Immunogenic Cell Death Inducers in Cancer Immunotherapy to Turn Cold Tumors into Hot Tumors
Abstract
1. Introduction
1.1. ICD and Cancer Immunotherapy: Clinical Trials in Adult Patients
1.2. ICD and Cancer Immunotherapy in Pediatric Cancers: Preclinical Studies
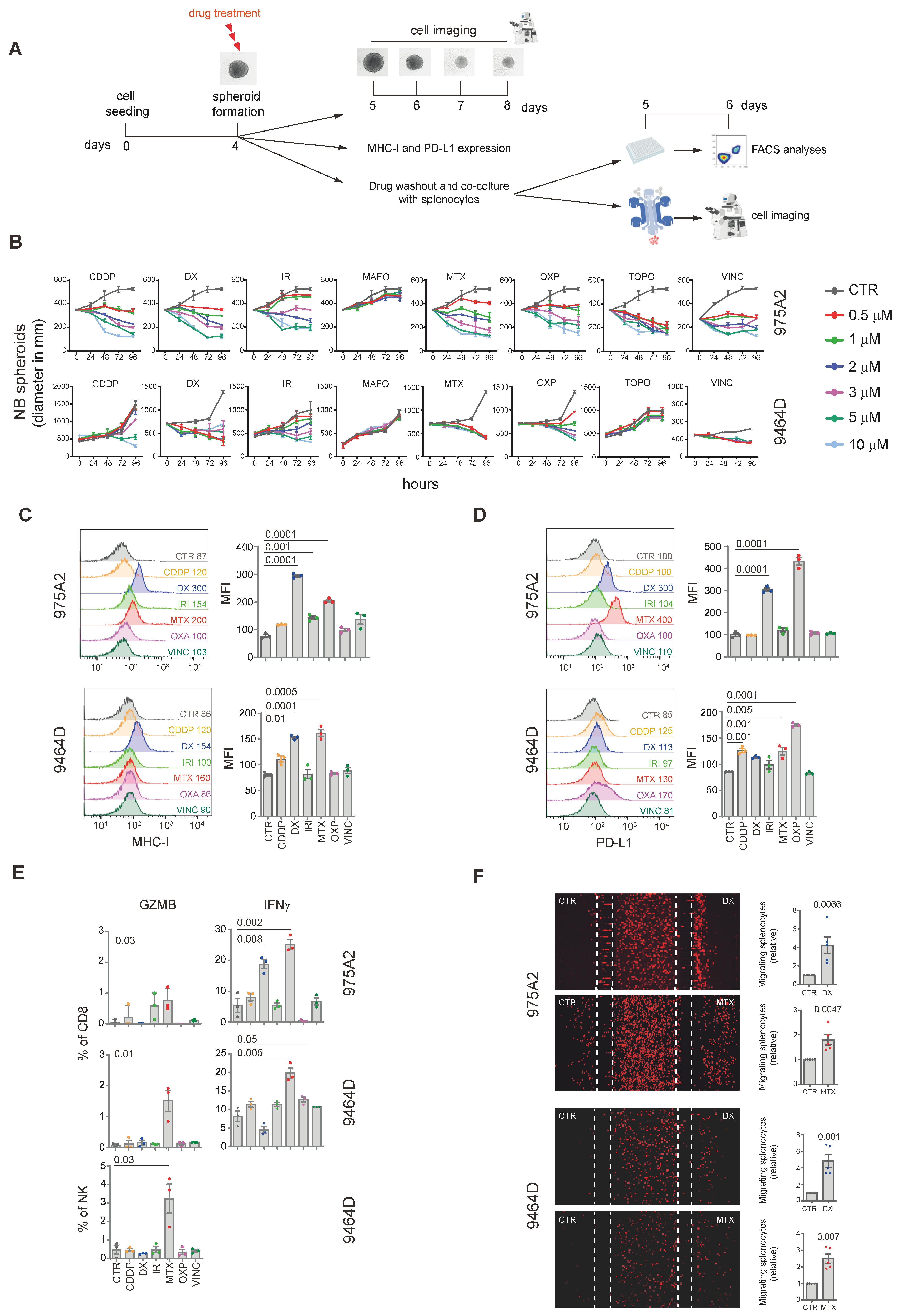
2. Results and Discussion
3D Tumor Spheroid Models: Advancing the Study of Immunomodulatory Agents in the Pediatric Tumor Microenvironment
3. Material and Methods
3.1. Mice, Cell Lines and Reagents
3.2. Tumor Spheroid Growth, Drug Treatment, and Co-Culture Experiments
3.3. Flow-Cytometry
3.4. Microfluidic Device Migration Assay
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naimi, A.; Mohammed, R.N.; Raji, A.; Chupradit, S.; Yumashev, A.V.; Suksatan, W.; Shalaby, M.N.; Thangavelu, L.; Kamrava, S.; Shomali, N.; et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun. Signal 2022, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Wienke, J.; Dierselhuis, M.P.; Tytgat, G.A.M.; Kunkele, A.; Nierkens, S.; Molenaar, J.J. The immune landscape of neuroblastoma: Challenges and opportunities for novel therapeutic strategies in pediatric oncology. Eur. J. Cancer 2021, 144, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, J.; Zhao, L.; Hollebecque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. PD-1 blockade synergizes with oxaliplatin-based, but not cisplatin-based, chemotherapy of gastric cancer. Oncoimmunology 2022, 11, 2093518. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Xiu, Z.; Guo, J.; Wang, L.; Zhou, Y.; Jiao, Y.; Sun, M.; Cai, J. Combination of Immune Checkpoint Inhibitors with Chemotherapy in Lung Cancer. Onco Targets Ther. 2020, 13, 7229–7241. [Google Scholar] [CrossRef]
- Fucikova, J.; Kepp, O.; Kasikova, L.; Petroni, G.; Yamazaki, T.; Liu, P.; Zhao, L.; Spisek, R.; Kroemer, G.; Galluzzi, L. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020, 11, 1013. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kepp, O.; Hett, E.; Kroemer, G.; Marincola, F.M. Immunogenic cell death in cancer: Concept and therapeutic implications. J. Transl. Med. 2023, 21, 162. [Google Scholar] [CrossRef]
- Ahmed, A.; Tait, S.W.G. Targeting immunogenic cell death in cancer. Mol. Oncol. 2020, 14, 2994–3006. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Arimoto, K.I.; Miyauchi, S.; Liu, M.; Zhang, D.E. Emerging role of immunogenic cell death in cancer immunotherapy. Front. Immunol. 2024, 15, 1390263. [Google Scholar] [CrossRef]
