The Proteome of Exosomes at Birth Predicts Insulin Resistance, Adrenarche and Liver Fat in Childhood
Abstract
1. Introduction
2. Results
2.1. Longitudinal Auxological, Endocrine–Metabolic and Imaging Variables
2.2. Exosome Characterization
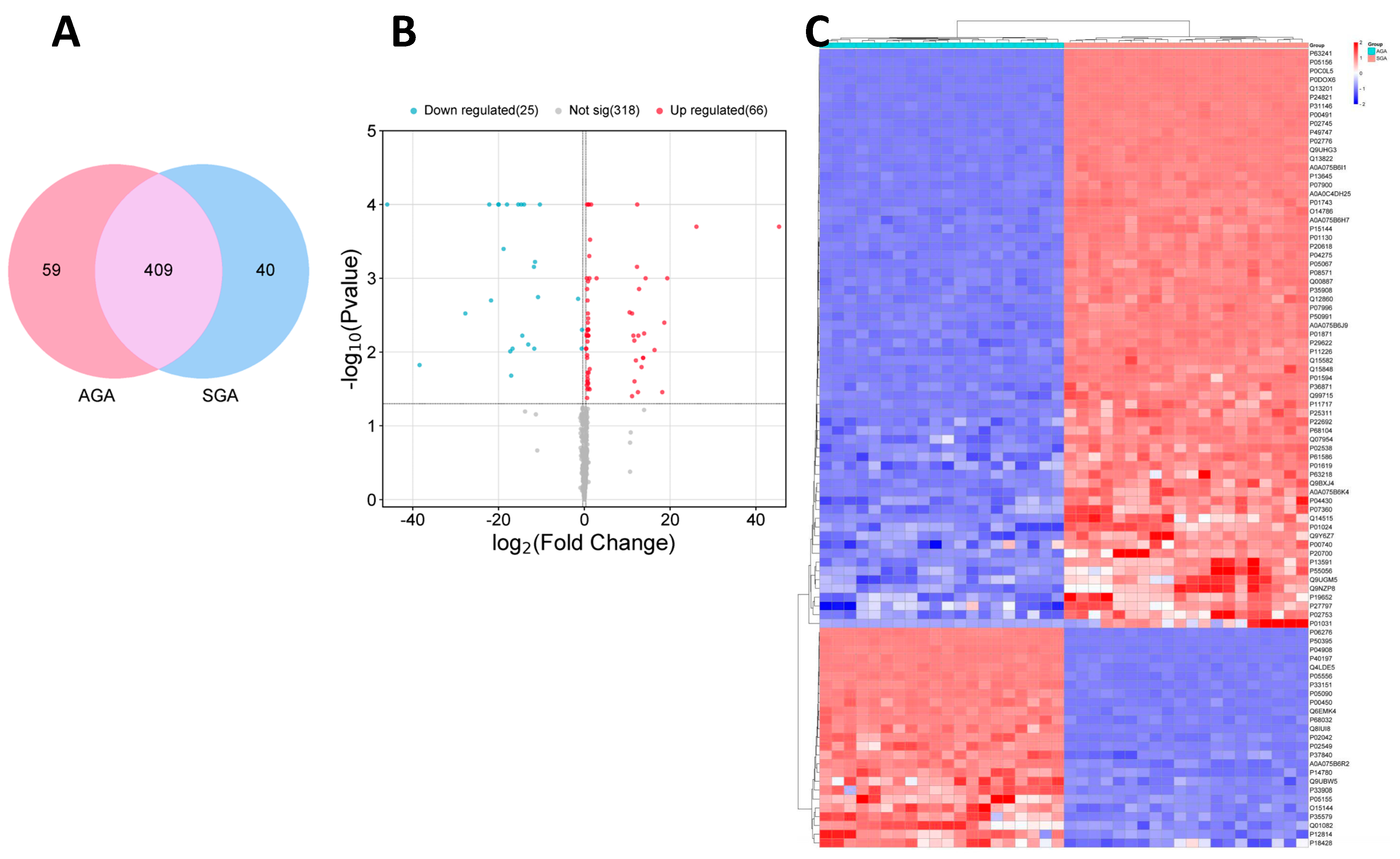
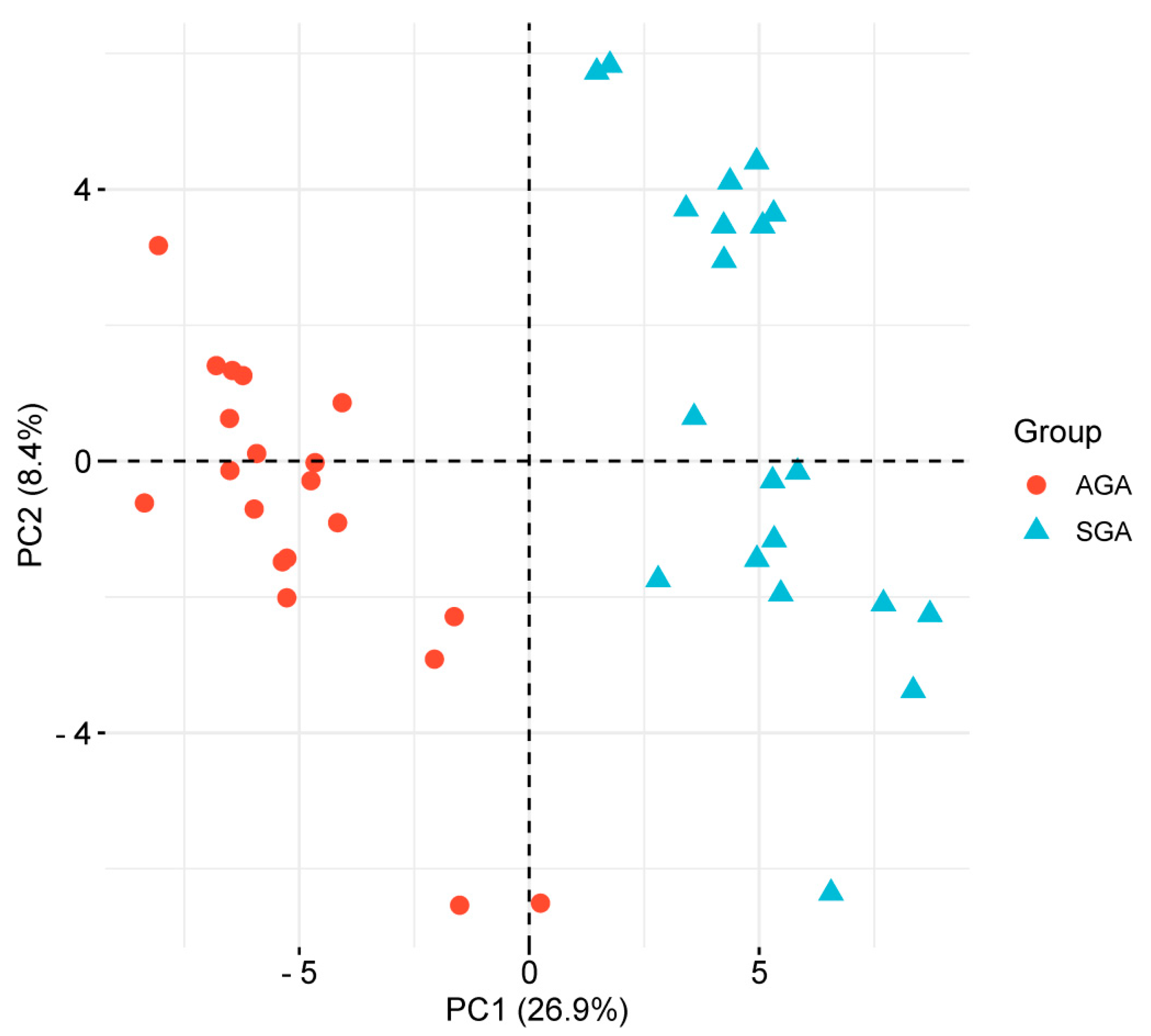
2.3. Label-Free Quantitative Proteomics
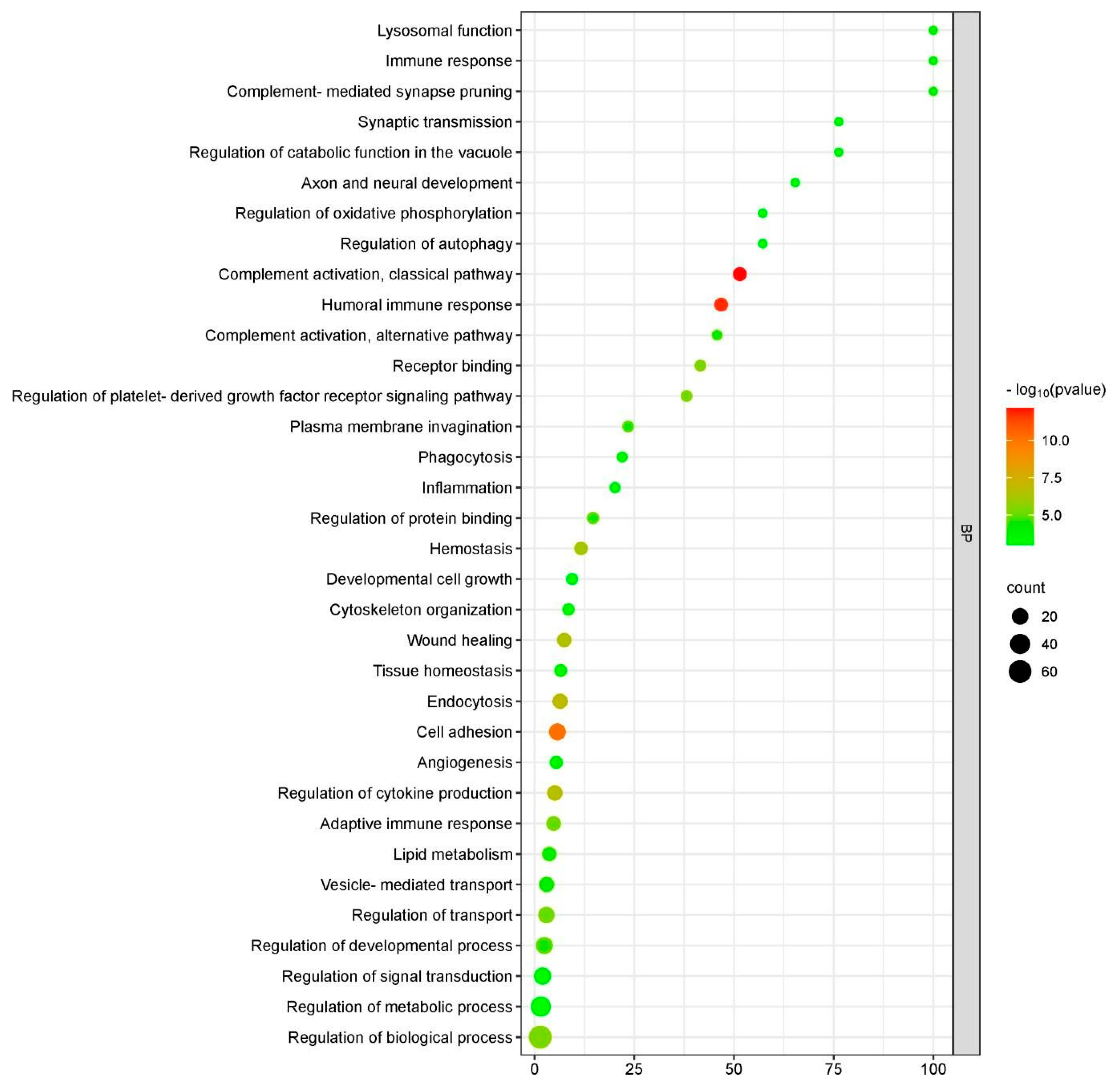
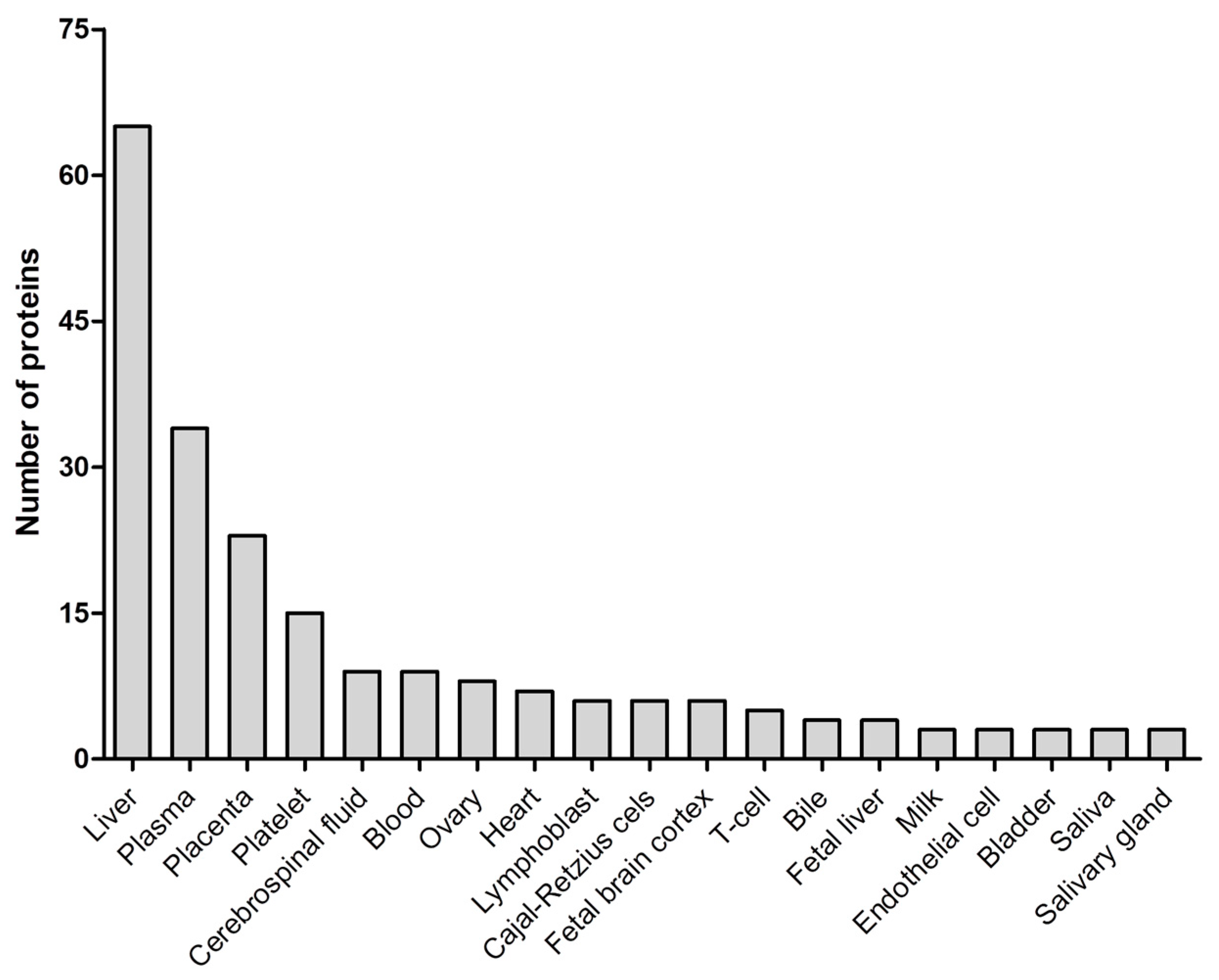
2.4. Functional Enrichment Analysis of Differentially Expressed Proteins (DEPs)
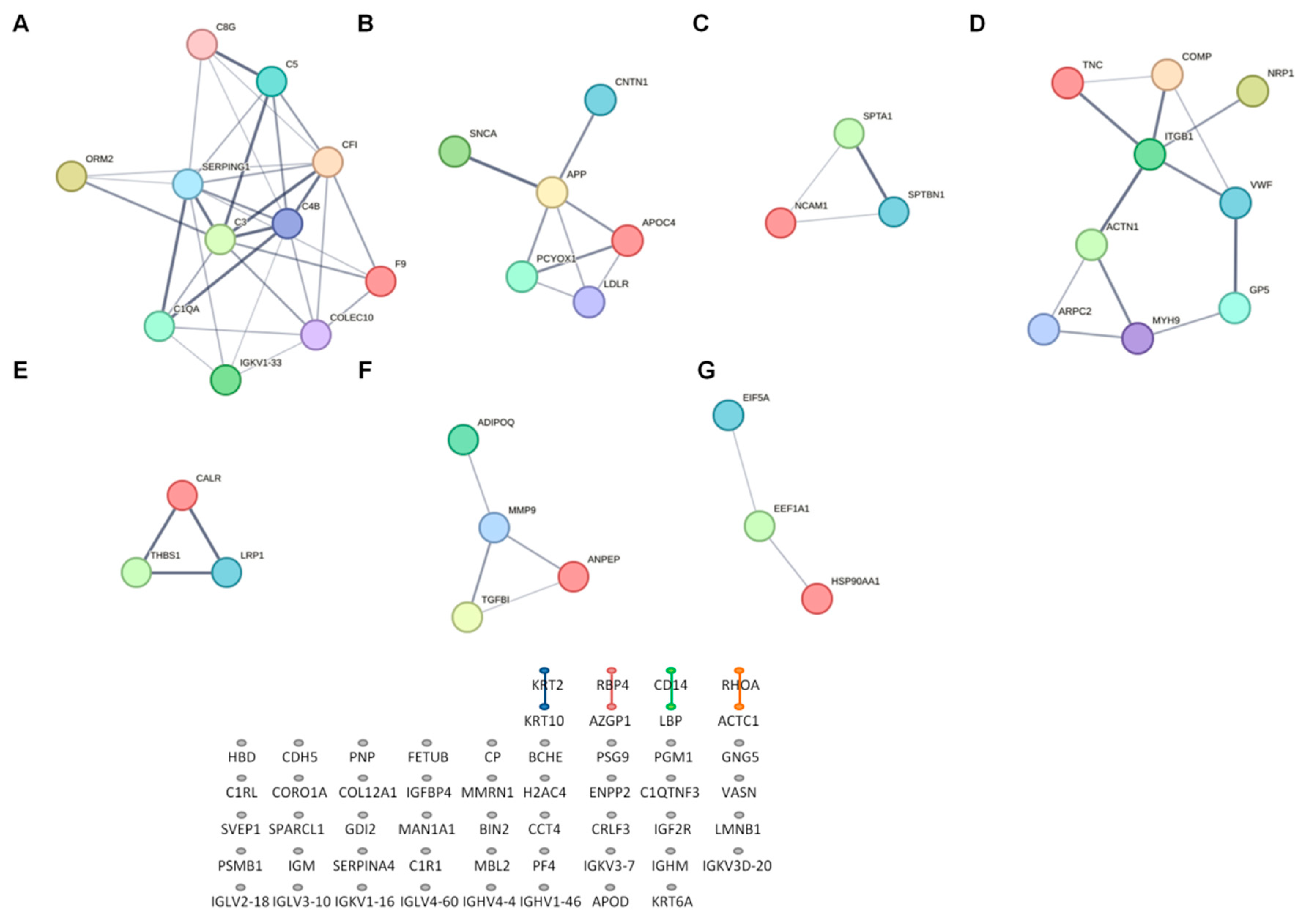
2.5. Network Analysis of Differentially Expressed Proteins (DEPs)
2.6. Receiver Operating Characteristics (ROC) Analysis
2.7. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Clinical, Endocrine–Metabolic, Body Composition and Body Fat Distribution Assessments
4.3. Exosome Isolation from Cord Blood
4.4. Proteomic Analysis of Cord-Blood-Derived Exosomes
4.4.1. Protein Extraction and In-StageTip Digestion (iST)
4.4.2. Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS Analysis)
4.4.3. Data Processing of LC-MS/MS Analysis
4.5. Bioinformatics Analysis
4.5.1. Gene Ontology (GO) Function and KEGG Pathway Analysis
4.5.2. Protein–Protein Interaction (PPI) Network Analysis
4.6. Statistical Analysis and Ethics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Ghafourian, M.; Mahdavi, R.; Akbari Jonoush, Z.; Sadeghi, M.; Ghadiri, N.; Farzaneh, M.; Mousavi Salehi, A. The implications of exosomes in pregnancy: Emerging as new diagnostic markers and therapeutics targets. Cell Commun. Signal. 2022, 20, 51. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S.; et al. Extracellular Vesicle and particle biomarkers define multiple human cancers. Cell 2020, 182, 1044–1061. [Google Scholar] [CrossRef] [PubMed]
- Nunez Lopez, Y.O.; Iliuk, A.; Petrilli, A.M.; Glass, C.; Casu, A.; Pratley, R.E. Proteomics and phosphoproteomics of circulating extracellular vesicles provide new insights into diabetes pathobiology. Int. J. Mol. Sci. 2022, 23, 5779. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Yang, S.; Chen, Y. Comparison of plasma exosome proteomes between obese and non-obese patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. 2023, 16, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Vinaiphat, A.; Sze, S.K. Proteomics for comprehensive characterization of extracellular vesicles in neurodegenerative disease. Exp. Neurol. 2022, 355, 114149. [Google Scholar] [CrossRef] [PubMed]
