Initiation of Progressive Morphological Transition Towards an Echino-Stomato-Spherocytic Phenotype by Phosphatidylserine Externalization and Its Implication in Thrombosis
Abstract
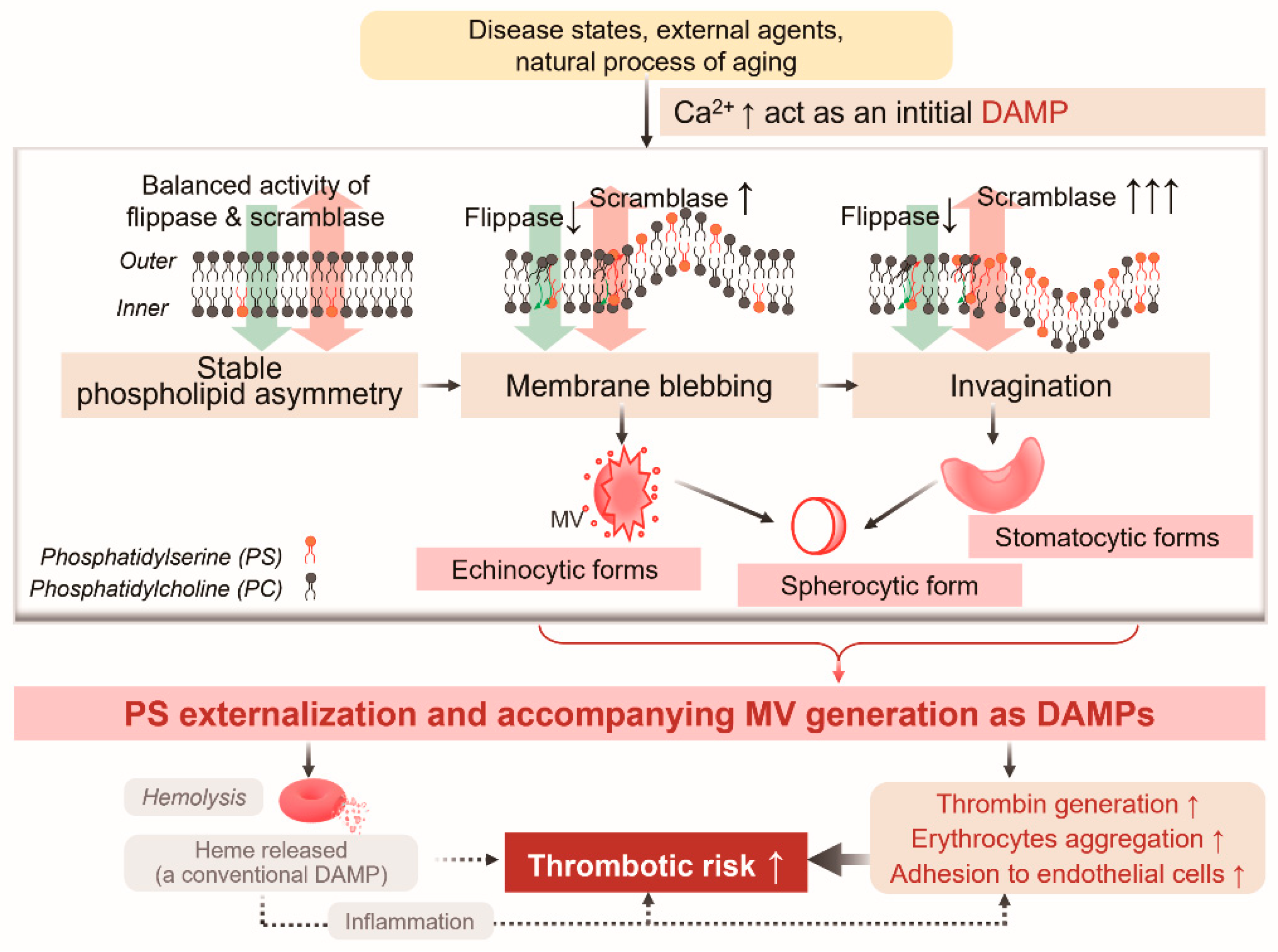
1. Background
2. Results
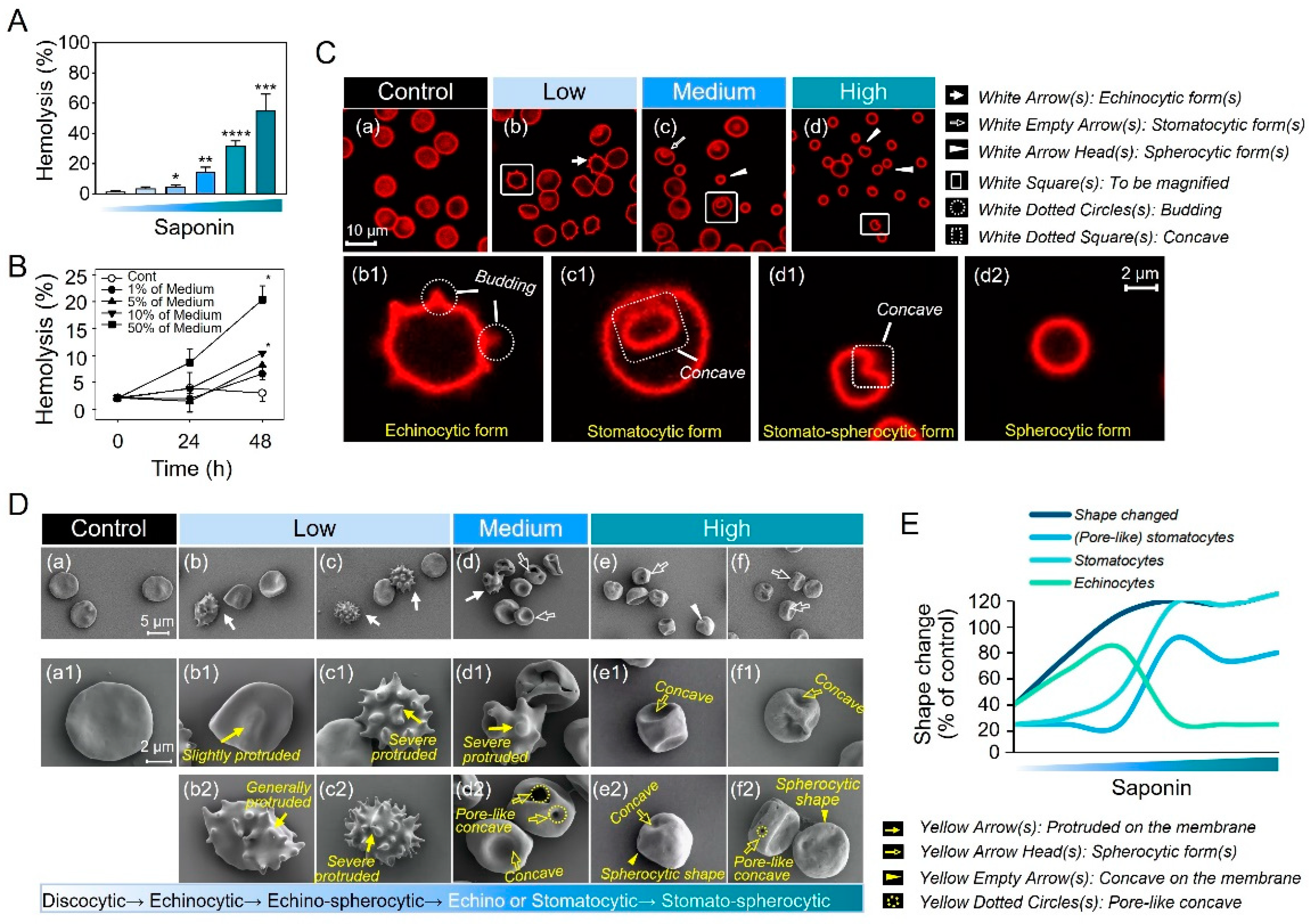
2.1. Progressive Morphological Transitions in a Saponin-Induced EST Model
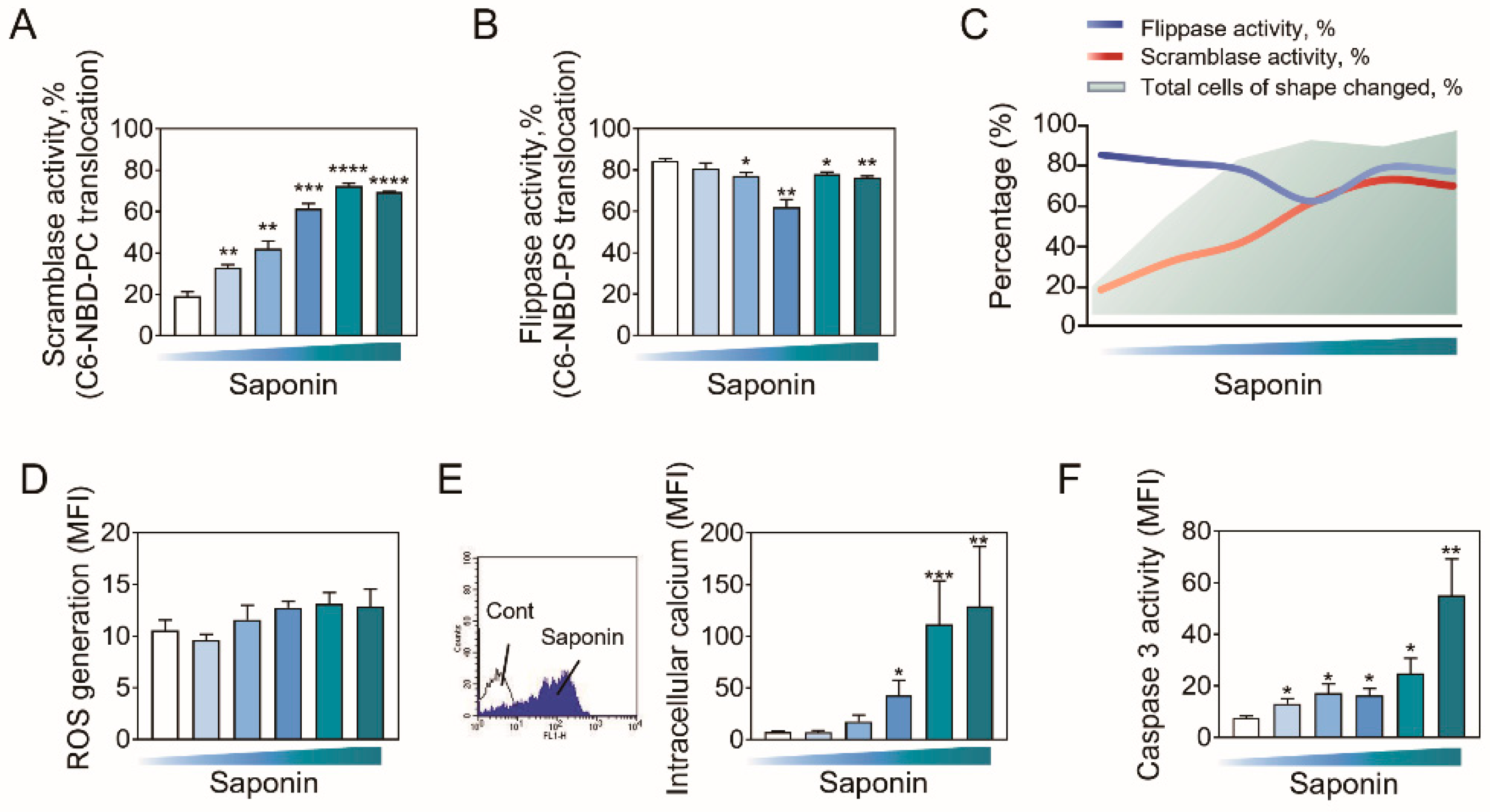
2.2. PS Externalization Accompanies the Entire Progression of Echino-Stomato-Spherocytic Shape Changes
2.3. Role of Phospholipid Translocases in Erythrocyte Morphological Alterations
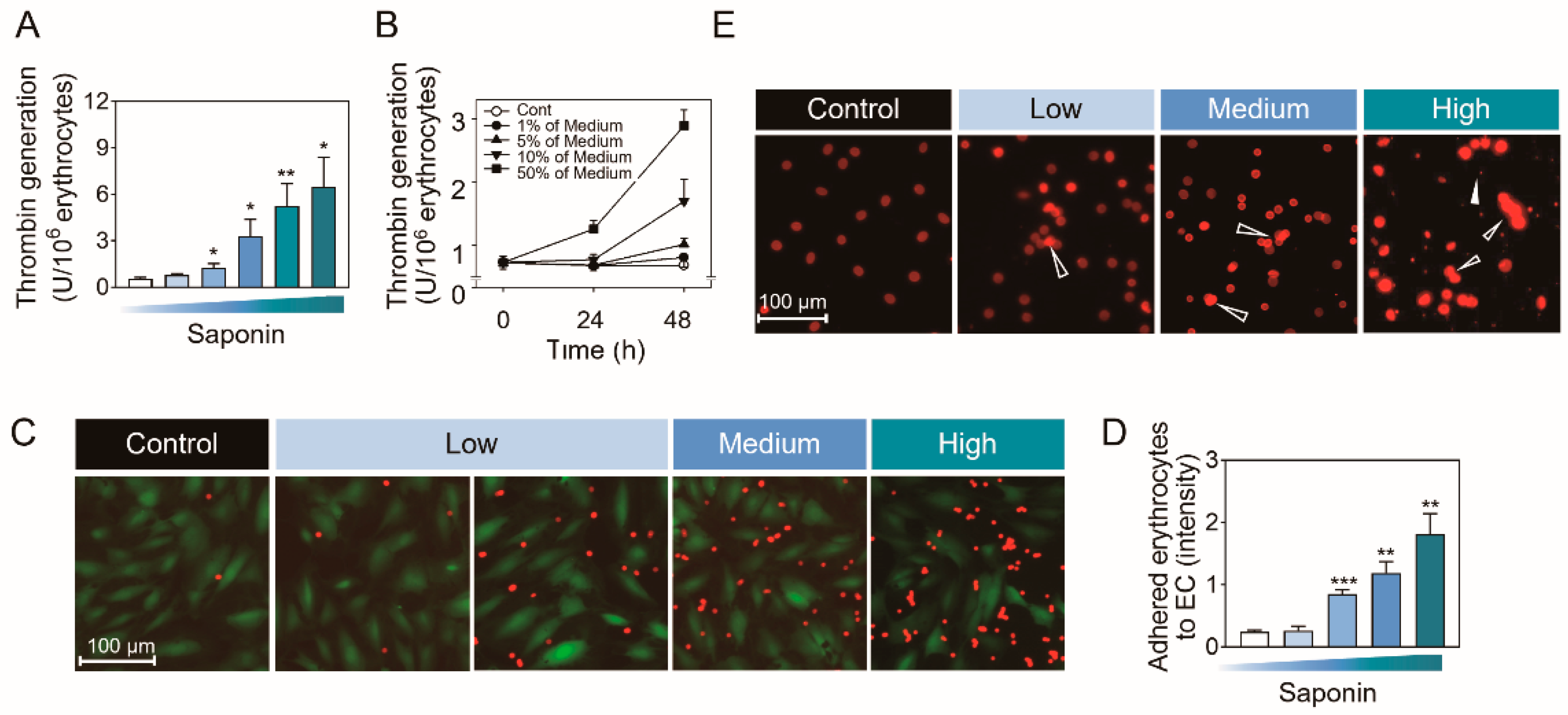
2.4. Biological Significances of PS Externalization-Associated Shape Changes
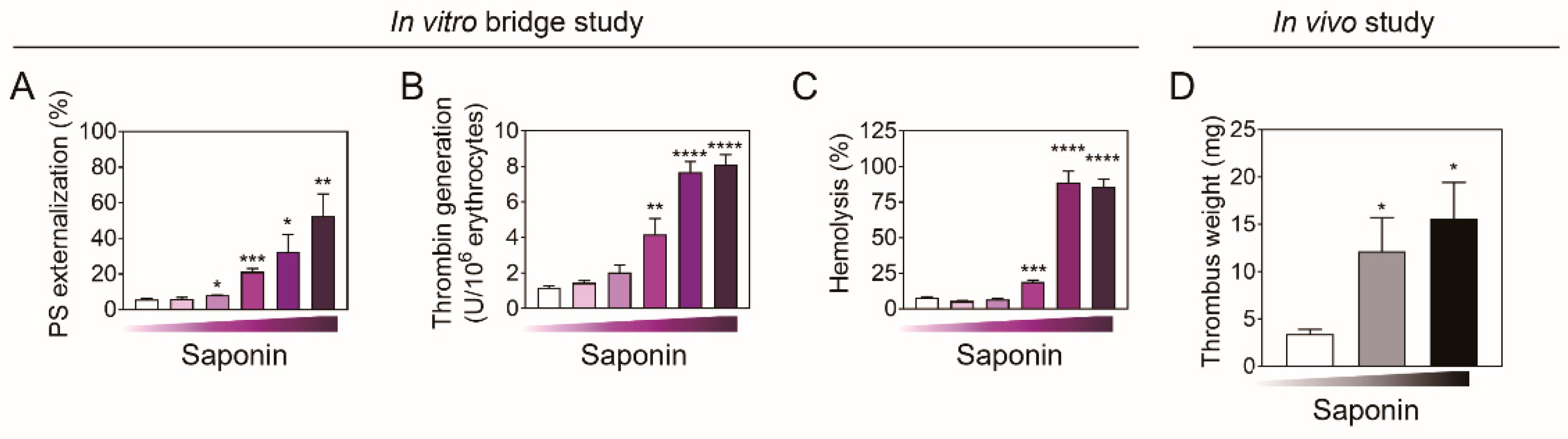
2.5. In Vivo Assessment Using Venous Thrombosis Rat Model
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Erythrocytes Preparation
4.3. Detection of Hemolytic Activity
4.4. Confocal Microscopic Observation
4.5. Flow Cytometric Analysis
4.6. SEM Observation
4.7. Prothrombinase Assay
4.8. Fluorescence Microscopy Observation
4.9. In Vivo Experiments
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Byrnes, J.R.; Wolberg, A.S. Red blood cells in thrombosis. Blood 2017, 130, 1795–1799. [Google Scholar] [CrossRef]