- Voorwerk, L.; Slagter, M.; Horlings, H.M.; Sikorska, K.; van de Vijver, K.K.; de Maaker, M.; Nederlof, I.; Kluin, R.J.C.; Warren, S.; Ong, S.; et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: The TONIC trial. Nat. Med. 2019, 25, 920–928, Erratum in Nat. Med. 2019, 25, 1175. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.T.; Matin, S.F.; Tam, A.L.; Sheth, R.A.; Ahrar, K.; Tidwell, R.S.; Rao, P.; Karam, J.A.; Wood, C.G.; Tannir, N.M.; et al. Pilot study of Tremelimumab with and without cryoablation in patients with metastatic renal cell carcinoma. Nat. Commun. 2021, 12, 6375. [Google Scholar] [CrossRef] [PubMed]
- Rossevold, A.H.; Andresen, N.K.; Bjerre, C.A.; Gilje, B.; Jakobsen, E.H.; Raj, S.X.; Falk, R.S.; Russnes, H.G.; Jahr, T.; Mathiesen, R.R.; et al. Atezolizumab plus anthracycline-based chemotherapy in metastatic triple-negative breast cancer: The randomized, double-blind phase 2b ALICE trial. Nat. Med. 2022, 28, 2573–2583. [Google Scholar] [CrossRef]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef]
- Vacchelli, E.; Ma, Y.; Baracco, E.E.; Sistigu, A.; Enot, D.P.; Pietrocola, F.; Yang, H.; Adjemian, S.; Chaba, K.; Semeraro, M.; et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science 2015, 350, 972–978. [Google Scholar] [CrossRef]
- Sistigu, A.; Yamazaki, T.; Vacchelli, E.; Chaba, K.; Enot, D.P.; Adam, J.; Vitale, I.; Goubar, A.; Baracco, E.E.; Remedios, C.; et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat. Med. 2014, 20, 1301–1309. [Google Scholar] [CrossRef]
- Kroemer, G.; Galassi, C.; Zitvogel, L.; Galluzzi, L. Immunogenic cell stress and death. Nat. Immunol. 2022, 23, 487–500. [Google Scholar] [CrossRef]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen presentation in cancer: Insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 2021, 21, 298–312. [Google Scholar] [CrossRef]
- Singleton, D.C.; Macann, A.; Wilson, W.R. Therapeutic targeting of the hypoxic tumour microenvironment. Nat. Rev. Clin. Oncol. 2021, 18, 751–772. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Adjemian, S.; Mattarollo, S.R.; Yamazaki, T.; Aymeric, L.; Yang, H.; Portela Catani, J.P.; Hannani, D.; Duret, H.; Steegh, K.; et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 2013, 38, 729–741. [Google Scholar] [CrossRef]
- Galluzzi, L.; Humeau, J.; Buque, A.; Zitvogel, L.; Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2020, 17, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Kepp, O. Small cell lung cancer responds to immunogenic chemotherapy followed by PD-1 blockade. Oncoimmunology 2021, 10, 1996686. [Google Scholar] [CrossRef] [PubMed]
- Correction: Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 2020, 8, e000337. [CrossRef]
- Kepp, O.; Zitvogel, L.; Kroemer, G. Lurbinectedin: An FDA-approved inducer of immunogenic cell death for the treatment of small-cell lung cancer. Oncoimmunology 2020, 9, 1795995. [Google Scholar] [CrossRef]
