Circulating Epithelial Tumor Cells (CETC/CTC) in Prostate Cancer: Potential Prognostic Marker for the Risk of Recurrence During Radiotherapy
Abstract
1. Introduction
2. Results
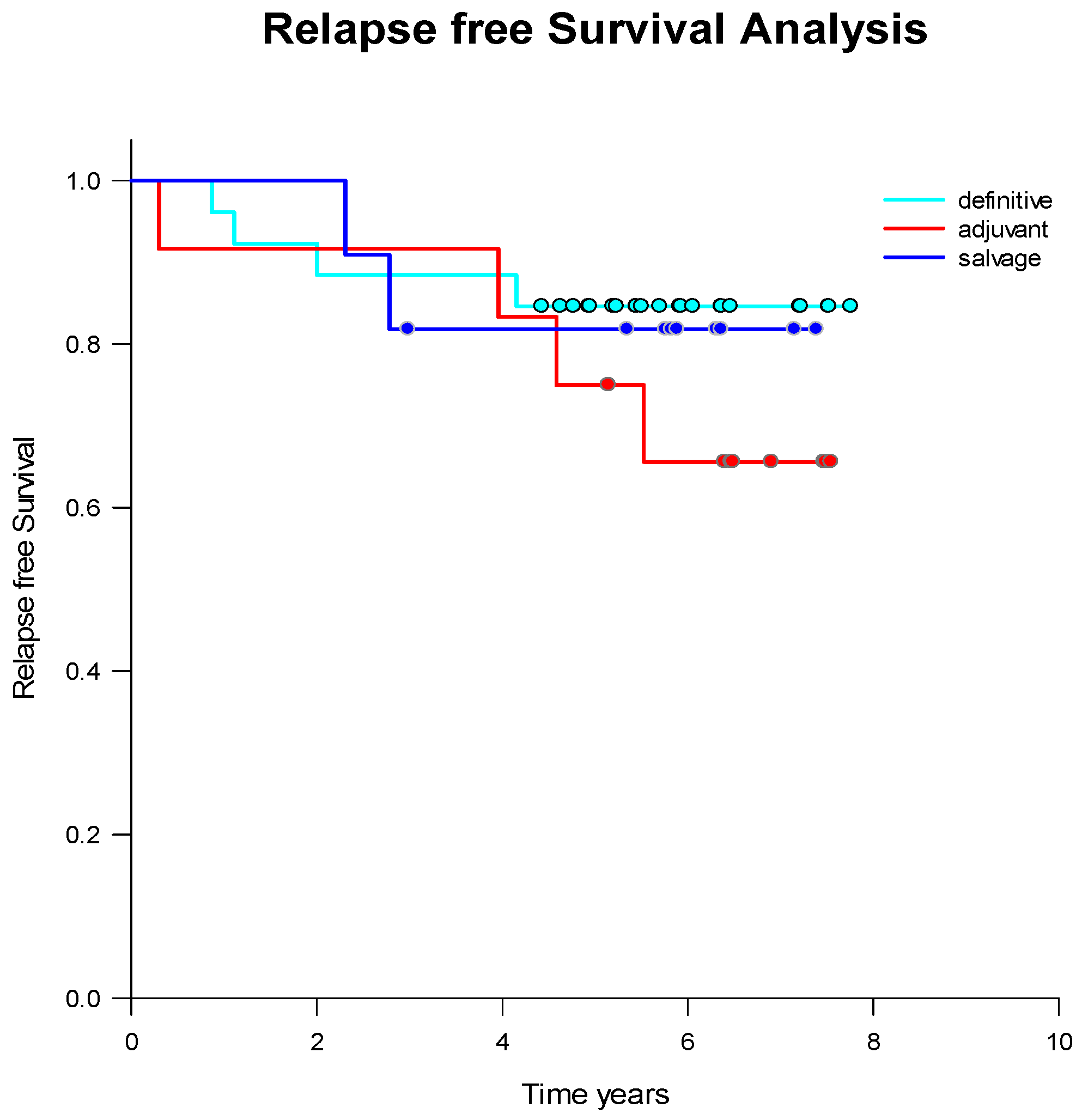
2.1. Treatment Strategies and Outcome
2.2. CETC/CTC Numbers
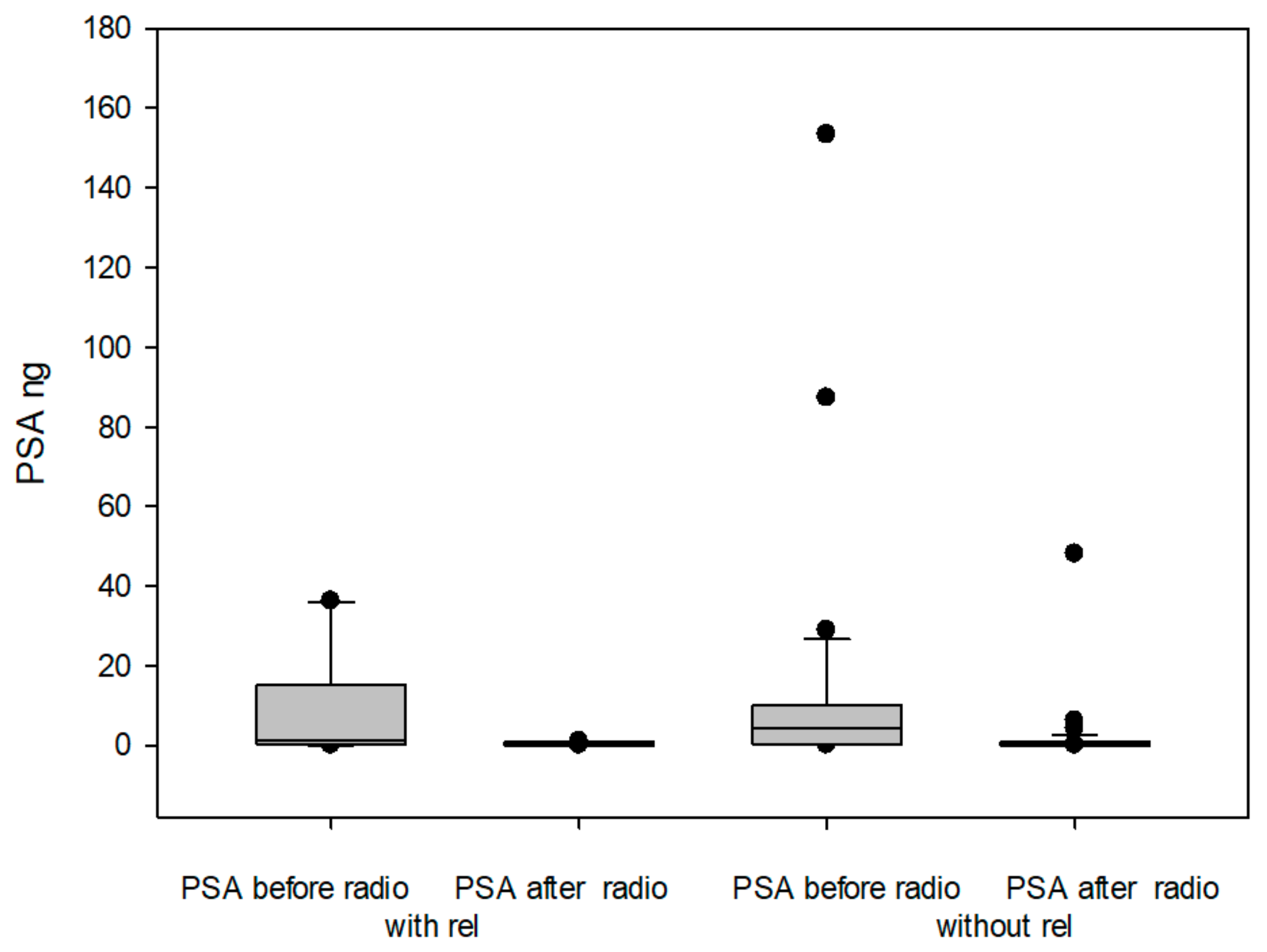
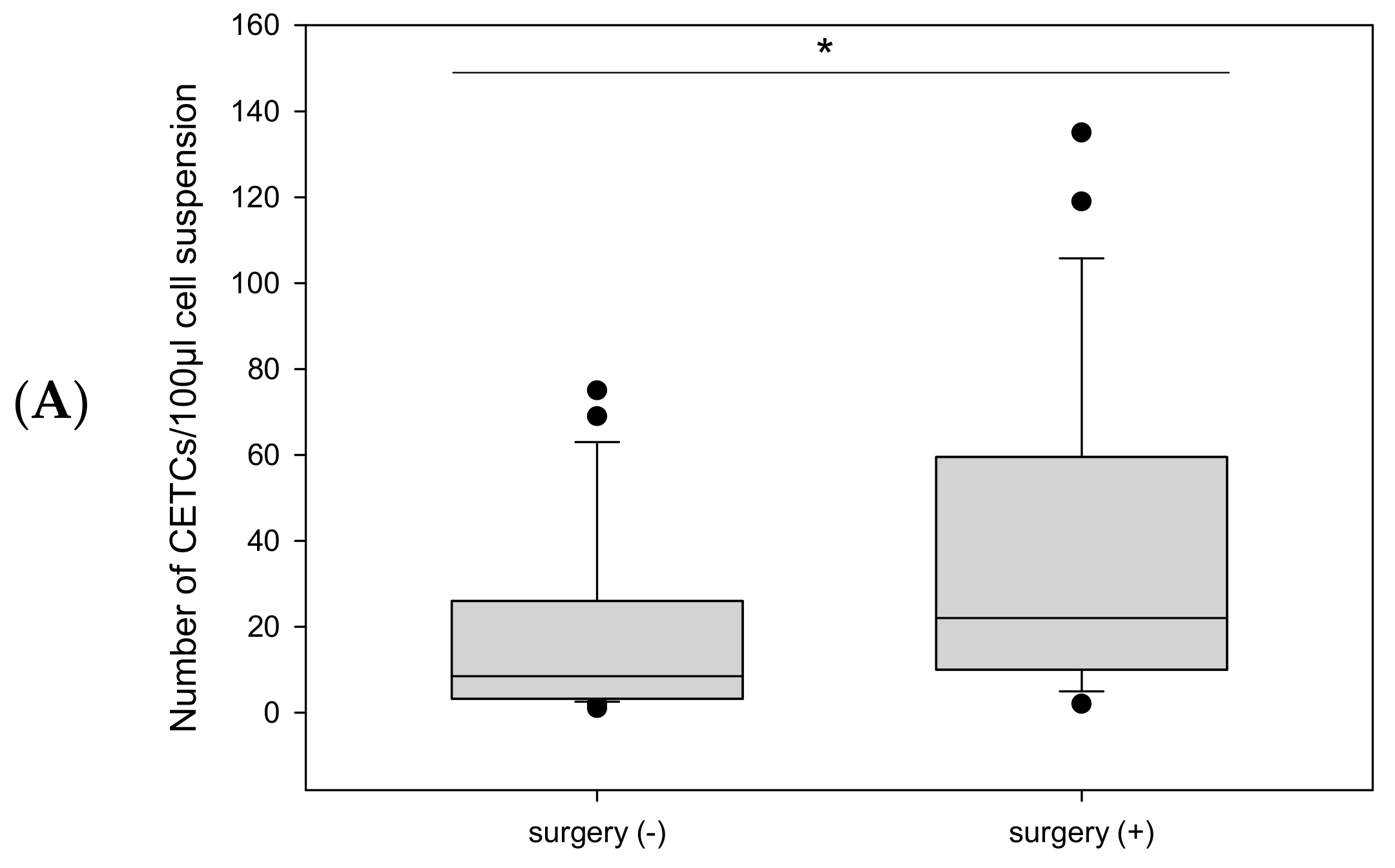
2.2.1. Before Radiotherapy, with and Without Surgery, and in Relation to Endocrine Therapy
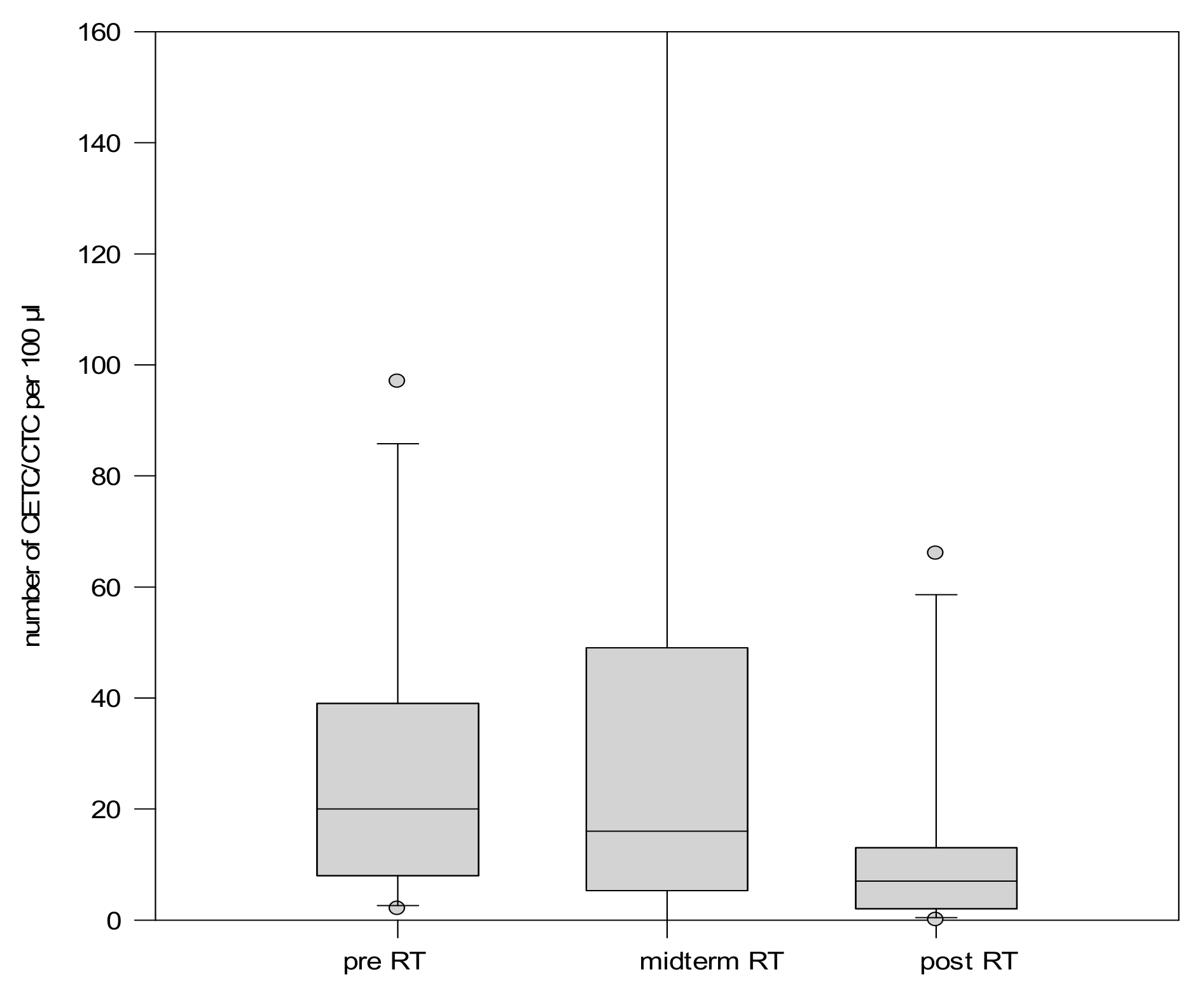
2.2.2. CETC/CTC Number During Radiotherapy
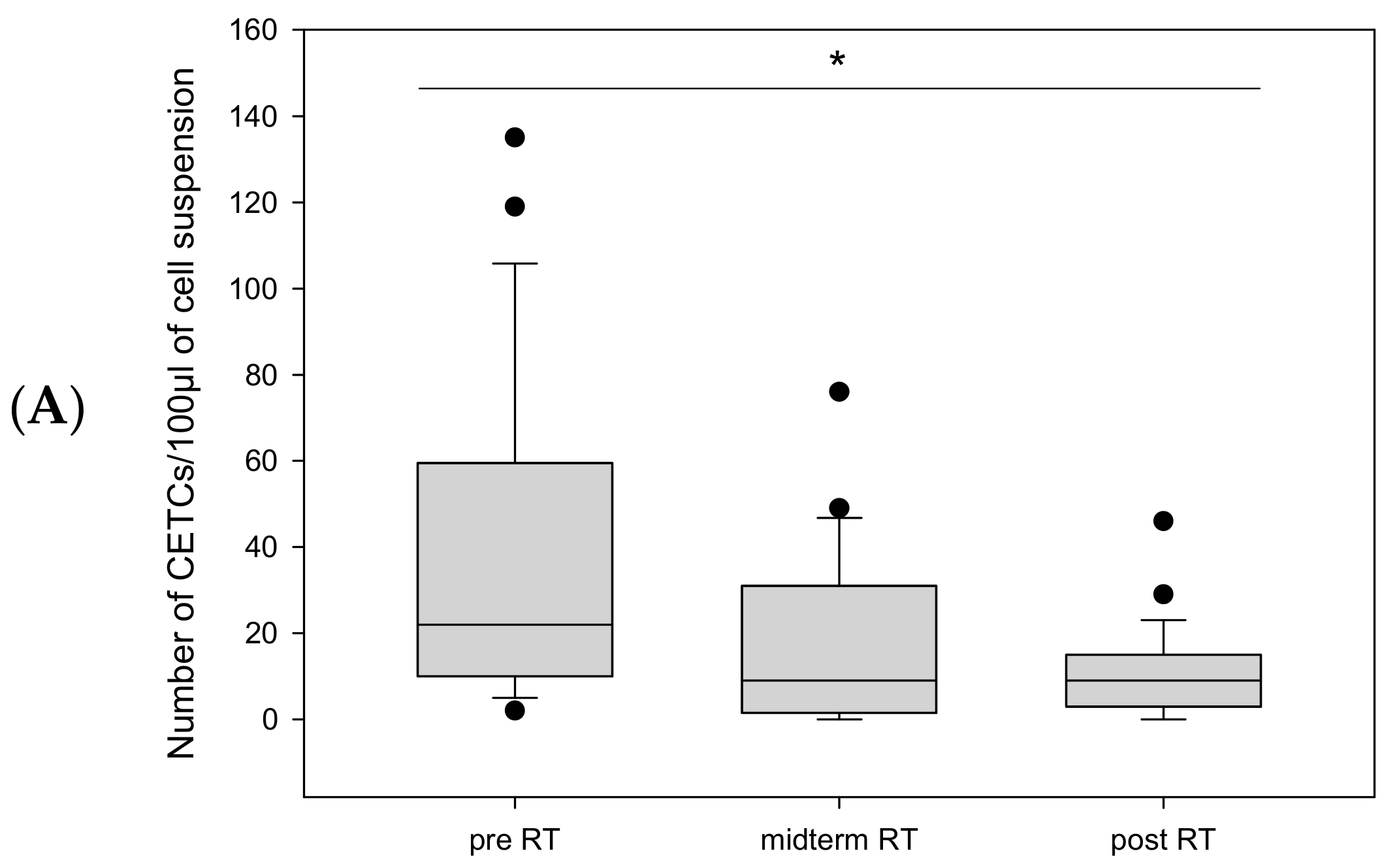
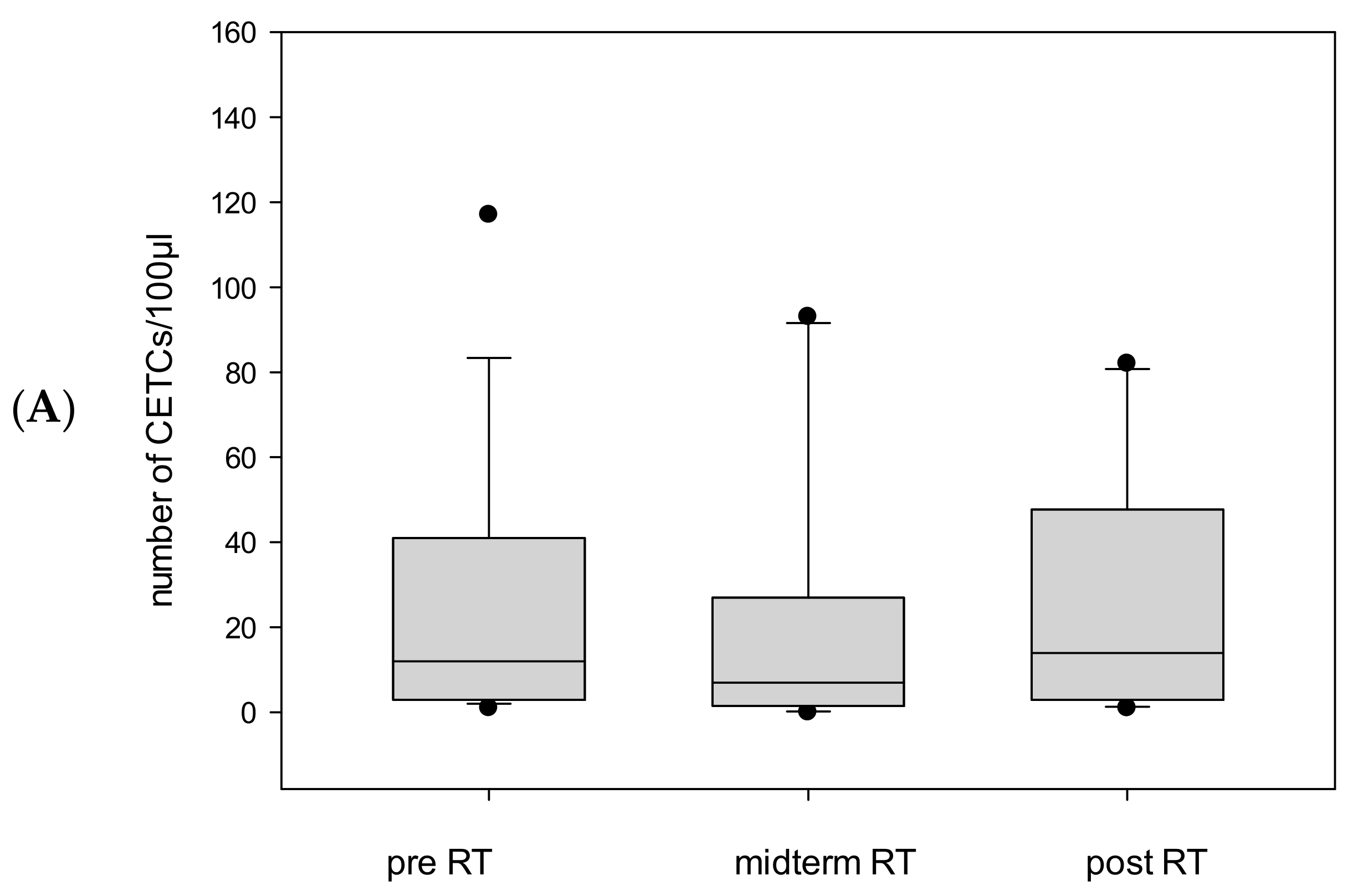
CETC/CTC Numbers in Patients Undergoing Adjuvant Radiotherapy
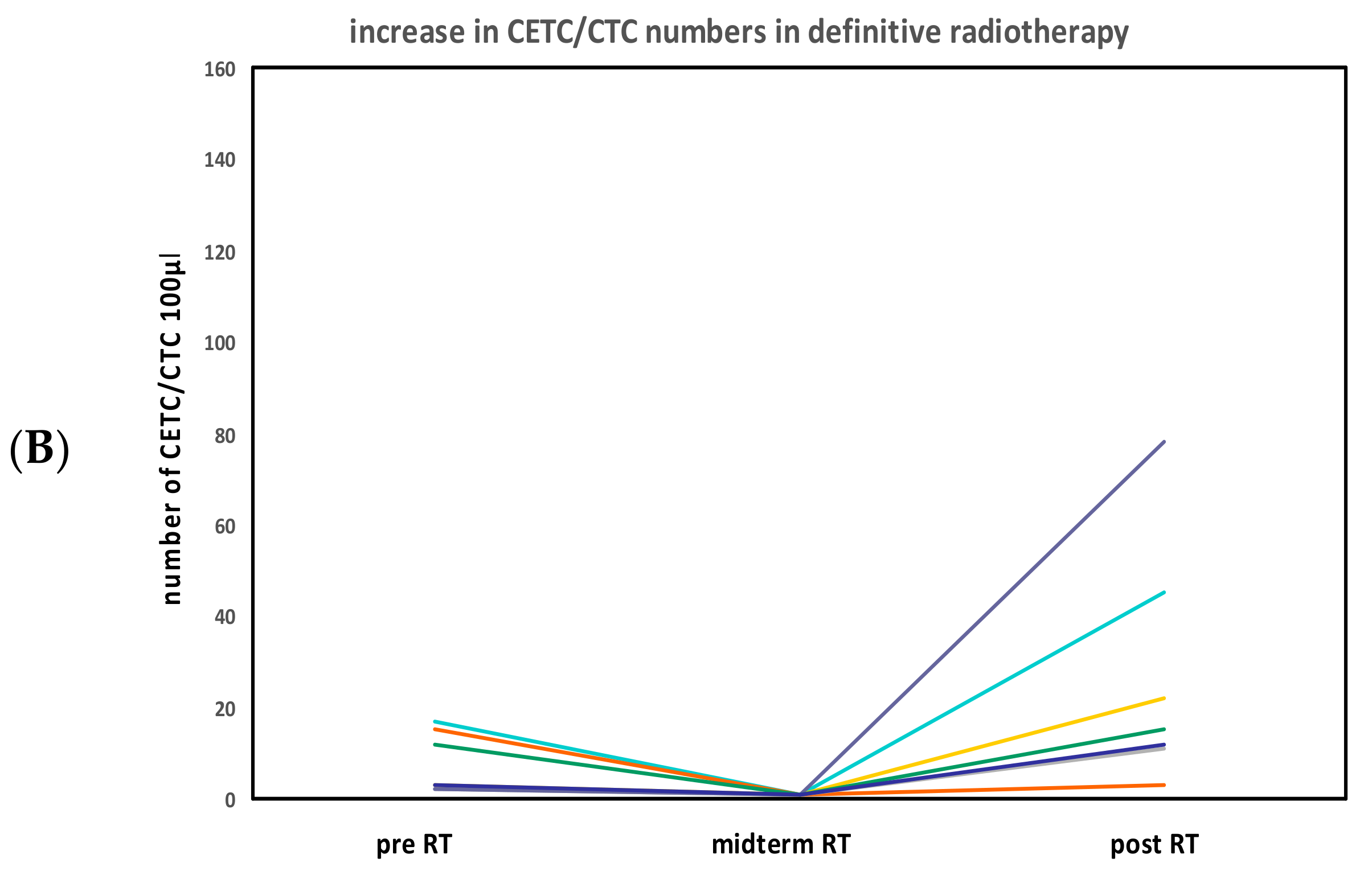
CETC/CTC Numbers in Patients Undergoing Definitive Radiotherapy
CETC/CTC Numbers in Patients Undergoing Salvage Radiotherapy
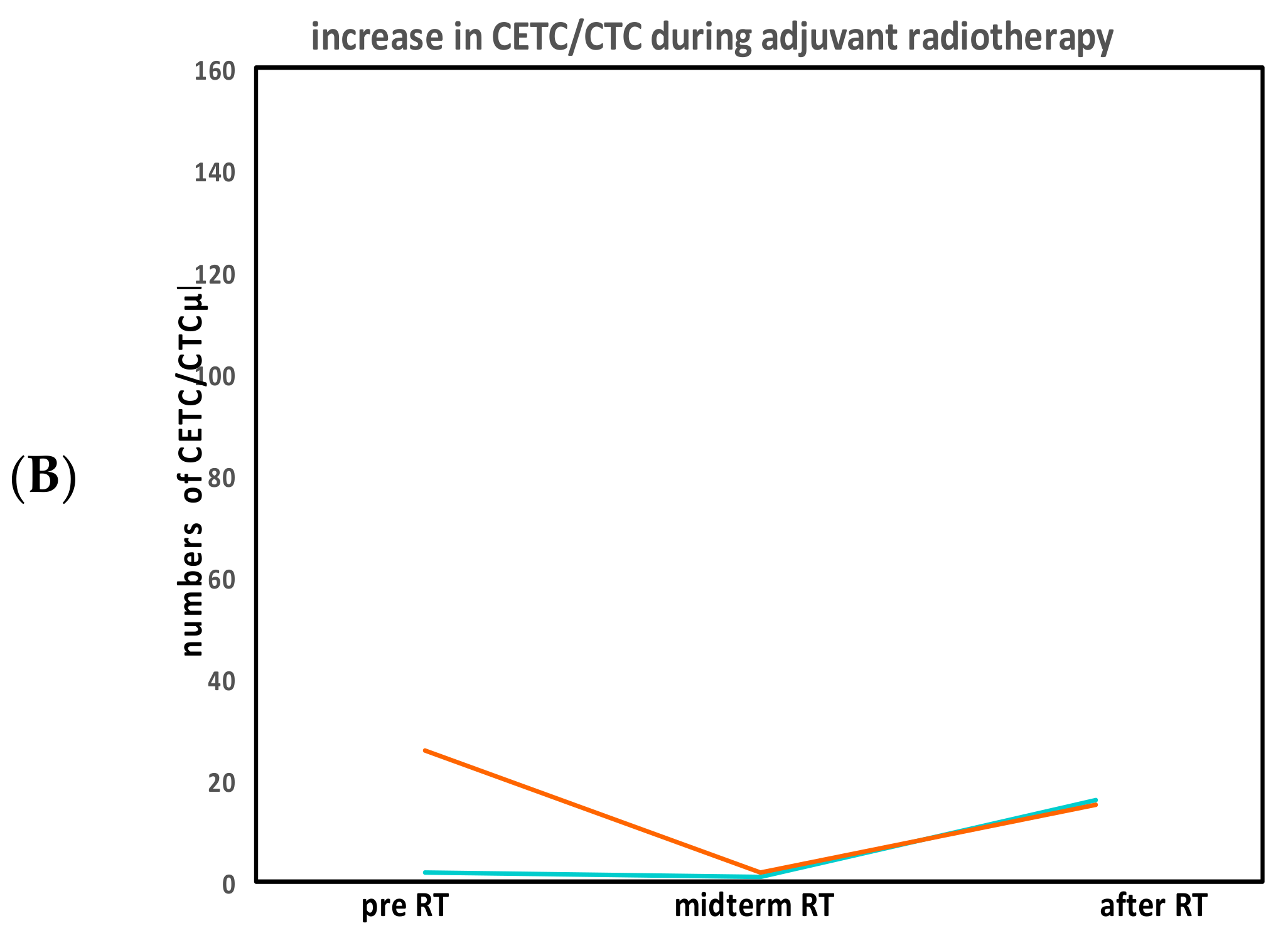
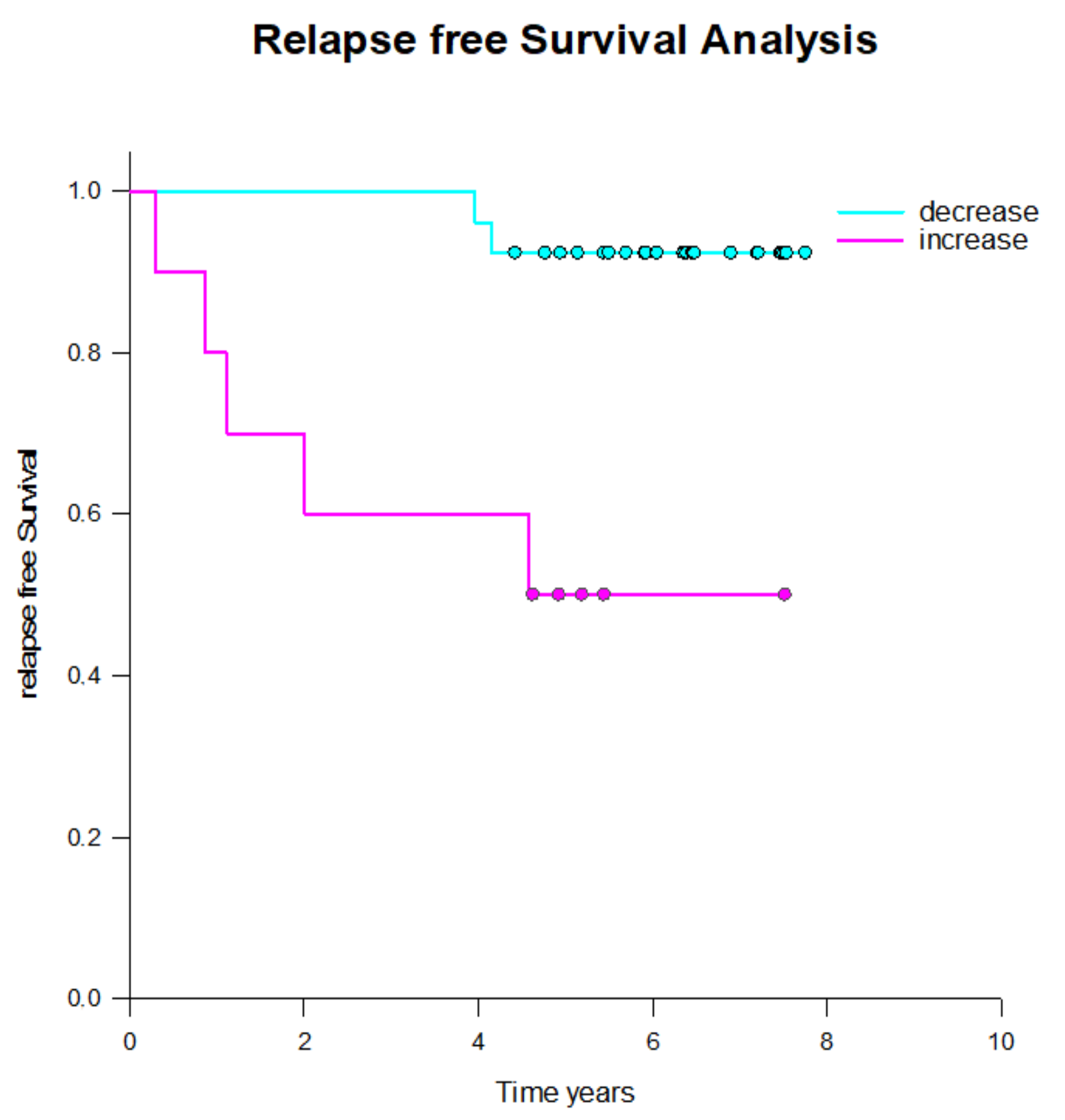
2.3. CETC/CTC Number Trajectory in Patients During Radiotherapy and Correlation with Relapse
3. Discussion
4. Materials and Methods
4.1. Inclusion Criteria
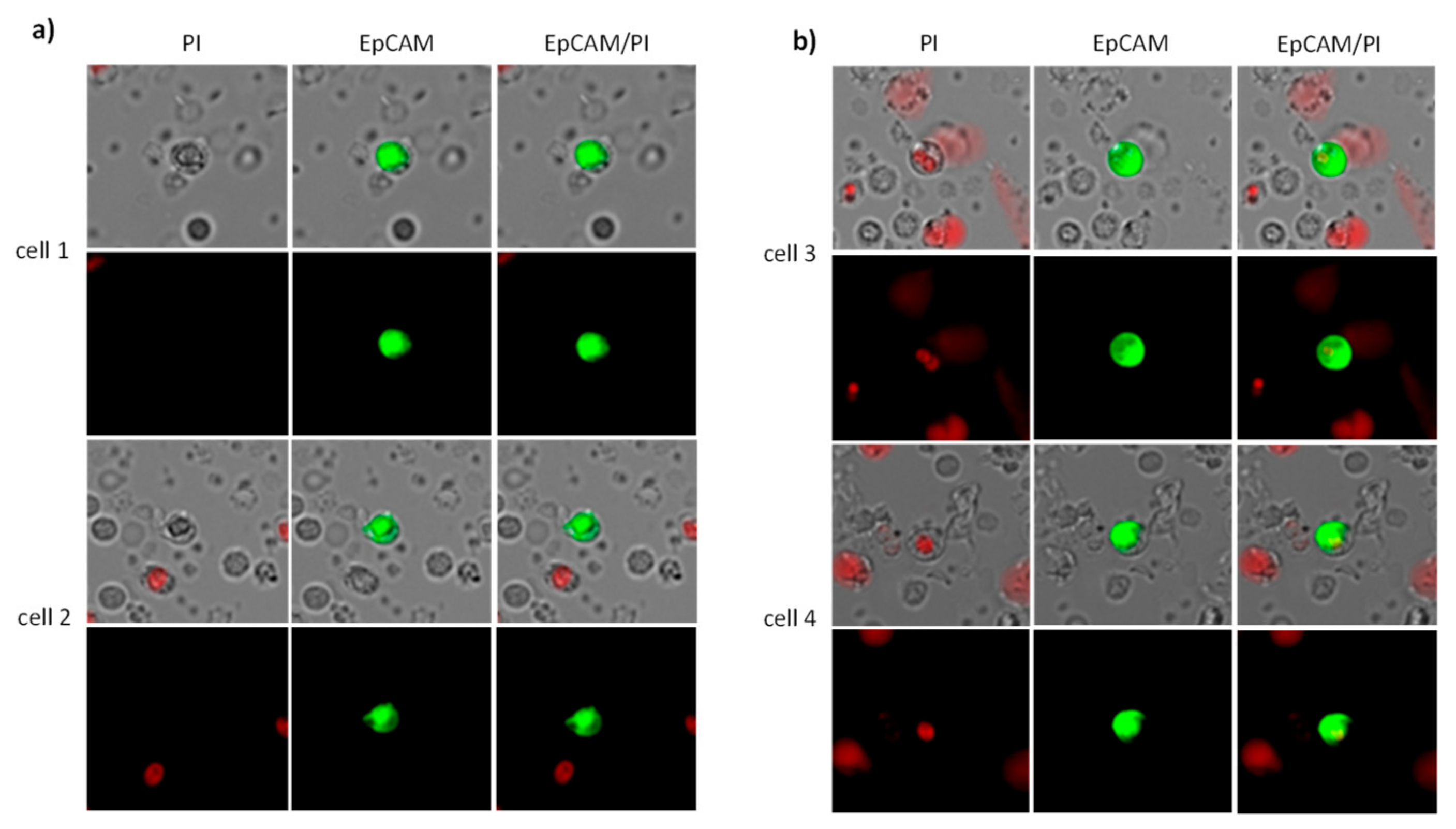
4.2. CETC/CTC Analysis
4.3. Secondary Antibody Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CETC/CTC | Circulating epithelial tumor cells |
| DFS | Disease-free survival |
| EpCAM | Epithelial cell adhesion molecule |
| FITC | Fluoroisothiocyanate |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| RT | Radiotherapy |
References
