Deferasirox Targets TAOK1 to Induce p53-Mediated Apoptosis in Esophageal Squamous Cell Carcinoma
Abstract
1. Introduction
2. Results
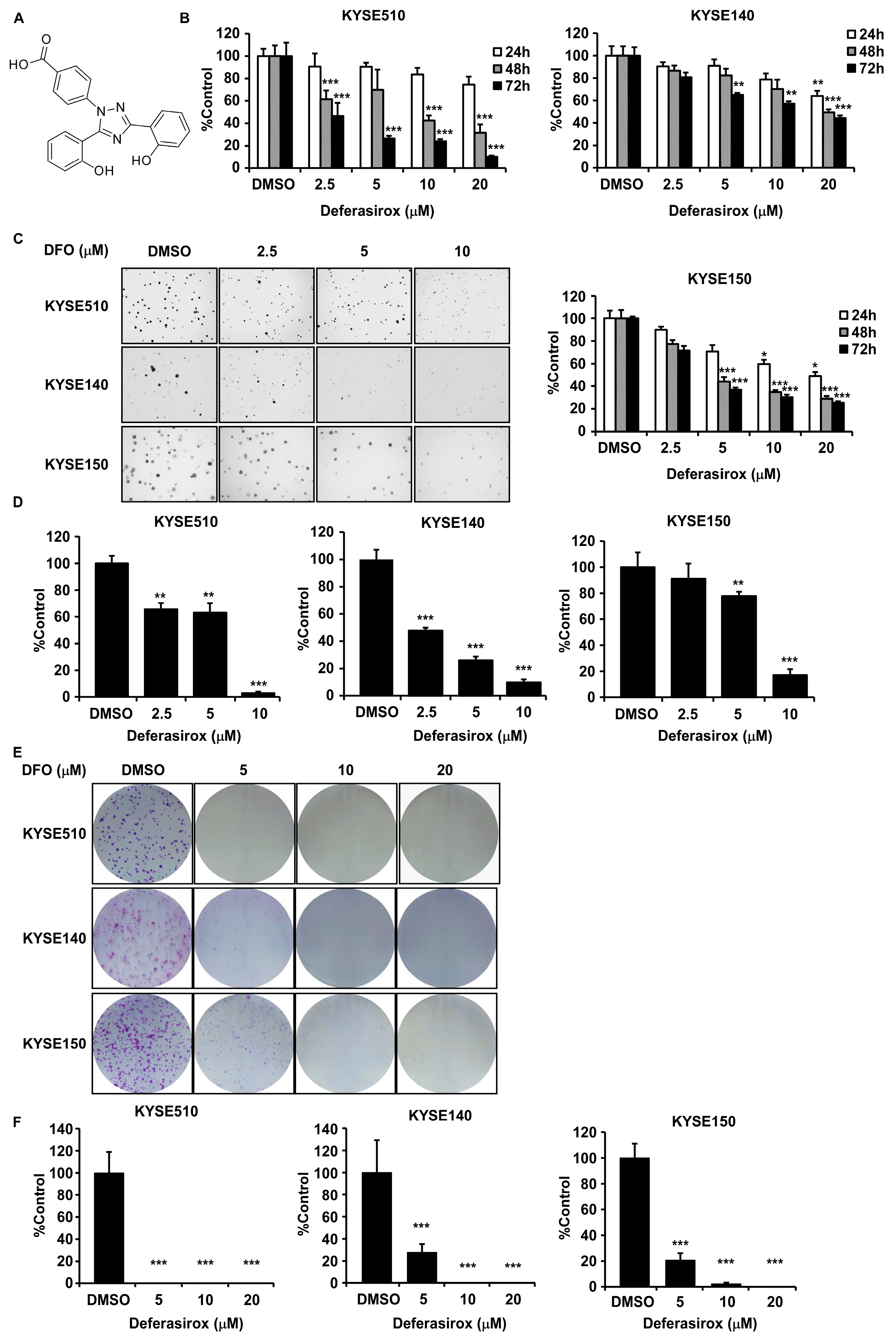
2.1. Deferasirox Significantly Inhibits ESCC Cell Proliferation and Colony Formation
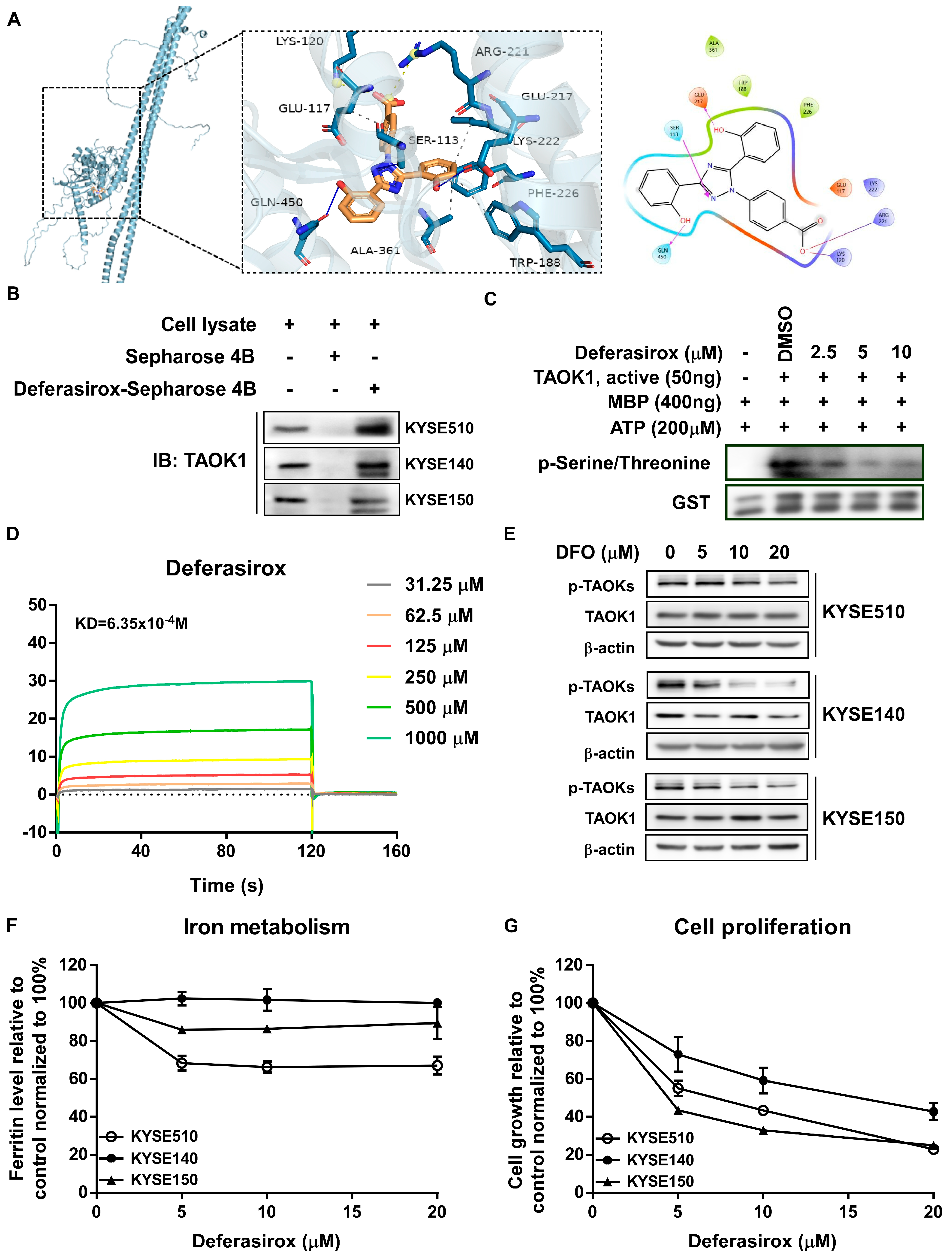
2.2. Deferasirox Directly Targets TAOK1 to Inhibit Its Kinase Activity
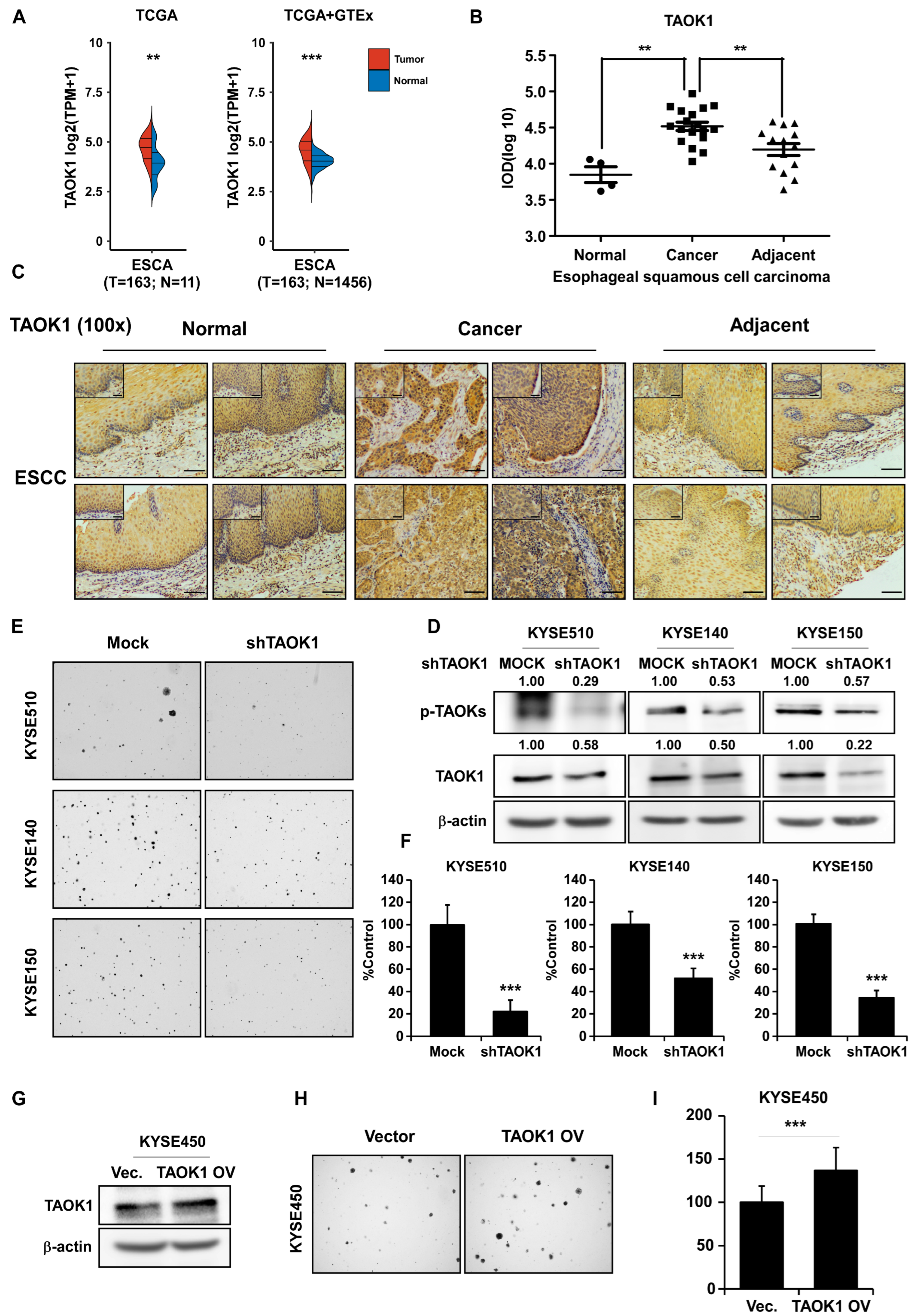
2.3. Targeting TAOK1 Effectively Attenuates ESCC Growth
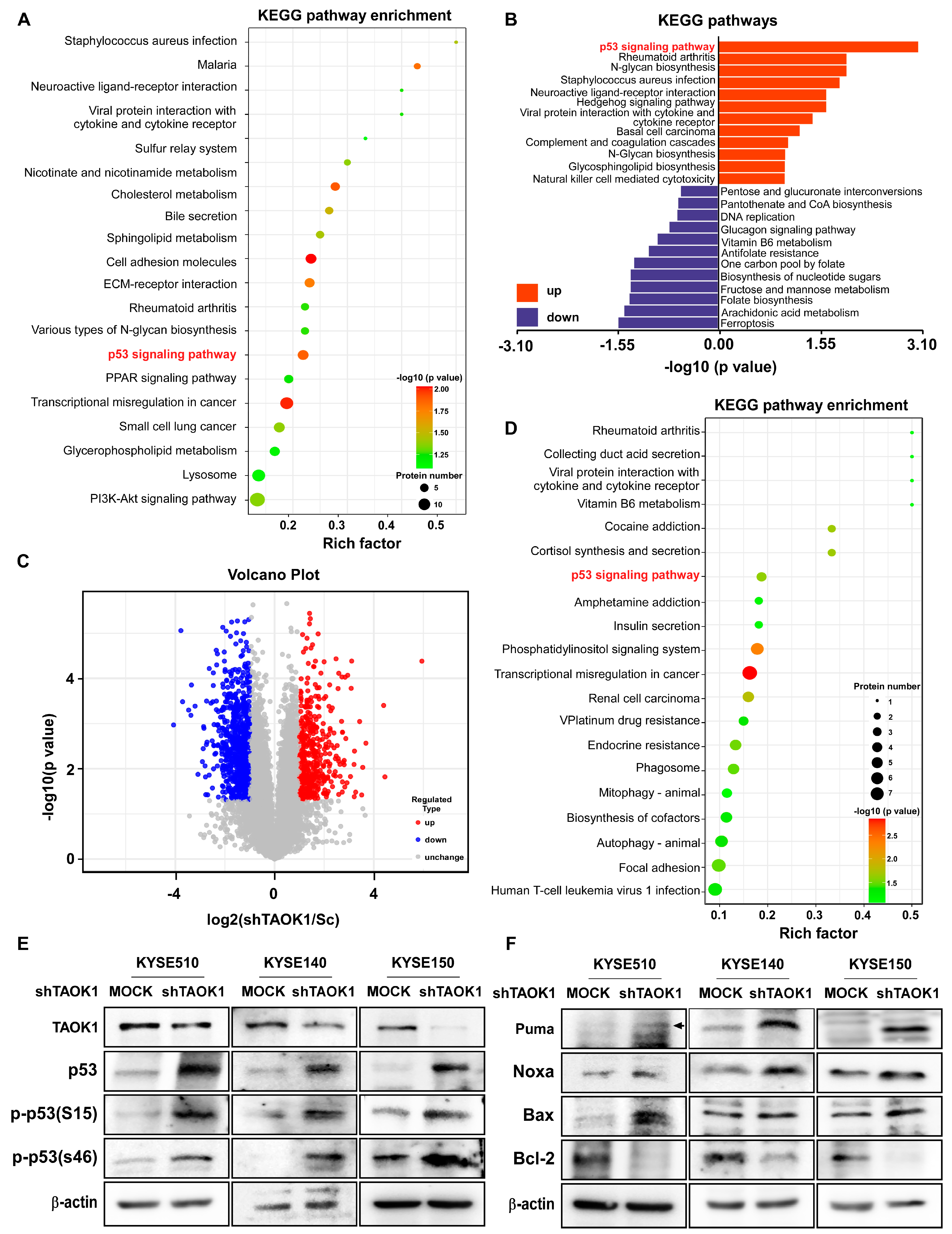
2.4. TAOK1 Knockdown Elevates p53-Mediated Apoptosis in ESCC
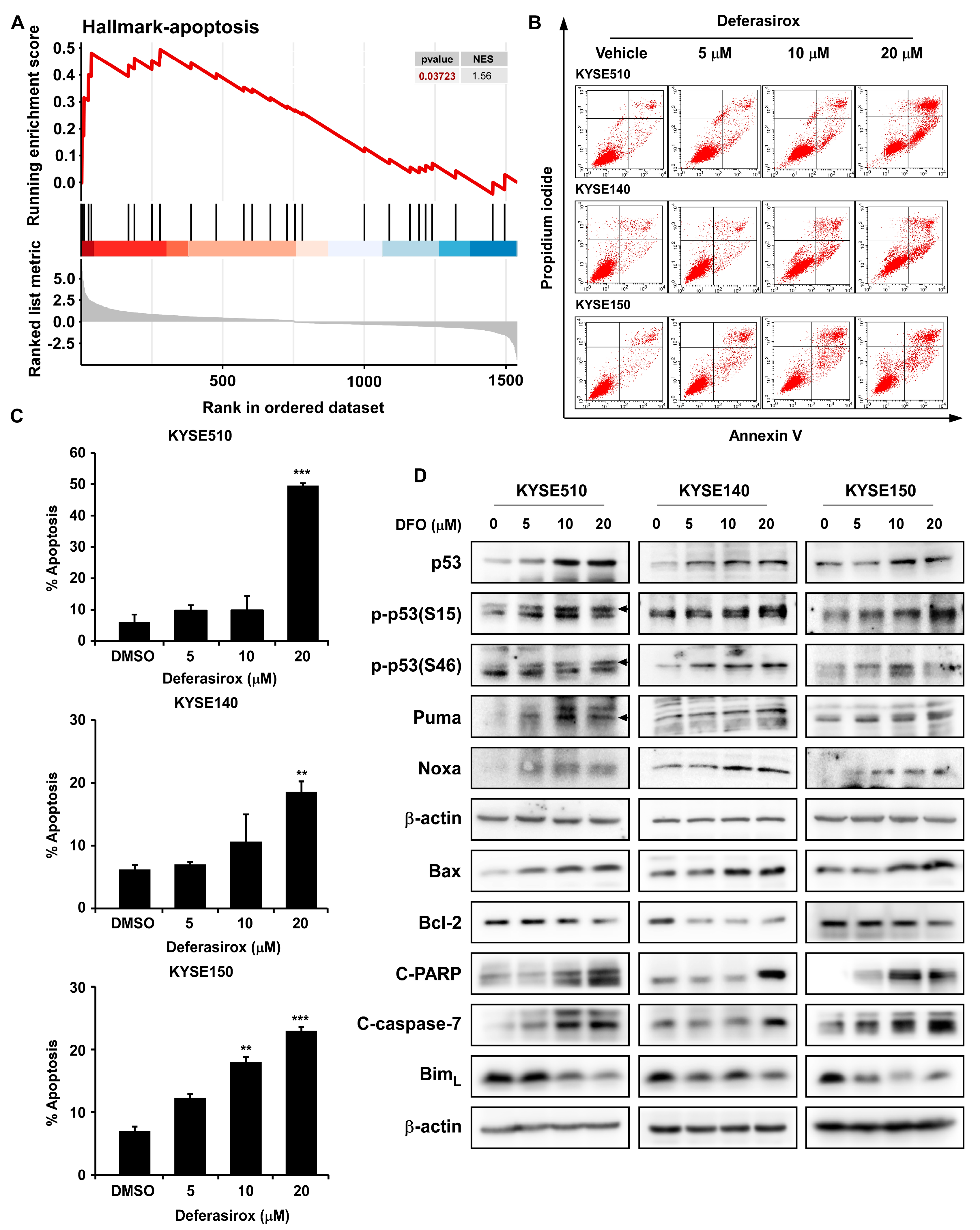
2.5. DFO Treatment Promotes Apoptosis in ESCC Cells by Inhibiting TAOK1
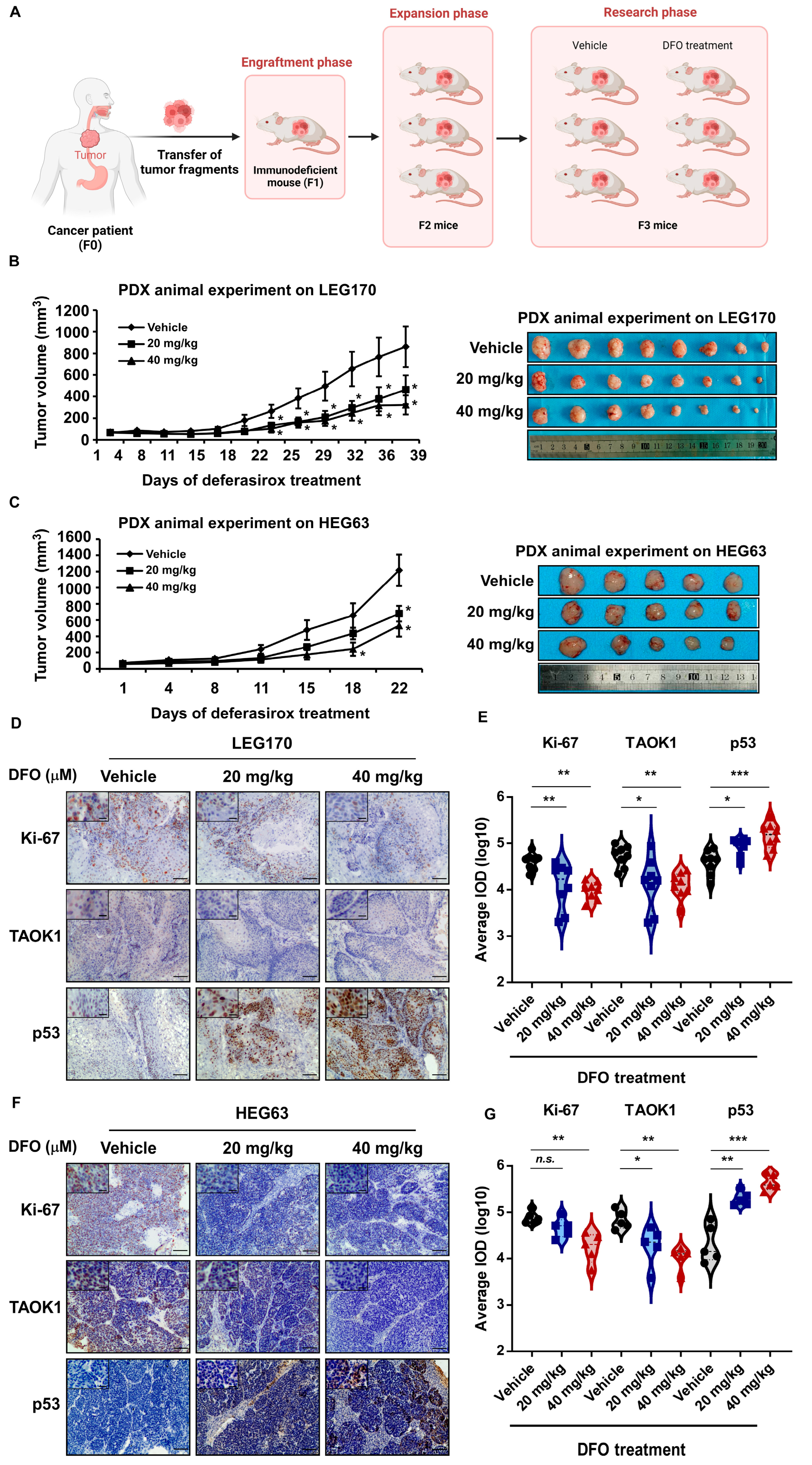
2.6. Deferasirox Inhibits ESCC PDX Growth In Vivo
3. Discussion
4. Materials and Methods
4.1. Reagents and Cells
4.2. Cell Proliferation Measurement