- Xie, W.; Forveille, S.; Iribarren, K.; Sauvat, A.; Senovilla, L.; Wang, Y.; Humeau, J.; Perez-Lanzon, M.; Zhou, H.; Martinez-Leal, J.F.; et al. Lurbinectedin synergizes with immune checkpoint blockade to generate anticancer immunity. Oncoimmunology 2019, 8, e1656502. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, L.; Pol, J.; Levesque, S.; Petrazzuolo, A.; Pfirschke, C.; Engblom, C.; Rickelt, S.; Yamazaki, T.; Iribarren, K.; et al. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat. Commun. 2019, 10, 1486. [Google Scholar] [CrossRef]
- Zitvogel, L.; Kroemer, G. Bortezomib Induces Immunogenic Cell Death in Multiple Myeloma. Blood Cancer Discov. 2021, 2, 405–407. [Google Scholar] [CrossRef]
- Yamazaki, T.; Buque, A.; Ames, T.D.; Galluzzi, L. PT-112 induces immunogenic cell death and synergizes with immune checkpoint blockers in mouse tumor models. Oncoimmunology 2020, 9, 1721810. [Google Scholar] [CrossRef]
- Vanmeerbeek, I.; Sprooten, J.; De Ruysscher, D.; Tejpar, S.; Vandenberghe, P.; Fucikova, J.; Spisek, R.; Zitvogel, L.; Kroemer, G.; Galluzzi, L.; et al. Trial watch: Chemotherapy-induced immunogenic cell death in immuno-oncology. Oncoimmunology 2020, 9, 1703449. [Google Scholar] [CrossRef] [PubMed]
- Floridi, A.; Bagnato, A.; Bianchi, C.; Fanciulli, M.; Silvestrini, B.; Caputo, A. Lonidamine-induced membrane permeability and the effect of adriamycin on the energy metabolism of Ehrlich ascites tumor cells. Ann. N. Y Acad. Sci. 1988, 551, 270–272. [Google Scholar] [CrossRef]
- Nishimura, J.; Deguchi, S.; Tanaka, H.; Yamakoshi, Y.; Yoshii, M.; Tamura, T.; Toyokawa, T.; Lee, S.; Muguruma, K.; Ohira, M. Induction of Immunogenic Cell Death of Esophageal Squamous Cell Carcinoma by 5-Fluorouracil and Cisplatin. Vivo 2021, 35, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Zsiros, E.; Lynam, S.; Attwood, K.M.; Wang, C.; Chilakapati, S.; Gomez, E.C.; Liu, S.; Akers, S.; Lele, S.; Frederick, P.J.; et al. Efficacy and Safety of Pembrolizumab in Combination With Bevacizumab and Oral Metronomic Cyclophosphamide in the Treatment of Recurrent Ovarian Cancer: A Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2021, 7, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Cocco, C.; Morandi, F.; Airoldi, I. Immune Checkpoints in Pediatric Solid Tumors: Targetable Pathways for Advanced Therapeutic Purposes. Cells 2021, 10, 927. [Google Scholar] [CrossRef]
- Davis, K.L.; Fox, E.; Merchant, M.S.; Reid, J.M.; Kudgus, R.A.; Liu, X.; Minard, C.G.; Voss, S.; Berg, S.L.; Weigel, B.J.; et al. Nivolumab in children and young adults with relapsed or refractory solid tumours or lymphoma (ADVL1412): A multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2020, 21, 541–550. [Google Scholar] [CrossRef]
- Geoerger, B.; Kang, H.J.; Yalon-Oren, M.; Marshall, L.V.; Vezina, C.; Pappo, A.; Laetsch, T.W.; Petrilli, A.S.; Ebinger, M.; Toporski, J.; et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): Interim analysis of an open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2020, 21, 121–133. [Google Scholar] [CrossRef]