- Leslie, S.W.; Soon-Sutton, T.L.; Skelton, W.P. Prostate Cancer. In StatPearls; StatPearls Publishing Copyright © 2022; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Metcalfe, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Zelefsky, M.J.; Pei, X.; Chou, J.F.; Schechter, M.; Kollmeier, M.; Cox, B.; Yamada, Y.; Fidaleo, A.; Sperling, D.; Happersett, L.; et al. Dose escalation for prostate cancer radiotherapy: Predictors of long-term biochemical tumor control and distant metastases-free survival outcomes. Eur. Urol. 2011, 60, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Bolla, M.; van Poppel, H.; Collette, L.; van Cangh, P.; Vekemans, K.; Da Pozzo, L.; de Reijke, T.M.; Verbaeys, A.; Bosset, J.-F.; van Velthoven, R.; et al. Postoperative radiotherapy after radical prostatectomy: A randomised controlled trial (EORTC trial 22911). Lancet 2005, 366, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, P.J.; Rojas, A.M.; Bownes, P.J.; Lowe, G.J.; Ostler, P.J.; Bryant, L. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother. Oncol. 2012, 103, 217–222. [Google Scholar] [CrossRef]
- Spratt, D.E.; Zumsteg, Z.S.; Ghadjar, P.; Kollmeier, M.A.; Pei, X.; Cohen, G.; Polkinghorn, W.; Yamada, Y.; Zelefsky, M.J. Comparison of high-dose (86.4 Gy) IMRT vs combined brachytherapy plus IMRT for intermediate-risk prostate cancer. BJU Int. 2014, 114, 360–367. [Google Scholar] [CrossRef]
- Zelefsky, M.J.; Poon, B.Y.; Eastham, J.; Vickers, A.; Pei, X.; Scardino, P.T. Longitudinal assessment of quality of life after surgery, conformal brachytherapy, and intensity-modulated radiation therapy for prostate cancer. Radiother. Oncol. 2016, 118, 85–91. [Google Scholar] [CrossRef]
