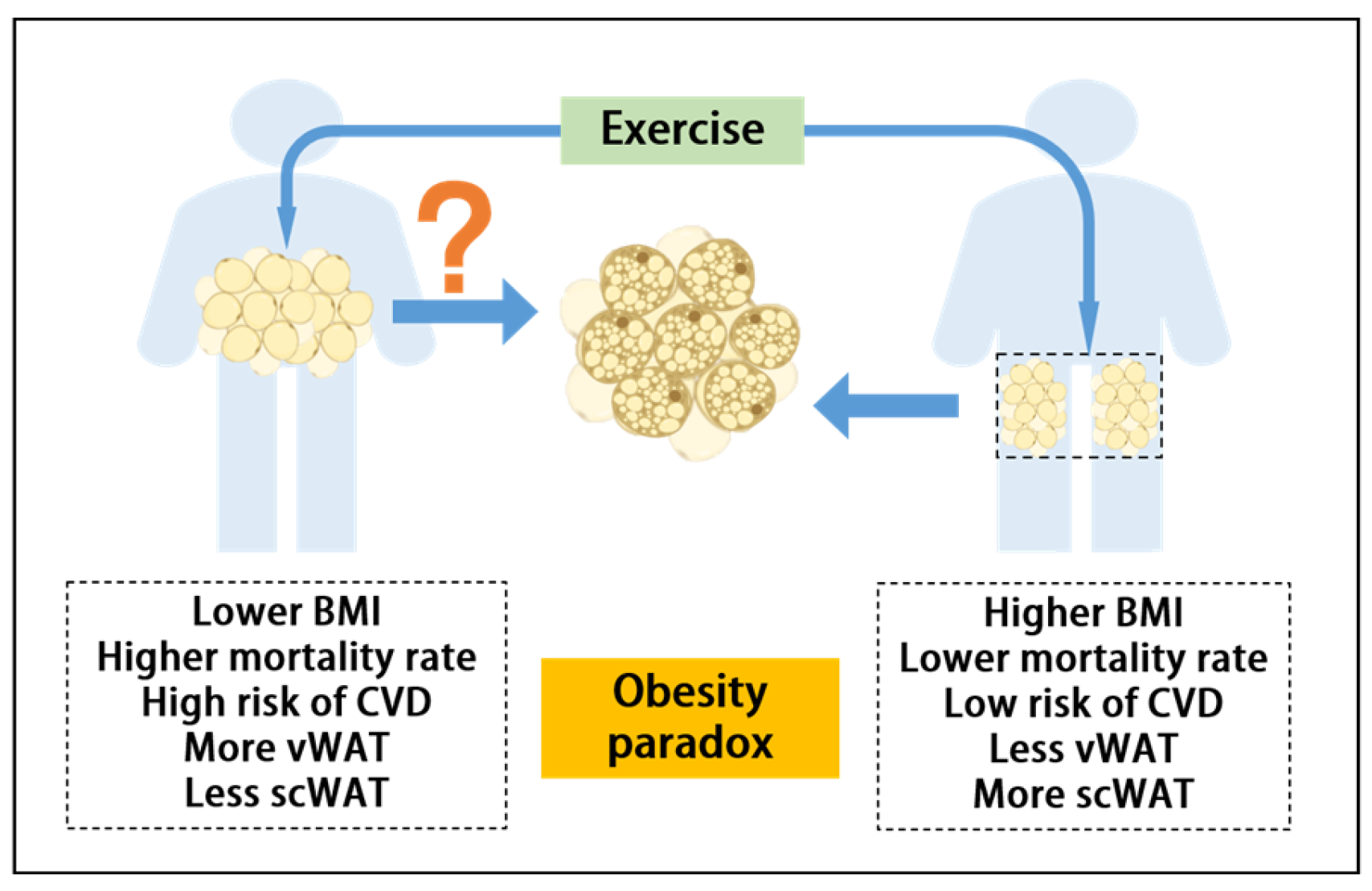
Can Exercise-Mediated Adipose Browning Provide an Alternative Explanation for the Obesity Paradox?
Abstract
1. Introduction
2. The Relationship Between AT and CVD
2.1. AT Distribution and Its Impact on CVD
2.2. AT Inflammation and CVD Risk
2.3. AT Adipokines and CVD Risk
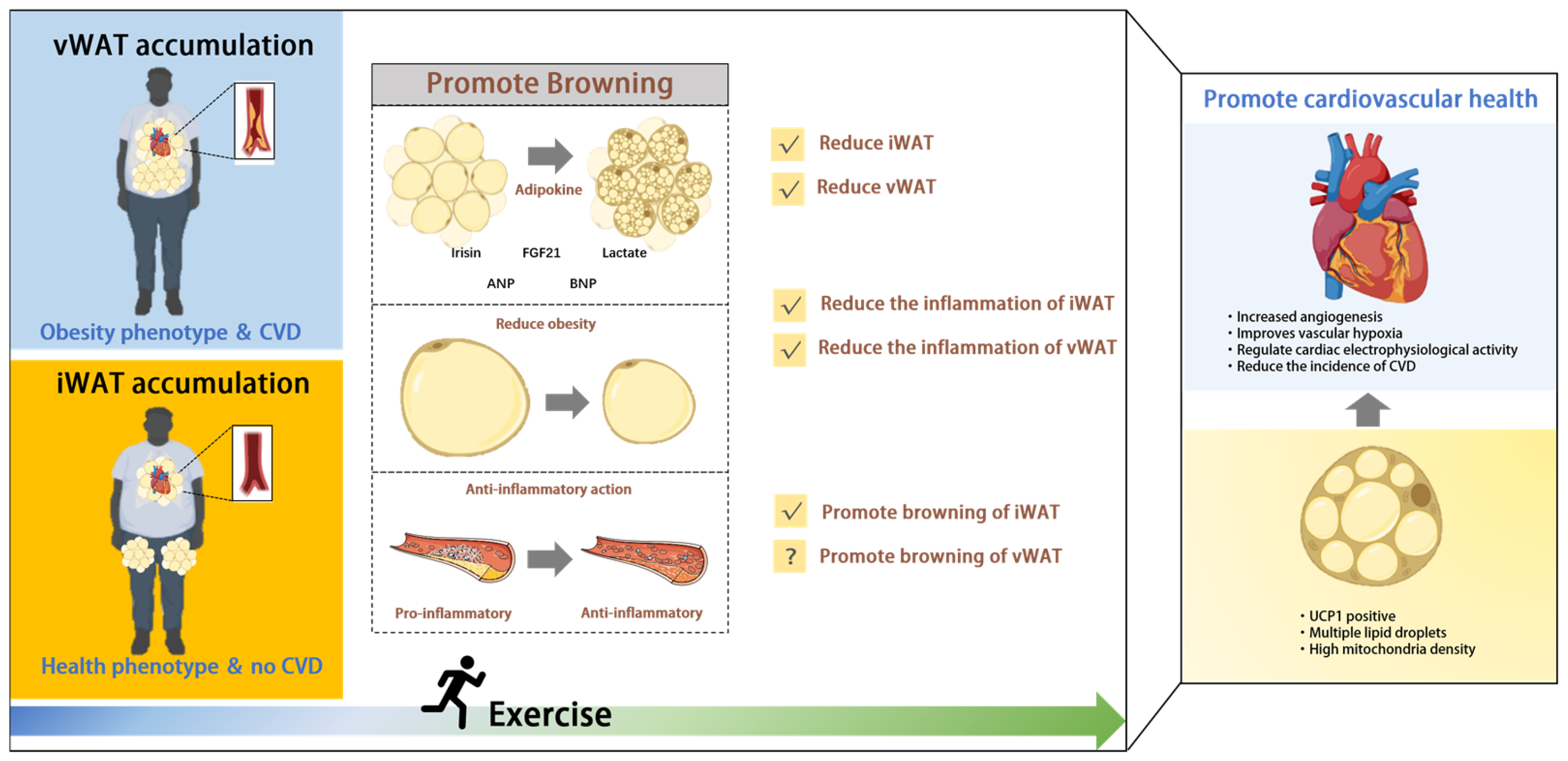
3. Exercise-Mediated Browning: A New Avenue for CVD Protection
3.1. Exercise-Mediated Browning Regulates AT Distribution
3.2. Exercise-Mediated Browning Reduces Inflammation
3.3. Browning-Associated Adipokines Benefit CVD
3.3.1. Irisin
3.3.2. FGF21
3.3.3. Lactate
| Hematological Factors | Functionality | Relationship with Cardiovascular Disease | Reference | |
|---|---|---|---|---|
| Noninflammatory factor | ||||
| 1 | Irisin PGC-1α | Increases energy expenditure and improves glucose homeostasis | Maintaining cardiovascular health and reducing the risk of atherosclerosis | [18,133,135] |
| 2 | FGF21 | Regulates blood sugar and increases metabolic levels | Protects the heart, but high concentrations are harmful | [21,138,140,141] |
| 3 | Lactate | Energy supply, metabolic boost | Reduces risk of atherosclerosis and improves ischemia | [145,146,147] |
| 4 | 12,13-diHOME | Increased intake of fatty acids | Regulates calcium circulation, enhances cardiac function, and enhances cardiac hemodynamics | [152,153,154] |
| 5 | Bcl2l13 | Induction of mitochondrial fracture and mitochondrial autophagy to ameliorate mitochondrial dysfunction | Improvement of HF | [155,156,157] |
| 6 | Apelin | Enhancement of mitochondrial function | Decreases in systolic and diastolic blood pressure and increases in blood flow regulate vascular tone, promote vascular development, and maintain cardiomyocyte homeostasis | [158,159,160] |
| 7 | BAIBA | Regulation of lipid metabolism | Treatment of metabolic syndrome and its cardiovascular complications and improvement of atherosclerosis | [161,162] |
| 8 | METRNL | Regulation of metabolism and inflammation | Associated with metabolic or CVD (type 2 diabetes, coronary heart disease) | [163,164,165,166] |
| 9 | Myostatin | Increased energy substrate uptake | Improvement of cardiovascular risk factors | [167,168,169] |
| 10 | Follistatin | Promotes energy metabolism, regulates insulin and glucagon | Myocardial injury protective factor | [170,171,172] |
| 11 | GABA | Inhibits insulin secretion | Treatment of CVD-related | [162,173] |
| 12 | SPARC | Regulates cell function and tissue remodeling | Affects hemodynamics and cardiac function | [174,175] |
| 13 | VEGF | Promoting exercise-induced neurogenesis | Promotes angiogenesis | [176,177] |
| Inflammatory factor | ||||
| 14 | IL-6 | Pro-lipolytic, anti-inflammatory, promotes glucose uptake | Improvement of glucose tolerance, affecting cardiometabolic diseases and CVDs | [86,178,179] |
| 15 | TNF-α | Induction of involvement in inflammation | Induction of ROS production leads to endothelial dysfunction in many pathophysiologic conditions | [180,181] |
| 16 | TGFβ1 | Immune cell chemokines that affect skeletal muscle growth | Regulation of cardiorespiratory fitness and remodeling | [182,183] |
4. Conclusions and Future Perspective
Funding
Conflicts of Interest
References
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A compass for future health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, M.; DeCleene, N.; Dorsey, H.; Fuster, V.; Johnson, C.O.; LeGrand, K.E.; Mensah, G.A.; Razo, C.; Stark, B.; Turco, J.V.; et al. Global Burden of Cardiovascular Diseases and Risks Collaboration, 1990–2021. J. Am. Coll. Cardiol. 2022, 80, 2372–2425. [Google Scholar] [CrossRef] [PubMed]
- Prillaman, M. Why BMI is flawed—And how to redefine obesity. Nature 2023, 622, 232–233. [Google Scholar] [CrossRef] [PubMed]
- Kenchaiah, S.; Evans, J.C.; Levy, D.; Wilson, P.W.; Benjamin, E.J.; Larson, M.G.; Kannel, W.B.; Vasan, R.S. Obesity and the Risk of Heart Failure. N. Engl. J. Med. 2002, 347, 305–313. [Google Scholar] [CrossRef]
- Horwich, T.B.; Fonarow, G.C.; Hamilton, M.A.; MacLellan, W.R.; Woo, M.A.; Tillisch, J.H. The relationship between obesity and mortality in patients with heart failure. J. Am. Coll. Cardiol. 2001, 38, 789–795. [Google Scholar] [CrossRef]
- Carbone, S.; Lavie, C.J.; Arena, R. Obesity and Heart Failure: Focus on the Obesity Paradox. Mayo Clin. Proc. 2017, 92, 266–279. [Google Scholar] [CrossRef]
- Lavie, C.J.; Sharma, A.; Alpert, M.A.; De Schutter, A.; Lopez-Jimenez, F.; Milani, R.V.; Ventura, H.O. Update on Obesity and Obesity Paradox in Heart Failure. Prog. Cardiovasc. Dis. 2016, 58, 393–400. [Google Scholar] [CrossRef]
- Bodhini, D.; Mohan, V. Mediators of insulin resistance & cardiometabolic risk: Newer insights. Indian J. Med. Res. 2018, 148, 127–129. [Google Scholar] [CrossRef]
- Emamat, H.; Jamshidi, A.; Farhadi, A.; Ghalandari, H.; Ghasemi, M.; Tangestani, H. The association between the visceral to subcutaneous abdominal fat ratio and the risk of cardiovascular diseases: A systematic review. BMC Public Health 2024, 24, 1827. [Google Scholar] [CrossRef]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al. Identification and Importance of Brown Adipose Tissue in Adult Humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef]
- Becher, T.; Palanisamy, S.; Kramer, D.J.; Eljalby, M.; Marx, S.J.; Wibmer, A.G.; Butler, S.D.; Jiang, C.S.; Vaughan, R.; Schöder, H.; et al. Brown adipose tissue is associated with cardiometabolic health. Nat. Med. 2021, 27, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Dodd, G.T.; Decherf, S.; Loh, K.; Simonds, S.E.; Wiede, F.; Balland, E.; Merry, T.L.; Münzberg, H.; Zhang, Z.-Y.; Kahn, B.B.; et al. Leptin and Insulin Act on POMC Neurons to Promote the Browning of White Fat. Cell 2015, 160, 88–104. [Google Scholar] [CrossRef]
- Otero-Díaz, B.; Rodríguez-Flores, M.; Sánchez-Muñoz, V.; Monraz-Preciado, F.; Ordoñez-Ortega, S.; Becerril-Elias, V.; Baay-Guzmán, G.; Obando-Monge, R.; García-García, E.; Palacios-González, B.; et al. Exercise Induces White Adipose Tissue Browning Across the Weight Spectrum in Humans. Front. Physiol. 2018, 9, 1781. [Google Scholar] [CrossRef]
- Kern, P.A.; Finlin, B.S.; Zhu, B.; Rasouli, N.; McGehee, R.E., Jr.; Westgate, P.M.; Dupont-Versteegden, E.E. The effects of temperature and seasons on subcutaneous white adipose tissue in humans: Evidence for thermogenic gene induction. J. Clin. Endocrinol. Metab. 2014, 99, E2772–E2779. [Google Scholar] [CrossRef]
