Shrimp Shapes a Nitrite Tolerance Trait via Regulating Autophagy and Apoptosis
Abstract
1. Introduction
2. Results
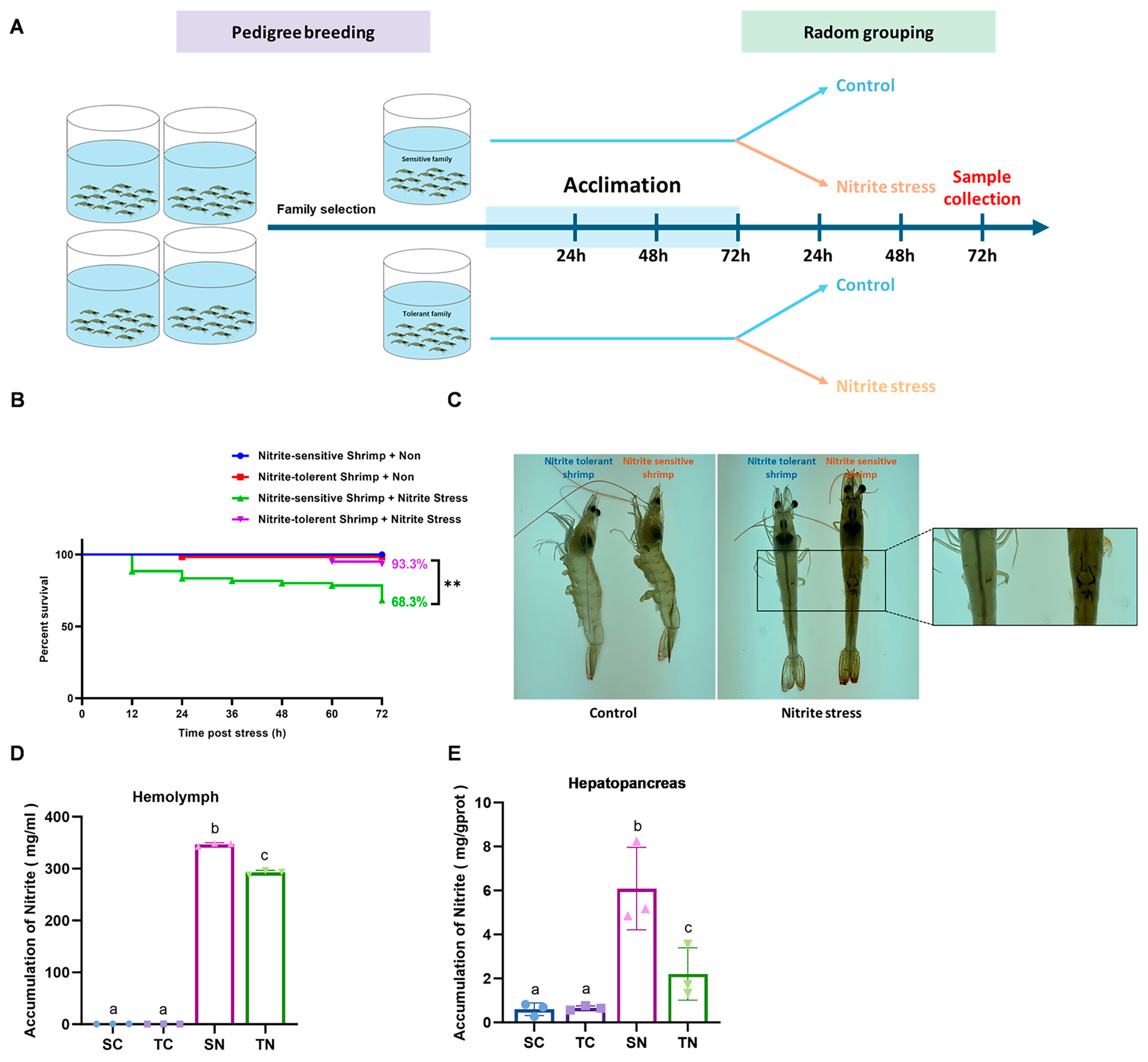
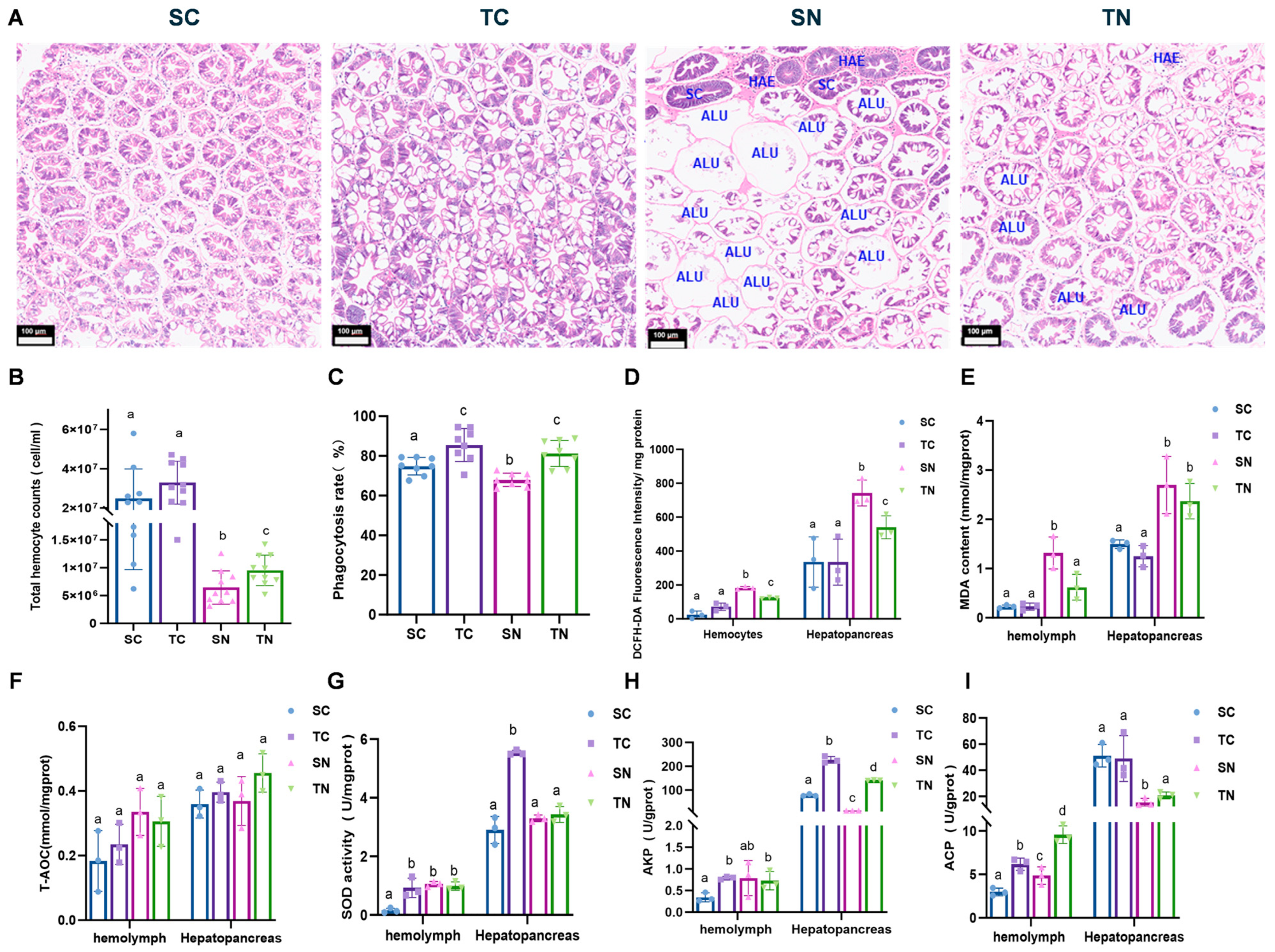
2.1. Physiological Changes of Nitrite-Tolerant and Nitrite-Sensitive Families Under Nitrite Stress
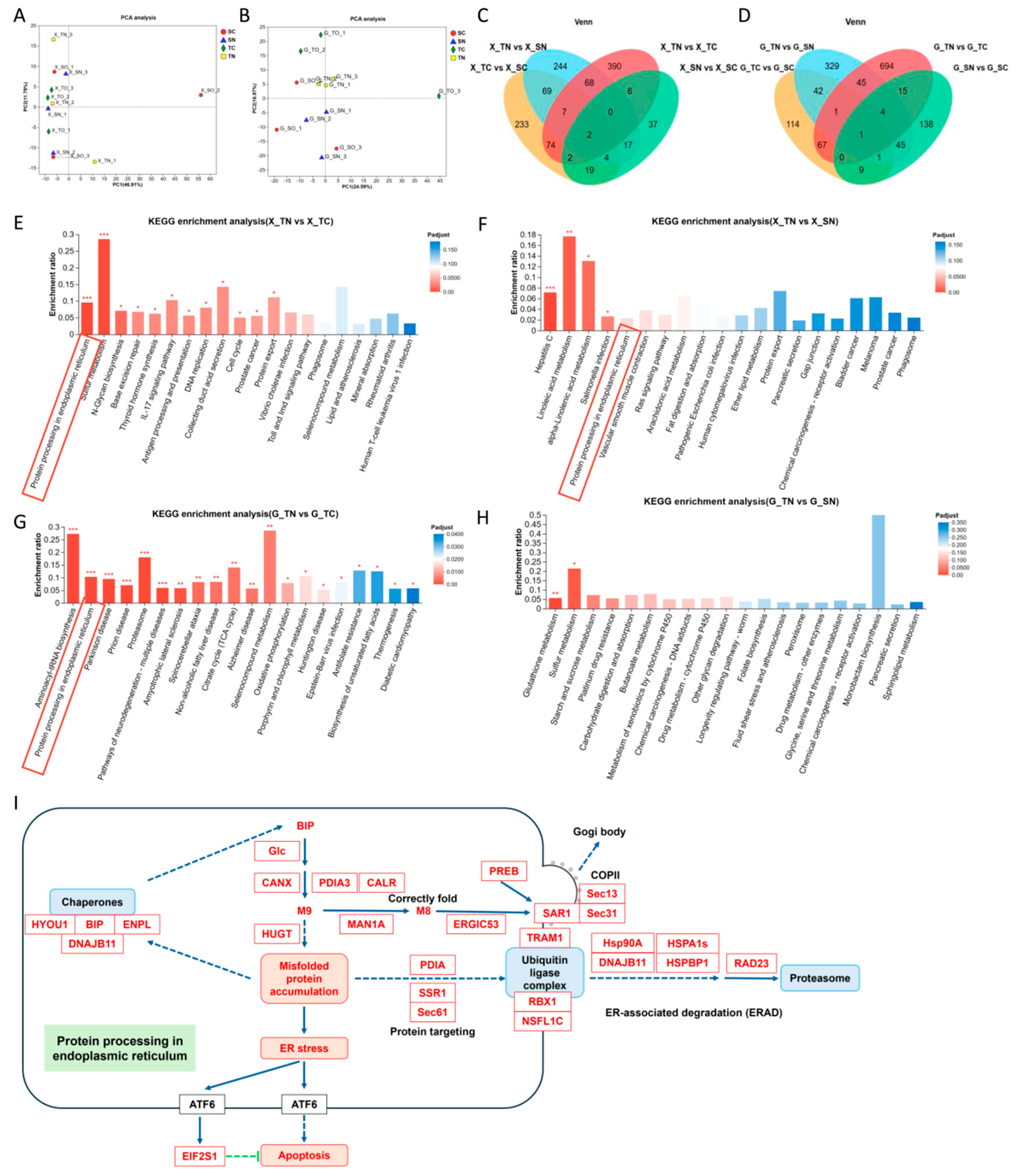
2.2. Differential Transcriptomic Profiles in Nitrite-Tolerant and Sensitive Families
2.3. Protein Processing in the Endoplasmic Reticulum Is Overexpressed in Tolerant Families Under Nitrite Stress
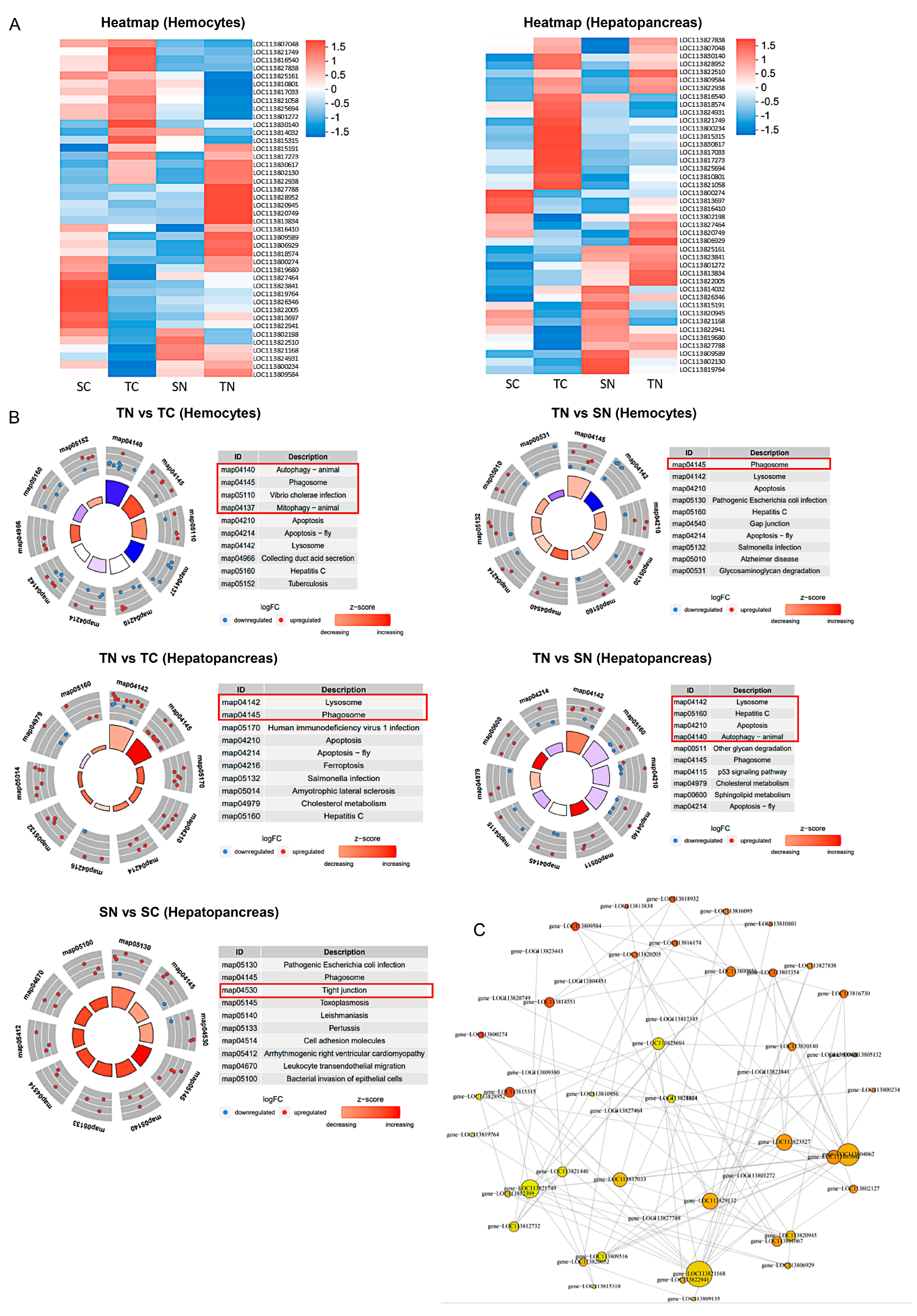
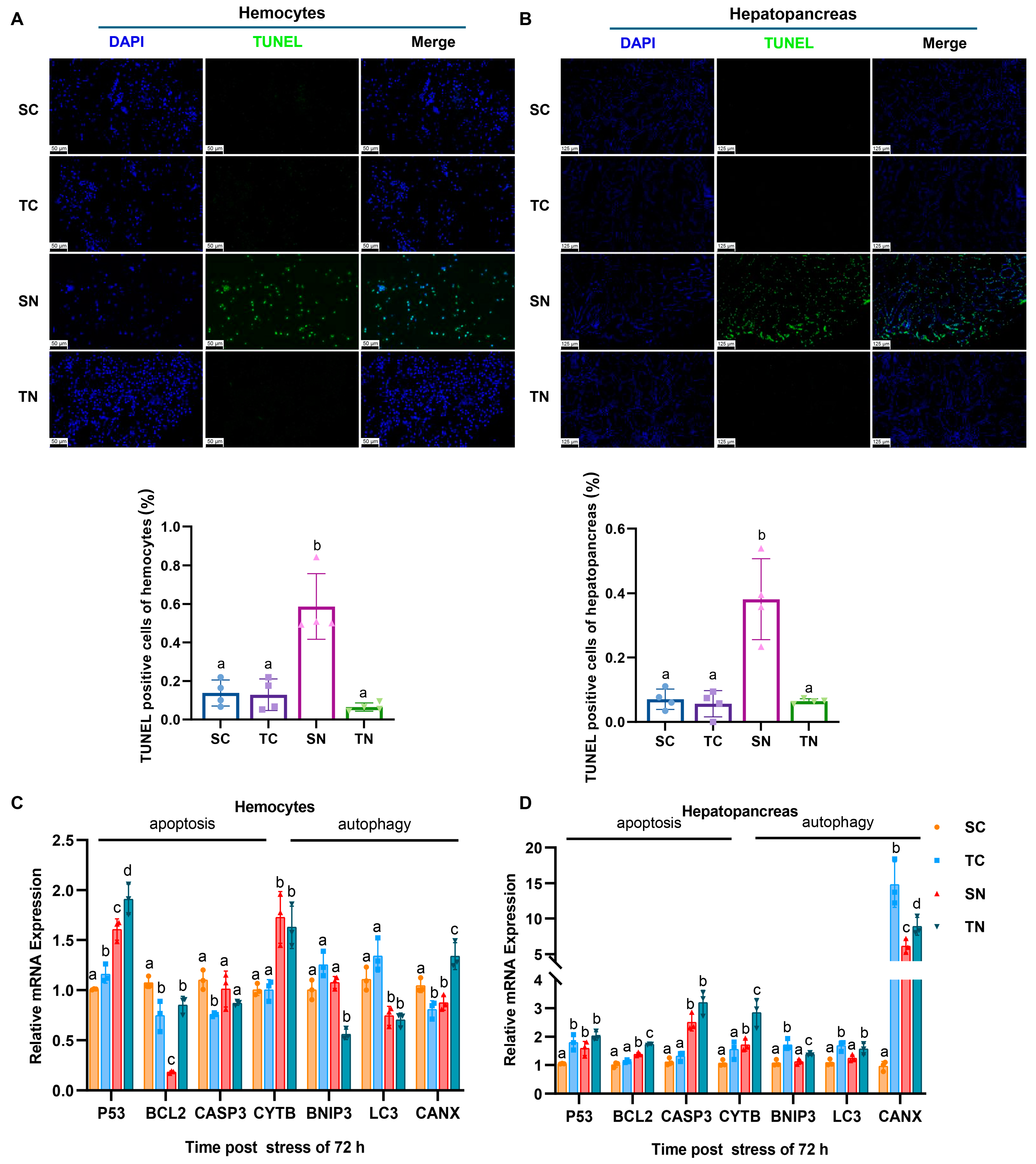
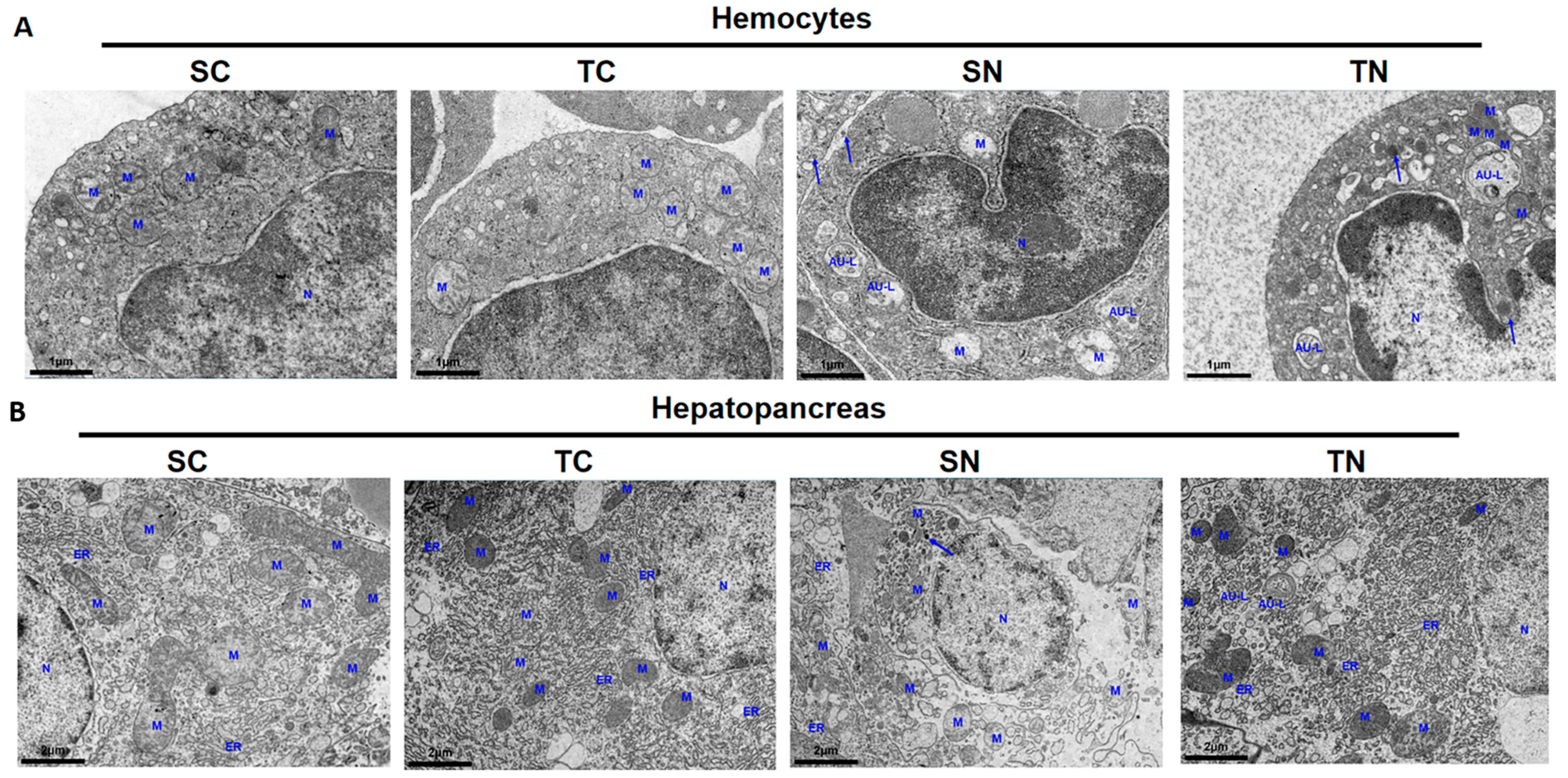
2.4. Effect of Nitrite Stress on Apoptosis and Autophagy Between Nitrite-Tolerant and Sensitive Shrimp Families
2.5. Apoptosis and Autophagy Analysis of Tolerant and Sensitive Shrimp Families Under Nitrite Stress
3. Discussion
4. Material and Methods
4.1. Ethics Statement
4.2. Animals and Treatment
4.3. Acute Nitrite Exposure and Sample Collection
4.4. Shrimp Survival Assay
4.5. Histological Analysis
4.6. Assay of Oxidative Damage and Antioxidant Enzymes
4.7. Total Hemocyte Count (THC) Analysis
4.8. Phagocytic Activity
4.9. Measurement of Intracellular ROS Contents
4.10. Transcriptome Sequencing and Data Analysis
4.11. Total RNA Extraction and Quantitative Real-Time PCR
4.12. Apoptotic Cell Analysis
4.13. Transmission Electron Microscopy (TEM)
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosas, C.; Cuzon, G.; Gaxiola, G.; Le Priol, Y.; Pascual, C.; Rossignyol, J.; Contreras, F.; Sanchez, A.; Van Wormhoudt, A. Metabolism and growth of juveniles of Litopenaeus vannamei: Effect of salinity and dietary carbohydrate levels. J. Exp. Mar. Bio. Ecol. 2001, 259, 1–22. [Google Scholar] [CrossRef]
- Paredes, M.G.; Bianco, K.A.; Menéndez-Helman, R.J.; Kristoff, G. Aquatic Contamination in Lugano Lake (Lugano Lake Ecological Reserve, Buenos Aires, Argentina) Cause Negative Effects on the Reproduction and Juvenile Survival of the Native Gastropod Biomphalaria straminea. Front. Physiol. 2022, 13, 954868. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Nader, M.M.; Salem, H.M.; El-Tahan, A.M.; Soliman, S.M.; Khafaga, A.F. Effect of environmental factors on growth performance of Nile tilapia (Oreochromis niloticus). Int. J. Biometeorol. 2022, 66, 2183–2194. [Google Scholar] [CrossRef]
- Paungfoo, C.; Prasertsan, P.; Burrell, P.C.; Intrasungkha, N.; Blackall, L.L. Nitrifying bacterial communities in an aquaculture wastewater treatment system using fluorescence in situ hybridization (FISH), 16S rRNA gene cloning, and phylogenetic analysis. Biotechnol. Bioeng. 2007, 97, 985–990. [Google Scholar] [CrossRef]
- Ruenglertpanyakul, W.; Attasat, S.; Wanichpongpan, P. Nutrient removal from shrimp farm effluent by aquatic plants. Water Sci. Technol. 2004, 50, 321–330. [Google Scholar] [CrossRef]