4.3. Anchorage-Independent Cell Growth Assay
4.4. Cell Apoptosis Assessment
4.5. Western Blot
4.6. Ex Vivo Pull-Down Assay
4.7. Surface Plasmon Resonance (SPR)
4.8. In Vitro Kinase Assay
4.9. Virtual Screening
4.10. Enzyme-Linked Immunosorbent Assay
4.11. Lentiviral Infection
4.12. Multi-Omics Sequencing
4.13. Patient-Derived Xenograft (PDX) Mouse Model
4.14. Hematoxylin–Eosin (H&E) Staining and Immunohistochemical (IHC) Analysis
4.15. Statistical Analysis
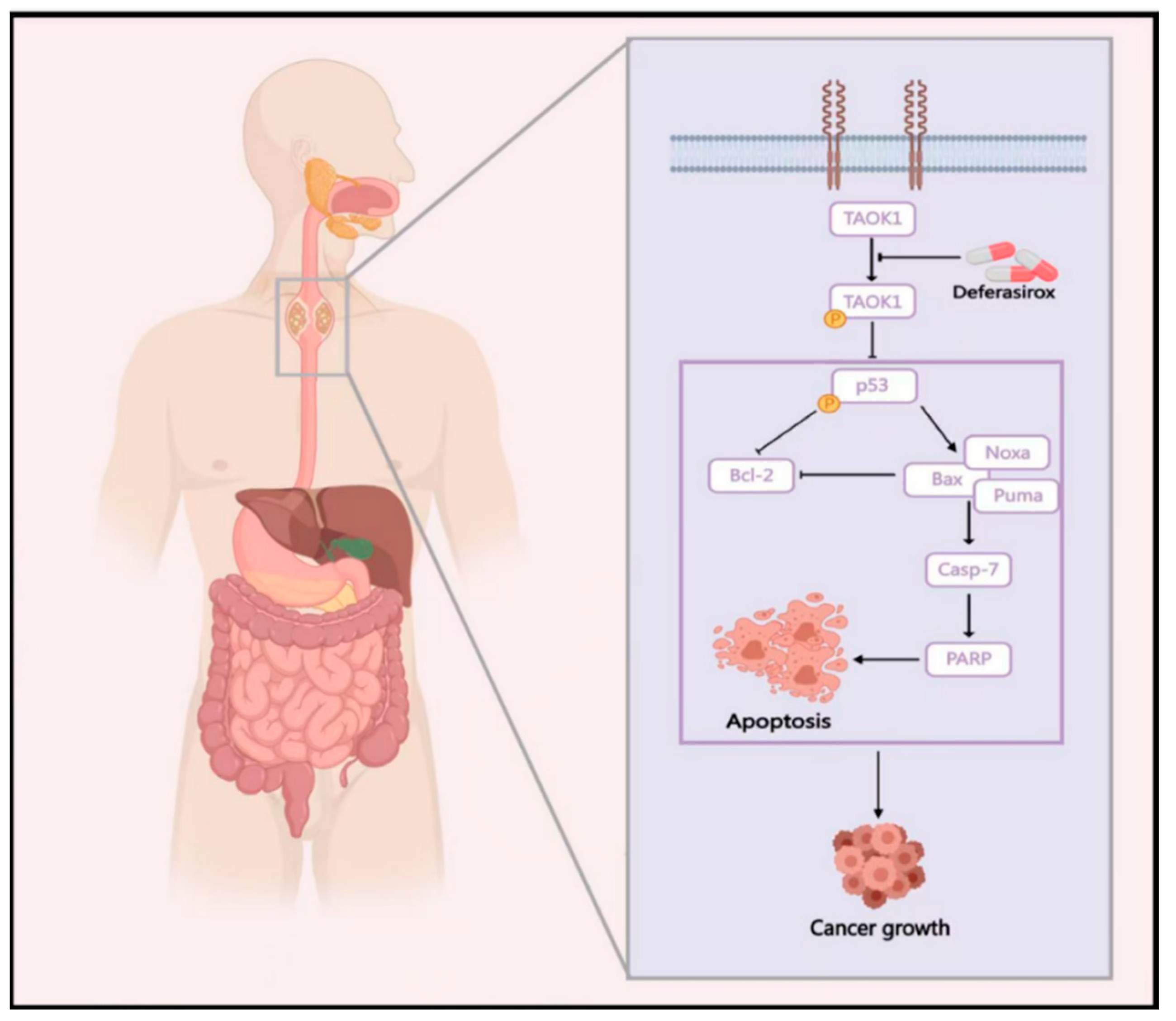
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Domper Arnal, M.J.; Ferrández Arenas, Á.; Lanas Arbeloa, Á. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J. Gastroenterol. 2015, 21, 7933–7943. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, A.K.; El-Serag, H.B. Esophageal carcinoma. N. Engl. J. Med. 2014, 371, 2499–2509. [Google Scholar] [CrossRef]