- Stanford, K.I.; Middelbeek, R.J.; Townsend, K.L.; Lee, M.-Y.; Takahashi, H.; So, K.; Hitchcox, K.M.; Markan, K.R.; Hellbach, K.; Hirshman, M.F.; et al. A Novel Role for Subcutaneous Adipose Tissue in Exercise-Induced Improvements in Glucose Homeostasis. Diabetes 2015, 64, 2002–2014. [Google Scholar] [CrossRef]
- Trevellin, E.; Scorzeto, M.; Olivieri, M.; Granzotto, M.; Valerio, A.; Tedesco, L.; Fabris, R.; Serra, R.; Quarta, M.; Reggiani, C.; et al. Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes 2014, 63, 2800–2811. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, X.; Hu, G.; Li, C.; Guo, L.; Zhang, L.; Sun, F.; Xia, Y.; Yan, W.; Cui, Z.; et al. Small Extracellular Vesicles From Brown Adipose Tissue Mediate Exercise Cardioprotection. Circ. Res. 2022, 130, 1490–1506. [Google Scholar] [CrossRef]
- Nigro, P.; Vamvini, M.; Yang, J.; Caputo, T.; Ho, L.-L.; Carbone, N.P.; Papadopoulos, D.; Conlin, R.; He, J.; Hirshman, M.F.; et al. Exercise training remodels inguinal white adipose tissue through adaptations in innervation, vascularization, and the extracellular matrix. Cell Rep. 2023, 42, 112392. [Google Scholar] [CrossRef]
- Mu, W.-J.; Zhu, J.-Y.; Chen, M.; Guo, L. Exercise-Mediated Browning of White Adipose Tissue: Its Significance, Mechanism and Effectiveness. Int. J. Mol. Sci. 2021, 22, 11512. [Google Scholar] [CrossRef] [PubMed]
- Parry-Williams, G.; Sharma, S. The effects of endurance exercise on the heart: Panacea or poison? Nat. Rev. Cardiol. 2020, 17, 402–412. [Google Scholar] [CrossRef]
- McKie, G.L.; Medak, K.D.; Knuth, C.M.; Shamshoum, H.; Townsend, L.K.; Peppler, W.T.; Wright, D.C. Housing temperature affects the acute and chronic metabolic adaptations to exercise in mice. J. Physiol. 2019, 597, 4581–4600. [Google Scholar] [CrossRef]
- Cho, E.; Jeong, D.Y.; Kim, J.G.; Lee, S. The Acute Effects of Swimming Exercise on PGC-1α-FNDC5/Irisin-UCP1 Expression in Male C57BL/6J Mice. Metabolites 2021, 11, 111. [Google Scholar] [CrossRef]
- Polkinghorne, M.D.; West, H.W.; Antoniades, C. Adipose Tissue in Cardiovascular Disease: From Basic Science to Clinical Translation. Ann. Rev. Physiol. 2024, 86, 175–198. [Google Scholar] [CrossRef]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human Epicardial Adipose Tissue Is a Source of Inflammatory Mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.-E.; Tchernof, A.; Després, J.-P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Vandanmagsar, B.; Youm, Y.-H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef]
- Neeland, I.J.; Gupta, S.; Ayers, C.R.; Turer, A.T.; Rame, J.E.; Das, S.R.; Berry, J.D.; Khera, A.; McGuire, D.K.; Vega, G.L.; et al. Relation of regional fat distribution to left ventricular structure and function. Circ. Cardiovasc. Imaging 2013, 6, 800–807. [Google Scholar] [CrossRef]
- Després, J.-P. Body Fat Distribution and Risk of Cardiovascular Disease: An update. Circulation 2012, 126, 1301–1313. [Google Scholar] [CrossRef]
- Yudkin, J.S.; Eringa, E.; Stehouwer, C.D.A. “Vasocrine” signalling from perivascular fat: A mechanism linking insulin resistance to vascular disease. Lancet 2005, 365, 1817–1820. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.E.; Clark, D.; Jones, D.W. Fat and cardiometabolic risk: Location, location, location. J. Clin. Hypertens. 2019, 21, 963–965. [Google Scholar] [CrossRef]
- Liu, J.; Fox, C.S.; Hickson, D.A.; May, W.D.; Hairston, K.G.; Carr, J.J.; Taylor, H.A. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: The Jackson Heart Study. J. Clin. Endocrinol. Metab. 2010, 95, 5419–5426. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.-P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Tousoulis, D. The molecular mechanisms of obesity paradox. Cardiovasc. Res. 2017, 113, 1074–1086. [Google Scholar] [CrossRef]
- Ahmadi, N.; Nabavi, V.; Yang, E.; Hajsadeghi, F.; Lakis, M.; Flores, F.; Zeb, I.; Bevinal, M.; Ebrahimi, R.; Budoff, M. Increased epicardial, pericardial, and subcutaneous adipose tissue is associated with the presence and severity of coronary artery calcium. Acad. Radiol. 2010, 17, 1518–1524. [Google Scholar] [CrossRef]
- Macotela, Y.; Emanuelli, B.; Mori, M.A.; Gesta, S.; Schulz, T.J.; Tseng, Y.-H.; Kahn, C.R. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes 2012, 61, 1691–1699. [Google Scholar] [CrossRef]
- Ding, H.; Zheng, S.; Garcia-Ruiz, D.; Hou, D.; Wei, Z.; Liao, Z.; Li, L.; Zhang, Y.; Han, X.; Zen, K.; et al. Fasting induces a subcutaneous-to-visceral fat switch mediated by microRNA-149-3p and suppression of PRDM16. Nat. Commun. 2016, 7, 11533. [Google Scholar] [CrossRef]
- Wang, Q.A.; Tao, C.; Gupta, R.K.; Scherer, P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 2013, 19, 1338–1344. [Google Scholar] [CrossRef]
- Crewe, C.; An, Y.A.; Scherer, P.E. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. J. Clin. Investig. 2017, 127, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, S.; Cantini, G.; Poli, G.; Francalanci, M.; Squecco, R.; Di Franco, A.; Borgogni, E.; Frontera, S.; Nesi, G.; Liotta, F.; et al. Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLoS ONE 2012, 7, e36569. [Google Scholar] [CrossRef] [PubMed]
- Karason, K.; Jamaly, S. Heart failure development in obesity: Mechanistic pathways. Eur. Hear. J. 2020, 41, 3485. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-Induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ. Res. 2016, 118, 1786–1807. [Google Scholar] [CrossRef]
- Khan, T.; Muise, E.S.; Iyengar, P.; Wang, Z.V.; Chandalia, M.; Abate, N.; Zhang, B.B.; Bonaldo, P.; Chua, S.; Scherer, P.E. Metabolic dysregulation and adipose tissue fibrosis: Role of collagen VI. Mol. Cell. Biol. 2009, 29, 1575–1591. [Google Scholar] [CrossRef]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L., Jr. Inflammation in Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef]
- Henrichot, E.; Juge-Aubry, C.E.; Pernin, A.; Pache, J.-C.; Velebit, V.; Dayer, J.-M.; Meda, P.; Chizzolini, C.; Meier, C.A. Production of Chemokines by Perivascular Adipose Tissue: A role in the pathogenesis of atherosclerosis? Arter. Thromb. Vasc. Biol. 2005, 25, 2594–2599. [Google Scholar] [CrossRef]