- Dearnaley, D.P.; Jovic, G.; Syndikus, I.; Khoo, V.; Cowan, R.A.; Graham, J.D.; Aird, E.G.; Bottomley, D.; Huddart, R.A.; Jose, C.C.; et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: Long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014, 15, 464–473. [Google Scholar] [CrossRef]
- Drozdz, S.; Schwedas, M.; Salz, H.; Foller, S.; Wendt, T.G. Prostate cancer treated with image-guided helical TomoTherapy® and image-guided LINAC-IMRT. Strahlenther. Onkol. 2016, 192, 223–231. [Google Scholar] [CrossRef]
- Weiner, A.B.; Kakani, P.; Armstrong, A.J.; Bossi, A.; Cornford, P.; Feng, F.; Kanabur, P.; Karnes, R.J.; McKay, R.R.; Morgan, T.M.; et al. Risk Stratification of Patients with Recurrence After Primary Treatment for Prostate Cancer: A Systematic Review. Eur. Urol. 2024, 86, 200–210. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Liquid biopsy and minimal residual disease—Latest advances and implications for cure. Nat. Rev. Clin. Oncol. 2019, 16, 409–424. [Google Scholar] [CrossRef]
- Trujillo, B.; Wu, A.; Wetterskog, D.; Attard, G. Blood-based liquid biopsies for prostate cancer: Clinical opportunities and challenges. Br. J. Cancer 2022, 127, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Ried, K.; Tamanna, T.; Matthews, S.; Eng, P.; Sali, A. New Screening Test Improves Detection of Prostate Cancer Using Circulating Tumor Cells and Prostate-Specific Markers. Front. Oncol. 2020, 10, 582. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Pyo, H.; Yoo, G.S.; Lee, H.M.; Jeon, S.S.; Seo, S.I.; Jeong, B.C.; Jeon, H.G.; Sung, H.H.; Kang, M.; et al. Prostate-specific antigen kinetics in hypofractionated radiation therapy alone for intermediate- and high-risk localized prostate cancer. Prostate Int. 2023, 11, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, A.; Khan, N.; Phillips, C.; Briones, J.; Kapoor, A.; Zalewski, P.; Fleshner, N.E.; Chow, E.; Emmenegger, U. Prevalence and Prognostic Implications of PSA Flares during Radium-223 Treatment among Men with Metastatic Castration Resistant Prostate Cancer. J. Clin. Med. 2023, 12, 5604. [Google Scholar] [CrossRef] [PubMed]
- Abeshouse, A.; Ahn, J.; Akbani, R.; Ally, A.; Amin, S.; Andry, C.D.; Annala, M.; Aprikian, A.; Armenia, J.; Arora, A.; et al. The Molecular Taxonomy of Primary Prostate Cancer. Cell 2015, 163, 1011–1025. [Google Scholar] [CrossRef]
- Lorente, D.; Omlin, A.; Zafeiriou, Z.; Nava-Rodrigues, D.; Pérez-López, R.; Pezaro, C.; Mehra, N.; Sheridan, E.; Figueiredo, I.; Riisnaes, R.; et al. Castration-Resistant Prostate Cancer Tissue Acquisition From Bone Metastases for Molecular Analyses. Clin. Genitourin. Cancer 2016, 14, 485–493. [Google Scholar] [CrossRef]
- Farha, M.W.; Salami, S.S. Biomarkers for prostate cancer detection and risk stratification. Ther. Adv. Urol. 2022, 14, 17562872221103988. [Google Scholar] [CrossRef]
- Pizon, M.; Zimon, D.; Carl, S.; Pachmann, U.; Pachmann, K.; Camara, O. Heterogeneity of circulating epithelial tumour cells from individual patients with respect to expression profiles and clonal growth (sphere formation) in breast cancer. Ecancermedicalscience 2013, 7, 343. [Google Scholar]