- Hoang, P.H.; Nguyen, T.M.; Le, N.C.T.; Phan, K.S.; Mai, T.T.T.; Ha, P.T. Characterization of isolated aerobic denitrifying bacteria and their potential use in the treatment of nitrogen-polluted aquaculture water. Curr. Microbiol. 2022, 79, 209. [Google Scholar] [CrossRef] [PubMed]
- San Diego-McGlone, M.L.; Azanza, R.V.; Villanoy, C.L.; Jacinto, G.S. Eutrophic waters, algal bloom and fish kill in fish farming areas in Bolinao, Pangasinan, Philippines. Mar. Pollut. Bull. 2008, 57, 295–301. [Google Scholar] [CrossRef]
- Biao, X.; Zhuhong, D.; Xiaorong, W. Impact of the intensive shrimp farming on the water quality of the adjacent coastal creeks from Eastern China. Mar. Pollut. Bull. 2004, 48, 543–553. [Google Scholar] [CrossRef]
- Svobodová, Z.; Machova, J.; Poleszczuk, G.; Hůda, J.; Hamáčková, J.; Kroupová, H.K. Nitrite poisoning of fish in aquaculture facilities with water-recirculating systems. Acta Vet. Brno 2005, 74, 129–137. [Google Scholar] [CrossRef]
- Wasielesky, W.J.; Poersch, L.H.; Martins, T.G.; Miranda-Filho, K.C. Chronic effects of nitrogenous compounds on survival and growth of juvenile pink shrimp. Braz. J. Biol. 2017, 77, 558–565. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Li, S.; Guo, X.; Fu, Y.; He, N.; Ruan, G.; Wang, Q.; Gao, W.; Fang, L. Impact of nitrite exposure on oxidative stress and antioxidative-related genes responses in the gills of Procambarus clarkii. Fish Shellfish Immunol. 2022, 131, 624–630. [Google Scholar] [CrossRef]
- Jensen, F.B. Nitrite disrupts multiple physiological functions in aquatic animals. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003, 135, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Tahon, J.P.; Van Hoof, D.; Vinckier, C.; Witters, R.; De Ley, M.; Lontie, R. The reaction of nitrite with the haemocyanin of Astacus leptodactylus. Biochem. J. 1988, 249, 891–896. [Google Scholar] [CrossRef]
- Li, Z.S.; Ma, S.; Shan, H.W.; Wang, T.; Xiao, W. Responses of hemocyanin and energy metabolism to acute nitrite stress in juveniles of the shrimp Litopenaeus vannamei. Ecotoxicol. Environ. Saf. 2019, 186, 109753. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Chen, J.C. Joint action of elevated ambient nitrite and nitrate on hemolymph nitrogenous compounds and nitrogen excretion of tiger shrimp Penaeus monodon. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 131, 303–314. [Google Scholar] [CrossRef]
- Lin, W.; Guo, H.; Li, Y.; Wang, L.; Zhang, D.; Hou, J.; Wu, X.; Li, L.; Li, D.; Zhang, X. Single and combined exposure of microcystin-LR and nitrite results in reproductive endocrine disruption via hypothalamic-pituitary-gonadal-liver axis. Chemosphere 2018, 211, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Petrova, E.; Gluhcheva, Y.; Pavlova, E.; Vladov, I.; Voyslavov, T.; Ivanova, J. Effect of acute sodium nitrite intoxication on some essential biometals in mouse spleen. J. Trace Elem. Med. Biol. 2020, 58, 126431. [Google Scholar] [CrossRef]