- Öhman, M.; Luo, W.; Wang, H.; Guo, C.; Abdallah, W.; Russo, H.; Eitzman, D. Perivascular visceral adipose tissue induces atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis 2011, 219, 33–39. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Margaritis, M.; Coutinho, P.; Digby, J.; Patel, R.; Psarros, C.; Ntusi, N.; Karamitsos, T.D.; Lee, R.; De Silva, R.; et al. Reciprocal effects of systemic inflammation and brain natriuretic peptide on adiponectin biosynthesis in adipose tissue of patients with ischemic heart disease. Arter. Thromb. Vasc. Biol. 2014, 34, 2151–2159. [Google Scholar] [CrossRef]
- Girón-Ulloa, A.; González-Domínguez, E.; Klimek, R.S.; Patiño-Martínez, E.; Vargas-Ayala, G.; Segovia-Gamboa, N.C.; Campos-Peña, V.; E Rodríguez-Arellano, M.A.; Meraz-Ríos, M.; Campos-Campos, S.F.; et al. Specific macrophage subsets accumulate in human subcutaneous and omental fat depots during obesity. Immunol. Cell Biol. 2020, 98, 868–882. [Google Scholar] [CrossRef] [PubMed]
- Ghigliotti, G.; Barisione, C.; Garibaldi, S.; Fabbi, P.; Brunelli, C.; Spallarossa, P.; Altieri, P.; Rosa, G.; Spinella, G.; Palombo, D.; et al. Adipose Tissue Immune Response: Novel Triggers and Consequences for Chronic Inflammatory Conditions. Inflammation 2014, 37, 1337–1353. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Kurobe, H.; Akaike, M.; Chikugo, F.; Hori, T.; Bando, Y.; Nishio, C.; Higashida, M.; Nakaya, Y.; Kitagawa, T.; et al. Enhanced Inflammation in Epicardial Fat in Patients With Coronary Artery Disease. Int. Hear. J. 2011, 52, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Asterholm, I.W.; Tao, C.; Morley, T.S.; Wang, Q.A.; Delgado-Lopez, F.; Wang, Z.V.; Scherer, P.E. Adipocyte Inflammation Is Essential for Healthy Adipose Tissue Expansion and Remodeling. Cell Metab. 2014, 20, 103–118. [Google Scholar] [CrossRef]
- Patel, V.B.; Shah, S.; Verma, S.; Oudit, G.Y. Epicardial adipose tissue as a metabolic transducer: Role in heart failure and coronary artery disease. Heart Fail. Rev. 2017, 22, 889–902. [Google Scholar] [CrossRef]
- Packer, M. Epicardial Adipose Tissue May Mediate Deleterious Effects of Obesity and Inflammation on the Myocardium. J. Am. Coll. Cardiol. 2018, 71, 2360–2372. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Antoniades, C. The role of adipose tissue in cardiovascular health and disease. Nat. Rev. Cardiol. 2018, 16, 83–99. [Google Scholar] [CrossRef]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919. [Google Scholar] [CrossRef]
- Nakamura, K.; Fuster, J.J.; Walsh, K. Adipokines: A link between obesity and cardiovascular disease. J. Cardiol. 2014, 63, 250–259. [Google Scholar] [CrossRef]
- Reilly, M.P.; Lehrke, M.; Wolfe, M.L.; Rohatgi, A.; Lazar, M.A.; Rader, D.J. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation 2005, 111, 932–939. [Google Scholar] [CrossRef]
- Panee, J. Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes. Cytokine 2012, 60, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tabata, M.; Kadomatsu, T.; Fukuhara, S.; Miyata, K.; Ito, Y.; Endo, M.; Urano, T.; Zhu, H.J.; Tsukano, H.; Tazume, H.; et al. Angiopoietin-like Protein 2 Promotes Chronic Adipose Tissue Inflammation and Obesity-Related Systemic Insulin Resistance. Cell Metab. 2009, 10, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Vasamsetti, S.B.; Natarajan, N.; Sadaf, S.; Florentin, J.; Dutta, P. Regulation of cardiovascular health and disease by visceral adipose tissue-derived metabolic hormones. J. Physiol. 2023, 601, 2099–2120. [Google Scholar] [CrossRef] [PubMed]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Ichiromiyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Ouchi, N.; Higuchi, A.; Ohashi, K.; Oshima, Y.; Gokce, N.; Shibata, R.; Akasaki, Y.; Shimono, A.; Walsh, K. Sfrp5 Is an Anti-Inflammatory Adipokine That Modulates Metabolic Dysfunction in Obesity. Science 2010, 329, 454–457. [Google Scholar] [CrossRef]
- Uemura, Y.; Shibata, R.; Ohashi, K.; Enomoto, T.; Kambara, T.; Yamamoto, T.; Ogura, Y.; Yuasa, D.; Joki, Y.; Matsuo, K.; et al. Adipose-derived factor CTRP9 attenuates vascular smooth muscle cell proliferation and neointimal formation. FASEB J. 2012, 27, 25–33. [Google Scholar] [CrossRef]
- Uemura, Y.; Shibata, R.; Kanemura, N.; Ohashi, K.; Kambara, T.; Hiramatsu-Ito, M.; Enomoto, T.; Yuasa, D.; Joki, Y.; Matsuo, K.; et al. Adipose-derived protein omentin prevents neointimal formation after arterial injury. FASEB J. 2015, 29, 141–151. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, X.; Zhu, Y.; Yin, L.; Quan, Z.; Ou, Y.; He, B. Causal associations of circulating adiponectin with cardiometabolic diseases and osteoporotic fracture. Sci. Rep. 2022, 12, 6689. [Google Scholar] [CrossRef]
- Brunetti, L.; Leone, S.; Orlando, G.; Ferrante, C.; Recinella, L.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; et al. Hypotensive effects of omentin-1 related to increased adiponectin and decreased interleukin-6 in intra-thoracic pericardial adipose tissue. Pharmacol. Rep. 2014, 66, 991–995. [Google Scholar] [CrossRef]
- Yamawaki, H.; Kuramoto, J.; Kameshima, S.; Usui, T.; Okada, M.; Hara, Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem. Biophys. Res. Commun. 2011, 408, 339–343. [Google Scholar] [CrossRef]
- Lee, M.-W.; Lee, M.; Oh, K.-J. Adipose Tissue-Derived Signatures for Obesity and Type 2 Diabetes: Adipokines, Batokines and MicroRNAs. J. Clin. Med. 2019, 8, 854. [Google Scholar] [CrossRef] [PubMed]
- Pischon, T.; Girman, C.J.; Hotamisligil, G.S.; Rifai, N.; Hu, F.B.; Rimm, E.B. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 2004, 291, 1730–1737. [Google Scholar] [CrossRef]
- Schulze, M.B.; Shai, I.; Rimm, E.B.; Li, T.; Rifai, N.; Hu, F.B. Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes 2005, 54, 534–539. [Google Scholar] [CrossRef]
- Sirico, F.; Bianco, A.; D’Alicandro, G.; Castaldo, C.; Montagnani, S.; Spera, R.; Di Meglio, F.; Nurzynska, D. Effects of Physical Exercise on Adiponectin, Leptin, and Inflammatory Markers in Childhood Obesity: Systematic Review and Meta-Analysis. Child. Obes. 2018, 14, 207–217. [Google Scholar] [CrossRef]
- Fayaz, E.; Damirchi, A.; Zebardast, N.; Babaei, P. Cinnamon extract combined with high-intensity endurance training alleviates metabolic syndrome via non-canonical WNT signaling. Nutrition 2019, 65, 173–178. [Google Scholar] [CrossRef]
- Sabouri, M.; Norouzi, J.; Zarei, Y.; Sangani, M.H.; Moghadam, B.H. Comparing High-Intensity Interval Training (HIIT) and Continuous Training on Apelin, APJ, NO, and Cardiotrophin-1 in Cardiac Tissue of Diabetic Rats. J. Diabetes Res. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Morland, C.; Andersson, K.A.; Haugen, Ø.P.; Hadzic, A.; Kleppa, L.; Gille, A.; Rinholm, J.E.; Palibrk, V.; Diget, E.H.; Kennedy, L.H.; et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 2017, 8, 15557. [Google Scholar] [CrossRef]