- Lin, Y.; Totsuka, Y.; Shan, B.; Wang, C.; Wei, W.; Qiao, Y.; Kikuchi, S.; Inoue, M.; Tanaka, H.; He, Y. Esophageal cancer in high-risk areas of China: Research progress and challenges. Ann. Epidemiol. 2017, 27, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Dan, I.; Watanabe, N.M.; Kusumi, A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 2001, 11, 220–230. [Google Scholar] [CrossRef]
- Zihni, C.; Mitsopoulos, C.; Tavares, I.A.; Ridley, A.J.; Morris, J.D. Prostate-derived sterile 20-like kinase 2 (PSK2) regulates apoptotic morphology via C-Jun N-terminal kinase and Rho kinase-1. J. Biol. Chem. 2006, 281, 7317–7323. [Google Scholar] [CrossRef]
- Chen, L. TAOK1 Promotes Proliferation and Invasion of Non-Small-Cell Lung Cancer Cells by Inhibition of WWC1. Comput. Math. Methods Med. 2022, 2022, 3157448. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, D.D.; Liu, J.B.; Yang, X.L.; Xin, R.; Jia, C.Y.; Wang, H.M.; Lu, G.X.; Wang, P.Y.; Liu, Y.; et al. Comprehensive analysis to identify DLEU2L/TAOK1 axis as a prognostic biomarker in hepatocellular carcinoma. Mol. Ther. Nucleic Acids 2021, 23, 702–718. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Qu, Y.; Jia, H.; Zhang, Y.; Liu, S.; Laster, K.V.; Choi, B.Y.; Tian, J.; Gu, T.; Chen, H.; et al. Targeting TAOK1 with resveratrol inhibits esophageal squamous cell carcinoma growth in vitro and in vivo. Mol. Carcinog. 2024, 63, 991–1008. [Google Scholar] [CrossRef]
- Koo, C.Y.; Giacomini, C.; Reyes-Corral, M.; Olmos, Y.; Tavares, I.A.; Marson, C.M.; Linardopoulos, S.; Tutt, A.N.; Morris, J.D.H. Targeting TAO Kinases Using a New Inhibitor Compound Delays Mitosis and Induces Mitotic Cell Death in Centrosome Amplified