- Navajas, R.; Ramos-Fernandez, A.; Herraiz, I.; Galindo, A.; Bartha, J.L.; Corrales, F.; Paradela, A. Quantitative proteomic analysis of serum-purified exosomes identifies putative pre-eclampsia-associated biomarkers. Clin. Proteomics 2022, 19, 5. [Google Scholar] [CrossRef]
- Bernea, E.G.; Suica, V.I.; Uyy, E.; Cerveanu-Hogas, A.; Boteanu, R.M.; Ivan, L.; Ceausu, I.; Mihai, D.A.; Ionescu-Tîrgoviște, C.; Antohe, F. Exosome proteomics reveals the deregulation of coagulation. complement and lipid metabolism proteins in gestational diabetes mellitus. Molecules 2022, 27, 5502. [Google Scholar] [CrossRef]
- Gallo, D.M.; Fitzgerald, W.; Romero, R.; Gomez-Lopez, N.; Gudicha, D.W.; Than, N.G.; Bosco, M.; Chaiworapongsa, T.; Jung, E.; Meyyazhagan, A.; et al. Proteomic profile of extracellular vesicles in maternal plasma of women with fetal death. J. Matern. Fetal Neonatal Med. 2023, 36, 2177529. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; Brunetti, G.; Ventura, A.; D’Aniello, M.; Pepe, T.; Giordano, P.; Monteduro, M.; Cavallo, L. Nonalcoholic fatty liver disease in prepubertal children born small for gestational age: Influence of rapid weight catch-up growth. Horm. Res. Paediatr. 2013, 79, 103–109. [Google Scholar] [CrossRef]
- Goedegebuure, W.J.; Van der Steen, M.; Smeets, C.C.J.; Kerkhof, G.F.; Hokken-Koelega, A.C.S. SGA-born adults with postnatal catch-up have a persistently unfavourable metabolic health profile and increased adiposity at age 32 years. Eur. J. Endocrinol. 2022, 187, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Jee, Y.H.; Jumani, S.; Mericq, V. The association of accelerated early growth. timing of puberty. and metabolic consequences in children. J. Clin. Endocrinol. Metab. 2023, 108, e663–e670. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, L.; de Zegher, F. Adolescent PCOS: A postpubertal central obesity syndrome. Trends Mol. Med. 2023, 29, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Clayton, P.E.; Cianfarani, S.; Czernichow, P.; Johannsson, G.; Rapaport, R.; Rogol, A. Management of the child born small for gestational age through to adulthood: A consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J. Clin. Endocrinol. Metab. 2007, 92, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Casano, P.; Quesada, T.; López-Bermejo, A.; de Zegher, F.; Villarroya, F.; Ibáñez, L. Circulating exosomes decrease in size and increase in number between birth and age 7: Relations to fetal growth and liver fat. Front. Endocrinol. 2023, 14, 1257768. [Google Scholar] [CrossRef]