- Hoier, B.; Hellsten, Y. Exercise-induced capillary growth in human skeletal muscle and the dynamics of VEGF. Microcirculation 2014, 21, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, N.; Gawli, K.; Pasupulleti, V.K.; Unniappan, S. Metabolic and Cardiovascular Actions of Nesfatin-1: Implications in Health and Disease. Curr. Pharm. Des. 2017, 23, 1453–1464. [Google Scholar] [CrossRef]
- Ghanbari-Niaki, A.; Hosseini, F.; Broom, D.R.; Tejenjari, B.; Rahmati-Ahmadabad, S. Combined Effects of High-Intensity Aerobic Exercise Training and Ziziphus jujuba Extract on Tissue Nesfatin-1 in Rats. Front. Endocrinol. 2022, 13, 845014. [Google Scholar] [CrossRef]
- Recinella, L.; Orlando, G.; Ferrante, C.; Chiavaroli, A.; Brunetti, L.; Leone, S. Adipokines: New Potential Therapeutic Target for Obesity and Metabolic, Rheumatic, and Cardiovascular Diseases. Front. Physiol. 2020, 11, 578966. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Gong, D.-W.; Tian, Z. FSTL1 as a Potential Mediator of Exercise-Induced Cardioprotection in Post-Myocardial Infarction Rats. Sci. Rep. 2016, 6, 32424. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, N.; Fujie, S.; Horii, N.; Uchida, M.; Kurihara, T.; Sanada, K.; Hamaoka, T.; Iemitsu, M. Aerobic exercise training-induced changes in serum C1q/TNF-related protein levels are associated with reduced arterial stiffness in middle-aged and older adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R94–R101. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Kim, T.H.; Kong, I.D. Exercise intervention lowers aberrant serum WISP-1 levels with insulin resistance in breast cancer survivors: A randomized controlled trial. Sci. Rep. 2020, 10, 10898. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, K.H.; Seo, K.W.; Bae, J.U.; Kim, Y.H.; Lee, S.J.; Lee, W.S.; Kim, C.D. Resistin derived from diabetic perivascular adipose tissue up-regulates vascular expression of osteopontin via the AP-1 signalling pathway. J. Pathol. 2014, 232, 87–97. [Google Scholar] [CrossRef]
- Pedersen, B.K. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef]
- Spartano, N.L.; Stevenson, M.D.; Xanthakis, V.; Larson, M.G.; Andersson, C.; Murabito, J.M.; Vasan, R.S. Associations of objective physical activity with insulin sensitivity and circulating adipokine profile: The Framingham Heart Study. Clin. Obes. 2017, 7, 59–69. [Google Scholar] [CrossRef]
- Ko, J.R.; Seo, D.Y.; Kim, T.N.; Park, S.H.; Kwak, H.-B.; Ko, K.S.; Rhee, B.D.; Han, J. Aerobic Exercise Training Decreases Hepatic Asprosin in Diabetic Rats. J. Clin. Med. 2019, 8, 666. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Yidilisi, A.; Xu, Y.; Dong, Q.; Jiang, J. Causal Associations Between Circulating Adipokines and Cardiovascular Disease: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2022, 107, e2572–e2580. [Google Scholar] [CrossRef]
- Numao, S.; Sasai, H.; Nomata, Y.; Matsuo, T.; Eto, M.; Tsujimoto, T.; Tanaka, K. Effects of exercise training on circulating retinol-binding protein 4 and cardiovascular disease risk factors in obese men. Obes. Facts 2012, 5, 845–855. [Google Scholar] [CrossRef]
- Moghadasi, M.; Domieh, A.M. Effects of Resistance versus Endurance Training on Plasma Lipocalin-2 in Young Men. Asian J. Sports Med. 2014, 5, 108–114. [Google Scholar] [PubMed]
- Kim, D.-I.; Lee, D.H.; Hong, S.; Jo, S.-W.; Won, Y.-S.; Jeon, J.Y. Six weeks of combined aerobic and resistance exercise using outdoor exercise machines improves fitness, insulin resistance, and chemerin in the Korean elderly: A pilot randomized controlled trial. Arch. Gerontol. Geriatr. 2018, 75, 59–64. [Google Scholar] [CrossRef]
- Dutheil, F.; Gordon, B.A.; Naughton, G.; Crendal, E.; Courteix, D.; Chaplais, E.; Thivel, D.; Lac, G.; Benson, A.C. Cardiovascular risk of adipokines: A review. J. Int. Med. Res. 2018, 46, 2082–2095. [Google Scholar] [CrossRef]
- Choi, K.M.; Kim, J.H.; Cho, G.J.; Baik, S.H.; Park, H.S.; Kim, S.M. Effect of exercise training on plasma visfatin and eotaxin levels. Eur. J. Endocrinol. 2007, 157, 437–442. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Llorens, S.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Talebi, M.; Shakibaei, M.; Samarghandian, S. An Overview of the Role of Adipokines in Cardiometabolic Diseases. Molecules 2020, 25, 5218. [Google Scholar] [CrossRef]
- Müller, I.I.; Schneider, M.; Müller, K.A.L.; Lunov, O.; Borst, O.; Simmet, T.; Gawaz, M. Protective role of Gremlin-1 in myocardial function. Eur. J. Clin. Investig. 2021, 51, e13539. [Google Scholar] [CrossRef]
- Saeidi, A.; Seifi-Ski-Shahr, F.; Soltani, M.; Daraei, A.; Shirvani, H.; Laher, I.; Hackney, A.C.; Johnson, K.E.; Basati, G.; Zouhal, H. Resistance training, gremlin 1 and macrophage migration inhibitory factor in obese men: A randomised trial. Arch. Physiol. Biochem. 2023, 129, 640–648. [Google Scholar] [CrossRef]
- Zhuang, Y.T.; Xu, D.Y.; Wang, G.Y.; Sun, J.L.; Huang, Y.; Wang, S.Z. IL-6 induced lncRNA MALAT1 enhances TNF-alpha expression in LPS-induced septic cardiomyocytes via activation of SAA3. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 302–309. [Google Scholar]
- Wang, Y.; Chen, D.; Zhang, Y.; Wang, P.; Zheng, C.; Zhang, S.; Yu, B.; Zhang, L.; Zhao, G.; Ma, B.; et al. Novel Adipokine, FAM19A5, Inhibits Neointima Formation After Injury Through Sphingosine-1-Phosphate Receptor 2. Circulation 2018, 138, 48–63. [Google Scholar] [CrossRef]
- Zeadin, M.; Butcher, M.; Werstuck, G.; Khan, M.; Yee, C.K.; Shaughnessy, S.G. Effect of leptin on vascular calcification in apolipoprotein E–deficient mice. Arter. Thromb. Vasc. Biol. 2009, 29, 2069–2075. [Google Scholar] [CrossRef]
- Bodary, P.F.; Gu, S.; Shen, Y.; Hasty, A.H.; Buckler, J.M.; Eitzman, D.T. Recombinant leptin promotes atherosclerosis and thrombosis in apolipoprotein E–deficient mice. Arter. Thromb. Vasc. Biol. 2005, 25, e119–e122. [Google Scholar] [CrossRef] [PubMed]
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Schieffer, B.; Selle, T.; Hilfiker, A.; Hilfiker-Kleiner, D.; Grote, K.; Tietge, U.J.; Trautwein, C.; Luchtefeld, M.; Schmittkamp, C.; Heeneman, S.; et al. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation 2004, 110, 3493–3500. [Google Scholar] [CrossRef]
