Augmented Bone Morphogenetic Protein Signaling During TMJ Development Alters Morphology in a Timepoint-Dependent Manner
Abstract
1. Introduction
2. Results
2.1. Wnt1 Cre;caBmpr1a Mice Show a More Severe Mandibular Phenotype Compared to P0 Cre;caBmpr1a Mice
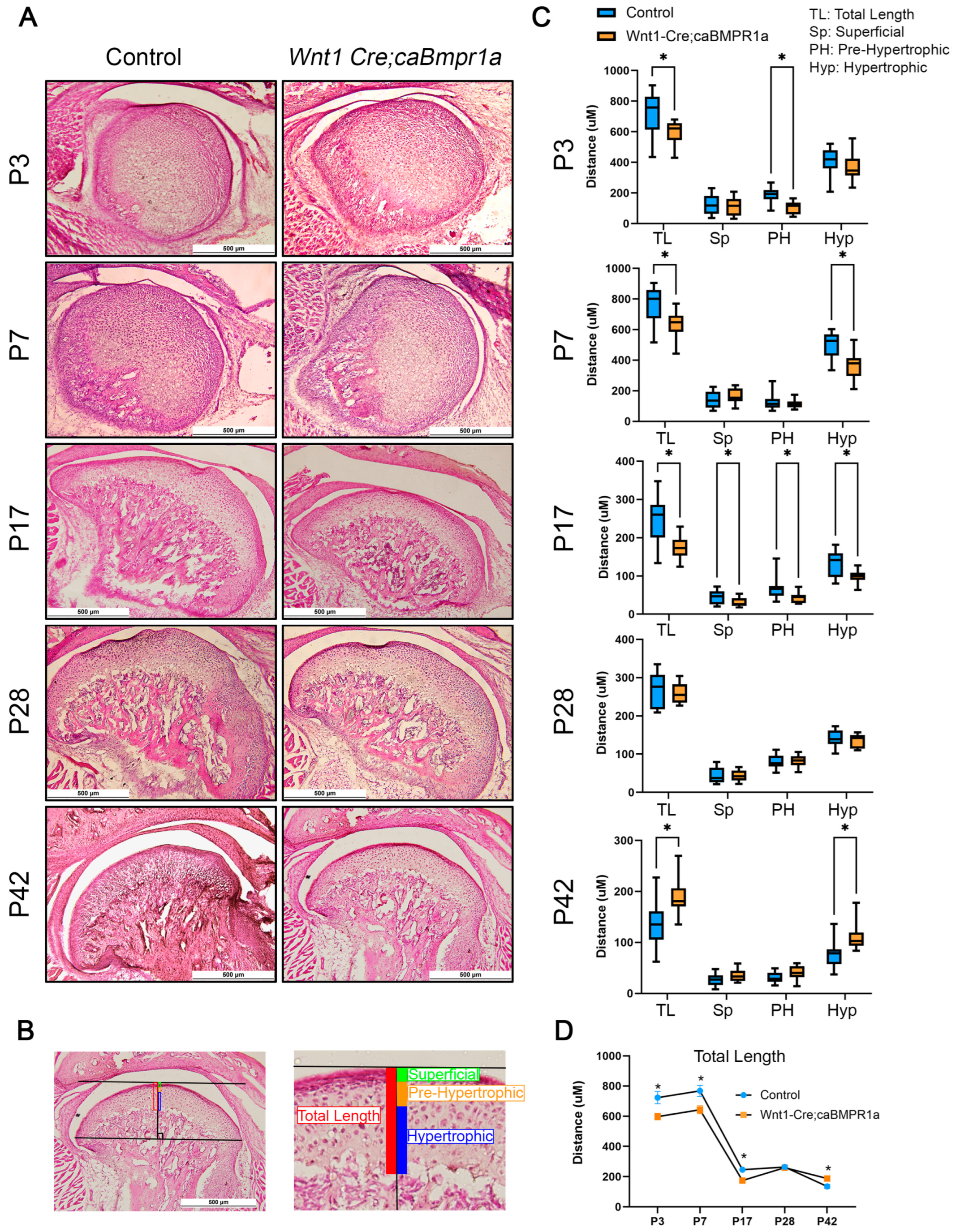
2.2. Wnt1 Cre;caBmpr1a Mice Have Smaller Mandibular Condyles Through Development That Are Recovered Post-Weaning
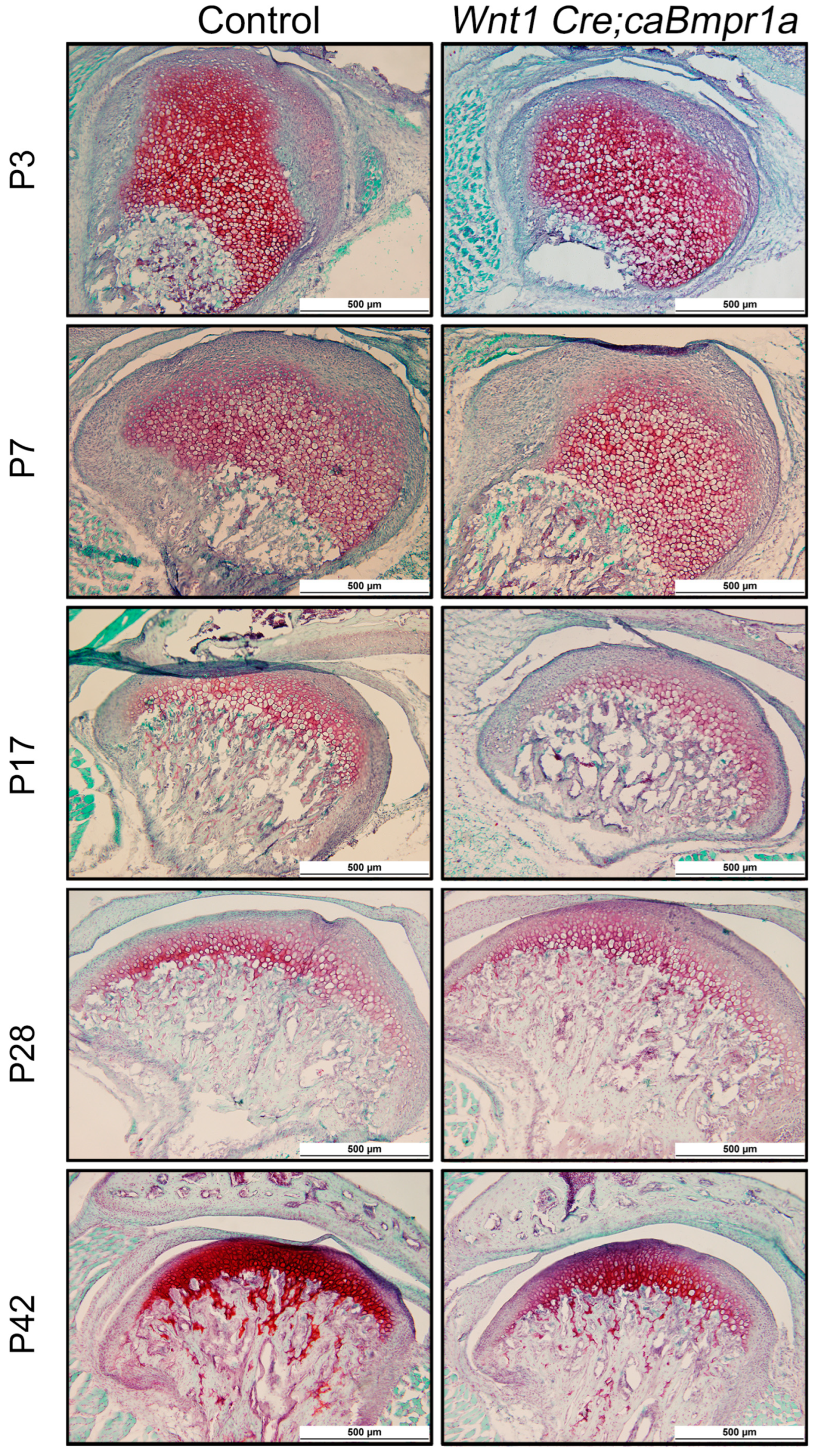
2.3. BMP Signaling in Neural Crest Cells Does Not Impact Extracellular Matrix Composition in the TMJ
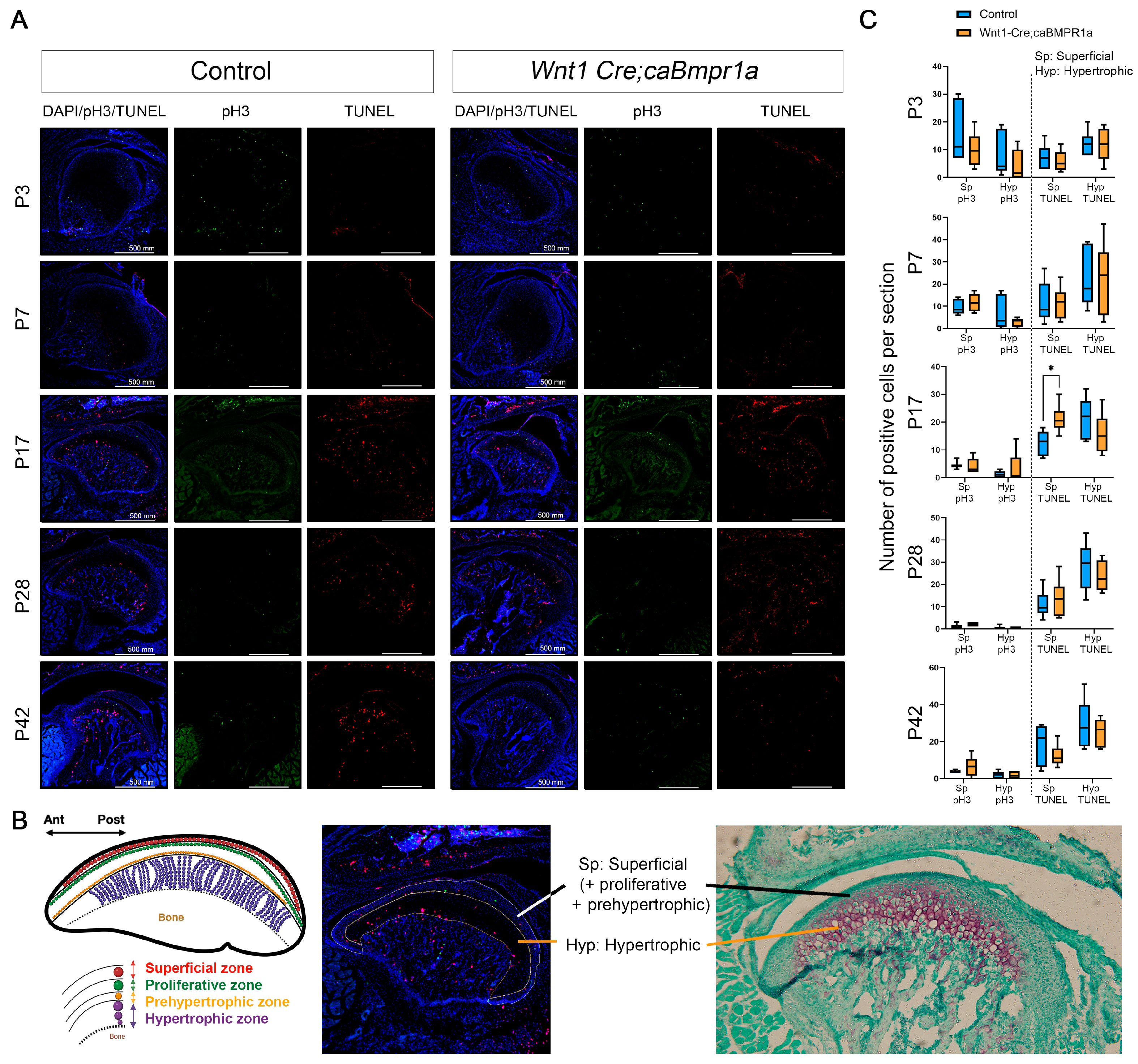
2.4. Cell Death and Proliferation Lead to Alterations in Wnt1 Cre;caBmpr1a Condylar Cartilage Throughout Development
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Micro-Computed Tomography
4.3. Histology and Immunohistochemistry
4.4. Experimental Groups
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TMJ | Temporomandibular joint |
| BMP | Bone morphogenetic protein |
| CNCCs | Cranial neural crest cells |
References
- Alomar, X.; Medrano, J.; Cabratosa, J.; Clavero, J.A.; Lorente, M.; Serra, I.; Monill, J.M.; Salvador, A. Anatomy of the Temporomandibular Joint. Semin. Ultrasound CT MR 2007, 28, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, G.; Al-Ani, Z. Temporomandibular joint anatomy, function and clinical relevance. Br. Dent. J. 2022, 233, 539–546. [Google Scholar] [CrossRef]
- Hinton, R.J.; Jing, J.; Feng, J.Q. Genetic influences on temporomandibular joint development and growth. Curr. Top. Dev. Biol. 2015, 115, 85–109. [Google Scholar] [PubMed]
- Veronica Iturriaga, V.; Thomas Bornhardt, T.; Velasquez, N. Temporomandibular Joint: Review of Anatomy and Clinical Implications. Dent. Clin. N. Am. 2023, 67, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Kuć, J.; Szarejko, K.D.; Gołȩbiewska, M. Smiling, Yawning, Jaw Functional Limitations and Oral Behaviors With Respect to General Health Status in Patients With Temporomandibular Disorder—Myofascial Pain With Referral. Front. Neurol. 2021, 12, 646293. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Koolstra, J.H. Biomechanics of the temporomandibular joint. J. Dent. Res. 2008, 87, 989–991. [Google Scholar] [CrossRef] [PubMed]