- de Zegher, F.; Sebastiani, G.; Diaz, M.; Sánchez-Infantes, D.; Lopez-Bermejo, A.; Ibáñez, L. Body composition and circulating high-molecular-weight adiponectin and IGF-I in infants born small for gestational age: Breast- versus formula-feeding. Diabetes 2012, 61, 1969–1973. [Google Scholar] [CrossRef]
- Díaz, M.; García, C.; Sebastiani, G.; de Zegher, F.; López-Bermejo, A.; Ibáñez, L. Placental and cord blood methylation of genes envolved in energy homeostasis: Association with fetal growth and neonatal body composition. Diabetes 2017, 66, 779–784. [Google Scholar] [CrossRef]
- López-Lera, A.; Corvillo, F.; Nozal, P.; Regueiro, J.R.; Sánchez-Corral, P.; López-Trascasa, M. Complement as a diagnostic tool in immunopathology. Semin. Cell. Dev. Biol. 2019, 85, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Mohlin, F.C.; Mercier, E.; Fremeaux-Bacchi, V.; Liszewski, M.K.; Atkinson, J.P.; Gris, J.C.; Blom, A.M. Analysis of genes coding for CD46. CD55. and C4b-binding protein in patients with idiopathic. recurrent. spontaneous pregnancy loss. Eur. J. Immunol. 2013, 43, 1617–1629. [Google Scholar] [CrossRef]
- Singh, J.; Ahmed, A.; Girardi, G. Role of complement component C1q in the onset of preeclampsia in mice. Hypertension 2011, 58, 716–724. [Google Scholar] [CrossRef]
- Chow, W.N.; Lee, Y.L.; Wong, P.C.; Chung, M.K.; Lee, K.F.; Yeung, W.S. Complement 3 deficiency impairs early pregnancy in mice. Mol. Reprod. Dev. 2009, 76, 647–655. [Google Scholar] [CrossRef]
- Phieler, J.; Garcia-Martin, R.; Lambris, J.D.; Chavakis, T. The role of the complement system in metabolic organs and metabolic diseases. Semin. Immunol. 2013, 25, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ma, Y.; Zhao, Z.; Zhen, S.; Wen, D. Complement C1q as a potential biomarker for obesity and metabolic syndrome in Chinese adolescents. Front. Endocrinol. 2020, 11, 586440. [Google Scholar] [CrossRef] [PubMed]
- de Zegher, F.; Malpique, R.; Garcia-Beltran, C.; Ibáñez, L. Towards a simple marker of hepato-visceral adiposity and insulin resistance: The Z-score change from weight-at-birth to BMI-in-childhood. Pediatr. Obes. 2019, 14, e12533. [Google Scholar] [CrossRef] [PubMed]