- Williams, E.M.; Eddy, F.B. Some effects of adrenaline on anion transport and nitrite-induced methaemoglobin formation in the rainbow trout (Salmo gairdneri Richardson). J. Exp. Zool. 1987, 241, 269–273. [Google Scholar] [CrossRef]
- Li, X.; Dai, X. Characterization and functional analysis of Litopenaeus vannamei Na(+)/K(+)/2Cl(-) cotransporter 1 under nitrite stress. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2024, 298, 111749. [Google Scholar] [CrossRef]
- Kır, M.; Sunar, M.C.; Topuz, M.; Sarıipek, M. Thermal acclimation capacity and standard metabolism of the Pacific white shrimp Litopenaeus vannamei (Boone, 1931) at different temperature and salinity combinations. J. Therm. Biol. 2023, 112, 103429. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Chen, L.; Qin, J.G.; Sun, X.; Li, E.; Gu, S.; Yu, N. Acute tolerance and metabolic responses of Chinese mitten crab (Eriocheir sinensis) juveniles to ambient nitrite. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 149, 419–426. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, Y.J.; Lee, K.M. Effects of Nitrite Exposure on the Hematological Properties, Antioxidant and Stress Responses of Juvenile Hybrid Groupers, Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀. Antioxidants 2022, 11, 545. [Google Scholar] [CrossRef]
- Liu, H.J.; Dong, M.; Jiang, W.D.; Wu, P.; Liu, Y.; Jin, X.W.; Kuang, S.Y.; Tang, L.; Zhang, L.; Feng, L.; et al. Acute nitrite exposure-induced oxidative damage, endoplasmic reticulum stress, autophagy and apoptosis caused gill tissue damage of grass carp (Ctenopharyngodon idella): Relieved by dietary protein. Ecotoxicol. Environ. Saf. 2022, 243, 113994. [Google Scholar] [CrossRef]
- Cheng, C.H.; Su, Y.L.; Ma, H.L.; Deng, Y.Q.; Feng, J.; Chen, X.L.; Jie, Y.K.; Guo, Z.X. Effect of nitrite exposure on oxidative stress, DNA damage and apoptosis in mud crab (Scylla paramamosain). Chemosphere 2020, 239, 124668. [Google Scholar] [CrossRef]
- Yang, S.; Luo, J.; Huang, Y.; Yuan, Y.; Cai, S. Effect of sub-lethal ammonia and nitrite stress on autophagy and apoptosis in hepatopancreas of Pacific whiteleg shrimp Litopenaeusvannamei. Fish Shellfish Immunol. 2022, 130, 72–78. [Google Scholar] [CrossRef]
- Liang, Q.; Dong, B.; Li, A.; Wu, L.; Zhang, Y.; Han, T.; Liu, X. scRNA-seq analysis reveals toxicity mechanisms in shrimp hemocytes subjected to nitrite stress. Chemosphere 2023, 316, 137853. [Google Scholar] [CrossRef]
- Zhang, T.T.; Ma, P.; Yin, X.Y.; Yang, D.Y.; Li, D.P.; Tang, R. Acute nitrite exposure induces dysfunction and oxidative damage in grass carp isolated hemocytes. J. Aquat. Anim. Health 2022, 34, 58–68. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, Q.Y.; Du, J.H.; Zhu, W.L.; Li, Q.Y.; Chen, X.L.; Chen, X.H.; Liu, H.; Zhou, X.Y.; Zhao, Y.Z.; et al. Integrated analysis of physiological, transcriptomic and metabolomic responses and tolerance mechanism of nitrite exposure in Litopenaeus vannamei. Sci. Total Environ. 2020, 711, 134416. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Elshopakey, G.E.; Abdelwarith, A.A.; Younis, E.M.; Davies, S.J.; Elbahnaswy, S. Alleviating effects of Gracilaria verrucosa supplement on non-specific immunity, antioxidant capacity and immune-related genes of pacific white shrimp (Litopenaeus vannamei) provoked with white spot syndrome virus. BMC Vet. Res. 2024, 20, 487. [Google Scholar] [CrossRef]