Breast Cancer Cells. Mol. Cancer Ther. 2017, 16, 2410–2421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Shen, Q.; Wang, Y.; Deng, X.; Fan, J.; Gu, X.; Fan, M.; Wei, K.; Cheng, C.R.; Zhang, W.D.; et al. Corylifol A ameliorates muscle atrophy by inhibiting TAOK1/p38-MAPK/FoxO3 pathway in cancer cachexia. J. Cachexia Sarcopenia Muscle 2023, 14, 2098–2113. [Google Scholar] [CrossRef]
- Steinbrueck, A.; Sedgwick, A.C.; Han, H.H.; Zhao, M.Y.; Sen, S.; Huang, D.Y.; Zang, Y.; Li, J.; He, X.P.; Sessler, J.L. In vitro studies of deferasirox derivatives as potential organelle-targeting traceable anti-cancer therapeutics. Chem. Commun. 2021, 57, 5678–5681. [Google Scholar] [CrossRef]
- Roatsch, M.; Hoffmann, I.; Abboud, M.I.; Hancock, R.L.; Tarhonskaya, H.; Hsu, K.F.; Wilkins, S.E.; Yeh, T.L.; Lippl, K.; Serrer, K.; et al. The Clinically Used Iron Chelator Deferasirox Is an Inhibitor of Epigenetic JumonjiC Domain-Containing Histone Demethylases. ACS Chem. Biol. 2019, 14, 1737–1750. [Google Scholar] [CrossRef]
- Puri, S.; Kumar, R.; Rojas, I.G.; Salvatori, O.; Edgerton, M. Iron Chelator Deferasirox Reduces Candida albicans Invasion of Oral Epithelial Cells and Infection Levels in Murine Oropharyngeal Candidiasis. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Tury, S.; Assayag, F.; Bonin, F.; Chateau-Joubert, S.; Servely, J.L.; Vacher, S.; Becette, V.; Caly, M.; Rapinat, A.; Gentien, D.; et al. The iron chelator deferasirox synergises with chemotherapy to treat triple-negative breast cancers. J. Pathol. 2018, 246, 103–114. [Google Scholar] [CrossRef]
- Zhou, N.; Cui, Y.; Zhu, R.; Kuang, Y.; Ma, W.; Hou, J.; Zhu, Y.; Chen, S.; Xu, X.; Tan, K.; et al. Deferasirox shows inhibition activity against cervical cancer In Vitro and In Vivo. Gynecol. Oncol. 2022, 166, 126–137. [Google Scholar] [CrossRef]