- Gabrielsson, B.G.; Johansson, J.M.; Lönn, M.; Jernås, M.; Olbers, T.; Peltonen, M.; Larsson, I.; Lönn, L.; Sjöström, L.; Carlsson, B.; et al. High expression of complement components in omental adipose tissue in obese men. Obes. Res. 2003, 11, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ojeda, F.J.; Méndez-Gutiérrez, A.; Aguilera, C.M.; Plaza-Díaz, J. Extracellular matrix remodeling of adipose tissue in obesity and metabolic diseases. Int. J. Mol. Sci. 2019, 20, 4888. [Google Scholar] [CrossRef] [PubMed]
- Merle, N.S.; Singh, P.; Rahman, J.; Kemper, C. Integrins meet complement: The evolutionary tip of an iceberg orchestrating metabolism and immunity. Br. J. Pharmacol. 2021, 178, 2754–2770. [Google Scholar] [CrossRef]
- Bedinger, D.H.; Adams, S.H. Metabolic. anabolic. and mitogenic insulin responses: A tissue-specific perspective for insulin receptor activators. Mol. Cell. Endocrinol. 2015, 415, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Kassouf, T.; Sumara, G. Impact of conventional and atypical MAPKs on the development of metabolic diseases. Biomolecules 2020, 10, 1256. [Google Scholar] [CrossRef]
- Jiao, P.; Feng, B.; Li, Y.; He, Q.; Xu, H. Hepatic ERK activity plays a role in energy metabolism. Mol. Cell. Endocrinol. 2013, 375, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Sabio, G.; Cavanagh-Kyros, J.; Barrett, T.; Jung, D.Y.; Ko, H.J.; Ong, H.; Morel, C.; Mora, A.; Reilly, J.; Kim, J.K.; et al. Role of the hypothalamic-pituitary-thyroid axis in metabolic regulation by JNK1. Genes Dev. 2010, 24, 256–264. [Google Scholar] [CrossRef]
- González-Terán, B.; Matesanz, N.; Nikolic, I.; Verdugo, M.A.; Sreeramkumar, V.; Hernández-Cosido, L.; Mora, A.; Crainiciuc, G.; Sáiz, M.L.; Bernardo, E.; et al. p38γ and p38δ reprogram liver metabolism by modulating neutrophil infiltration. EMBO J. 2016, 35, 536–552. [Google Scholar] [CrossRef] [PubMed]
- Madsen, R.R.; Toker, A. PI3K signaling through a biochemical systems lens. J. Biol. Chem. 2023, 299, 105224. [Google Scholar] [CrossRef]
- Savova, M.S.; Mihaylova, L.V.; Tews, D.; Wabitsch, M.; Georgiev, M.I. Targeting PI3K/AKT signaling pathway in obesity. Biomed. Pharmacother. 2023, 159, 114244. [Google Scholar] [CrossRef] [PubMed]