- Haal, B.K. Immobilization and cartilage transformation into bone in the embryonic chick. Anat. Rec. 1972, 173, 391–403. [Google Scholar] [CrossRef]
- Hinton, R.J. Genes that regulate morphogenesis and growth of the temporomandibular joint: A review. Dev. Dyn. 2014, 243, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, I.; Toriya, N.; Nakao, Y. Growth of the mandible and biological characteristics of the mandibular condylar cartilage. Jpn. Dent. Sci. Rev. 2013, 49, 139–150. [Google Scholar] [CrossRef]
- Galea, G.L.; Zein, M.R.; Allen, S.; Francis-West, P. Making and shaping endochondral and intramembranous bones. Dev. Dyn. 2021, 250, 414–449. [Google Scholar] [CrossRef]
- Kronenberg, H.M. Developmental regulation of the growth plate. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef]
- Franz-Odendaal, T.A. Induction and patterning of intramembranous bone. Front. Biosci. 2011, 16, 2734–2746. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cao, X.U. BMP signaling and skeletogenesis. Ann. N. Y. Acad. Sci. 2006, 1068, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Jing, J.; Ye, L.; Liu, X.; Harris, S.E.; Hinton, R.J.; Feng, J.Q. Chondrogenesis and osteogenesis are one continuous developmental and lineage defined biological process. Sci. Rep. 2017, 7, 10020. [Google Scholar] [CrossRef] [PubMed]
- Karadede, B.; Karadede, B.; Karadede, M.İ. Growth, Development, and Ossification of Mandible and Temporomandibular Joint. In Imaging of the Temporomandibular Joint; Springer: Cham, Switzerland, 2019; Chapter 3; pp. 43–57. [Google Scholar] [CrossRef]
- Luder, H.U. Structure and growth activities of the mandibular condyle in monkeys (Macaca fascicularis): II. Synergistic behavior of cell dynamics and metabolism. Am. J. Anat. 1987, 178, 185–192. [Google Scholar] [CrossRef]
- Yoon, B.S.; Lyons, K.M. Multiple functions of BMPs in chondrogenesis. J. Cell. Biochem. 2004, 93, 93–103. [Google Scholar] [CrossRef]