- Lira, F.S.; Rosa, J.C.E.; Lima-Silva, A.; A. Souza, H.; Caperuto, E.C.; Seelaender, M.C.; Damaso, A.R.; Oyama, L.M.; Santos, R.V. Sedentary subjects have higher PAI-1 and lipoproteins levels than highly trained athletes. Diabetol. Metab. Syndr. 2010, 2, 1–5. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, M.; Wu, X.; Zhang, Y.; Xia, Y. Muscle-to-tumor crosstalk: The effect of exercise-induced myokine on cancer progression. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188761. [Google Scholar] [CrossRef]
- Raffin, J.; Angioni, D.; Giudici, K.V.; Valet, P.; Aggarwal, G.; Nguyen, A.D.E.; Morley, J.; Guyonnet, S.; Rolland, Y.; Vellas, B.; et al. Physical Activity, Body Mass Index, and Blood Progranulin in Older Adults: Cross-Sectional Associations in the MAPT Study. J. Gerontol. Ser. A 2022, 77, 1141–1149. [Google Scholar] [CrossRef]
- Bennett, D.A.; Du, H.; Clarke, R.; Guo, Y.; Yang, L.; Bian, Z.; Chen, Y.; Millwood, I.; Yu, C.; He, P.; et al. Association of Physical Activity With Risk of Major Cardiovascular Diseases in Chinese Men and Women. JAMA Cardiol. 2017, 2, 1349–1358. [Google Scholar] [CrossRef]
- Aldiss, P.; Betts, J.; Sale, C.; Pope, M.; Budge, H.; Symonds, M.E. Exercise-induced ‘browning’ of adipose tissues. Metabolism 2018, 81, 63–70. [Google Scholar] [CrossRef]
- Carey, V.J.; Walters, E.E.; Colditz, G.A.; Solomon, C.G.; Willet, W.C.; Rosner, B.A.; Speizer, F.E.; Manson, J.E. Body Fat Distribution and Risk of Non-Insulin-dependent Diabetes Mellitus in Women: The Nurses’ Health Study. Am. J. Epidemiol. 1997, 145, 614–619. [Google Scholar] [CrossRef]
- Wang, Y.; Rimm, E.B.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am. J. Clin. Nutr. 2005, 81, 555–563. [Google Scholar] [CrossRef]
- Zhang, C.; Rexrode, K.M.; van Dam, R.M.; Li, T.Y.; Hu, F.B. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: Sixteen years of follow-up in US women. Circulation 2008, 117, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Garg, A.; Abate, N.; Peshock, R.M.; Stray-Gundersen, J.; Grundy, S.M. Relationship of anterior and posterior subcutaneous abdominal fat to insulin sensitivity in nondiabetic men. Obes. Res. 1997, 5, 93–99. [Google Scholar] [CrossRef]
- Snijder, M.B.; Dekker, J.M.; Visser, M.; Bouter, L.M.; DA Stehouwer, C.; Kostense, P.J.; Yudkin, J.S.; Heine, R.J.; Nijpels, G.; Seidell, J.C. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: The Hoorn Study. Am. J. Clin. Nutr. 2003, 77, 1192–1197. [Google Scholar] [CrossRef]
- Xiong, Y.; Wu, Z.; Zhang, B.; Wang, C.; Mao, F.; Liu, X.; Hu, K.; Sun, X.; Jin, W.; Kuang, S. Fndc5 loss-of-function attenuates exercise-induced browning of white adipose tissue in mice. FASEB J. 2019, 33, 5876–5886. [Google Scholar] [CrossRef]
- Stallknecht, B.; Vinten, J.; Ploug, T.; Galbo, H. Increased activities of mitochondrial enzymes in white adipose tissue in trained rats. Am. J. Physiol. Metab. 1991, 261, E410–E414. [Google Scholar] [CrossRef]
- Sutherland, L.N.; Bomhof, M.R.; Capozzi, L.C.; Basaraba, S.A.; Wright, D.C. Exercise and adrenaline increase PGC-1α mRNA expression in rat adipose tissue. J. Physiol. 2009, 587, 1607–1617. [Google Scholar] [CrossRef]
- Wu, M.V.; Bikopoulos, G.; Hung, S.; Ceddia, R.B. Thermogenic capacity is antagonistically regulated in classical brown and white subcutaneous fat depots by high fat diet and endurance training in rats: Impact on whole-body energy expenditure. J. Biol. Chem. 2014, 289, 34129–34140. [Google Scholar] [CrossRef]
- Rönn, T.; Volkov, P.; Davegårdh, C.; Dayeh, T.; Hall, E.; Olsson, A.H.; Nilsson, E.; Tornberg, A.; Nitert, M.D.; Eriksson, K.-F.; et al. A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue. PLoS Genet. 2013, 9, e1003572. [Google Scholar] [CrossRef]
- Ruschke, K.; Fishbein, L.; Dietrich, A.; Klöting, N.; Tönjes, A.; Oberbach, A.; Fasshauer, M.; Jenkner, J.; Schön, M.R.; Stumvoll, M.; et al. Gene expression of PPARγ and PGC-1α in human omental and subcutaneous adipose tissues is related to insulin resistance markers and mediates beneficial effects of physical training. Eur. J. Endocrinol. 2010, 162, 515–523. [Google Scholar] [CrossRef]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef]
- Sindhu, S.; Thomas, R.; Kochumon, S.; Wilson, A.; Abu-Farha, M.; Bennakhi, A.; Al-Mulla, F.; Ahmad, R. Increased Adipose Tissue Expression of Interferon Regulatory Factor (IRF)-5 in Obesity: Association with Metabolic Inflammation. Cells 2019, 8, 1418. [Google Scholar] [CrossRef]
- Li, B.-Y.; Peng, W.-Q.; Liu, Y.; Guo, L.; Tang, Q.-Q. HIGD1A links SIRT1 activity to adipose browning by inhibiting the ROS/DNA damage pathway. Cell Rep. 2023, 42, 112731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-C.; Qin, F.; Wang, X.-J.; Liu, Z.-F.; Zhu, L.; Zeng, A.; Zhang, M.-Z.; Yu, N.-Z.; Long, X. Adipose-Derived Stromal Cells Attenuate Adipose Inflammation in Obesity through Adipocyte Browning and Polarization of M2 Macrophages. Mediat. Inflamm. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.G.; Murholm, M.; Carey, A.L.; Biensø, R.S.; Basse, A.L.; Allen, T.L.; Hidalgo, J.; Kingwell, B.A.; Febbraio, M.A.; Hansen, J.B.; et al. Role of IL-6 in Exercise Training- and Cold-Induced UCP1 Expression in Subcutaneous White Adipose Tissue. PLoS ONE 2014, 9, e84910. [Google Scholar] [CrossRef]
- Stanford, K.I.; Middelbeek, R.J.; Townsend, K.L.; An, D.; Nygaard, E.B.; Hitchcox, K.M.; Markan, K.R.; Nakano, K.; Hirshman, M.F.; Tseng, Y.-H.; et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Investig. 2013, 123, 215–223. [Google Scholar] [CrossRef]
- Adachi, Y.; Ueda, K.; Nomura, S.; Ito, K.; Katoh, M.; Katagiri, M.; Yamada, S.; Hashimoto, M.; Zhai, B.; Numata, G.; et al. Beiging of perivascular adipose tissue regulates its inflammation and vascular remodeling. Nat. Commun. 2022, 13, 5117. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, Y.; Xu, Y.; Wang, X.; Zhou, Z.; Wu, K.; Meng, Q.; Wang, L.; Yang, Y.; Gao, H.; et al. Inflammatory markers link triglyceride-glucose index and obesity indicators with adverse cardiovascular events in patients with hypertension: Insights from three cohorts. Cardiovasc. Diabetol. 2025, 24, 11. [Google Scholar] [CrossRef]
- Christman, M.A.; Goetz, D.J.; Dickerson, E.; McCall, K.D.; Lewis, C.J.; Benencia, F.; Silver, M.J.; Kohn, L.D.; Malgor, R. Wnt5a is expressed in murine and human atherosclerotic lesions. Am. J. Physiol. Circ. Physiol. 2008, 294, H2864–H2870. [Google Scholar] [CrossRef]