- Elchebly, M.; Payette, P.; Michaliszyn, E.; Cromlish, W.; Collins, S.; Loy, A.L.; Normandin, D.; Cheng, A.; Himms-Hagen, J.; Chan, C.C.; et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 1999, 283, 1544–1548. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.T.; Kim, P.T.; Peacock, J.W.; Yau, T.Y.; Mui, A.L.; Chung, S.W.; Sossi, V.; Doudet, D.; Green, D.; Ruth, T.J.; et al. Pten (phosphatase and tensin homologue gene) haploinsufficiency promotes insulin hypersensitivity. Diabetologia 2007, 50, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cao, I.; Song, M.S.; Hobbs, R.M.; Laurent, G.; Giorgi, C.; de Boer, V.C.; Anastasiou, D.; Ito, K.; Sasaki, A.T.; Rameh, L.; et al. Systemic elevation of PTEN induces a tumor-suppressive metabolic state. Cell 2012, 149, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Foukas, L.C.; Bilanges, B.; Bettedi, L.; Pearce, W.; Ali, K.; Sancho, S.; Withers, D.J.; Vanhaesebroeck, B. Long-term p110α PI3K inactivation exerts a beneficial effect on metabolism. EMBO Mol. Med. 2013, 5, 563–571. [Google Scholar] [CrossRef]
- Ortega-Molina, A.; Lopez-Guadamillas, E.; Mattison, J.A.; Mitchell, S.J.; Muñoz-Martin, M.; Iglesias, G.; Gutierrez, V.M.; Vaughan, K.L.; Szarowicz, M.D.; González-García, I.; et al. Pharmacological inhibition of PI3K reduces adiposity and metabolic syndrome in obese mice and rhesus monkeys. Cell Metab. 2015, 21, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Milner, R.D.; Hill, D.J. Fetal growth control: The role of insulin and related peptides. Clin. Endocrinol. 1984, 21, 415–433. [Google Scholar] [CrossRef]
- Mericq, V.; Ong, K.K.; Bazaes, R.; Peña, V.; Avila, A.; Salazar, T.; Soto, N.; Iñiguez, G.; Dunger, D.B. Longitudinal changes in insulin sensitivity and secretion from birth to age three years in small- and appropriate-for-gestational-age children. Diabetologia 2005, 48, 2609–2614. [Google Scholar] [CrossRef]
- Ibáñez, L.; Lopez-Bermejo, A.; Diaz, M.; de Zegher, F. Catch-up growth in girls born small for gestational age precedes childhood progression to high adiposity. Fertil. Steril. 2011, 96, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, G.; Díaz, M.; Bassols, J.; Aragonés, G.; López-Bermejo, A.; de Zegher, F.; Ibáñez, L. The sequence of prenatal growth restraint and post-natal catch-up growth leads to a thicker intima- media and more pre-peritoneal and hepatic fat by age 3–6 years. Pediatr. Obes. 2016, 11, 251–257. [Google Scholar] [CrossRef]