- Majumdar, M.K.; Wang, E.; Morris, E.A. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J. Cell. Physiol. 2001, 189, 275–284. [Google Scholar] [CrossRef]
- Denker, A.E.; Haas, A.R.; Nicoll, S.B.; Tuan, R.S. Chondrogenic differentiation of murine C3H10T1/2 multipotential mesenchymal cells: I. Stimulation by bone morphogenetic protein-2 in high-density micromass cultures. Differentiation 1999, 64, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Pizette, S.; Niswander, L. BMPs are required at two steps of limb chondrogenesis: Formation of prechondrogenic condensations and their differentiation into chondrocytes. Dev. Biol. 2000, 219, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Ueharu, H.; Pan, H.; Liu, X.; Ishii, M.; Pongetti, J.; Kulkarni, A.K.; Adegbenro, F.E.; Wurn, J.; Maxson, R.E.; Sun, H.; et al. Augmentation of BMP signaling in cranial neural crest cells leads to premature cranial sutures fusion through endochondral ossification in mice. J. Bone Miner. Res. Plus 2023, 7, e10716. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, Y.; Yu, P.B.; Kamiya, N.; Pan, H.; Fukuda, T.; Scott, G.J.; Ray, M.K.; Yamamura, K.-I.; Mishina, Y. Augmentation of Smad-dependent BMP signaling in neural crest cells causes craniosynostosis in mice. J. Bone Miner. Res. 2013, 28, 1422–1433. [Google Scholar] [CrossRef] [PubMed]
- Santagati, F.; Rijli, F.M. Cranial neural crest and the building of the vertebrate head. Nat. Rev. Neurosci. 2003, 4, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Le Douarin, N.M.; Creuzet, S.; Couly, G.; Dupin, E. Neural crest cell plasticity and its limits. Development 2004, 131, 4637–4650. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Arias, A.C.; Liu, L.; Chen, Y.; Bronner, M.E.; Maxson, R.E. A stable cranial neural crest cell line from mouse. Stem Cells Dev. 2012, 21, 3069–3080. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ishan, M.; Yang, J.; Kishigami, S.; Fukuda, T.; Scott, G.; Ray, M.K.; Sun, C.; Chen, S.; Komatsu, Y.; et al. Specific and spatial labeling of P0-Cre versus Wnt1-Cre in cranial neural crest in early mouse embryos. Genesis 2017, 55, e23034. [Google Scholar] [CrossRef]