- Apovian, C.M.; Bigornia, S.; Mott, M.; Meyers, M.R.; Ulloor, J.; Gagua, M.; McDonnell, M.; Hess, D.; Joseph, L.; Gokce, N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arter. Thromb. Vasc. Biol. 2008, 28, 1654–1659. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef]
- Emanuele, E.; Minoretti, P.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Garatachea, N.; Lucia, A. Serum Irisin Levels, Precocious Myocardial Infarction, and Healthy Exceptional Longevity. Am. J. Med. 2014, 127, 888–890. [Google Scholar] [CrossRef] [PubMed]
- Elsen, M.; Raschke, S.; Eckel, J. Browning of white fat: Does irisin play a role in humans? J. Endocrinol. 2014, 222, R25–R38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T.; et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Xiang, G.; Yue, L.; Zhang, J.; Zhao, L. Circulating irisin levels are positively associated with endothelium-dependent vasodilation in newly diagnosed type 2 diabetic patients without clinical angiopathy. Atherosclerosis 2014, 235, 328–333. [Google Scholar] [CrossRef]
- Saadeldin, M.K.; Elshaer, S.S.; Emara, I.A.; Maged, M.; Abdel-Aziz, A.K. Serum sclerostin and irisin as predictive markers for atherosclerosis in Egyptian type II diabetic female patients: A case control study. PLoS ONE 2018, 13, e0206761. [Google Scholar] [CrossRef]
- Rao, S.; Pandey, A.; Garg, S.; Park, B.; Mayo, H.; Després, J.-P.; Kumbhani, D.; de Lemos, J.A.; Neeland, I.J. Effect of Exercise and Pharmacological Interventions on Visceral Adiposity: A Systematic Review and Meta-analysis of Long-term Randomized Controlled Trials. Mayo Clin. Proc. 2019, 94, 211–224. [Google Scholar] [CrossRef]
- Panagiotou, G.; Mu, L.; Na, B.; Mukamal, K.J.; Mantzoros, C.S. Circulating irisin, omentin-1, and lipoprotein subparticles in adults at higher cardiovascular risk. Metabolism 2014, 63, 1265–1271. [Google Scholar] [CrossRef]
- Reilly, S.M.; Abu-Odeh, M.; Ameka, M.; DeLuca, J.H.; Naber, M.C.; Dadpey, B.; Ebadat, N.; Gomez, A.V.; Peng, X.; Poirier, B.; et al. FGF21 is required for the metabolic benefits of IKKε/TBK1 inhibition. J. Clin. Investig. 2021, 131, e145546. [Google Scholar] [CrossRef]
- Lee, C.H.; Woo, Y.C.; Chow, W.S.; Cheung, C.Y.Y.; Fong, C.H.Y.; Yuen, M.M.A.; Xu, A.; Tse, H.F.; Lam, K.S.L. Role of Circulating Fibroblast Growth Factor 21 Measurement in Primary Prevention of Coronary Heart Disease Among Chinese Patients With Type 2 Diabetes Mellitus. J. Am. Hear. Assoc. 2017, 6, e005344. [Google Scholar] [CrossRef]
- Ong, K.L.; Hui, N.; Januszewski, A.S.; Kaakoush, N.O.; Xu, A.; Fayyad, R.; DeMicco, D.A.; Jenkins, A.J.; Keech, A.C.; Waters, D.D.; et al. High plasma FGF21 levels predicts major cardiovascular events in patients treated with atorvastatin (from the Treating to New Targets [TNT] Study). Metabolism 2019, 93, 93–99. [Google Scholar] [CrossRef]
- Cheng, J.; Su, X.; Qiao, L.; Zhai, C.; Chen, W. Circulating level of fibroblast growth factor 21 is independently associated with the risks of unstable angina pectoris. Biosci. Rep. 2018, 38, BSR20181099. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.; Mikami, T. Exercise-Induced Lactate Release Mediates Mitochondrial Biogenesis in the Hippocampus of Mice via Monocarboxylate Transporters. Front. Physiol. 2021, 12, 736905. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jin, Z.; Zheng, H.; Yan, L.-J. Sources and implications of NADH/NAD+ redox imbalance in diabetes and its complications. Diabetes Metab. Syndr. Obes. 2016, 9, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Bai, M.; Wang, S.; Chen, L.; Li, Z.; Li, C.; Cao, P.; Chen, Y. Lactate Is a Key Mediator That Links Obesity to Insulin Resistance via Modulating Cytokine Production From Adipose Tissue. Diabetes 2022, 71, 637–652. [Google Scholar] [CrossRef]
- Yao, Z.; Yan, Y.; Zheng, X.; Wang, M.; Zhang, H.; Li, H.; Chen, W. Dietary Lactate Supplementation Protects against Obesity by Promoting Adipose Browning in Mice. J. Agric. Food Chem. 2020, 68, 14841–14849. [Google Scholar] [CrossRef]
- Wu, P.; Zhu, T.; Huang, Y.; Fang, Z.; Luo, F. Current understanding of the contribution of lactate to the cardiovascular system and its therapeutic relevance. Front. Endocrinol. 2023, 14, 1205442. [Google Scholar] [CrossRef]
- Végran, F.; Boidot, R.; Michiels, C.; Sonveaux, P.; Feron, O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011, 71, 2550–2560. [Google Scholar] [CrossRef]
- Li, Z.; Solomonidis, E.G.; Meloni, M.; Taylor, R.S.; Duffin, R.; Dobie, R.; Magalhaes, M.S.; Henderson, B.E.P.; Louwe, P.A.; D’Amico, G.; et al. Single-cell transcriptome analyses reveal novel targets modulating cardiac neovascularization by resident endothelial cells following myocardial infarction. Eur. Heart J. 2019, 40, 2507–2520. [Google Scholar] [CrossRef]
- Oka, T.; Akazawa, H.; Naito, A.T.; Komuro, I. Angiogenesis and Cardiac Hypertrophy: Maintenance of cardiac function and causative roles in heart failure. Circ. Res. 2014, 114, 565–571. [Google Scholar] [CrossRef]
- Lafontan, M.; Langin, D. Lipolysis and lipid mobilization in human adipose tissue. Prog. Lipid Res. 2009, 48, 275–297. [Google Scholar] [CrossRef]
- Sakurai, T.; Davenport, R.; Stafford, S.; Grosse, J.; Ogawa, K.; Cameron, J.; Parton, L.; Sykes, A.; Mack, S.; Bousba, S.; et al. Identification of a novel GPR81-selective agonist that suppresses lipolysis in mice without cutaneous flushing. Eur. J. Pharmacol. 2014, 727, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pinckard, K.M.; Shettigar, V.K.; Wright, K.R.; Abay, E.; Baer, L.A.; Vidal, P.; Dewal, R.S.; Das, D.; Duarte-Sanmiguel, S.; Hernández-Saavedra, D.; et al. A Novel Endocrine Role for the BAT-Released Lipokine 12,13-diHOME to Mediate Cardiac Function. Circulation 2021, 143, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Lynes, M.D.; Leiria, L.O.; Lundh, M.; Bartelt, A.; Shamsi, F.; Huang, T.L.; Takahashi, H.; Hirshman, M.F.; Schlein, C.; Lee, A.; et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med. 2017, 23, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Lynes, M.D.; Takahashi, H.; Baer, L.A.; Arts, P.J.; May, F.J.; Lehnig, A.C.; Middelbeek, R.J.; Richard, J.J.; So, K.; et al. 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab. 2018, 27, 1357. [Google Scholar] [CrossRef]
- Meng, F.; Sun, N.; Liu, D.; Jia, J.; Xiao, J.; Dai, H. BCL2L13: Physiological and pathological meanings. Cell. Mol. Life Sci. 2020, 78, 2419–2428. [Google Scholar] [CrossRef]