- Pachmann, K.; Camara, O.; Kavallaris, A.; Krauspe, S.; Malarski, N.; Gajda, M.; Kroll, T.; Jorke, C.; Hammer, U.; Altendorf-Hofmann, A.; et al. Monitoring the response of circulating epithelial tumor cells to adjuvant chemotherapy in breast cancer allows detection of patients at risk of early relapse. J. Clin. Oncol. 2008, 26, 1208–1215. [Google Scholar] [CrossRef]
- Mäurer, M.; Schott, D.; Pizon, M.; Drozdz, S.; Wendt, T.; Wittig, A.; Pachmann, K. Increased Circulating Epithelial Tumor Cells (CETC/CTC) over the Course of Adjuvant Radiotherapy Is a Predictor of Less Favorable Outcome in Patients with Early-Stage Breast Cancer. Curr. Oncol. 2023, 30, 261–273. [Google Scholar] [CrossRef]
- Mäurer, M.; Pachmann, K.; Wendt, T.; Schott, D.; Wittig, A. Prospective Monitoring of Circulating Epithelial Tumor Cells (CETC) Reveals Changes in Gene Expression during Adjuvant Radiotherapy of Breast Cancer Patients. Curr. Oncol. 2021, 28, 3507–3524. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xia, B.-R.; Jin, W.-L.; Lou, G. Circulating tumor cells in precision oncology: Clinical applications in liquid biopsy and 3D organoid model. Cancer Cell Int. 2019, 19, 341. [Google Scholar] [CrossRef] [PubMed]
- Capuozzo, M.; Ferrara, F.; Santorsola, M.; Zovi, A.; Ottaiano, A. Circulating Tumor Cells as Predictive and Prognostic Biomarkers in Solid Tumors. Cells 2023, 12, 2590. [Google Scholar] [CrossRef] [PubMed]
- Goldkorn, A.; Tangen, C.; Plets, M.; Bsteh, D.; Xu, T.; Pinski, J.K.; Ingles, S.; Triche, T.J.; MacVicar, G.R.; Vaena, D.A.; et al. Circulating Tumor Cell Count and Overall Survival in Patients with Metastatic Hormone-Sensitive Prostate Cancer. JAMA Netw. Open 2024, 7, e2437871. [Google Scholar] [CrossRef]