- Danielian, P.S.; Muccino, D.; Rowitch, D.H.; Michael, S.K.; McMahon, A.P. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998, 8, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Abe, K.; Mantani, A.; Hitoshi, Y.; Suzuki, M.; Osuzu, F.; Kuratani, S.; Yamamura, K.I. A novel transgenic technique that allows specific marking of the neural crest cell lineage in mice. Dev. Biol. 1999, 212, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Uptegrove, A.; Chen, C.; Sahagun-Bisson, M.; Kulkarni, A.K.; Louie, K.W.; Ueharu, H.; Mishina, Y.; Omi-Sugihara, M. IInfluence of bone morphogenetic protein (BMP) signaling and masticatory load on morphological alterations of the mouse mandible during postnatal development. Arch. Oral. Biol. 2025, 169, 106096. [Google Scholar] [CrossRef]
- Kiliaridis, S.; Thilander, B.; Kjellberg, H.; Topouzelis, N.; Zafiriadis, A. Effect of low masticatory function on condylar growth: A morphometric study in the rat. Am. J. Orthod. Dentofac. Orthop. 1999, 116, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P. Morphometric integration and modularity in configurations of landmarks: Tools for evaluating a priori hypotheses. Evol. Dev. 2009, 11, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Spassov, A.; Toro-Ibacache, V.; Krautwald, M.; Brinkmeier, H.; Kupczik, K. Congenital muscle dystrophy and diet consistency affect mouse skull shape differently. J. Anat. 2017, 231, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hou, Y.; Zhang, P.; Lu, H.; Wang, W.; Ma, W. Changes of the condylar cartilage and subchondral bone in the temporomandibular joints of rats under unilateral mastication and expression of Insulin-like Growth Factor-1. J. Stomatol. Oral. Maxillofac. Surg. 2022, 123, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, C.; Coutts, R.D.; Healey, R.M.; Kubo, T.; Hirasawa, Y.; Amiel, D. Method of histomorphometric assessment of glycosaminoglycans in articular cartilage. J. Orthop. Res. 1997, 15, 670–674. [Google Scholar] [CrossRef]