- Olatunya, O.S.; Lanaro, C.; Longhini, A.L.; Penteado, C.F.F.; Fertrin, K.Y.; Adekile, A.; Saad, S.T.O.; Costa, F.F. Red blood cells microparticles are associated with hemolysis markers and may contribute to clinical events among sickle cell disease patients. Ann. Hematol. 2019, 98, 2507–2521. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W.; Litvinov, R.I. Red blood cells: The forgotten player in hemostasis and thrombosis. J. Thromb. Haemost. 2019, 17, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Setty, B.N.; Betal, S.G. Microvascular endothelial cells express a phosphatidylserine receptor: A functionally active receptor for phosphatidylserine-positive erythrocytes. Blood 2008, 111, 905–914. [Google Scholar] [CrossRef]
- An, R.; Man, Y.; Cheng, K.; Zhang, T.; Chen, C.; Wang, F.; Abdulla, F.; Kucukal, E.; Wulftange, W.J.; Goreke, U.; et al. Sickle red blood cell-derived extracellular vesicles activate endothelial cells and enhance sickle red cell adhesion mediated by von Willebrand factor. Br. J. Haematol. 2023, 201, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef]
- Canesin, G.; Hejazi, S.M.; Swanson, K.D.; Wegiel, B. Heme-Derived Metabolic Signals Dictate Immune Responses. Front. Immunol. 2020, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; An, G.J.; Kim, K.; Ngo, T.; Shin, S.; Bae, O.N.; Lim, K.M.; Chung, J.H. Ginsenoside Rg3, a component of ginseng, induces pro-thrombotic activity of erythrocytes via hemolysis-associated phosphatidylserine exposure. Food Chem. Toxicol. 2019, 131, 110553. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.G.; Wortis, M.; Mukhopadhyay, R. Stomatocyte-discocyte-echinocyte sequence of the human red blood cell: Evidence for the bilayer- couple hypothesis from membrane mechanics. Proc. Natl. Acad. Sci. USA 2002, 99, 16766–16769. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.W.; Kuchel, R.P.; Koh, J.M.; Szekely, D.; Mirtschin, P.J.; Kuchel, P.W. Cytoskeletal rearrangements in human red blood cells induced by snake venoms: Light microscopy of shapes and NMR studies of membrane function. Cell Biol. Int. 2012, 36, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Kim, K.; Ngo, T.; Kim, I.; Bae, O.N.; Lim, K.M.; Chung, J.H. Silver nanoparticles promote procoagulant activity of red blood cells: A potential risk of thrombosis in susceptible population. Part. Fibre Toxicol. 2019, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Melzak, K.A.; Uhlig, S.; Kirschhöfer, F.; Brenner-Weiss, G.; Bieback, K. The Blood Bag Plasticizer Di-2-Ethylhexylphthalate Causes Red Blood Cells to Form Stomatocytes, Possibly by Inducing Lipid Flip-Flop. Transfus. Med. Hemotherapy 2018, 45, 413–422. [Google Scholar] [CrossRef]
- Manno, S.; Takakuwa, Y.; Mohandas, N. Identification of a functional role for lipid asymmetry in biological membranes: Phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc. Natl. Acad. Sci. USA 2002, 99, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.C.; Cohen, A.R.; Pressel, S.L.; Aygun, B.; Imran, H.; Luchtman-Jones, L.; Thompson, A.A.; Fuh, B.; Schultz, W.H.; Davis, B.R.; et al. Organ iron accumulation in chronically transfused children with sickle cell anaemia: Baseline results from the TWiTCH trial. Br. J. Haematol. 