- Murakawa, T.; Yamaguchi, O.; Hashimoto, A.; Hikoso, S.; Takeda, T.; Oka, T.; Yasui, H.; Ueda, H.; Akazawa, Y.; Nakayama, H.; et al. Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat. Commun. 2015, 6, 7527. [Google Scholar] [CrossRef]
- Ju, L.; Chen, S.; Alimujiang, M.; Bai, N.; Yan, H.; Fang, Q.; Han, J.; Ma, X.; Yang, Y.; Jia, W. A novel role for Bc12113 in promoting beige adipocyte biogenesis. Biochem. Biophys. Res. Commun. 2018, 506, 485–491. [Google Scholar] [CrossRef]
- Gao, S.; Chen, H. Therapeutic potential of apelin and Elabela in cardiovascular disease. Biomed. Pharmacother. 2023, 166, 115268. [Google Scholar] [CrossRef]
- Mughal, A.; O’Rourke, S.T. Vascular effects of apelin: Mechanisms and therapeutic potential. Pharmacol. Ther. 2018, 190, 139–147. [Google Scholar] [CrossRef]
- Than, A.; He, H.L.; Chua, S.H.; Xu, D.; Sun, L.; Leow, M.K.-S.; Chen, P. Apelin Enhances Brown Adipogenesis and Browning of White Adipocytes. J. Biol. Chem. 2015, 290, 14679–14691. [Google Scholar] [CrossRef]
- Tanianskii, D.A.; Jarzebska, N.; Birkenfeld, A.L.; O’sullivan, J.F.; Rodionov, R.N. Beta-Aminoisobutyric Acid as a Novel Regulator of Carbohydrate and Lipid Metabolism. Nutrients 2019, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, N.; Chaturvedi, P.; Tyagi, S.C. Browning of White Fat: Novel Insight Into Factors, Mechanisms, and Therapeutics. J. Cell. Physiol. 2017, 232, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.-W.; Hu, W.-J.; Li, Z.-Y.; Miao, C.-Y. Involvement of the secreted protein Metrnl in human diseases. Acta Pharmacol. Sin. 2020, 41, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Dadmanesh, M.; Aghajani, H.; Fadaei, R.; Ghorban, K. Lower serum levels of Meteorin-like/Subfatin in patients with coronary artery disease and type 2 diabetes mellitus are negatively associated with insulin resistance and inflammatory cytokines. PLoS ONE 2018, 13, e0204180. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ji, H.; Yao, M.; Wang, L.; Wang, Y.; Zhou, P.; Liu, Y.; Zheng, X.; He, H.; Wang, L.; et al. Serum Metrnl is associated with the presence and severity of coronary artery disease. J. Cell. Mol. Med. 2019, 23, 271–280. [Google Scholar] [CrossRef]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like Is a Hormone that Regulates Immune-Adipose Interactions to Increase Beige Fat Thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef]
- Ataeinosrat, A.; Saeidi, A.; Abednatanzi, H.; Rahmani, H.; Daloii, A.A.; Pashaei, Z.; Hojati, V.; Basati, G.; Mossayebi, A.; Laher, I.; et al. Intensity Dependent Effects of Interval Resistance Training on Myokines and Cardiovascular Risk Factors in Males With Obesity. Front. Endocrinol. 2022, 13, 895512. [Google Scholar] [CrossRef]
- Allen, D.L.; Hittel, D.S.; Mcpherron, A.C. Expression and Function of Myostatin in Obesity, Diabetes, and Exercise Adaptation. Med. Sci. Sports Exerc. 2011, 43, 1828–1835. [Google Scholar] [CrossRef]
- Gavaldà-Navarro, A.; Villarroya, J.; Cereijo, R.; Giralt, M.; Villarroya, F. The endocrine role of brown adipose tissue: An update on actors and actions. Rev. Endocr. Metab. Disord. 2022, 23, 31–41. [Google Scholar] [CrossRef]
- Oshima, Y.; Ouchi, N.; Sato, K.; Izumiya, Y.; Pimentel, D.R.; Walsh, K. Follistatin-Like 1 Is an Akt-Regulated Cardioprotective Factor That Is Secreted by the Heart. Circulation 2008, 117, 3099–3108. [Google Scholar] [CrossRef]
- Braga, M.; Reddy, S.T.; Vergnes, L.; Pervin, S.; Grijalva, V.; Stout, D.; David, J.; Li, X.; Tomasian, V.; Reid, C.B.; et al. Follistatin promotes adipocyte differentiation, browning, and energy metabolism. J. Lipid Res. 2014, 55, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Braga, M.; Pervin, S. Regulation of brown adipocyte metabolism by myostatin/follistatin signaling. Front. Cell Dev. Biol. 2014, 2, 60. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.D.; Boström, P.; O’sullivan, J.F.; Schinzel, R.T.; Lewis, G.D.; Dejam, A.; Lee, Y.-K.; Palma, M.J.; Calhoun, S.; Georgiadi, A.; et al. β-aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014, 19, 96–108. [Google Scholar] [CrossRef]
- Mukherjee, S.; Choi, M.J.; Kim, S.W.; Yun, J.W. Secreted protein acidic and rich in cysteine (SPARC) regulates thermogenesis in white and brown adipocytes. Mol. Cell. Endocrinol. 2020, 506, 110757. [Google Scholar] [CrossRef]
- Veith, C.; Vartürk-Özcan, I.; Wujak, M.; Hadzic, S.; Wu, C.-Y.; Knoepp, F.; Kraut, S.; Petrovic, A.; Gredic, M.; Pak, O.; et al. SPARC, a Novel Regulator of Vascular Cell Function in Pulmonary Hypertension. Circulation 2022, 145, 916–933. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kim, Y.H.; Son, J.E.; Lee, J.H.; Kim, S.; Choe, M.S.; Moon, J.H.; Zhong, J.; Fu, K.; Lenglin, F.; et al. Intermittent fasting promotes adipose thermogenesis and metabolic homeostasis via VEGF-mediated alternative activation of macrophage. Cell Res. 2017, 27, 1309–1326. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Townsend, L.K.; Wright, D.C. Looking on the “brite” side exercise-induced browning of white adipose tissue. Pflügers Arch. Eur. J. Physiol. 2018, 471, 455–465. [Google Scholar] [CrossRef]
- Sharif, K.; Watad, A.; Bragazzi, N.L.; Lichtbroun, M.; Amital, H.; Shoenfeld, Y. Physical activity and autoimmune diseases: Get moving and manage the disease. Autoimmun. Rev. 2018, 17, 53–72. [Google Scholar] [CrossRef]
- Zhang, H.; Park, Y.; Wu, J.; Chen, X.P.; Lee, S.; Yang, J.; Dellsperger, K.C.; Zhang, C. Role of TNF-α in vascular dysfunction. Clin. Sci. 2009, 116, 219–230. [Google Scholar] [CrossRef]
- Rhee, M.; Kim, J.-W.; Lee, M.-W.; Yoon, K.-H.; Lee, S.-H. Preadipocyte factor 1 regulates adipose tissue browning via TNF-α-converting enzyme-mediated cleavage. Metabolism 2019, 101, 153977. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, C.; Zeng, X.; Resch, J.M.; Jedrychowski, M.P.; Yang, Z.; Desai, B.N.; Banks, A.S.; Lowell, B.B.; Mathis, D.; et al. γδ T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature 2020, 578, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Calvier, L.; Chouvarine, P.; Legchenko, E.; Hoffmann, N.; Geldner, J.; Borchert, P.; Jonigk, D.; Mozes, M.M.; Hansmann, G. PPARγ Links BMP2 and TGFβ1 Pathways in Vascular Smooth Muscle Cells, Regulating Cell Proliferation and Glucose Metabolism. Cell Metab. 2017, 25, 1118–1134.e7. [Google Scholar] [CrossRef]
- Nakhuda, A.; Josse, A.R.; Gburcik, V.; Crossland, H.; Raymond, F.; Metairon, S.; Good, L.; Atherton, P.J.; Phillips, S.M.; Timmons, J.A. Biomarkers of browning of white adipose tissue and their regulation during exercise- and diet-induced weight loss. Am. J. Clin. Nutr. 2016, 104, 557–565. [Google Scholar] [CrossRef]