- Jing, J.; Hinton, R.J.; Mishina, Y.; Liu, Y.; Zhou, X.; Feng, J.Q. Critical role of Bmpr1a in mandibular condyle growth. Connect. Tissue Res. 2014, 55 (Suppl. S1), 73–78. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Wu, W.; Liu, C.; Yang, L.; Sun, C.; Ye, W.; Li, X.; Chen, J.; Long, F.; Chen, Y. BMPRIA mediated signaling is essential for temporomandibular joint development in mice. PLoS ONE 2014, 9, e101000. [Google Scholar] [CrossRef] [PubMed]
- Omi, M.; Koneru, T.; Lyu, Y.; Haraguchi, A.; Kamiya, N.; Mishina, Y. Increased BMP-Smad signaling does not affect net bone mass in long bones. Front. Physiol. 2023, 14, 1145763. [Google Scholar] [CrossRef] [PubMed]
- Mishina, Y.; Starbuck, M.W.; Gentile, M.A.; Fukuda, T.; Kasparcova, V.; Seedor, J.G.; Hanks, M.C.; Amling, M.; Pinero, G.J.; Harada, S.-I.; et al. Bone morphogenetic protein type IA receptor signaling regulates postnatal osteoblast function and bone remodeling. J. Biol. Chem. 2004, 279, 27560–27566. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.G.; Kotchen, J.M.; Kotchen, T.A.; Cowley, T.; Dasgupta, M.; Cowley Jr, A.W. Temporomandibular disorders and associated clinical comorbidities. Clin. J. Pain. 2011, 27, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Hayano, S.; Komatsu, Y.; Pan, H.; Mishina, Y. Augmented BMP signaling in the neural crest inhibits nasal cartilage morphogenesis by inducing p53-mediated apoptosis. Development 2015, 142, 1357–1367. [Google Scholar] [CrossRef]
- Ueharu, H.; Pan, H.; Hayano, S.; Guerra, K.Z.; Yang, J.; Mishina, Y. Augmentation of bone morphogenetic protein signaling in cranial neural crest cells in mice deforms skull base due to premature fusion of intersphenoidal synchondrosis. Genesis 2023, 61, e23509. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, L.; Pan, H.; Ueharu, H.; Toda, M.; Yang, Q.; Hallett, S.A.; Olson, L.E.; Mishina, Y. A BMP-controlled metabolic/epigenetic signaling cascade directs midfacial morphogenesis. J. Clin. Investig. 2024, 138. [Google Scholar] [CrossRef] [PubMed]