- Schott, D.S.; Pizon, M.; Pachmann, U.; Pachmann, K.; Schobert, R.; Wittig, A.; Mäurer, M. Influence of adjuvant radiotherapy on circulating epithelial tumor cells and circulating cancer stem cells in primary non-metastatic breast cancer. Transl. Oncol. 2021, 14, 101009. [Google Scholar] [CrossRef]
- Shaheen, H.; Salans, M.A.; Mohamad, O.; Coleman, P.W.; Ahmed, S.; Roach, M., 3rd. Age 70 +/− 5 Years and Cancer-Specific Outcomes After Treatment of Localized Prostate Cancer: A Systematic Review. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 672–681. [Google Scholar] [CrossRef]
- Terlizzi, M.; Bossi, A. ProtecT trial: What’s new after 15 years of follow-up: Re: Hamdy FC and colleagues. N. Engl. J. Med. 2023 Apr 27. Urologia 2024, 91, 3–4. [Google Scholar] [CrossRef]
- Urabe, F.; Kimura, S.; Tashiro, K.; Kido, M.; Sasaki, H.; Aoki, M.; Kimura, T.; Miki, K.; Egawa, S. Prognostic value of PSA bounce in prostate cancer following definitive radiation therapy: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2021, 24, 976–985. [Google Scholar] [CrossRef]
- Danila, D.C.; Fleisher, M.; Scher, H.I. Circulating tumor cells as biomarkers in prostate cancer. Clin. Cancer Res. 2011, 17, 3903–3912. [Google Scholar] [CrossRef]
- Matuszczak, M.; Schalken, J.A.; Salagierski, M. Prostate Cancer Liquid Biopsy Biomarkers’ Clinical Utility in Diagnosis and Prognosis. Cancers 2021, 13, 3373. [Google Scholar] [CrossRef]