2016, 172, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Bettiol, A.; Galora, S.; Argento, F.R.; Fini, E.; Emmi, G.; Mattioli, I.; Bagni, G.; Fiorillo, C.; Becatti, M. Erythrocyte oxidative stress and thrombosis. Expert. Rev. Mol. Med. 2022, 24, e31. [Google Scholar] [CrossRef]
- Lim, K.M.; Kim, S.; Noh, J.Y.; Kim, K.; Jang, W.H.; Bae, O.N.; Chung, S.M.; Chung, J.H. Low-level mercury can enhance procoagulant activity of erythrocytes: A new contributing factor for mercury-related thrombotic disease. Environ. Health Perspect. 2010, 118, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Song, J.S.; Lim, K.M.; Kang, S.; Noh, J.Y.; Kim, K.; Bae, O.N.; Chung, J.H. Procoagulant and prothrombotic effects of the herbal medicine, Dipsacus asper and its active ingredient, dipsacus saponin C, on human platelets. J. Thromb. Haemost. 2012, 10, 895–906. [Google Scholar] [CrossRef]
- Banerjee, T.; Kuypers, F.A. Reactive oxygen species and phosphatidylserine externalization in murine sickle red cells. Br. J. Haematol. 2004, 124, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, A.; Makhro, A.; Wang, J.; Lipp, P.; Kaestner, L. Calcium in red blood cells-a perilous balance. Int. J. Mol. Sci. 2013, 14, 9848–9872. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Fujii, T.; Imao, T.; Ishihara, K.; Kuba, H.; Nagata, S. Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members. J. Biol. Chem. 2013, 288, 13305–13316. [Google Scholar] [CrossRef]