- Melaiu, O.; Lucarini, V.; Giovannoni, R.; Fruci, D.; Gemignani, F. News on immune checkpoint inhibitors as immunotherapy strategies in adult and pediatric solid tumors. Semin. Cancer Biol. 2022, 79, 18–43. [Google Scholar] [CrossRef]
- Maris, J.M.; Hogarty, M.D.; Bagatell, R.; Cohn, S.L. Neuroblastoma. Lancet 2007, 369, 2106–2120. [Google Scholar] [CrossRef]
- Aiken, T.J.; Erbe, A.K.; Zebertavage, L.; Komjathy, D.; Feils, A.S.; Rodriguez, M.; Stuckwisch, A.; Gillies, S.D.; Morris, Z.S.; Birstler, J.; et al. Mechanism of effective combination radio-immunotherapy against 9464D-GD2, an immunologically cold murine neuroblastoma. J. Immunother. Cancer 2022, 10, e004834. [Google Scholar] [CrossRef] [PubMed]
- Mina, M.; Boldrini, R.; Citti, A.; Romania, P.; D’Alicandro, V.; De Ioris, M.; Castellano, A.; Furlanello, C.; Locatelli, F.; Fruci, D. Tumor-infiltrating T lymphocytes improve clinical outcome of therapy-resistant neuroblastoma. Oncoimmunology 2015, 4, e1019981. [Google Scholar] [CrossRef] [PubMed]
- Louault, K.; De Clerck, Y.A.; Janoueix-Lerosey, I. The neuroblastoma tumor microenvironment: From an in-depth characterization towards novel therapies. EJC Paediatr. Oncol. 2024, 3, 100161. [Google Scholar] [CrossRef]
- Lorenzi, S.; Forloni, M.; Cifaldi, L.; Antonucci, C.; Citti, A.; Boldrini, R.; Pezzullo, M.; Castellano, A.; Russo, V.; van der Bruggen, P.; et al. IRF1 and NF-kB restore MHC class I-restricted tumor antigen processing and presentation to cytotoxic T cells in aggressive neuroblastoma. PLoS ONE 2012, 7, e46928. [Google Scholar] [CrossRef]
- Melaiu, O.; Mina, M.; Chierici, M.; Boldrini, R.; Jurman, G.; Romania, P.; D’Alicandro, V.; Benedetti, M.C.; Castellano, A.; Liu, T.; et al. PD-L1 Is a Therapeutic Target of the Bromodomain Inhibitor JQ1 and, Combined with HLA Class I, a Promising Prognostic Biomarker in Neuroblastoma. Clin. Cancer Res. 2017, 23, 4462–4472. [Google Scholar] [CrossRef]
- Tempora, P.; D’Amico, S.; Gragera, P.; Damiani, V.; Krol, K.; Scaldaferri, V.; Pandey, K.; Chung, S.; Lucarini, V.; Giorda, E.; et al. Combining ERAP1 silencing and entinostat therapy to overcome resistance to cancer immunotherapy in neuroblastoma. J. Exp. Clin. Cancer Res. 2024, 43, 292. [Google Scholar] [CrossRef]
- Lucarini, V.; Melaiu, O.; D’Amico, S.; Pastorino, F.; Tempora, P.; Scarsella, M.; Pezzullo, M.; De Ninno, A.; D’Oria, V.; Cilli, M.; et al. Combined mitoxantrone and anti-TGFbeta treatment with PD-1 blockade enhances antitumor immunity by remodelling the tumor immune landscape in neuroblastoma. J. Exp. Clin. Cancer Res. 2022, 41, 326. [Google Scholar] [CrossRef]