- Lydiatt, D.D.; Davis, L.F. The effects of immobilization on the rabbit temporomandibular joint. J. Oral. Maxillofac. Surg. 1985, 43, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Glineburg, R.W.; Laskin, D.M.; Blaustein, D.I. The effects of immobilization on the primate temporomandibular joint: A histologic and histochemical study. J. Oral. Maxillofac. Surg. 1982, 40, 3–8. [Google Scholar] [CrossRef]
- Hichijo, N.; Kawai, N.; Mori, H.; Sano, R.; Ohnuki, Y.; Okumura, S.; Langenbach, G.E.J.; Tanaka, E. Effects of the masticatory demand on the rat mandibular development. J. Oral. Rehabil. 2014, 41, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Yasuno, K.; Ito, A.; Yoshida, M.; Fukunaga, T.; Honda, T.; Tsumaki, H.; Yamaguchi, K.; Mizoguchi, I. Influence of feeding a soft diet on proteoglycan expression in rat temporomandibular joint discs. J. Oral. Biosci. 2024, 66, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Iura, A.; McNerny, E.G.; Zhang, Y.; Kamiya, N.; Tantillo, M.; Lynch, M.; Kohn, D.H.; Mishina, Y. Mechanical loading synergistically increases trabecular bone volume and improves mechanical properties in the mouse when BMP signaling is specifically ablated in osteoblasts. PLoS ONE 2015, 10, e0141345. [Google Scholar] [CrossRef]
- Panciera, T.; Azzolin, L.; Michelangelo Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Wang, K.C.; Zhipeng Meng, Z. Mechanoregulation of YAP and TAZ in Cellular Homeostasis and Disease Progression. Front. Cell Dev. Biol. 2021, 9, 673599. [Google Scholar] [CrossRef]
- Brandão, A.S.; Bensimon-Brit, A.; Lourenço, R.; Borbinha, J.; Soares, A.R.; Mateus, R.; Jacinto, A. Yap induces osteoblast differentiation by modulating Bmp signalling during zebrafish caudal fin regeneration. J. Cell Sci. 2019, 132, jcs.231993. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tang, L.; Le, T.P.; Nguyen, B.H.; Chen, W.; Zheng, M.; Yamaguchi, H.; Dawson, B.; You, S.; Martinez-Traverso, I.M.; et al. Yap and Taz promote osteogenesis and prevent chondrogenesis in neural crest cells in vitro and in vivo. Sci. Signal 2022, 15, eabn9009. [Google Scholar] [CrossRef] [PubMed]