- Nagata, S.; Suzuki, J.; Segawa, K.; Fujii, T. Exposure of phosphatidylserine on the cell surface. Cell Death Differ. 2016, 23, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Sakuragi, T.; Nagata, S. Regulation of phospholipid distribution in the lipid bilayer by flippases and scramblases. Nat. Rev. Mol. Cell Biol. 2023, 24, 576–596. [Google Scholar] [CrossRef] [PubMed]
- Mischitelli, M.; Jemaa, M.; Almasry, M.; Faggio, C.; Lang, F. Stimulation of erythrocyte cell membrane scrambling by quinine. Cell Physiol. Biochem. 2016, 40, 657–667. [Google Scholar] [CrossRef]
- Krishnacoumar, B.; Stenzel, M.; Garibagaoglu, H.; Omata, Y.; Sworn, R.L.; Hofmann, T.; Ipseiz, N.; Czubala, M.A.; Steffen, U.; Maccataio, A.; et al. Caspase-8 promotes scramblase-mediated phosphatidylserine exposure and fusion of osteoclast precursors. Bone Res. 2024, 12, 40. [Google Scholar] [CrossRef]
- Azab, W.; Gramatica, A.; Herrmann, A.; Osterrieder, N. Binding of alphaherpesvirus glycoprotein H to surface α4β1-integrins activates calcium-signaling pathways and induces phosphatidylserine exposure on the plasma membrane. mBio 2015, 6, e01552-15. [Google Scholar] [CrossRef] [PubMed]
- Oğuz, O.; Adan, A. Involvement of Sphingolipid Metabolism Enzymes in Resveratrol-Mediated Cytotoxicity in Philadelphia-Positive Acute Lymphoblastic Leukemia. Nutr. Cancer 2022, 74, 2508–2521. [Google Scholar] [CrossRef]