- Ibáñez, L.; Lopez-Bermejo, A.; Díaz, M.; Suárez, L.; de Zegher, F. Low-birth weight children develop lower sex hormone binding globulin and higher dehydroepiandrosterone sulfate levels and aggravate their visceral adiposity and hypoadiponectinemia between six and eight years of age. J. Clin. Endocrinol. Metab. 2009, 94, 3696–3699. [Google Scholar] [CrossRef]
- Augsburger, P.; Liimatta, J.; Flück, C.E. Update on adrenarche-still a mystery. J. Clin. Endocrinol. Metab. 2024, 109, 1403–1422. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, L.; López-Bermejo, A.; Díaz, M.; Marcos, M.V.; de Zegher, F. Early metformin therapy (age 8-12 years) in girls with precocious pubarche to reduce hirsutism. androgen excess. and oligomenorrhea in adolescence. J. Clin. Endocrinol. Metab. 2011, 96, E1262–E1267. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhang, J.; Wei, H.; Zhang, H.; Guan, Y.; Wang, X.; Tong, X. Reduction of the PI3K/Akt related signaling activities in skeletal muscle tissues involves insulin resistance in intrauterine growth restriction rats with catch-up growth. PLoS ONE 2019, 14, e0216665. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.B.; Martin-Gronert, M.S.; Storgaard, H.; Madsbad, S.; Vaag, A.; Ozanne, S.E. Altered PI3-kinase/Akt signalling in skeletal muscle of young men with low birth weight. PLoS ONE 2008, 3, e3738. [Google Scholar] [CrossRef]
- Pagano, G.; Pacini, G.; Musso, G.; Gambino, R.; Mecca, F.; Depetris, N.; Cassader, M.; David, E.; Cavallo-Perin, P.; Rizzetto, M. Nonalcoholic steatohepatitis. insulin resistance. and metabolic syndrome: Further evidence for an etiologic association. Hepatology 2002, 35, 367–372. [Google Scholar] [CrossRef]
- Azzu, V.; Vacca, M.; Virtue, S.; Allison, M.; Vidal-Puig, A. Adipose tissue-liver cross talk in the control of whole-body metabolism: Implications in nonalcoholic fatty liver disease. Gastroenterology 2020, 158, 1899–1912. [Google Scholar] [CrossRef]
- Bugianesi, E.; Bizzarri, C.; Rosso, C.; Mosca, A.; Panera, N.; Veraldi, S.; Dotta, A.; Giannone, G.; Raponi, M.; Cappa, M.; et al. Low birthweight increases the likelihood of severe steatosis in pediatric non-alcoholic fatty liver disease. Am. J. Gastroenterol. 2017, 112, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Marcos, L.V.; Lou-Bonafonte, J.M.; Martinez-Gracia, M.V.; Arnal, C.; Navarro, M.A.; Osada, J. Prenylcysteine oxidase 1. a pro-oxidant enzyme of low density lipoproteins. Front. Biosci. 2018, 23, 1020–1037. [Google Scholar]