- Webb, E.R.; Moreno-Vincente, J.; Easton, A.; Lanati, S.; Taylor, M.; James, S.; Williams, E.L.; English, V.; Penfold, C.; Beers, S.A.; et al. Cyclophosphamide depletes tumor infiltrating T regulatory cells and combined with anti-PD-1 therapy improves survival in murine neuroblastoma. iScience 2022, 25, 104995. [Google Scholar] [CrossRef]
- Melaiu, O.; Chierici, M.; Lucarini, V.; Jurman, G.; Conti, L.A.; De Vito, R.; Boldrini, R.; Cifaldi, L.; Castellano, A.; Furlanello, C.; et al. Cellular and gene signatures of tumor-infiltrating dendritic cells and natural-killer cells predict prognosis of neuroblastoma. Nat. Commun. 2020, 11, 5992. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Aref, A.R.; Lizotte, P.H.; Ivanova, E.; Stinson, S.; Zhou, C.W.; Bowden, M.; Deng, J.; Liu, H.; Miao, D.; et al. Ex Vivo Profiling of PD-1 Blockade Using Organotypic Tumor Spheroids. Cancer Discov. 2018, 8, 196–215. [Google Scholar] [CrossRef]
- Goliachenko, A.M.; P’Ianov Iu, V.; Pokanevich, V.V.; Iatsiuk, A.S. Evaluation of the scope of physicians’ duties in a hospital. Sov. Zdr. 1987, 10, 20–23. [Google Scholar]
- Kaptein, P.; Slingerland, N.; Metoikidou, C.; Prinz, F.; Brokamp, S.; Machuca-Ostos, M.; de Roo, G.; Schumacher, T.N.M.; Yeung, Y.A.; Moynihan, K.D.; et al. CD8-Targeted IL2 Unleashes Tumor-Specific Immunity in Human Cancer Tissue by Reviving the Dysfunctional T-cell Pool. Cancer Discov. 2024, 14, 1226–1251. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucarini, V.; Melaiu, O.; Gragera, P.; Król, K.; Scaldaferri, V.; Damiani, V.; De Ninno, A.; Nardozi, D.; Businaro, L.; Masuelli, L.; et al. Immunogenic Cell Death Inducers in Cancer Immunotherapy to Turn Cold Tumors into Hot Tumors. Int. J. Mol. Sci. 2025, 26, 1613. https://doi.org/10.3390/ijms26041613
Lucarini V, Melaiu O, Gragera P, Król K, Scaldaferri V, Damiani V, De Ninno A, Nardozi D, Businaro L, Masuelli L, et al. Immunogenic Cell Death Inducers in Cancer Immunotherapy to Turn Cold Tumors into Hot Tumors. International Journal of Molecular Sciences. 2025; 26(4):1613. https://doi.org/10.3390/ijms26041613
Chicago/Turabian StyleLucarini, Valeria, Ombretta Melaiu, Paula Gragera, Kamila Król, Valentina Scaldaferri, Verena Damiani, Adele De Ninno, Daniela Nardozi, Luca Businaro, Laura Masuelli, and et al. 2025. "Immunogenic Cell Death Inducers in Cancer Immunotherapy to Turn Cold Tumors into Hot Tumors" International Journal of Molecular Sciences 26, no. 4: 1613. https://doi.org/10.3390/ijms26041613
APA StyleLucarini, V., Melaiu, O., Gragera, P., Król, K., Scaldaferri, V., Damiani, V., De Ninno, A., Nardozi, D., Businaro, L., Masuelli, L., Bei, R., Cifaldi, L., & Fruci, D. (2025). Immunogenic Cell Death Inducers in Cancer Immunotherapy to Turn Cold Tumors into Hot Tumors. International Journal of Molecular Sciences, 26(4), 1613. https://doi.org/10.3390/ijms26041613