- She, Y.; Ren, R.; Jiang, N. Mechanical stress can regulate temporomandibular joint cavitation via signalling pathways. Dev. Biol. 2024, 507, 1–8. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Snider, T.N.; Louie, K.A.W.; Zuzo, G.; de Oliveira Ruellas, A.C.; Solem, R.C.; Cevidanes, L.H.; Zhang, H.; Mishina, Y. Quantification of three dimensional morphology of craniofacial mineralized tissue defects in Tgfbr2/Osx Cre mice. Oral. Sci. Int. 2021, 18, 193–202. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Midla, S.C.; Omi-Sugihara, M.; Cha, M.; Chen, C.; Cavalcante, R.C.; Pan, H.; Mishina, Y.; Ueharu, H. Augmented Bone Morphogenetic Protein Signaling During TMJ Development Alters Morphology in a Timepoint-Dependent Manner. Int. J. Mol. Sci. 2025, 26, 1655. https://doi.org/10.3390/ijms26041655
Midla SC, Omi-Sugihara M, Cha M, Chen C, Cavalcante RC, Pan H, Mishina Y, Ueharu H. Augmented Bone Morphogenetic Protein Signaling During TMJ Development Alters Morphology in a Timepoint-Dependent Manner. International Journal of Molecular Sciences. 2025; 26(4):1655. https://doi.org/10.3390/ijms26041655
Chicago/Turabian StyleMidla, Susannah C., Maiko Omi-Sugihara, Madeline Cha, Coral Chen, Rafael Correia Cavalcante, Haichun Pan, Yuji Mishina, and Hiroki Ueharu. 2025. "Augmented Bone Morphogenetic Protein Signaling During TMJ Development Alters Morphology in a Timepoint-Dependent Manner" International Journal of Molecular Sciences 26, no. 4: 1655. https://doi.org/10.3390/ijms26041655
APA StyleMidla, S. C., Omi-Sugihara, M., Cha, M., Chen, C., Cavalcante, R. C., Pan, H., Mishina, Y., & Ueharu, H. (2025). Augmented Bone Morphogenetic Protein Signaling During TMJ Development Alters Morphology in a Timepoint-Dependent Manner. International Journal of Molecular Sciences, 26(4), 1655. https://doi.org/10.3390/ijms26041655