- Banfi, C.; Baetta, R.; Barbieri, S.S.; Brioschi, M.; Guarino, A.; Ghilardi, S.; Sandrini, L.; Eligini, S.; Polvani, G.; Bergman, O.; et al. Prenylcysteine oxidase 1. an emerging player in atherosclerosis. Commun. Biol. 2021, 4, 1109. [Google Scholar] [CrossRef] [PubMed]
- Banfi, C.; Mallia, A.; Ghilardi, S.; Brioschi, M.; Gianazza, E.; Eligini, S.; Sahlén, P.; Baetta, R. Prenylcysteine oxidase 1 Is a key regulator of adipogenesis. Antioxidants 2023, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Gao, J.; Kosinski, P.A.; Elliman, S.J.; Hughes, T.E.; Gromada, J.; Kemp, D.M. Heat shock protein 90 (HSP90) inhibitors activate the heat shock factor 1 (HSF1) stress response pathway and improve glucose regulation in diabetic mice. Biochem. Biophys. Res. Commun. 2013, 430, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Kuan, Y.C.; Hashidume, T.; Shibata, T.; Uchida, K.; Shimizu, M.; Inoue, J.; Sato, R. Heat Shock Protein 90 modulates lipid homeostasis by regulating the stability and function of Sterol Regulatory Element-binding Protein (SREBP) and SREBP cleavage-activating protein. J. Biol. Chem. 2017, 292, 3016–3028. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Campderrós, L.; Guimaraes, M.P.; López-Bermejo, A.; de Zegher, F.; Villarroya, F.; Ibáñez, L. Circulating growth-and-differentiation factor-15 in early life: Relation to prenatal and postnatal growth and adiposity measurements. Pediatr. Res. 2020, 87, 897–902. [Google Scholar] [CrossRef]
- Díaz, M.; Blasco-Roset, A.; Villarroya, J.; López-Bermejo, A.; de Zegher, F.; Villarroya, F.; Ibáñez, L. Circulating diazepam-binding inhibitor in infancy: Relation to markers of adiposity and metabolic health. Pediatr. Obes. 2021, 16, e12802. [Google Scholar] [CrossRef]
- Díaz, M.; Carreras-Badosa, G.; Villarroya, J.; Gavaldà-Navarro, A.; Bassols, J.; de Zegher, F.; López-Bermejo, A.; Villarroya, F.; Ibáñez, L. Circulating GDF15 concentrations in girls with low birth weight: Effects of prolonged metformin treatment. Pediatr. Res. 2023, 93, 964–968. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage. supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
| Category | Term | Count | p Value |
|---|---|---|---|
| KEGG_PATHWAY | Complement and coagulation cascades | 10 | 1.6 × 10−9 |
| KEGG_PATHWAY | Phagosome | 8 | 3.1 × 10−5 |
| KEGG_PATHWAY | ECM–receptor interaction | 6 | 1.8 × 10−4 |