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014, 281, 739–749. [Google Scholar] [CrossRef]
- Zuriaga, M.A.; Fuster, J.J.; Gokce, N.; Walsh, K. Humans and Mice Display Opposing Patterns of “Browning” Gene Expression in Visceral and Subcutaneous White Adipose Tissue Depots. Front. Cardiovasc. Med. 2017, 4, 27. [Google Scholar] [CrossRef]
- Van den Beukel, J.C.; Grefhorst, A.; Hoogduijn, M.J.; Steenbergen, J.; Mastroberardino, P.G.; Dor, F.J.M.F.; Themmen, A.P.N. Women have more potential to induce browning of perirenal adipose tissue than men. Obesity 2015, 23, 1671–1679. [Google Scholar] [CrossRef]
- Nigro, P.; Middelbeek, R.J.; Alves, C.R.; Rovira-Llopis, S.; Ramachandran, K.; Rowland, L.A.; Møller, A.B.; Takahashi, H.; Alves-Wagner, A.B.; Vamvini, M.; et al. Exercise Training Promotes Sex-Specific Adaptations in Mouse Inguinal White Adipose Tissue. Diabetes 2021, 70, 1250–1264. [Google Scholar] [CrossRef]
- Taylor, J.L.; Holland, D.J.; Keating, S.E.; Leveritt, M.D.; Gomersall, S.R.; Rowlands, A.V.; Bailey, T.G.; Coombes, J.S. Short-term and Long-term Feasibility, Safety, and Efficacy of High-Intensity Interval Training in Cardiac Rehabilitation: The FITR heart study randomized clinical trial. JAMA Cardiol. 2020, 5, 1382–1389. [Google Scholar] [CrossRef]
- Wewege, M.A.; Ahn, D.; Yu, J.; Liou, K.; Keech, A. High-Intensity Interval Training for Patients With Cardiovascular Disease—Is It Safe? A Systematic Review. J. Am. Hear. Assoc. 2018, 7, e009305. [Google Scholar] [CrossRef]
- Gomes-Neto, M.; Durães, A.R.; dos Reis, H.F.C.; Neves, V.R.; Martinez, B.P.O.; Carvalho, V. High-intensity interval training versus moderate-intensity continuous training on exercise capacity and quality of life in patients with coronary artery disease: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2017, 24, 1696–1707. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Wang, Y.; Liu, H.; Kong, Z.; Qi, F. Effects of High-Intensity Interval vs. Moderate-Intensity Continuous Training on Cardiac Rehabilitation in Patients With Cardiovascular Disease: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 845225. [Google Scholar] [CrossRef] [PubMed]
- Hannan, A.L.; Hing, W.; Simas, V.; Climstein, M.; Coombes, J.S.; Jayasinghe, R.; Byrnes, J.; Furness, J. High-intensity interval training versus moderate-intensity continuous training within cardiac rehabilitation: A systematic review and meta-analysis. Open Access J. Sports Med. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Picoli, C.D.C.; Gilio, G.R.; Henriques, F.; Leal, L.G.; Besson, J.C.; Lopes, M.A.; Franzói de Moraes, S.M.; Hernandes, L.; Batista Junior, M.L.; Peres, S.B. Resistance exercise training induces subcutaneous and visceral adipose tissue browning in Swiss mice. J. Appl. Physiol. 2020, 129, 66–74. [Google Scholar] [CrossRef]
| Adipokines | Function in CVD | Reference | |
|---|---|---|---|
| Beneficial | |||
| 1 | Omentin-1 | Reduces inflammatory response from TNF-α | [69,70,71] |
| 2 | Adiponectin | Protects cardiovascular smooth muscle and inhibits cholesterol uptake by LDL receptor | [72,73,74] |
| 3 | SFRP5 | Reduces atherosclerosis | [71,75] |
| 4 | Cardiotrophin-1 | Maintains blood cholesterol levels | [71,76] |
| 5 | VEGF | Promotes angiogenesis | [77,78] |
| 6 | Nesfatin-1 | Prevention of ischemia-reperfusion injury and regulation of arterial blood pressure | [79,80] |
| 7 | FSTL1 | Prevention of ventricular hypertrophy, cardiomyocyte apoptosis, and promotion of proliferation | [81,82] |
| 8 | CTRPs | CTRP-1 and CTRP-3 prevent coronary artery disease and ischemic injury | [81,83] |
| 9 | WISP1 | Promotes myocardial repair and prevents apoptosis | [81,84] |
| Pernicious | |||
| 10 | Resistin | Promotes coronary artery calcification | [85] |
| 11 | TNF-α | Diminished vascular elasticity, endothelial cell apoptosis, impaired myocardial function | [86] |
| 12 | FABP-4 | Promotes hypertension, atherosclerosis, impairs myocardial contraction | [71,87] |
| 13 | Asprosin | Increased triglyceride levels | [71,88] |
| 14 | RBP4 | Pro-inflammation and ventricular hypertrophy | [89,90] |
| 15 | Lipocalin-2 | Pro-inflammatory, positively associated with atherosclerosis, myocardial infarction | [81,91] |
| 16 | Chemerin | Pro-atherosclerotic, positively correlated with cholesterol levels | [71,81,92] |
| 17 | Visfatin | Atherosclerosis, myocardial infarction | [93,94] |
| 18 | Apelin | Causes high blood pressure, HF, and impairs myocardial contraction | [95] |
| 19 | Gremlin-1 | Promotes macrophage migration | [96,97] |
| 20 | SAA3 | Predictive marker of CVD, up-regulation of TNF-α | [93,98] |
| 21 | FAM19A5 | Inhibition of angiogenesis | [99] |
| Lack or too much is harmful | |||
| 22 | Leptin | Increases macrophages, promotes atherosclerosis, controls blood pressure | [100,101] |
| 23 | Interleukin | IL-1, causes hypotension IL-6, associated with atherosclerosis IL-4/13, promotes M2 macrophage recruitment | [86,90,102,103] |
| 24 | Vaspin | Pro-atherosclerosis; prevention of vascular endothelial cell apoptosis | [81] |
| 25 | PAI-1 | Reduction of myocardial fibrosis | [93,104] |
| 26 | SPARC | Promotes myocardial injury and fibrosis | [81,105] |
| 27 | PGRN | Anti-inflammatory by lowering TNF and promoting myocardial repair after ischemia | [81,106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Li, X.; Liang, C.; Yan, Y. Can Exercise-Mediated Adipose Browning Provide an Alternative Explanation for the Obesity Paradox? Int. J. Mol. Sci. 2025, 26, 1790. https://doi.org/10.3390/ijms26051790
Zhao J, Li X, Liang C, Yan Y. Can Exercise-Mediated Adipose Browning Provide an Alternative Explanation for the Obesity Paradox? International Journal of Molecular Sciences. 2025; 26(5):1790. https://doi.org/10.3390/ijms26051790
Chicago/Turabian StyleZhao, Jiani, Xuehan Li, Chunyu Liang, and Yi Yan. 2025. "Can Exercise-Mediated Adipose Browning Provide an Alternative Explanation for the Obesity Paradox?" International Journal of Molecular Sciences 26, no. 5: 1790. https://doi.org/10.3390/ijms26051790
APA StyleZhao, J., Li, X., Liang, C., & Yan, Y. (2025). Can Exercise-Mediated Adipose Browning Provide an Alternative Explanation for the Obesity Paradox? International Journal of Molecular Sciences, 26(5), 1790. https://doi.org/10.3390/ijms26051790