- Zeng, Q.; Yang, Q.; Chai, Y.; Wei, W.; Luo, M.; Li, W. Polystyrene microplastics enhanced copper-induced acute immunotoxicity in red swamp crayfish (Procambarus clarkii). Ecotoxicol. Environ. Saf. 2023, 249, 114432. [Google Scholar] [CrossRef]
- Gunderson, M.P.; Nguyen, B.T.; Cervantes Reyes, J.C.; Holden, L.L.; French, J.M.T.; Smith, B.D.; Lineberger, C. Response of phase I and II detoxification enzymes, glutathione, metallothionein and acetylcholine esterase to mercury and dimethoate in signal crayfish (Pacifastacus leniusculus). Chemosphere 2018, 208, 749–756. [Google Scholar] [CrossRef]
- Xiao, J.; Luo, S.S.; Du, J.H.; Liu, Q.Y.; Huang, Y.; Wang, W.F.; Chen, X.L.; Chen, X.H.; Liu, H.; Zhou, X.Y.; et al. Transcriptomic analysis of gills in nitrite-tolerant and -sensitive families of Litopenaeus vannamei. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 253, 109212. [Google Scholar] [CrossRef] [PubMed]
- Cybulsky, A.V. Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat. Rev. Nephrol. 2017, 13, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ou-Yang, K.; He, Y.; Wang, X.; Wang, L.; Yang, Q.; Li, D.; Li, L. Nitrite induces hepatic glucose and lipid metabolism disorders in zebrafish through mitochondrial dysfunction and ERs response. Aquat. Toxicol. 2024, 273, 107015. [Google Scholar] [CrossRef]
- Myers, A.L.; Harris, C.M.; Choe, K.M.; Brennan, C.A. Inflammatory production of reactive oxygen species by Drosophila hemocytes activates cellular immune defenses. Biochem. Biophys. Res. Commun. 2018, 505, 726–732. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, X.; Li, M.; Wang, R.; Qian, Y.; Hong, M. Effect of nitrite exposure on haematological status, oxidative stress, immune response and apoptosis in yellow catfish (Pelteobagrus fulvidraco). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 238, 108867. [Google Scholar] [CrossRef]
- Zhang, T.; Yao, C.; Hu, Z.; Li, D.; Tang, R. Protective Effect of Selenium on the Oxidative Damage of Kidney Cells Induced by Sodium Nitrite in Grass Carp (Ctenopharyngodon idellus). Biol. Trace Elem. Res. 2022, 200, 3876–3884. [Google Scholar] [CrossRef]
- Tseng, I.T.; Chen, J.C. The immune response of white shrimp Litopenaeus vannamei and its susceptibility to Vibrio alginolyticus under nitrite stress. Fish Shellfish Immunol. 2004, 17, 325–333. [Google Scholar] [CrossRef]
- Paludan, S.R.; Pradeu, T.; Masters, S.L.; Mogensen, T.H. Constitutive immune mechanisms: Mediators of host defence and immune regulation. Nat. Rev. Immunol. 2021, 21, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Okado, K.; Shinzawa, N.; Aonuma, H.; Nelson, B.; Fukumoto, S.; Fujisaki, K.; Kawazu, S.; Kanuka, H. Rapid recruitment of innate immunity regulates variation of intracellular pathogen resistance in Drosophila. Biochem. Biophys. Res. Commun. 2009, 379, 6–10. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, J.C. Accumulation of nitrite in the tissues of Penaeus monodon exposed to elevated ambient nitrite after different time periods. Arch. Environ. Contam. Toxicol. 2000, 39, 183–192. [Google Scholar] [CrossRef]
- Simmons, A.E.; Karimi, I.; Talwar, M.; Simmons, T.W. Effects of nitrite on development of embryos and early larval stages of the zebrafish (Danio rerio). Zebrafish 2012, 9, 200–206. [Google Scholar] [CrossRef]
- Gam, L.T.H.; Jensen, F.B.; Damsgaard, C.; Huong, D.T.T.; Phuong, N.T.; Bayley, M. Extreme nitrite tolerance in the clown knifefish Chitala ornata is linked to up-regulation of methaemoglobin reductase activity. Aquat. Toxicol. 2017, 187, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Chen, X.; Hou, Y.; Jiang, T.; Liu, H.; Yang, J. Combined transcriptome and metabolome analysis reveals the mechanism of high nitrite tolerance in freshwater mussel Anodonta woodiana. Comp. Biochem. Physiol. Part D Genomics Proteomics 2024, 52, 101359. [Google Scholar] [CrossRef]
- Lefevre, S.; Jensen, F.B.; Huong do, T.T.; Wang, T.; Phuong, N.T.; Bayley, M. Effects of nitrite exposure on functional haemoglobin levels, bimodal respiration, and swimming performance in the facultative air-breathing fish Pangasianodon hypophthalmus. Aquat. Toxicol. 2011, 104, 86–93. [Google Scholar] [CrossRef]
- Díaz, A.C.; Velurtas, S.M.; Espino, M.L.; Fenucci, J.L. Effect of dietary astaxanthin on free radical scavenging capacity and nitrite stress tolerance of postlarvae shrimp, Pleoticus muelleri. J. Agric. Food Chem. 2014, 62, 12326–12331. [Google Scholar] [CrossRef]
- Wang, X.; Song, Q.; Wang, Z.; Han, F. A novel extracellular copper/zinc superoxide dismutase identified from Nibea albiflora and its characteristics under ammonia/nitrite stress. Int. J. Biol. Macromol. 2018, 115, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jin, Y.; Yu, Y.; Xiang, J.; Li, F. Cadmium-induced oxidative stress, metabolic dysfunction and metal bioaccumulation in adult palaemonid shrimp Palaemon macrodactylus (Rathbun, 1902). Ecotoxicol. Environ. Saf. 2021, 208, 111591. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhao, Z.; Li, W. Nitrite exposure leads to glycolipid metabolic disorder via the heme-HO pathway in teleost. Ecotoxicol. Environ. Saf. 2024, 281, 116653. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, Y.; Zhuo, H.; Li, J.; Fu, S.; Zhou, X.; Wu, G.; Guo, C.; Liu, J. Integrated histological, physiological, and transcriptome analysis reveals the post-exposure recovery mechanism of nitrite in Litopenaeus vannamei. Ecotoxicol. Environ. Saf. 2024, 281, 116673. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Z.; Zheng, Z.; Yao, D.; Zhao, Y.; Chen, X.; Zhang, Y.; Aweya, J.J. The CCR1 and CCR5 C-C chemokine receptors in Penaeus vannamei are annexed by bacteria to attenuate shrimp survival. Dev. Comp. Immunol. 2023, 139, 104561. [Google Scholar] [CrossRef]