| KEGG_PATHWAY | Lipid and atherosclerosis | 6 | 9.1 × 10−3 |
| KEGG_PATHWAY | Regulation of actin cytoskeleton | 7 | 2.4 × 10−3 |
| KEGG_PATHWAY | PI3K-Akt signaling pathway | 7 | 2.0 × 10−2 |
| KEGG_PATHWAY | Tight junction | 5 | 1.9 × 10−3 |
| KEGG_PATHWAY | Focal adhesion | 7 | 1.3 × 10−3 |
| KEGG_PATHWAY | Efferocytosis | 4 | 6.7 × 10−2 |
| REACTOME_PATHWAY | Innate immune system | 26 | 5.8 × 10−9 |
| REACTOME_PATHWAY | Hemostasis | 16 | 8.9 × 10−6 |
| REACTOME_PATHWAY | Extracellular matrix organization | 10 | 1.4 × 10−4 |
| REACTOME_PATHWAY | Nervous system development | 12 | 1.2 × 10−3 |
| REACTOME_PATHWAY | Metabolism of proteins | 25 | 2.3 × 10−3 |
| REACTOME_PATHWAY | Vesicle-mediated transport | 12 | 4.0 × 10−3 |
| REACTOME_PATHWAY | Signal transduction | 27 | 1.1 × 10−2 |
| REACTOME_PATHWAY | Membrane trafficking | 9 | 5.3 × 10−2 |
| REACTOME_PATHWAY | Cell–cell communication | 4 | 7.8 × 10−2 |
| Liver Fat at Age 7 Years | |||
|---|---|---|---|
| Beta | Significance | R2 | |
| PCYOX1 | 0.432 | 0.007 | 0.328 |
| HSP90AA1 | 0.689 | 0.005 | 0.377 |
| IGF-1 at age 2 years | 0.465 | 0.017 | 0.110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, M.; Quesada-López, T.; Villarroya, F.; Casano, P.; López-Bermejo, A.; de Zegher, F.; Ibáñez, L. The Proteome of Exosomes at Birth Predicts Insulin Resistance, Adrenarche and Liver Fat in Childhood. Int. J. Mol. Sci. 2025, 26, 1721. https://doi.org/10.3390/ijms26041721
Díaz M, Quesada-López T, Villarroya F, Casano P, López-Bermejo A, de Zegher F, Ibáñez L. The Proteome of Exosomes at Birth Predicts Insulin Resistance, Adrenarche and Liver Fat in Childhood. International Journal of Molecular Sciences. 2025; 26(4):1721. https://doi.org/10.3390/ijms26041721
Chicago/Turabian StyleDíaz, Marta, Tania Quesada-López, Francesc Villarroya, Paula Casano, Abel López-Bermejo, Francis de Zegher, and Lourdes Ibáñez. 2025. "The Proteome of Exosomes at Birth Predicts Insulin Resistance, Adrenarche and Liver Fat in Childhood" International Journal of Molecular Sciences 26, no. 4: 1721. https://doi.org/10.3390/ijms26041721
APA StyleDíaz, M., Quesada-López, T., Villarroya, F., Casano, P., López-Bermejo, A., de Zegher, F., & Ibáñez, L. (2025). The Proteome of Exosomes at Birth Predicts Insulin Resistance, Adrenarche and Liver Fat in Childhood. International Journal of Molecular Sciences, 26(4), 1721. https://doi.org/10.3390/ijms26041721