- Wu, Y.; Ran, L.; Yang, Y.; Gao, X.; Peng, M.; Liu, S.; Sun, L.; Wan, J.; Wang, Y.; Yang, K.; et al. Deferasirox alleviates DSS-induced ulcerative colitis in mice by inhibiting ferroptosis and improving intestinal microbiota. Life Sci. 2023, 314, 121312. [Google Scholar] [CrossRef] [PubMed]
- Ford, S.J.; Obeidy, P.; Lovejoy, D.B.; Bedford, M.; Nichols, L.; Chadwick, C.; Tucker, O.; Lui, G.Y.; Kalinowski, D.S.; Jansson, P.J.; et al. Deferasirox (ICL670A) effectively inhibits oesophageal cancer growth in vitro and in vivo. Br. J. Pharmacol. 2013, 168, 1316–1328. [Google Scholar] [CrossRef]
- Jubashi, A.; Kotani, D.; Kojima, T.; Takebe, N.; Shitara, K. Current landscape of targeted therapy in esophageal squamous cell carcinoma. Curr. Probl. Cancer 2024, 53, 101152. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.W.; Lin, T.E.; HuangFu, W.C.; Chang, C.D.; Tu, H.J.; Chen, L.C.; Yen, S.C.; Sung, T.Y.; Huang, W.J.; Yang, C.R.; et al. Identification of a dual TAOK1 and MAP4K5 inhibitor using a structure-based virtual screening approach. J. Enzym. Inhib. Med. Chem. 2021, 36, 98–108. [Google Scholar] [CrossRef]
- Huck, B.R.; Mochalkin, I. Recent progress towards clinically relevant ATP-competitive Akt inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 2838–2848. [Google Scholar] [CrossRef] [PubMed]
- Galanello, R.; Campus, S.; Origa, R. Deferasirox: Pharmacokinetics and clinical experience. Expert Opin. Drug Metab. Toxicol. 2012, 8, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, K.; Ikeda, M.; Miyamoto, H.D.; Furusawa, S.; Abe, K.; Watanabe, M.; Kanamura, T.; Fujita, S.; Nishimura, R.; