Fetoscopic Endoluminal Tracheal Occlusion-Synergic Therapies in the Prenatal Treatment of Congenital Diaphragmatic Hernia
Abstract
1. Introduction
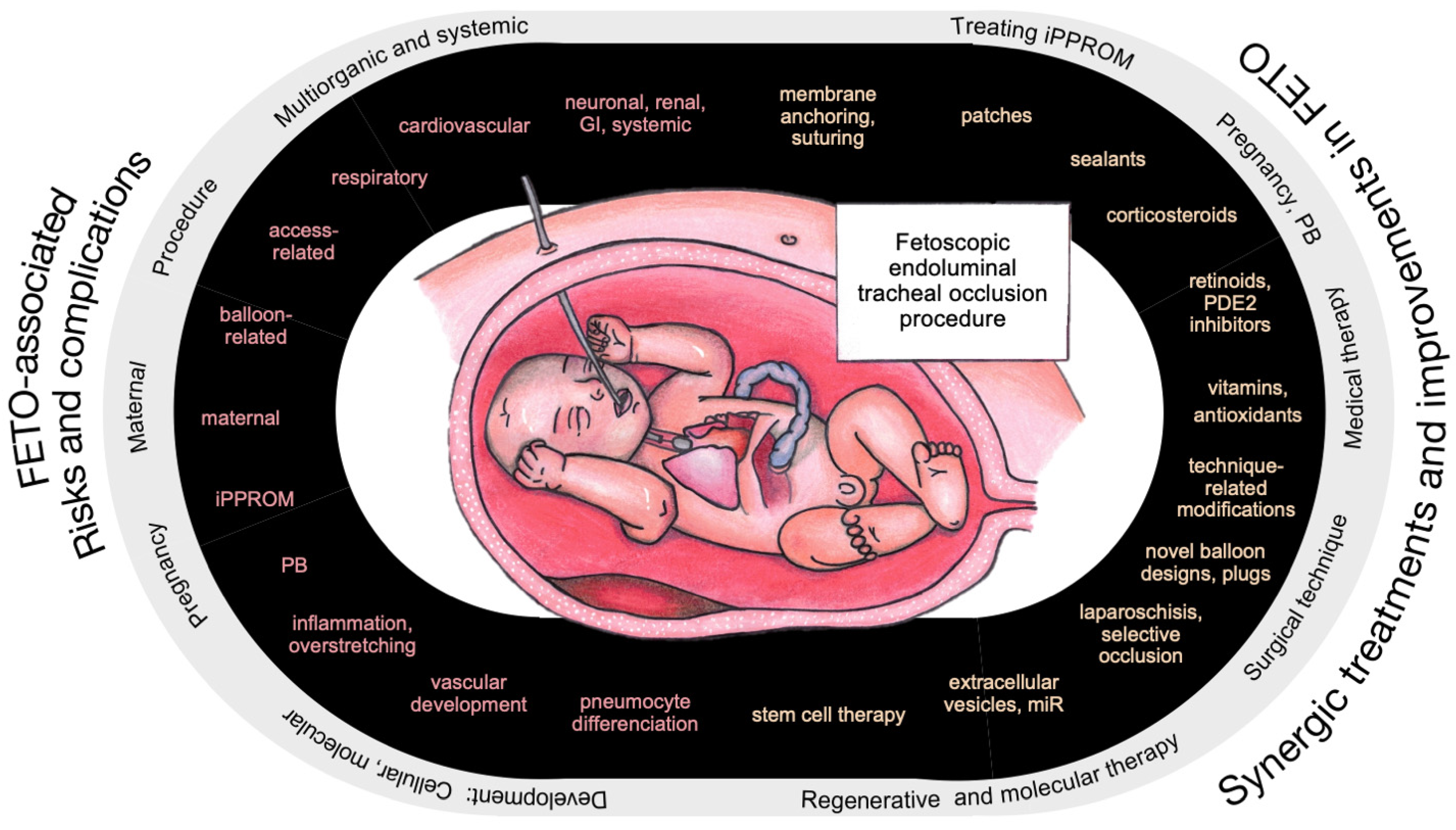
2. FETO-Associated Risks, Challenges, and Pitfalls
2.1. Procedure-Related/Technical Complications
2.2. Pregnancy and Maternal Risks
2.3. Fetal FETO-Associated Lesions
2.4. Effect of FETO on Lung Development
2.5. Effects of FETO at Molecular Level
3. Synergic Treatment Options and Overcoming Complications
3.1. Treating PPROM and Preventing PB
3.2. Synergic Medical Therapies
3.3. Role of EVs and Regenerative Medicine in CDH Treatment
3.4. Novel Ways to Deliver Mediators
3.5. Surgical- and Technique-Related Modifications of FETO
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE2 | Alveolar epithelial type II (cells) |
| AFSC-EVs | Amniotic-fluid-stem-cell-derived extracellular vesicles |
| AKI | Acute kidney injury |
| ATP | Adenosine triphosphate |
| CDH | Congenital diaphragmatic hernia |
| cGMP | Cyclic guanosine monophosphate |
| DAMPs | Damage-associated molecular patterns |
| ECM | Extracellular matrix |
| ECMO | Extracorporeal membrane oxygenation |
| EGFR | Epithelial growth factor |
| ERK | Extracellular signal-regulated kinase |
| EVs | Extracellular vesicles |
| EXIT | Ex utero intrapartum therapy |
| FETO | Fetoscopic endoluminal tracheal occlusion |
| FETO-LAP | Fetoscopic laparoschisis |
| Fgf10 | Fibroblast growth factor 10 |
| FgfR2 | Fibroblast growth factor receptor 2 |
| GA | Gestational age |
| GERD | Gastroesophageal reflux disease |
| GI | gastrointestinal |
| HSP | Heat shock protein |
| IGF | Insulin-like growth factor |
| IL | Interleukin |
| KGF | Keratinocyte growth factor |
| miR | MicroRNA |
| MRI | Magnetic resonance imaging |
| MSC-EVs | Mesenchyomal-stem-cell-derived extracellular vesicles |
| mTOR | Mammalian target of rapamycin |
| MWT | Medial wall thickness |
| NO | Nitrogen oxide |
| PB | Preterm birth |
| PCNA | Proliferating cell nuclear antigen |
| PDE | Phosphodiesterase |
| PDGF | Platelet-derived growth factor |
| PPROM | Preterm prelabor rupture of membrane |
| RA | Retinoic acid |
| RhoA | Ras homolog family member A |
| ROS | Reactive oxygen species |
| Spry2 | Sprouty homolog 2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| TGF-β | Transforming growth factor β |
| TNF-α | Tumor necrosis factor-α |
| TO | Tracheal occlusion |
| VEGF | Vascular endothelial growth factor |
| YAP | Yes-associated protein |
References
- Eastwood, M.P.; Russo, F.M.; Toelen, J.; Deprest, J. Medical Interventions to Reverse Pulmonary Hypoplasia in the Animal Model of Congenital Diaphragmatic Hernia: A Systematic Review. Pediatr. Pulmonol. 2015, 50, 820–838. [Google Scholar] [CrossRef] [PubMed]
- Danzer, E.; Rintoul, N.E.; van Meurs, K.P.; Deprest, J. Prenatal Management of Congenital Diaphragmatic Hernia. Semin. Fetal Neonatal Med. 2022, 27, 101406. [Google Scholar] [CrossRef]
- Ameis, D.; Khoshgoo, N.; Keijzer, R. Abnormal Lung Development in Congenital Diaphragmatic Hernia. Semin. Pediatr. Surg. 2017, 26, 123–128. [Google Scholar] [CrossRef]
- Holden, K.I.; Rintoul, N.E.; McNamara, P.J.; Harting, M.T. Congenital Diaphragmatic Hernia-Associated Pulmonary Hypertension. Semin. Pediatr. Surg. 2024, 33, 151437. [Google Scholar] [CrossRef]
- Burgos, C.M.; Frenckner, B.; Luco, M.; Harting, M.T.; Lally, P.A.; Lally, K.P. Prenatally versus Postnatally Diagnosed Congenital Diaphragmatic Hernia—Side, Stage, and Outcome. J. Pediatr. Surg. 2019, 54, 651–655. [Google Scholar] [CrossRef]
- Gallot, D.; Boda, C.; Ughetto, S.; Perthus, I.; Robert-Gnansia, E.; Francannet, C.; Laurichesse-Delmas, H.; Jani, J.; Coste, K.; Deprest, J.; et al. Prenatal Detection and Outcome of Congenital Diaphragmatic Hernia: A French Registry-based Study. Ultrasound Obstet. Gynecol. 2007, 29, 276–283. [Google Scholar] [CrossRef]
- Russo, F.M.; Cordier, A.; De Catte, L.; Saada, J.; Benachi, A.; Deprest, J. Proposal for Standardized Prenatal Ultrasound Assessment of the Fetus with Congenital Diaphragmatic Hernia by the European Reference Network on Rare Inherited and Congenital Anomalies (ERNICA). Prenat. Diagn. 2018, 38, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Jani, J.; Nicolaides, K.H.; Keller, R.L.; Benachi, A.; Peralta, C.F.A.; Favre, R.; Moreno, O.; Tibboel, D.; Lipitz, S.; Eggink, A.; et al. Observed to Expected Lung Area to Head Circumference Ratio in the Prediction of Survival in Fetuses with Isolated Diaphragmatic Hernia. Ultrasound Obstet. Gynecol. 2007, 30, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.M.; De Coppi, P.; Allegaert, K.; Toelen, J.; van der Veeken, L.; Attilakos, G.; Eastwood, M.P.; David, A.L.; Deprest, J. Current and Future Antenatal Management of Isolated Congenital Diaphragmatic Hernia. Semin. Fetal Neonatal Med. 2017, 22, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Perrone, E.E.; Deprest, J.A. Fetal Endoscopic Tracheal Occlusion for Congenital Diaphragmatic Hernia: A Narrative Review of the History, Current Practice, and Future Directions. Transl. Pediatr. 2021, 10, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- DeKoninck, P.; Gratacos, E.; Van Mieghem, T.; Richter, J.; Lewi, P.; Ancel, A.M.; Allegaert, K.; Nicolaides, K.; Deprest, J. Results of Fetal Endoscopic Tracheal Occlusion for Congenital Diaphragmatic Hernia and the Set up of the Randomized Controlled TOTAL Trial. Early Hum. Dev. 2011, 87, 619–624. [Google Scholar] [CrossRef]
- Persico, N.; Fabietti, I.; Ciralli, F.; Gentilino, V.; D’Ambrosi, F.; Boito, S.; Ossola, M.W.; Colnaghi, M.; Condò, V.; Macchini, F.; et al. Fetoscopic Endoluminal Tracheal Occlusion in Fetuses with Severe Diaphragmatic Hernia: A Three-Year Single-Center Experience. Fetal Diagn. Ther. 2017, 41, 215–219. [Google Scholar] [CrossRef]
- Manfroi, A.; Bernardes, L.S.; de Oliveira, L.M.C.; Peres, S.V.; de Carvalho, W.B.; Tannuri, A.C.A.; da Silva, M.M.; del Bigio, J.Z.; de Amorim Filho, A.G.; de Carvalho, M.H.B.; et al. Congenital Diaphragmatic Hernia Treated via Fetal Endoscopic Tracheal Occlusion Improves Outcome in a Middle-Income Country. J. Perinat. Med. 2024, 52, 751–758. [Google Scholar] [CrossRef]
- Kosiński, P.; Wielgoś, M. Congenital Diaphragmatic Hernia: Pathogenesis, Prenatal Diagnosis and Management—Literature Review. Ginekol. Pol. 2017, 88, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Deprest, J.A.; Nicolaides, K.H.; Benachi, A.; Gratacos, E.; Ryan, G.; Persico, N.; Sago, H.; Johnson, A.; Wielgoś, M.; Berg, C.; et al. Randomized Trial of Fetal Surgery for Severe Left Diaphragmatic Hernia. N. Engl. J. Med. 2021, 385, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Deprest, J.A.; Benachi, A.; Gratacos, E.; Nicolaides, K.H.; Berg, C.; Persico, N.; Belfort, M.; Gardener, G.J.; Ville, Y.; Johnson, A.; et al. Randomized Trial of Fetal Surgery for Moderate Left Diaphragmatic Hernia. N. Engl. J. Med. 2021, 385, 119–129. [Google Scholar] [CrossRef]
- Gien, J.; Kinsella, J.P.; Behrendt, N.J.; Zaretsky, M.V.; Galan, H.L.; Liechty, K.W. Improved Survival for Infants with Severe Congenital Diaphragmatic Hernia. J. Perinatol. 2022, 42, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Idelson, A.; Tenenbaum-Gavish, K.; Danon, D.; Duvdevani, N.-R.; Bromiker, R.; Klinger, G.; Orbach-Zinger, S.; Almog, A.; Sharabi-Nov, A.; Meiri, H.; et al. Fetal Surgery Using Fetoscopic Endoluminal Tracheal Occlusion for Severe Congenital Diaphragmatic Hernia: A Single-Center Experience. Arch. Gynecol. Obstet. 2023, 310, 345–351. [Google Scholar] [CrossRef]
- Cruz-Martínez, R.; Shazly, S.; Martínez-Rodríguez, M.; Gámez-Varela, A.; Luna-García, J.; Juárez-Martínez, I.; López-Briones, H.; Coronel-Cruz, F.; Villalobos-Gómez, R.; Ibarra-Rios, D.; et al. Impact of Fetal Endoscopic Tracheal Occlusion in Fetuses with Congenital Diaphragmatic Hernia and Moderate Lung Hypoplasia. Prenat. Diagn. 2022, 42, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Keijzer, R.; Liu, J.; Deimling, J.; Tibboel, D.; Post, M. Dual-Hit Hypothesis Explains Pulmonary Hypoplasia in the Nitrofen Model of Congenital Diaphragmatic Hernia. Am. J. Pathol. 2000, 156, 1299–1306. [Google Scholar] [CrossRef]
- Holden, K.I.; Harting, M.T. Recent Advances in the Treatment of Complex Congenital Diaphragmatic Hernia—A Narrative Review. Transl. Pediatr. 2023, 12, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Olutoye, O.O., II; Short, W.D.; Gilley, J.; Hammond, J.D., II; Belfort, M.A.; Lee, T.C.; King, A.; Espinoza, J.; Joyeux, L.; Lingappan, K.; et al. The Cellular and Molecular Effects of Fetoscopic Endoluminal Tracheal Occlusion in Congenital Diaphragmatic Hernia. Front. Pediatr. 2022, 10, 925106. [Google Scholar] [CrossRef]
- Blumenfeld, Y.J.; Belfort, M.A. New Approaches to Congenital Diaphragmatic Hernia. Curr. Opin. Obstet. Gynecol. 2020, 32, 121–127. [Google Scholar] [CrossRef]
- Van der Veeken, L.; Russo, F.M.; De Catte, L.; Gratacos, E.; Benachi, A.; Ville, Y.; Nicolaides, K.; Berg, C.; Gardener, G.; Persico, N.; et al. Fetoscopic Endoluminal Tracheal Occlusion and Reestablishment of Fetal Airways for Congenital Diaphragmatic Hernia. Gynecol. Surg. 2018, 15, 9. [Google Scholar] [CrossRef]
- Danzer, E.; Davey, M.G.; Kreiger, P.A.; Ruchelli, E.D.; Johnson, M.P.; Adzick, N.S.; Flake, A.W.; Hedrick, H.L. Fetal Tracheal Occlusion for Severe Congenital Diaphragmatic Hernia in Humans: A Morphometric Study of Lung Parenchyma and Muscularization of Pulmonary Arterioles. J. Pediatr. Surg. 2008, 43, 1767–1775. [Google Scholar] [CrossRef]
- Doné, E.; Gratacos, E.; Nicolaides, K.H.; Allegaert, K.; Valencia, C.; Castañon, M.; Martinez, J.-M.; Jani, J.; Van Mieghem, T.; Greenough, A.; et al. Predictors of Neonatal Morbidity in Fetuses with Severe Isolated Congenital Diaphragmatic Hernia Undergoing Fetoscopic Tracheal Occlusion. Ultrasound Obstet. Gynecol. 2013, 42, 77–83. [Google Scholar] [CrossRef]
- Jiménez, J.A.; Eixarch, E.; DeKoninck, P.; Bennini, J.R.; Devlieger, R.; Peralta, C.F.; Gratacos, E.; Deprest, J. Balloon Removal after Fetoscopic Endoluminal Tracheal Occlusion for Congenital Diaphragmatic Hernia. Am. J. Obstet. Gynecol. 2017, 217, 78.e1–78.e11. [Google Scholar] [CrossRef]
- Spiers, A.; Legendre, G.; Biquard, F.; Descamps, P.; Corroenne, R. Ex Utero Intrapartum Technique (EXIT): Indications, Procedure Methods and Materno-Fetal Complications—A Literature Review. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102252. [Google Scholar] [CrossRef]
- Ruano, R.; Yoshisaki, C.T.; da Silva, M.M.; Ceccon, M.E.J.; Grasi, M.S.; Tannuri, U.; Zugaib, M. A Randomized Controlled Trial of Fetal Endoscopic Tracheal Occlusion versus Postnatal Management of Severe Isolated Congenital Diaphragmatic Hernia. Ultrasound Obstet. Gynecol. 2012, 39, 20–27. [Google Scholar] [CrossRef]
- Basurto, D.; Watananirun, K.; Cordier, A.-G.; Otaño, J.; Carriere, D.; Scuglia, M.; de Luna Freire Vargas, A.M.; Prat, J.; Russo, F.M.; Debeer, A.; et al. Tracheomalacia and Tracheomegaly in Infants and Children with Congenital Diaphragmatic Hernia Managed with and without Fetoscopic Endoluminal Tracheal Occlusion (FETO): A Multicentre, Retrospective Cohort Study. Lancet Child. Adolesc. Health 2024, 8, 580–588. [Google Scholar] [CrossRef]
- Saura, L.; Castañón, M.; Prat, J.; Albert, A.; Caceres, F.; Moreno, J.; Gratacós, E. Impact of Fetal Intervention on Postnatal Management of Congenital Diaphragmatic Hernia. Eur. J. Pediatr. Surg. 2007, 17, 404–407. [Google Scholar] [CrossRef]
- Beck, V.; Lewi, P.; Gucciardo, L.; Devlieger, R. Preterm Prelabor Rupture of Membranes and Fetal Survival after Minimally Invasive Fetal Surgery: A Systematic Review of the Literature. Fetal Diagn. Ther. 2012, 31, 1–9. [Google Scholar] [CrossRef]
- Swiatkowska-Freund, M.; Traczyk-Łos, A.; Partyka, A.; Obara, K.; Damdinsuren, A.; Preis, K. Perinatal Outcome in Preterm Premature Rupture of Membranes before 37 Weeks of Gestation. Ginekol. Pol. 2019, 90, 645–650. [Google Scholar] [CrossRef]
- Gratacós, E. Fetoscopy and Risk of Iatrogenic Preterm Premature Rupture of Membranes: Not as High as It May Seem (in Experienced Hands). Fetal Diagn. Ther. 2012, 31, 10–11. [Google Scholar] [CrossRef]
- Chmait, R.; Chon, A.; Korst, L.; Llanes, A.; Kontopoulos, E.; Quintero, R. Risks of Preterm Premature Rupture of Membranes and Preterm Birth Post Fetoscopy Based on Location of Trocar Insertion Site. Am. J. Perinatol. 2018, 35, 801–808. [Google Scholar] [CrossRef]
- Forde, B.; Habli, M. Unique Considerations. Obstet. Gynecol. Clin. N. Am. 2020, 47, 653–669. [Google Scholar] [CrossRef]
- Amberg, B.J.; Hodges, R.J.; Rodgers, K.A.; Crossley, K.J.; Hooper, S.B.; DeKoninck, P.L.J. Why Do the Fetal Membranes Rupture Early after Fetoscopy? A Review. Fetal Diagn. Ther. 2021, 48, 493–503. [Google Scholar] [CrossRef]
- Jha, P.; Feldstein, V.A.; Revzin, M.V.; Katz, D.S.; Moshiri, M. Role of Imaging in Obstetric Interventions: Criteria, Considerations, and Complications. RadioGraphics 2021, 41, E1243–E1264. [Google Scholar] [CrossRef]
- Ruano, R.; Duarte, S.A.; Pimenta, E.J.d.A.; Takashi, E.; da Silva, M.M.; Tannuri, U.; Zugaib, M. Comparison between Fetal Endoscopic Tracheal Occlusion Using a 1.0-Mm Fetoscope and Prenatal Expectant Management in Severe Congenital Diaphragmatic Hernia. Fetal Diagn. Ther. 2011, 29, 64–70. [Google Scholar] [CrossRef]
- Jani, J.C.; Nicolaides, K.H.; Gratacós, E.; Valencia, C.M.; Doné, E.; Martinez, J.-M.; Gucciardo, L.; Cruz, R.; Deprest, J.A. Severe Diaphragmatic Hernia Treated by Fetal Endoscopic Tracheal Occlusion. Ultrasound Obstet. Gynecol. 2009, 34, 304–310. [Google Scholar] [CrossRef]
- Araujo Júnior, E.; Tonni, G.; Martins, W.; Ruano, R. Procedure-Related Complications and Survival Following Fetoscopic Endotracheal Occlusion (FETO) for Severe Congenital Diaphragmatic Hernia: Systematic Review and Meta-Analysis in the FETO Era. Eur. J. Pediatr. Surg. 2017, 27, 297–305. [Google Scholar] [CrossRef]
- Valenzuela, I.; van der Merwe, J.; De Catte, L.; Devlieger, R.; Deprest, J.; Lewi, L. Foetal Therapies and Their Influence on Preterm Birth. Semin. Immunopathol. 2020, 42, 501–514. [Google Scholar] [CrossRef]
- Vogel, J.P.; Chawanpaiboon, S.; Moller, A.-B.; Watananirun, K.; Bonet, M.; Lumbiganon, P. The Global Epidemiology of Preterm Birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 3–12. [Google Scholar] [CrossRef]
- Sacco, A.; Van der Veeken, L.; Bagshaw, E.; Ferguson, C.; Van Mieghem, T.; David, A.L.; Deprest, J. Maternal Complications Following Open and Fetoscopic Fetal Surgery: A Systematic Review and Meta-analysis. Prenat. Diagn. 2019, 39, 251–268. [Google Scholar] [CrossRef]
- Gregoir, C.; Engels, A.C.; Gomez, O.; DeKoninck, P.; Lewi, L.; Gratacos, E.; Deprest, J.A. Fertility, Pregnancy and Gynecological Outcomes after Fetoscopic Surgery for Congenital Diaphragmatic Hernia. Hum. Reprod. 2016, 31, 2024–2030. [Google Scholar] [CrossRef]
- Enriquez, G.; Cadavid, L.; Garcés-Iñigo, E.; Castellote, A.; Piqueras, J.; Peiró, J.L.; Carreras, E. Tracheobronchomegaly Following Intrauterine Tracheal Occlusion for Congenital Diaphragmatic Hernia. Pediatr. Radiol. 2012, 42, 916–922. [Google Scholar] [CrossRef]
- Zani, A.; Sellars, M.; Allen, P.; Tyraskis, A.; Nicolaides, K.; Greenough, A.; Patel, S.; Davenport, M.; Ade-Ajayi, N. Tracheomegaly in Infants with Severe Congenital Diaphragmatic Hernia Treated with Fetal Endoluminal Tracheal Occlusion. J. Pediatr. 2014, 164, 1311–1315. [Google Scholar] [CrossRef]
- Losty, P.D. Congenital Diaphragmatic Hernia: Where and What Is the Evidence? Semin. Pediatr. Surg. 2014, 23, 278–282. [Google Scholar] [CrossRef]
- Tho, A.L.W.; Rath, C.P.; Tan, J.K.G.; Rao, S.C. Prevalence of Symptomatic Tracheal Morbidities after Fetoscopic Endoluminal Tracheal Occlusion: A Systematic Review and Meta-Analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2024, 109, 52–58. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Maskatia, S.A.; Loar, R.W.; Colquitt, J.L.; Mehollin-Ray, A.R.; Ruano, R.; Belfort, M.A.; Olutoye, O.O.; Kailin, J.A. The Impact of Fetal Endoscopic Tracheal Occlusion in Isolated Left-sided Congenital Diaphragmatic Hernia on Left-sided Cardiac Dimensions. Prenat. Diagn. 2018, 38, 812–820. [Google Scholar] [CrossRef]
- Alves da Rocha, L.; Byrne, F.A.; Keller, R.L.; Miniati, D.; Brook, M.M.; Silverman, N.H.; Moon-Grady, A.J. Left Heart Structures in Human Neonates with Congenital Diaphragmatic Hernia and the Effect of Fetal Endoscopic Tracheal Occlusion. Fetal Diagn. Ther. 2014, 35, 36–43. [Google Scholar] [CrossRef]
- Arattu Thodika, F.M.S.; Dassios, T.; Deep, A.; Greenough, A. Acute Kidney Injury in Infants with Congenital Diaphragmatic Hernia. J. Perinatol. 2022, 42, 925–929. [Google Scholar] [CrossRef]
- Koivusalo, A.I.; Pakarinen, M.P.; Lindahl, H.G.; Rintala, R.J. The Cumulative Incidence of Significant Gastroesophageal Reflux in Patients with Congenital Diaphragmatic Hernia—A Systematic Clinical, PH-Metric, and Endoscopic Follow-up Study. J. Pediatr. Surg. 2008, 43, 279–282. [Google Scholar] [CrossRef]
- Macchini, F.; Morandi, A.; Mazzoleni, S.; Ichino, M.; Cavallaro, G.; Raffaeli, G.; Ferrari, C.; Gangi, S.; Mosca, F.; Fabietti, I.; et al. Is Fetal Endoscopic Tracheal Occlusion (FETO) a Predisposing Factor for Acid Gastro-Esophageal Reflux in Infants With Congenital Diaphragmatic Hernia? Front. Pediatr. 2020, 8, 467. [Google Scholar] [CrossRef]
- Cordier, A.G.; Laup, L.; Letourneau, A.; Le Sache, N.; Fouquet, V.; Senat, M.V.; Perrotin, F.; Rosenblatt, J.; Sananes, N.; Jouannic, J.M.; et al. Prenatal Stomach Position Predicts Gastrointestinal Morbidity at 2 Years in Fetuses with Left-sided Congenital Diaphragmatic Hernia. Ultrasound Obstet. Gynecol. 2021, 57, 959–967. [Google Scholar] [CrossRef]
- Danzer, E.; Gerdes, M.; D’Agostino, J.A.; Partridge, E.A.; Hoffman-Craven, C.H.; Bernbaum, J.; Rintoul, N.E.; Flake, A.W.; Adzick, N.S.; Hedrick, H.L. Preschool Neurological Assessment in Congenital Diaphragmatic Hernia Survivors: Outcome and Perinatal Factors Associated with Neurodevelopmental Impairment. Early Hum. Dev. 2013, 89, 393–400. [Google Scholar] [CrossRef]
- Sferra, S.R.; Penikis, A.B.; Guo, M.; Baschat, A.A.; Mogayzel, P.J.; Burton, V.J.; Kunisaki, S.M. Neurodevelopmental Outcomes in Children After Fetoscopic Endoluminal Tracheal Occlusion for Severe Congenital Diaphragmatic Hernia: Results From a Multidisciplinary Clinic. J. Pediatr. Surg. 2024, 59, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Sferra, S.R.; Nies, M.K.; Miller, J.L.; Garcia, A.V.; Hodgman, E.I.; Penikis, A.B.; Engwall-Gill, A.J.; Burton, V.J.; Rice, J.L.; Mogayzel, P.J.; et al. Morbidity in Children after Fetoscopic Endoluminal Tracheal Occlusion for Severe Congenital Diaphragmatic Hernia: Results from a Multidisciplinary Clinic. J. Pediatr. Surg. 2023, 58, 14–19. [Google Scholar] [CrossRef]
- Heerema, A.E.; Rabban, J.T.; Sydorak, R.M.; Harrison, M.R.; Jones, K.D. Lung Pathology in Patients with Congenital Diaphragmatic Hernia Treated with Fetal Surgical Intervention, Including Tracheal Occlusion. Pediatr. Dev. Pathol. 2003, 6, 536–546. [Google Scholar] [CrossRef]
- Wu, J.; Ge, X.; Verbeken, E.K.; Gratacós, E.; Yesildaglar, N.; Deprest, J.A. Pulmonary Effects of in Utero Tracheal Occlusion Are Dependent on Gestational Age in a Rabbit Model of Diaphragmatic Hernia. J. Pediatr. Surg. 2002, 37, 11–17. [Google Scholar] [CrossRef]
- Dobrinskikh, E.; Al-Juboori, S.I.; Oria, M.; Reisz, J.A.; Zheng, C.; Peiro, J.L.; Marwan, A.I. Heterogeneous Response in Rabbit Fetal Diaphragmatic Hernia Lungs After Tracheal Occlusion. J. Surg. Res. 2020, 250, 23–38. [Google Scholar] [CrossRef]
- Davey, M. Surfactant Levels in Congenital Diaphragmatic Hernia. PLoS Med. 2007, 4, e243. [Google Scholar] [CrossRef] [PubMed]
- Willson, D.F.; Chess, P.R.; Wang, Z.; Notter, R.H. Pulmonary Surfactant: Biology and Therapy. In The Respiratory Tract in Pediatric Critical Illness and Injury; Springer: London, UK, 2009; pp. 1–14. [Google Scholar]
- Pelizzo, G.; Mimmi, M.C.; Peiro, J.L.; Marotta, M.; Amoroso, F.; Fusillo, M.; Carlini, V.; Calcaterra, V. Congenital Diaphragmatic Hernia: Endotracheal Fluid Phospholipidic Profile Following Tracheal Occlusion in an Experimental Model. J. Perinat. Med. 2017, 45, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.G.; Biard, J.; Robinson, L.; Tsai, J.; Schwarz, U.; Danzer, E.; Adzick, N.S.; Flake, A.W.; Hedrick, H.L. Surfactant Protein Expression Is Increased in the Ipsilateral but Not Contralateral Lungs of Fetal Sheep with Left-sided Diaphragmatic Hernia. Pediatr. Pulmonol. 2005, 39, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Bütter, A.; Bratu, I.; Flageole, H.; Laberge, J.-M.; Kovacs, L.; Faucher, D.; Piedoboeuf, B. Fetal Tracheal Occlusion in Lambs with Congenital Diaphragmatic Hernia: Role of Exogenous Surfactant at Birth. Pediatr. Res. 2005, 58, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Sevilmis, Y.D.; Olutoye, O.O.; Peiffer, S.; Mehl, S.C.; Belfort, M.A.; Rhee, C.J.; Garcia-Prats, J.A.; Vogel, A.M.; Lee, T.C.; Keswani, S.G.; et al. Surfactant Therapy in Congenital Diaphragmatic Hernia and Fetoscopic Endoluminal Tracheal Occlusion. J. Surg. Res. 2024, 296, 239–248. [Google Scholar] [CrossRef]
- Bratu, I.; Flageole, H.; Laberge, J.-M.; Possmayer, F.; Harbottle, R.; Kay, S.; Khalife, S.; Piedboeuf, B. Surfactant Levels after Reversible Tracheal Occlusion and Prenatal Steroids in Experimental Diaphragmatic Hernia. J. Pediatr. Surg. 2001, 36, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.G.; Hedrick, H.L.; Bouchard, S.; Mendoza, J.M.; Schwarz, U.; Adzick, N.S.; Flake, A.W. Temporary Tracheal Occlusion in Fetal Sheep with Lung Hypoplasia Does Not Improve Postnatal Lung Function. J. Appl. Physiol. 2003, 94, 1054–1062. [Google Scholar] [CrossRef][Green Version]
- Kanai, M.; Kitano, Y.; von Allmen, D.; Davies, P.; Adzick, N.S.; Flake, A.W. Fetal Tracheal Occlusion in the Rat Model of Nitrofen-Induced Congenital Diaphragmatic Hernia: Tracheal Occlusion Reverses the Arterial Structural Abnormality. J. Pediatr. Surg. 2001, 36, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Luks, F.I.; Wild, Y.K.; Piasecki, G.J.; De Paepe, M.E. Short-Term Tracheal Occlusion Corrects Pulmonary Vascular Anomalies in the Fetal Lamb with Diaphragmatic Hernia. Surgery 2000, 128, 266–272. [Google Scholar] [CrossRef] [PubMed]
- DeKoninck, P.L.J.; Crossley, K.J.; Kashyap, A.J.; Skinner, S.M.; Thio, M.; Rodgers, K.A.; Deprest, J.A.; Hooper, S.B.; Hodges, R.J. Effects of Tracheal Occlusion on the Neonatal Cardiopulmonary Transition in an Ovine Model of Diaphragmatic Hernia. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F609–F616. [Google Scholar] [CrossRef] [PubMed]
- Varisco, B.M.; Sbragia, L.; Chen, J.; Scorletti, F.; Joshi, R.; Wong, H.R.; Lopes-Figueira, R.; Oria, M.; Peiro, J. Excessive Reversal of Epidermal Growth Factor Receptor and Ephrin Signaling Following Tracheal Occlusion in Rabbit Model of Congenital Diaphragmatic Hernia. Mol. Med. 2016, 22, 398–411. [Google Scholar] [CrossRef]
- Peiro, J.L.; Oria, M.; Aydin, E.; Joshi, R.; Cabanas, N.; Schmidt, R.; Schroeder, C.; Marotta, M.; Varisco, B.M. Proteomic Profiling of Tracheal Fluid in an Ovine Model of Congenital Diaphragmatic Hernia and Fetal Tracheal Occlusion. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2018, 315, L1028–L1041. [Google Scholar] [CrossRef]
- Wallace, M.J.; Thiel, A.M.; Lines, A.M.; Polglase, G.R.; Sozo, F.; Hooper, S.B. Role of Platelet-Derived Growth Factor-B, Vascular Endothelial Growth Factor, Insulin-like Growth Factor-II, Mitogen-Activated Protein Kinase and Transforming Growth Factor-Β1 in Expansion-Induced Lung Growth in Fetal Sheep. Reprod. Fertil. Dev. 2006, 18, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Dilai, S.; Lingampally, A.; Chao, C.-M.; Danopoulos, S.; Carraro, G.; Mukhametshina, R.; Wilhelm, J.; Baumgart-Vogt, E.; Al Alam, D.; et al. A Comprehensive Analysis of Fibroblast Growth Factor Receptor 2b Signaling on Epithelial Tip Progenitor Cells During Early Mouse Lung Branching Morphogenesis. Front. Genet. 2019, 9, 746. [Google Scholar] [CrossRef] [PubMed]
- Unbekandt, M.; del Moral, P.-M.; Sala, F.G.; Bellusci, S.; Warburton, D.; Fleury, V. Tracheal Occlusion Increases the Rate of Epithelial Branching of Embryonic Mouse Lung via the FGF10-FGFR2b-Sprouty2 Pathway. Mech. Dev. 2008, 125, 314–324. [Google Scholar] [CrossRef]
- Stanton, A.E.; Goodwin, K.; Sundarakrishnan, A.; Jaslove, J.M.; Gleghorn, J.P.; Pavlovich, A.L.; Nelson, C.M. Negative Transpulmonary Pressure Disrupts Airway Morphogenesis by Suppressing Fgf10. Front. Cell Dev. Biol. 2021, 9, 725785. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.S.; Osada, H.; Finch, P.W.; Taylor, W.G.; Rudikoff, S.; Aaronson, S.A. Purification and Characterization of a Newly Identified Growth Factor Specific for Epithelial Cells. Proc. Natl. Acad. Sci. USA 1989, 86, 802–806. [Google Scholar] [CrossRef]
- Danilenko, D.M. Preclinical and Early Clinical Development of Keratinocyte Growth Factor, an Epithelial- Specific Tissue Growth Factor. Toxicol. Pathol. 1999, 27, 64–71. [Google Scholar] [CrossRef]
- Saada, J.; Oudrhiri, N.; Bonnard, A.; de Lagausie, P.; Aissaoui, A.; Hauchecorne, M.; Oury, J.; Aigrain, Y.; Peuchmaur, M.; Lehn, J.; et al. Combining Keratinocyte Growth Factor Transfection into the Airways and Tracheal Occlusion in a Fetal Sheep Model of Congenital Diaphragmatic Hernia. J. Gene Med. 2010, 12, 413–422. [Google Scholar] [CrossRef] [PubMed]
- McCabe, A.J.; Carlino, U.; Holm, B.A.; Glick, P.L. Upregulation of Keratinocyte Growth Factor in the Tracheal Ligation Lamb Model of Congenital Diaphragmatic Hernia. J. Pediatr. Surg. 2001, 36, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.J.; Brownfield, D.G.; Krasnow, M.A. Alveolar Progenitor and Stem Cells in Lung Development, Renewal and Cancer. Nature 2014, 507, 190–194. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; He, H.; Liu, C.; Li, W.; Xie, L.; Zhang, Y. Csk/Src/EGFR Signaling Regulates Migration of Myofibroblasts and Alveolarization. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2016, 310, L562–L571. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Lo, H.-W. Landscape of EGFR Signaling Network in Human Cancers: Biology and Therapeutic Response in Relation to Receptor Subcellular Locations. Cancer Lett. 2012, 318, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, G.; Wang, Y. Strategies for Overcoming Resistance to EGFR Family Tyrosine Kinase Inhibitors. Cancer Treat. Rev. 2011, 37, 456–464. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bartram, U.; Speer, C.P. The Role of Transforming Growth Factor β in Lung Development and Disease. Chest 2004, 125, 754–765. [Google Scholar] [CrossRef]
- Vuckovic, A.; Herber-Jonat, S.; Flemmer, A.W.; Ruehl, I.M.; Votino, C.; Segers, V.; Benachi, A.; Martinovic, J.; Nowakowska, D.; Dzieniecka, M.; et al. Increased TGF-β: A Drawback of Tracheal Occlusion in Human and Experimental Congenital Diaphragmatic Hernia? Am. J. Physiol.-Lung Cell. Mol. Physiol. 2016, 310, L311–L327. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Jank, M.; Patel, D.; Ozturk, A.; Aubert, O.; Ai, X.; Yamataka, A.; Keijzer, R. Yes-Associated Protein Is Dysregulated in Human Congenital Diaphragmatic Hernia Patients during Mid and End Gestation. Pediatr. Surg. Int. 2024, 41, 16. [Google Scholar] [CrossRef]
- Aubert, O.; Miyake, Y.; Amonkar, G.M.; Dinwoodie, O.M.; Varisco, B.M.; Marotta, M.; Zhao, C.; Wagner, R.; Chen, Y.-W.; Moscatello, A.; et al. Fetal Tracheal Occlusion Corelates with Normalized YAP Expression and Alveolar Epithelial Differentiation in CDH. Am. J. Respir. Cell Mol. Biol. 2024. [Google Scholar] [CrossRef]
- Sanz-López, E.; Maderuelo, E.; Peláez, D.; Chimenti, P.; Lorente, R.; Muñoz, M.A.; Sánchez-Luna, M. Changes in the Expression of Vascular Endothelial Growth Factor after Fetal Tracheal Occlusion in an Experimental Model of Congenital Diaphragmatic Hernia. Crit. Care Res. Pract. 2013, 2013, 958078. [Google Scholar] [CrossRef]
- Hara, A.; Chapin, C.J.; Ertsey, R.; Kitterman, J.A. Changes in Fetal Lung Distension Alter Expression of Vascular Endothelial Growth Factor and Its Isoforms in Developing Rat Lung. Pediatr. Res. 2005, 58, 30–37. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Gonçalves, F.L.L.; Regis, A.C.; Gallindo, R.M.; Sbragia, L. Prenatal Retinoic Acid Improves Lung Vascularization and VEGF Expression in CDH Rat. Am. J. Obstet. Gynecol. 2012, 207, 76.e25–76.e32. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.P.; Schlueter, M.; Soifer, S.J.; Gutierrez, J.A. Cyclic Mechanical Stretch Induces VEGF and FGF-2 Expression in Pulmonary Vascular Smooth Muscle Cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2002, 282, L897–L903. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.L.L.; Figueira, R.L.; Gallindo, R.M.; Simões, A.L.B.; Coleman, A.; Peiró, J.L.; Sbragia, L. Tracheal Occlusion and Ventilation Changes the Nitric Oxide Pathway in Congenital Diaphragmatic Hernia Model. J. Surg. Res. 2016, 203, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Marwan, A.I.; Shabeka, U.; Dobrinskikh, E. Suggested Mechanisms of Tracheal Occlusion Mediated Accelerated Fetal Lung Growth: A Case for Heterogeneous Topological Zones. Front. Pediatr. 2017, 5, 295. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Lieckfeldt, P.; Piyadasa, H.; Markel, M.; Riedel, J.; Stefanovici, C.; Peukert, N.; Patel, D.; Derraugh, G.; Min, S.A.L.; et al. Proteomic Profiling of Hypoplastic Lungs Suggests an Underlying Inflammatory Response in the Pathogenesis of Abnormal Lung Development in Congenital Diaphragmatic Hernia. Ann. Surg. 2023, 278, e411–e421. [Google Scholar] [CrossRef] [PubMed]
- Flake, M.D.A.W.; Harrison, M.D.M.R. Fetal Surgery. Annu. Rev. Med. 1995, 46, 67–78. [Google Scholar] [CrossRef]
- Codsi, E.; Audibert, F. Fetal Surgery: Past, Present, and Future Perspectives. J. Obstet. Gynaecol. Can. 2019, 41, S287–S289. [Google Scholar] [CrossRef] [PubMed]
- Tchirikov, M.; Buchmann, J.; Bergner, M. Successful Closure of Small Iatrogenic Chorioamniotic Membranes Defect After Fetoscopy with Laser: A Case Report. J. Reprod. Med. 2017, 62, 194–199. [Google Scholar] [PubMed]
- Chmait, R.H.; Kontopoulos, E.V.; Chon, A.H.; Korst, L.M.; Llanes, A.; Quintero, R.A. Amniopatch Treatment of Iatrogenic Preterm Premature Rupture of Membranes (IPPROM) after Fetoscopic Laser Surgery for Twin–Twin Transfusion Syndrome. J. Matern.-Fetal Neonatal Med. 2017, 30, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.-H.; Kuk, J.-Y.; Cha, H.-H.; Choi, S.-J.; Oh, S.; Roh, C.-R.; Kim, J.-H. Amniopatch Treatment for Preterm Premature Rupture of Membranes before 23 Weeks’ Gestation and Factors Associated with Its Success. Taiwan. J. Obstet. Gynecol. 2017, 56, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, E.; Kawamura, Y.; Chigusa, Y.; Mogami, H.; Ueda, A.; Hamanishi, J.; Mandai, M. Intracervical Elastomeric Sealant in an Ex Vivo Model. J. Matern.-Fetal Neonatal Med. 2021, 34, 1109–1111. [Google Scholar] [CrossRef] [PubMed]
- Devaud, Y.R.; Stäuble, S.; Moehrlen, U.; Weisskopf, M.; Vonzun, L.; Zimmermann, R.; Ehrbar, M.; Ochsenbein-Kölble, N. Minimally Invasive Precise Application of Bioadhesives to Prevent IPPROM on a Pregnant Sheep Model. Fetal Diagn. Ther. 2021, 48, 785–793. [Google Scholar] [CrossRef]
- Byju, A.G.; Diemer, A.; Luk, C.; Heffernan, M.J.; Belfort, M.A.; Simons, B.W.; Koh, C.J.; Haridas, B.; Espinoza, J. The ChorioAnchor: Design and Testing of a Novel Chorioamniotic Anchoring Device to Enable Percutaneous Fetoscopic Surgery. Fetal Diagn. Ther. 2022, 49, 347–360. [Google Scholar] [CrossRef]
- Micheletti, T.; Eixarch, E.; Febas, G.; Berdun, S.; Parra, J.; Hernansanz, A.; Borrós, S.; Gratacos, E. Intraamniotic Sealing of Fetoscopic Membrane Defects in Ex Vivo and in Vivo Sheep Models Using an Integrated Semirigid Bioadhesive Patch. Am. J. Obstet. Gynecol. MFM 2022, 4, 100593. [Google Scholar] [CrossRef] [PubMed]
- Avilla-Royo, E.; Seehusen, F.; Devaud, Y.R.; Monné Rodriguez, J.M.; Strübing, N.; Weisskopf, M.; Messersmith, P.B.; Vonzun, L.; Moehrlen, U.; Ehrbar, M.; et al. In Vivo Sealing of Fetoscopy-Induced Fetal Membrane Defects by Mussel Glue. Fetal Diagn. Ther. 2022, 49, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Devaud, Y.R.; Avilla-Royo, E.; Lionetti, L.; Tronnier, H.; Seehusen, F.; Monné Rodriguez, J.M.; Moehrlen, U.; Weisskopf, M.; Vonzun, L.; Strübing, N.; et al. Tissue Glue-Based Sealing Patch for the in Vivo Prevention of Iatrogenic Prelabor Preterm Rupture of Fetal Membranes. Fetal Diagn. Ther. 2023, 50, 332–343. [Google Scholar] [CrossRef]
- Bergh, E.P.; Mann, L.K.; Won, J.H.; Nobles, A.; Johnson, A.; Papanna, R. Anchoring Device to Prevent Membrane Detachment and Preterm Premature Rupture of Membranes after Fetal Intervention. Ultrasound Obstet. Gynecol. 2024, 64, 374–380. [Google Scholar] [CrossRef]
- Kashyap, A.; DeKoninck, P.; Crossley, K.; Thio, M.; Polglase, G.; Russo, F.; Deprest, J.; Hooper, S.; Hodges, R. Antenatal Medical Therapies to Improve Lung Development in Congenital Diaphragmatic Hernia. Am. J. Perinatol. 2018, 35, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Jeanty, C.; Kunisaki, S.M.; MacKenzie, T.C. Novel Non-Surgical Prenatal Approaches to Treating Congenital Diaphragmatic Hernia. Semin. Fetal Neonatal Med. 2014, 19, 349–356. [Google Scholar] [CrossRef]
- Lally, K.P.; Bagolan, P.; Hosie, S.; Lally, P.A.; Stewart, M.; Cotten, C.M.; Van Meurs, K.P.; Alexander, G. Corticosteroids for Fetuses with Congenital Diaphragmatic Hernia: Can We Show Benefit? J. Pediatr. Surg. 2006, 41, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Reddy, U.M.; Deshmukh, U.; Dude, A.; Harper, L.; Osmundson, S.S. Society for Maternal-Fetal Medicine Consult Series #58: Use of Antenatal Corticosteroids for Individuals at Risk for Late Preterm Delivery. Am. J. Obstet. Gynecol. 2021, 225, B36–B42. [Google Scholar] [CrossRef]
- Baschat, A.A.; Desiraju, S.; Bernier, M.L.; Kunisaki, S.M.; Miller, J.L. Management Advances for Congenital Diaphragmatic Hernia: Integrating Prenatal and Postnatal Perspectives. Transl. Pediatr. 2024, 13, 643–662. [Google Scholar] [CrossRef] [PubMed]
- Bunt, J.E.; Carnielli, V.P.; Seidner, S.R.; Ikegami, M.; Darcos Wattimena, J.L.; Sauer, P.J.; Jobe, A.H.; Zimmermann, L.J. Metabolism of Endogenous Surfactant in Premature Baboons and Effect of Prenatal Corticosteroids. Am. J. Respir. Crit. Care Med. 1999, 160, 1481–1485. [Google Scholar] [CrossRef][Green Version]
- Fernandes-Silva, H.; Araújo-Silva, H.; Correia-Pinto, J.; Moura, R.S. Retinoic Acid: A Key Regulator of Lung Development. Biomolecules 2020, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.J.; Babiuk, R.P.; Thebaud, B. Etiology of Congenital Diaphragmatic Hernia: The Retinoid Hypothesis. Pediatr. Res. 2003, 53, 726–730. [Google Scholar] [CrossRef]
- Nakazawa, N.; Takayasu, H.; Montedonico, S.; Puri, P. Altered Regulation of Retinoic Acid Synthesis in Nitrofen-Induced Hypoplastic Lung. Pediatr. Surg. Int. 2007, 23, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Noble, B.R.; Babiuk, R.P.; Clugston, R.D.; Underhill, T.M.; Sun, H.; Kawaguchi, R.; Walfish, P.G.; Blomhoff, R.; Gundersen, T.E.; Greer, J.J. Mechanisms of Action of the Congenital Diaphragmatic Hernia-Inducing Teratogen Nitrofen. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2007, 293, L1079–L1087. [Google Scholar] [CrossRef]
- Vijfhuize, S.; Schaible, T.; Kraemer, U.; Cohen-Overbeek, T.; Tibboel, D.; Reiss, I. Management of Pulmonary Hypertension in Neonates with Congenital Diaphragmatic Hernia. Eur. J. Pediatr. Surg. 2012, 22, 374–383. [Google Scholar] [CrossRef]
- Lakshminrusimha, S.; Keszler, M. Persistent Pulmonary Hypertension of the Newborn. Neoreviews 2015, 16, e680–e692. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.M.; Da Cunha, M.G.M.C.M.; Jimenez, J.; Lesage, F.; Eastwood, M.P.; Toelen, J.; Deprest, J. Complementary Effect of Maternal Sildenafil and Fetal Tracheal Occlusion Improves Lung Development in the Rabbit Model of Congenital Diaphragmatic Hernia. Ann. Surg. 2022, 275, e586–e595. [Google Scholar] [CrossRef] [PubMed]
- Bratu, I.; Flageole, H.; Laberge, J.-M.; Chen, M.-F.; Piedboeuf, B. Pulmonary Structural Maturation and Pulmonary Artery Remodeling after Reversible Fetal Ovine Tracheal Occlusion in Diaphragmatic Hernia. J. Pediatr. Surg. 2001, 36, 739–744. [Google Scholar] [CrossRef]
- Davey, M.G.; Danzer, E.; Schwarz, U.; Robinson, L.; Shegu, S.; Adzick, N.S.; Flake, A.W.; Hedrick, H.L. Prenatal Glucocorticoids Improve Lung Morphology and Partially Restores Surfactant MRNA Expression in Lambs with Diaphragmatic Hernia Undergoing Fetal Tracheal Occlusion. Pediatr. Pulmonol. 2006, 41, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.G.; Danzer, E.; Schwarz, U.; Adzick, N.S.; Flake, A.W.; Hedrick, H.L. Prenatal Glucocorticoids and Exogenous Surfactant Therapy Improve Respiratory Function in Lambs with Severe Diaphragmatic Hernia Following Fetal Tracheal Occlusion. Pediatr. Res. 2006, 60, 131–135. [Google Scholar] [CrossRef][Green Version]
- Davey, M.; Shegu, S.; Danzer, E.; Ruchelli, E.; Adzick, S.; Flake, A.; Hedrick, H.L. Pulmonary Arteriole Muscularization in Lambs with Diaphragmatic Hernia after Combined Tracheal Occlusion/Glucocorticoid Therapy. Am. J. Obstet. Gynecol. 2007, 197, 381.e1–381.e7. [Google Scholar] [CrossRef]
- Mayer, S.; Klaritsch, P.; Sbragia, L.; Toelen, J.; Till, H.; Deprest, J.A. Maternal Administration of Betamethasone Inhibits Proliferation Induced by Fetal Tracheal Occlusion in the Nitrofen Rat Model for Congenital Diaphragmatic Hernia: A Placebo-Controlled Study. Pediatr. Surg. Int. 2008, 24, 1287–1295. [Google Scholar] [CrossRef]
- Roubliova, X.I.; Lewi, P.J.; Verbeken, E.K.; Vaast, P.; Jani, J.C.; Lu, H.; Tibboel, D.; Deprest, J.A. The Effect of Maternal Betamethasone and Fetal Tracheal Occlusion on Pulmonary Vascular Morphometry in Fetal Rabbits with Surgically Induced Diaphragmatic Hernia: A Placebo Controlled Morphologic Study. Prenat. Diagn. 2009, 29, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.F.; Gonçalves, F.L.L.; Figueira, R.L.; Scorletti, F.; Peiró, J.L.; Sbragia, L. Combined Antenatal Therapy with Retinoic Acid and Tracheal Occlusion in a Rat Model of Congenital Diaphragmatic Hernia. Pediatr. Surg. Int. 2016, 32, 591–598. [Google Scholar] [CrossRef]
- Delabaere, A.; Marceau, G.; Coste, K.; Blanchon, L.; Déchelotte, P.-J.; Blanc, P.; Sapin, V.; Gallot, D. Effects of Tracheal Occlusion with Retinoic Acid Administration on Normal Lung Development. Prenat. Diagn. 2017, 37, 427–434. [Google Scholar] [CrossRef]
- Delabaere, A.; Blanchon, L.; Coste, K.; Clairefond, G.; Belville, C.; Blanc, P.; Marceau, G.; Sapin, V.; Gallot, D. Retinoic Acid and Tracheal Occlusion for Diaphragmatic Hernia Treatment in Rabbit Fetuses. Prenat. Diagn. 2018, 38, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Lee, K.W.A.; Chan, L.K.W.; Hung, L.C.; Phoebe, L.K.W.; Park, Y.; Yi, K.-H. Clinical Applications of Exosomes: A Critical Review. Int. J. Mol. Sci. 2024, 25, 7794. [Google Scholar] [CrossRef] [PubMed]
- Khalaj, K.; Figueira, R.L.; Antounians, L.; Gandhi, S.; Wales, M.; Montalva, L.; Biouss, G.; Zani, A. Treatment with Amniotic Fluid Stem Cell Extracellular Vesicles Promotes Fetal Lung Branching and Cell Differentiation at Canalicular and Saccular Stages in Experimental Pulmonary Hypoplasia Secondary to Congenital Diaphragmatic Hernia. Stem Cells Transl. Med. 2022, 11, 1089–1102. [Google Scholar] [CrossRef]
- Pereira-Terra, P.; Deprest, J.A.; Kholdebarin, R.; Khoshgoo, N.; DeKoninck, P.; Munck, A.A.B.-D.; Wang, J.; Zhu, F.; Rottier, R.J.; Iwasiow, B.M.; et al. Unique Tracheal Fluid MicroRNA Signature Predicts Response to FETO in Patients With Congenital Diaphragmatic Hernia. Ann. Surg. 2015, 262, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Bardill, J.R.; Karimpour-Fard, A.; Breckenfelder, C.C.; Sucharov, C.C.; Eason, C.R.; Gallagher, L.T.; Khailova, L.; Wright, C.J.; Gien, J.; Galan, H.L.; et al. MicroRNAs in Congenital Diaphragmatic Hernia: Insights into Prenatal and Perinatal Biomarkers and Altered Molecular Pathways. Am. J. Obstet. Gynecol. MFM 2024, 6, 101535. [Google Scholar] [CrossRef] [PubMed]
- Monroe, M.N.; Zhaorigetu, S.; Gupta, V.S.; Jin, D.; Givan, K.D.; Curylo, A.L.; Olson, S.D.; Cox, C.S.; Segura, A.; Buja, L.M.; et al. Extracellular Vesicles Influence the Pulmonary Arterial Extracellular Matrix in Congenital Diaphragmatic Hernia. Pediatr. Pulmonol. 2020, 55, 2402–2411. [Google Scholar] [CrossRef] [PubMed]
- Zhaorigetu, S.; Bair, H.; Jin, D.; Gupta, V.S.; Pandit, L.M.; Bryan, R.M.; Lally, K.P.; Olson, S.D.; Cox, C.S.; Harting, M.T. Extracellular Vesicles Attenuate Nitrofen-Mediated Human Pulmonary Artery Endothelial Dysfunction: Implications for Congenital Diaphragmatic Hernia. Stem Cells Dev. 2020, 29, 967–980. [Google Scholar] [CrossRef]
- Fabietti, I.; Nardi, T.; Favero, C.; Dioni, L.; Cantone, L.; Pergoli, L.; Hoxha, M.; Pinatel, E.; Mosca, F.; Bollati, V.; et al. Extracellular Vesicles and Their MiRNA Content in Amniotic and Tracheal Fluids of Fetuses with Severe Congenital Diaphragmatic Hernia Undergoing Fetal Intervention. Cells 2021, 10, 1493. [Google Scholar] [CrossRef] [PubMed]
- Antounians, L.; Catania, V.D.; Montalva, L.; Liu, B.D.; Hou, H.; Chan, C.; Matei, A.C.; Tzanetakis, A.; Li, B.; Figueira, R.L.; et al. Fetal Lung Underdevelopment Is Rescued by Administration of Amniotic Fluid Stem Cell Extracellular Vesicles in Rodents. Sci. Transl. Med. 2021, 13, eaax5941. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Yokoi, A.; Yoshida, K.; Kitagawa, M.; Asano-Inami, E.; Miura, M.; Yasui, T.; Tano, S.; Ushida, T.; Imai, K.; et al. Amniotic Fluid-derived Small Extracellular Vesicles for Predicting Postnatal Severe Outcome of Congenital Diaphragmatic Hernia. J. Extracell. Biol. 2024, 3, e160. [Google Scholar] [CrossRef]
- Figueira, R.L.; Khoshgoo, N.; Doktor, F.; Khalaj, K.; Islam, T.; Moheimani, N.; Blundell, M.; Antounians, L.; Post, M.; Zani, A. Antenatal Administration of Extracellular Vesicles Derived From Amniotic Fluid Stem Cells Improves Lung Function in Neonatal Rats With Congenital Diaphragmatic Hernia. J. Pediatr. Surg. 2024, 59, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Doktor, F.; Lo, E.; Fortuna, V.; Khalaj, K.; Garcia, M.; Figueira, R.L.; Lacher, M.; Antounians, L.; Zani, A. Sex-Specific Differences in the Severity of Pulmonary Hypoplasia in Experimental Congenital Diaphragmatic Hernia and Implications for Extracellular Vesicle-Based Therapy. Pediatr. Surg. Int. 2024, 40, 278. [Google Scholar] [CrossRef] [PubMed]
- Antounians, L.; Figueira, R.L.; Kukreja, B.; Litvack, M.L.; Zani-Ruttenstock, E.; Khalaj, K.; Montalva, L.; Doktor, F.; Obed, M.; Blundell, M.; et al. Fetal Hypoplastic Lungs Have Multilineage Inflammation That Is Reversed by Amniotic Fluid Stem Cell Extracellular Vesicle Treatment. Sci. Adv. 2024, 10, eadn5405. [Google Scholar] [CrossRef]
- Doktor, F.; Figueira, R.L.; Fortuna, V.; Biouss, G.; Stasiewicz, K.; Obed, M.; Khalaj, K.; Antounians, L.; Zani, A. Amniotic Fluid Stem Cell Extracellular Vesicles Promote Lung Development via TGF-Beta Modulation in a Fetal Rat Model of Oligohydramnios. J. Control. Release 2025, 377, 427–441. [Google Scholar] [CrossRef]
- Ullrich, S.J.; Yung, N.K.; Bauer-Pisani, T.J.; Maassel, N.L.; Guerra, M.E.; Freedman-Weiss, M.; Ahle, S.L.; Ricciardi, A.S.; Sauler, M.; Saltzman, W.M.; et al. In Utero Delivery of MiRNA Induces Epigenetic Alterations and Corrects Pulmonary Pathology in Congenital Diaphragmatic Hernia. Mol. Ther. Nucleic Acids 2023, 32, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Tse, W.H.; Wang, J.Q.; Patel, D.; Ozturk, A.; Yamataka, A.; Keijzer, R. Microinjection With Nanoparticles to Deliver Drugs in Prenatal Lung Explants—A Pilot Study for Prenatal Therapy in Congenital Diaphragmatic Hernia. J. Pediatr. Surg. 2024, 59, 847–853. [Google Scholar] [CrossRef]
- Sananès, N.; Regnard, P.; Mottet, N.; Miry, C.; Fellmann, L.; Haelewyn, L.; Delaine, M.; Schneider, A.; Debry, C.; Favre, R. Evaluation of a New Balloon for Fetal Endoscopic Tracheal Occlusion in the Nonhuman Primate Model. Prenat. Diagn. 2019, 39, 403–408. [Google Scholar] [CrossRef]
- Sananès, N.; Basurto, D.; Cordier, A.-G.; Elie, C.; Russo, F.M.; Benachi, A.; Deprest, J. Fetoscopic Endoluminal Tracheal Occlusion with Smart-TO Balloon: Study Protocol to Evaluate Effectiveness and Safety of Non-Invasive Removal. PLoS ONE 2023, 18, e0273878. [Google Scholar] [CrossRef] [PubMed]
- Muensterer, O.J.; Nicola, T.; Farmer, S.; Harmon, C.M.; Ambalavanan, N. Temporary Fetal Tracheal Occlusion Using a Gel Plug in a Rabbit Model of Congenital Diaphragmatic Hernia. J. Pediatr. Surg. 2012, 47, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Elattal, R.; Rich, B.S.; Harmon, C.M.; Muensterer, O.J. Pulmonary Alveolar and Vascular Morphometry after Gel Plug Occlusion of the Trachea in a Fetal Rabbit Model of CDH. Int. J. Surg. 2013, 11, 558–561. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Campiglio, C.E.; Villonio, M.; Dellacà, R.L.; Mosca, F.; Draghi, L. An Injectable, Degradable Hydrogel Plug for Tracheal Occlusion in Congenital Diaphragmatic Hernia (CDH). Mater. Sci. Eng. C 2019, 99, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Jelin, E.B.; Etemadi, M.; Encinas, J.; Schecter, S.C.; Chapin, C.; Wu, J.; Guevara-Gallardo, S.; Nijagal, A.; Gonzales, K.D.; Ferrier, W.T.; et al. Dynamic Tracheal Occlusion Improves Lung Morphometrics and Function in the Fetal Lamb Model of Congenital Diaphragmatic Hernia. J. Pediatr. Surg. 2011, 46, 1150–1157. [Google Scholar] [CrossRef]
- Baba, J.S.; McKnight, T.E.; Ericson, M.N.; Johnson, A.; Moise, K.J.; Evans, B.M. Characterization of a Reversible Thermally-Actuated Polymer-Valve: A Potential Dynamic Treatment for Congenital Diaphragmatic Hernia. PLoS ONE 2018, 13, e0209855. [Google Scholar] [CrossRef]
- McGann, K.C.; Arca, M.J.; Pulhamus, M.; Livingston, M.H. Left Congenital Diaphragmatic Hernia and Gastroschisis in a Term Male Infant. BMJ Case Rep. 2021, 14, e239181. [Google Scholar] [CrossRef] [PubMed]
- Porreco, R.P.; Chang, J.H.T.; Quissell, B.J.; Morgan, M.A. Palliative Fetal Surgery for Diaphragmatic Hernia. Am. J. Obstet. Gynecol. 1994, 170, 833–834. [Google Scholar] [CrossRef] [PubMed]
- Moise, K.J.; Belfort, M.; Saade, G. Iatrogenic Gastroschisis in the Treatment of Diaphragmatic Hernia. Am. J. Obstet. Gynecol. 1995, 172, 715. [Google Scholar] [CrossRef]
- Chun, Y.-S.; Jung, S.-J. The Effect Analysis and Comparison between Gastroschisis and Tracheal Ligation on Experimental Diaphragmatic Hernia in Fetal Rabbits. J. Pediatr. Surg. 2007, 42, 2030–2034. [Google Scholar] [CrossRef]
- Kohl, T.; Riehle, N.; Messroghli, L.; Maus, S.; Otto, C.; Klinke, M.; Martel, R.; Beck, G.; Boettcher, M.; Schaible, T. Case Report: Fetoscopic Laparoschisis (FETO-LAP)—A New Therapeutic Route to Explore for Fetuses with Severe Diaphragmatic Hernias. Children 2023, 10, 1758. [Google Scholar] [CrossRef] [PubMed]
- Biard, J.-M.; Schwarz, U.; Davey, M.G.; Danzer, E.; Johnson, M.P.; Flake, A.W.; Adzick, S.N.; Hedrick, H.L. Main Bronchus Occlusion for Treatment of Congenital Diaphragmatic Hernia in Fetal Lambs. J. Pediatr. Surg. 2008, 43, 620–626. [Google Scholar] [CrossRef]

| Human Studies | Conclusions |
|---|---|
| Tchirikov et al., 2017 [100] | Small iatrogenic amniotic membrane defects successfully treated by laser coagulation technique. |
| Chmait et al., 2017 [101] | “Amniopatch”: higher GA at delivery and higher perinatal survival rates in almost two-thirds of cases. |
| Sung JH et al., 2017 [102] | In the iatrogenic PPROM group, the “Amniopatch” had a 36.4% success rate. Larger volumes of amniotic fluid before the procedure were key predictors of procedural success. |
| Experimental Studies | Conclusions |
| Kondoh et al., 2021 [103] | Intracervical elastomeric sealant (fibrin glue) demonstrated good fluid leakage prevention in an ex vivo model. |
| Devaud et al., 2021 [104] | Histoacryl® and Glubran2® tissue adhesives with umbrella-shaped receptors successfully sealed membrane defects in a sheep model. |
| Byju et al., 2022 [105] | The percutaneously delivered, resorbable “ChorioAnchor” device can secure the chorioamniotic membranes to the uterine wall—fulfills its engineering specifications during the initial phases of implantation. |
| Micheletti et al., 2022 [106] | In vivo and ex vivo sheep models: a fetoscopic applied semirigid silicone-hydroxypropyl methylcellulose patch sealed membrane defects. |
| Avilla-Royo et al., 2022 [107] | Mussel-inspired biomimetic glue had promising properties for the sealing of fetal membrane defects in an ovine model. |
| Devaud et al., 2023 [108] | Cyanoacrylate-based sealing patches led to a watertight seal at 10 or 24 days post-treatment in an ovine model. |