- Daleke, D.L. Regulation of transbilayer plasma membrane phospholipid asymmetry. J. Lipid Res. 2003, 44, 233–242. [Google Scholar] [CrossRef]
- Bardyn, M.; Rappaz, B.; Jaferzadeh, K.; Crettaz, D.; Tissot, J.D.; Moon, I.; Turcatti, G.; Lion, N.; Prudent, M. Red blood cells ageing markers: A multi-parametric analysis. Blood Transfus. 2017, 15, 239–248. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, A.; Ricci, E.; Scuderi, F.; Micalizzi, C.; Di Rocco, M. Stomatocytes and macrothrombocytopenia: A blood film for a rare disease. EJHaem 2020, 1, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Song, L.; Shen, L.; Zhang, K.; Lv, Y.; Gao, M.; Ma, J.; Wan, Y.; Gai, Z.; Liu, Y. Mutational Characteristics of Causative Genes in Chinese Hereditary Spherocytosis Patients: A Report on Fourteen Cases and a Review of the Literature. Front. Pharmacol. 2021, 12, 644352. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Yao, C.; Bian, Y.; Pi, J. Pb-Induced Eryptosis May Provoke Thrombosis Prior to Hemolysis. Int. J. Mol. Sci. 2022, 23, 7008. [Google Scholar] [CrossRef]
- Ramadas, N.; Sparkenbaugh, E.M. The APC-EPCR-PAR1 axis in sickle cell disease. Front. Med. 2023, 10, 1141020. [Google Scholar] [CrossRef]
- Reddy, P.H.; Kshirsagar, S.; Bose, C.; Pradeepkiran, J.A.; Hindle, A.; Singh, S.P.; Reddy, A.P. Rlip overexpression reduces oxidative stress and mitochondrial dysfunction in Alzheimer’s disease: Mechanistic insights. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166759. [Google Scholar] [CrossRef] [PubMed]
- Bou-Fakhredin, R.; Cappellini, M.D.; Taher, A.T.; De Franceschi, L. Hypercoagulability in hemoglobinopathies: Decoding the thrombotic threat. Am. J. Hematol. 2025, 100, 103–115. [Google Scholar] [CrossRef]
- Tripodi, A. Usefulness of Thrombin Generation. Hamostaseologie 2020, 40, 509–514. [Google Scholar] [CrossRef]
- Kim, E.H.; Choi, S.; Kim, D.; Park, H.J.; Bian, Y.; Choi, S.H.; Chung, H.Y.; Bae, O.N. Amine-modified nanoplastics promote the procoagulant activation of isolated human red blood cellserythrocytes and thrombus formation in rats. Part. Fibre Toxicol. 2022, 19, 60. [Google Scholar] [CrossRef]
- Soares, M.M.; King, S.W.; Thorpe, P.E. Targeting inside-out phosphatidylserine as a therapeutic strategy for viral diseases. Nat. Med. 2008, 14, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, R.J., Jr.; Balu-Iyer, S.; Loyall, J.; Sacca, A.J.; Shenoy, G.N.; Peng, P.; Iyer, V.; Fathallah, A.M.; Berenson, C.S.; Wallace, P.K.; et al. Extracellular Vesicles Present in Human Ovarian Tumor Microenvironments Induce a Phosphatidylserine-Dependent Arrest in the T-cell Signaling Cascade. Cancer Immunol. Res. 2015, 3, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Munoz, L.E.; Franz, S.; Pausch, F.; Fürnrohr, B.; Sheriff, A.; Vogt, B.; Kern, P.M.; Baum, W.; Stach, C.; von Laer, D.; et al. The influence on the immunomodulatory effects of dying and dead cells of Annexin V. J. Leukoc. Biol. 2007, 81, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Risinger, M.; Kalfa, T.A. Red cell membrane disorders: Structure meets function. Blood 2020, 136, 1250–1261. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, Y.; Jin, Q.; Chung, H.-Y.; Lim, K.-M.; Xu, Y. Initiation of Progressive Morphological Transition Towards an Echino-Stomato-Spherocytic Phenotype by Phosphatidylserine Externalization and Its Implication in Thrombosis. Int. J. Mol. Sci. 2025, 26, 1747. https://doi.org/10.3390/ijms26041747
Bian Y, Jin Q, Chung H-Y, Lim K-M, Xu Y. Initiation of Progressive Morphological Transition Towards an Echino-Stomato-Spherocytic Phenotype by Phosphatidylserine Externalization and Its Implication in Thrombosis. International Journal of Molecular Sciences. 2025; 26(4):1747. https://doi.org/10.3390/ijms26041747
Chicago/Turabian StyleBian, Yiying, Qiushuo Jin, Han-Young Chung, Kyung-Min Lim, and Yuanyuan Xu. 2025. "Initiation of Progressive Morphological Transition Towards an Echino-Stomato-Spherocytic Phenotype by Phosphatidylserine Externalization and Its Implication in Thrombosis" International Journal of Molecular Sciences 26, no. 4: 1747. https://doi.org/10.3390/ijms26041747
APA StyleBian, Y., Jin, Q., Chung, H.-Y., Lim, K.-M., & Xu, Y. (2025). Initiation of Progressive Morphological Transition Towards an Echino-Stomato-Spherocytic Phenotype by Phosphatidylserine Externalization and Its Implication in Thrombosis. International Journal of Molecular Sciences, 26(4), 1747. https://doi.org/10.3390/ijms26041747