- Guo, H.; Ye, C.X.; Wang, A.L.; Xian, J.A.; Liao, S.A.; Miao, Y.T.; Zhang, S.P. Trascriptome analysis of the Pacific white shrimp Litopenaeus vannamei exposed to nitrite by RNA-seq. Fish Shellfish Immunol. 2013, 35, 2008–2016. [Google Scholar] [CrossRef]
- Liu, F.; Li, S.; Yu, Y.; Sun, M.; Xiang, J.; Li, F. Effects of ammonia stress on the hemocytes of the Pacific white shrimp Litopenaeus vannamei. Chemosphere 2020, 239, 124759. [Google Scholar] [CrossRef]
- Averill-Bates, D. Reactive oxygen species and cell signaling. Review. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2024, 1871, 119573. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Chen, W.; Hu, R.; Feng, H. The injury and therapy of reactive oxygen species in intracerebral hemorrhage looking at mitochondria. Oxid. Med. Cell. Longev. 2016, 2016, 2592935. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, Z.; Sun, C.; Yang, D.; Zhou, Z.; Peng, X.; Zheng, L.; Tang, C. Exercise intervention mitigates pathological liver changes in NAFLD zebrafish by activating SIRT1/AMPK/NRF2 signaling. Int. J. Mol. Sci. 2021, 22, 10940. [Google Scholar] [CrossRef]
- Basit, F.; Bhat, J.A.; Alyemeni, M.N.; Shah, T.; Ahmad, P. Nitric oxide mitigates vanadium toxicity in soybean (Glycine max L.) by modulating reactive oxygen species (ROS) and antioxidant system. J. Hazard. Mater. 2023, 451, 131085. [Google Scholar] [CrossRef] [PubMed]
- Ohlemiller, K.K.; McFadden, S.L.; Ding, D.L.; Flood, D.G.; Reaume, A.G.; Hoffman, E.K.; Scott, R.W.; Wright, J.S.; Putcha, G.V.; Salvi, R.J. Targeted deletion of the cytosolic Cu/Zn-superoxide dismutase gene (Sod1) increases susceptibility to noise-induced hearing loss. Audiol. Neurotol. 1999, 4, 237–246. [Google Scholar] [CrossRef]
- He, Z.; Yu, S.; Mei, G.; Zheng, M.; Wang, M.; Dai, Y.; Tang, B.; Li, N. Maternally transmitted milk containing recombinant human catalase provides protection against oxidation for mouse offspring during lactation. Free Radical Bio. Med. 2008, 45, 1135–1142. [Google Scholar] [CrossRef]
- Liu, X.; Bian, D.D.; Jiang, Q.; Jiang, J.J.; Jin, Y.; Chen, F.X.; Zhang, D.Z.; Liu, Q.N.; Tang, B.P.; Dai, L.S. Insights into chlorantraniliprole exposure via activating cytochrome P450-mediated xenobiotic metabolism pathway in the Procambarus clarkii: Identification of P450 genes involved in detoxification. Int. J. Biol. Macromol. 2024, 277, 134231. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhang, J.; Wang, Y.; Liu, Q.; Xiong, D. Nitrite stress disrupts the structural integrity and induces oxidative stress response in the intestines of Pacific white shrimp Litopenaeus vannamei. J. Exp Zool. A Ecol. Integr. Physiol. 2018, 329, 43–50. [Google Scholar] [CrossRef]