| Bergh et al., 2024 [109] | Suturing device tested ex vivo and in vivo: anchored amniotic membranes to the underlying myometrium. |
| Corticosteroids | Conclusions |
|---|---|
| Bratu et al., 2001 [68] | Reversible tracheal occlusion (TO) and prenatal betamethasone led to similar pulmonary architectures to the controls and offered no added benefit in terms of surfactant production. |
| Bratu et al., 2001 [123] | Reversible TO and antenatal glucocorticoids prevented the thinning of the small pulmonary arteries and enhanced the lung growth and structural maturity. |
| Davey et al., 2006 [124] | Glucocorticoids reduced the lung liquid volume during TO, which also increased the AE2 cell density and surfactant protein mRNA expression. |
| Davey et al., 2006 [125] | TO plus glucocorticoid or surfactant significantly improved the respiratory gas exchange, lung compliance, and ventilatory efficiency index. The total lung capacity was normalized only when the glucocorticoids and surfactant were administered together. |
| Davey et al., 2007 [126] | TO and prenatal glucocorticoid treatments reduced the medial pulmonary arteriole hypertrophy by 19% in a severe congenital diaphragmatic hernia (CDH) fetal sheep model. |
| Mayer et al., 2008 [127] | Prenatal betamethasone inhibited lung proliferation in TO-treated nitrofen-induced CDH rat fetuses. |
| Roubliova et al., 2009 [128] | TO and betamethasone had a cumulative effect on reducing peripheric muscularization. |
| Retinoids | Conclusions |
| Schmidt et al., 2016 [129] | TO and retinoic acid (RA) together had no additional benefit in reducing the median pulmonary arteriole wall thickness or in increasing the VEGF and its receptors. |
| Delabaere et al., 2017 [130] | Liposomes and Miglyol could be used as vehicles for delivering RA into fetal airways. Tracheal RA opposed the effects of TO and improved the surfactant production in rabbit fetuses with normal lungs. |
| Delabaere et al., 2018 [131] | TO and RA had synergic effects on vascular measurements, proportional medial thickness, and endothelin-1 receptor type-A gene expression, and restored pneumocyte differentiation. |
| Phosphodiesterase Inhibitors | Conclusions |
| Russo et al., 2022 [122] | TO and maternal sildenafil had complementary effects on the vascular and parenchymal lung development. They also counteracted the reduced gene expression of VEGF and surfactant proteins A and B induced by TO (tested in a rabbit model). |
| EVs | Conclusions |
|---|---|
| Pereira-Terra et al., 2015 [136] | Fetal CDH lungs presented elevated expressions of miR-10a and miR-200b; miR-200b was elevated at balloon removal and in FETO survivors; this miR inhibited the TGF-β-induced SMAD signaling. |
| Monroe MN et al., 2020 [138] | Mesenchymal-stem-cell-derived extracellular vesicles (MSC-EVs) reversed extracellular matrix (ECM) remodeling in the CDH pulmonary vasculature: bolstered structural aspects of the pulmonary artery ECM and mitigated pathological disorganization, as exhibited by an increased medial wall thickness and stiffness. |
| Zhaorigetu S et al., 2020 [139] | MSC-EV treatment improved the cellular responses, including key endothelial dysfunction proteins in a nitrofen-induced CDH model. In vivo, MSC-EV exposure enhanced the pulmonary artery contractile response and reduced the pulmonary vascular dysfunction. |
| Fabietti I et al., 2021 [140] | Higher extracellular vesicle (EV) counts in the amniotic fluid of non-survivors and tracheal fluid collected during TO reversal indicated established pro-inflammatory lung reactivity in utero, potentially linked to poorer postnatal outcomes. The regulation of EV-derived miR-223-3p, miR-379-5p, miR-503-5p, and miR-889-3p was related to postnatal survival. Their target genes were possibly associated with altered lung function. |
| Antounians L et al., 2021 [141] | Overexpression of miR17-92 cluster in amniotic-fluid-stem-cell-derived EVs (AFSC-EVs) was observed in EV-treated primary lung epithelial cells. |
| Khalaj K et al., 2022 [135] | AFSC-EVs improved the airspace density and branching morphogenesis, and enhanced the alveolar cell markers during canalicular and saccular stages; they also restored the cell markers of ciliated epithelial, club, and pulmonary neuroendocrine cells at the saccular stage to control levels, along with lipofibroblasts and PDGFRA+ markers.AFSC-EVs transferred the miR-17-92 cluster to rescue branching morphogenesis and partially restored autophagy. |
| Matsuo S et al., 2024 [142] | AFSC-EV-derived miRs could prenatally predict severe CDH cases with a high accuracy; changes in these miR profiles could reflect the status of the lungs. |
| Figueira R et al., 2024 [143] | The administration of AFSC-EVs led to improvements in lung mechanics (resistance, elastance, compliance, tissue damping), as well as collagen deposition and branching morphogenesis. |
| Doktor F et al., 2024 [144] | The administration of AFSC-EVs facilitated lung growth (reduced mean linear intercept), vascularization (increased Enos and Cd31), and decreased inflammation (TNF-α, IL-1b). |
| Antounians L et al., 2024 [145] | AFSC-EVs injected into rats with CDH enhanced lung branching and epithelial differentiation; this treatment also reversed the inflammatory response with macrophage enrichment exhibited by these lungs. |
| Doktor F et al., 2025 [146] | AFSC-EV administration facilitated lung branching and patterning of airway progenitor cells, partly via miR-93-5p release. It blocked SMAD 7, leading to pSMAD2/3 upregulation and TGF-β signaling restoration. Antagomir 93-5p-treated oligohydramnios lungs showed different results: decreased TGF-β signaling and branching morphogenesis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bara, Z.; Gozar, H.; Nagy, N.; Gurzu, S.; Derzsi, Z.; Forró, T.; Kovács, E.; Jung, I. Fetoscopic Endoluminal Tracheal Occlusion-Synergic Therapies in the Prenatal Treatment of Congenital Diaphragmatic Hernia. Int. J. Mol. Sci. 2025, 26, 1639. https://doi.org/10.3390/ijms26041639
Bara Z, Gozar H, Nagy N, Gurzu S, Derzsi Z, Forró T, Kovács E, Jung I. Fetoscopic Endoluminal Tracheal Occlusion-Synergic Therapies in the Prenatal Treatment of Congenital Diaphragmatic Hernia. International Journal of Molecular Sciences. 2025; 26(4):1639. https://doi.org/10.3390/ijms26041639
Chicago/Turabian StyleBara, Zsolt, Horea Gozar, Nándor Nagy, Simona Gurzu, Zoltán Derzsi, Timea Forró, Evelyn Kovács, and Ioan Jung. 2025. "Fetoscopic Endoluminal Tracheal Occlusion-Synergic Therapies in the Prenatal Treatment of Congenital Diaphragmatic Hernia" International Journal of Molecular Sciences 26, no. 4: 1639. https://doi.org/10.3390/ijms26041639
APA StyleBara, Z., Gozar, H., Nagy, N., Gurzu, S., Derzsi, Z., Forró, T., Kovács, E., & Jung, I. (2025). Fetoscopic Endoluminal Tracheal Occlusion-Synergic Therapies in the Prenatal Treatment of Congenital Diaphragmatic Hernia. International Journal of Molecular Sciences, 26(4), 1639. https://doi.org/10.3390/ijms26041639