- Pachmann, K.; Clement, J.H.; Schneider, C.P.; Willen, B.; Camara, O.; Pachmann, U.; Hoffken, K. Standardized quantification of circulating peripheral tumor cells from lung and breast cancer. Clin. Chem. Lab. Med. 2005, 43, 617–627. [Google Scholar] [CrossRef]

| Patient Number (%) | |
|---|---|
| Male | 52 (100.0%) |
| Age | Mean 69.5 (min. 53–max. 82) years |
| Stage | |
| I | 4 (8.00%) |
| II | 28 (54.00%) |
| III | 11 (21.00%) |
| IV | 6 (11.00%) |
| n.a. 1 | 3 (6.00%) |
| T stage | |
| T1 | 8 (15.0%) |
| T2 | 24 (46.0%) |
| T3 | 15 (29.0%) |
| T4 | 1 (2.0%) |
| n.a. | 4 (8.0%) |
| N stage | |
| negative | 40 (78.0%) |
| positive | 6 (11.0%) |
| n.a. | 6 (11.0%) |
| Grading | |
| 1 | 9 (17.0%) |
| 2 | 17 (33.0%) |
| 3 | 17 (33.0%) |
| n.a. | 9 (17.0%) |
| Gleason Score | |
| 2 + 3 = 5 | 1 (2.00%) |
| 3 + 3 = 6 | 11 (21.00%) |
| 3 + 4 = 7 | 13 (25.00%) |
| 4 + 3 = 7 | 5 (10.00%) |
| 3 + 5 = 8 | 3 (6.00%) |
| 4 + 4 = 8 | 7 (13.00%) |
| 4 + 5 = 9 | 7 (13.00%) |
| 5 + 5 = 10 | 2 (4.00%) |
| n.a. | 3 (6.00%) |
| RT-Treatment | |
| Definitive | 29 (56%) |
| Adjuvant | 12 (23%) |
| Salvage RT | 11 (21%) |
| Risk groups | |
| Low risk | 12 (23.00%) |
| Intermediate risk | 18 (35.00%) |
| High risk | 19 (36.00%) |
| n.a. | 3 (6.00%) |
| PSA prior to RT | |
| <10 ng/mL | 36 (69.2%) |
| >10 ng/mL | 13 (25%) |
| n.a. | 3 (5.8%) |
| PSA after RT | |
| <10 ng/mL | 48 (92.3%) |
| >10 ng/mL | 1 (1.9%) |
| n.a. | 3 (5.8%) |
| Endocrine treatment | |
| Yes | 24 (46.00%) |
| No | 25 (48.00%) |
| n.a. | 3 (6.00%) |
| Surgery | |
| Yes | 25 (48.00%) |
| No | 25 (48.00%) |
| n.a. | 2 (4.00%) |