- Mi, L.; Min, X.; Shi, M.; Liu, L.; Zhang, Y.; Zhu, Y.; Li, P.; Chai, Y.; Chen, F.; Deng, Q.; et al. Neutrophil extracellular traps aggravate neuronal endoplasmic reticulum stress and apoptosis via TLR9 after traumatic brain injury. Cell Death Dis. 2023, 14, 374. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Wu, H. Ubiquitination-Proteasome System (UPS) and Autophagy Two Main Protein Degradation Machineries in Response to Cell Stress. Cells 2022, 11, 851. [Google Scholar] [CrossRef]
- Hwang, J.; Qi, L. Quality control in the endoplasmic reticulum: Crosstalk between ERAD and UPR pathways. Trends Biochem. Sci. 2018, 43, 593–605. [Google Scholar] [CrossRef]
- Song, S.; Tan, J.; Miao, Y.; Zhang, Q. Crosstalk of ER stress-mediated autophagy and ER-phagy: Involvement of UPR and the core autophagy machinery. J. Cell. Physiol. 2018, 233, 3867–3874. [Google Scholar] [CrossRef]
- Hetz, C.; Saxena, S. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 2017, 13, 477–491. [Google Scholar] [CrossRef]
- Lu, M.; Echeverri, F.; Moyer, B.D. Endoplasmic reticulum retention, degradation, and aggregation of olfactory G-protein coupled receptors. Traffic 2003, 4, 416–433. [Google Scholar] [CrossRef] [PubMed]
- Merighi, A.; Lossi, L. Endoplasmic reticulum stress signaling and neuronal cell death. Int. J. Mol. Sci. 2022, 23, 15186. [Google Scholar] [CrossRef]
- Wang, W.T.; Sun, L.; Sun, C.H. PDIA3-regulted inflammation and oxidative stress contribute to the traumatic brain injury (TBI) in mice. Biochem. Biophys. Res. Commun. 2019, 518, 657–663. [Google Scholar] [CrossRef]
- Kajimoto, K.; Minami, Y.; Harashima, H. Cytoprotective role of the fatty acid binding protein 4 against oxidative and endoplasmic reticulum stress in 3T3-L1 adipocytes. FEBS Open Bio. 2014, 4, 602–610. [Google Scholar] [PubMed]
- Ponsero, A.J.; Igbaria, A.; Darch, M.A.; Miled, S.; Outten, C.E.; Winther, J.R.; Palais, G.; D′Autréaux, B.; Delaunay-Moisan, A.; Toledano, M.B. Endoplasmic reticulum transport of glutathione by Sec61 is regulated by Ero1 and Bip. Mol. Cell 2017, 67, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Bettigole, S.E.; Glimcher, L.H. Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 2015, 33, 107–138. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Dang, Y.; Xu, Q.; Yu, L.; Liu, C.; Yuan, Y.; Wang, J. Microcystin-LR induced developmental toxicity and apoptosis in zebrafish (Danio rerio) larvae by activation of ER stress response. Chemosphere 2016, 157, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Li, C.; Wang, F.; Shan, H. Responses and correlation among ER stress, Ca2+ homeostasis, and fatty acid metabolism in Penaeus vannamei under ammonia stress. Aquat. Toxicol. 2024, 267, 106837. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.H.; Dong, B.B.; Liang, Q.J. Deficiency of PvDRAM2 increased the nitrite sensitivity of Pacific white shrimp (Penaeus vannamei) by inhibiting autophagy. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2025, 287, 110068. [Google Scholar] [CrossRef]
- Liu, X.; Hussain, R.; Mehmood, K.; Tang, Z.; Zhang, H.; Li, Y. Mitochondrial-Endoplasmic Reticulum Communication-Mediated Oxidative Stress and Autophagy. BioMed Res. Int. 2022, 2022, 6459585. [Google Scholar] [CrossRef]
- Xian, J.A.; Wang, A.L.; Ye, C.X.; Chen, X.D.; Wang, W.N. Phagocytic activity, respiratory burst, cytoplasmic free-Ca(2+) concentration and apoptotic cell ratio of haemocytes from the black tiger shrimp, Penaeus monodon under acute copper stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 152, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.F.; Zhu, X.Y.; Huang, J.H.; Nan, Y.X.; Duan, Y.F.; Zhang, J.S. Toxic effects of microplastics and nitrite exposure on intestinal histology, digestion, immunity, and microbial community of shrimp Litopenaeus vannamei. Mar. Pollut. Bull. 2024, 200, 116077. [Google Scholar] [CrossRef]
- Romano, N.; Zeng, C. Subchronic exposure to nitrite, potassium and their combination on survival, growth, total haemocyte count and gill structure of juvenile blue swimmer crabs, Portunus pelagicus. Ecotoxicol. Environ. Saf. 2009, 72, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Shiau, S.Y. Dietary vitamin C and its derivatives affect immune responses in grass shrimp, Penaeus monodon. Fish Shellfish Immunol. 2002, 12, 119–129. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Burden, C.J.; Qureshi, S.E.; Wilson, S.R. Error estimates for the analysis of differential expression from RNA-seq count data. PeerJ 2014, 2, e576. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.L.; Zanella, C.L.; Janssen, Y.M.; Timblin, C.R.; Jimenez, L.A.; Vacek, P.; Taatjes, D.J.; Mossman, B.T. Novel cell imaging techniques show induction of apoptosis and proliferation in mesothelial cells by asbestos. Am. J. Respir. Cell Mol. Bio. 1997, 17, 265–271. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Ma, Z.; Liu, Q.; Li, Q.; Peng, M.; Yang, C.; Zhang, B.; Chen, T.; Huang, Y.; Zheng, Z.; et al. Shrimp Shapes a Nitrite Tolerance Trait via Regulating Autophagy and Apoptosis. Int. J. Mol. Sci. 2025, 26, 1641. https://doi.org/10.3390/ijms26041641
Zhou L, Ma Z, Liu Q, Li Q, Peng M, Yang C, Zhang B, Chen T, Huang Y, Zheng Z, et al. Shrimp Shapes a Nitrite Tolerance Trait via Regulating Autophagy and Apoptosis. International Journal of Molecular Sciences. 2025; 26(4):1641. https://doi.org/10.3390/ijms26041641
Chicago/Turabian StyleZhou, Liping, Zhentao Ma, Qingyun Liu, Qiangyong Li, Min Peng, Chunling Yang, Bin Zhang, Tiancong Chen, Yuliu Huang, Zhihong Zheng, and et al. 2025. "Shrimp Shapes a Nitrite Tolerance Trait via Regulating Autophagy and Apoptosis" International Journal of Molecular Sciences 26, no. 4: 1641. https://doi.org/10.3390/ijms26041641
APA StyleZhou, L., Ma, Z., Liu, Q., Li, Q., Peng, M., Yang, C., Zhang, B., Chen, T., Huang, Y., Zheng, Z., Huang, A., Chen, X., Zhang, Y., Zhao, X., & Zhao, Y. (2025). Shrimp Shapes a Nitrite Tolerance Trait via Regulating Autophagy and Apoptosis. International Journal of Molecular Sciences, 26(4), 1641. https://doi.org/10.3390/ijms26041641