| Adjuvant RT | Definitive RT | Salvage RT | |
|---|---|---|---|
| No Relapse | 8 | 25 | 9 |
| Local recurrence (Prostate) | - | 1 | - |
| Biochemical recurrence | 3 | 1 | 1 |
| Distant metastasis | 1 | 2 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schott, D.; Pizon, M.; Drozdz, S.; Mäurer, I.; Wurschi, G.; Pachmann, K.; Mäurer, M. Circulating Epithelial Tumor Cells (CETC/CTC) in Prostate Cancer: Potential Prognostic Marker for the Risk of Recurrence During Radiotherapy. Int. J. Mol. Sci. 2025, 26, 1548. https://doi.org/10.3390/ijms26041548
Schott D, Pizon M, Drozdz S, Mäurer I, Wurschi G, Pachmann K, Mäurer M. Circulating Epithelial Tumor Cells (CETC/CTC) in Prostate Cancer: Potential Prognostic Marker for the Risk of Recurrence During Radiotherapy. International Journal of Molecular Sciences. 2025; 26(4):1548. https://doi.org/10.3390/ijms26041548
Chicago/Turabian StyleSchott, Dorothea, Monika Pizon, Sonia Drozdz, Irina Mäurer, Georg Wurschi, Katharina Pachmann, and Matthias Mäurer. 2025. "Circulating Epithelial Tumor Cells (CETC/CTC) in Prostate Cancer: Potential Prognostic Marker for the Risk of Recurrence During Radiotherapy" International Journal of Molecular Sciences 26, no. 4: 1548. https://doi.org/10.3390/ijms26041548
APA StyleSchott, D., Pizon, M., Drozdz, S., Mäurer, I., Wurschi, G., Pachmann, K., & Mäurer, M. (2025). Circulating Epithelial Tumor Cells (CETC/CTC) in Prostate Cancer: Potential Prognostic Marker for the Risk of Recurrence During Radiotherapy. International Journal of Molecular Sciences, 26(4), 1548. https://doi.org/10.3390/ijms26041548







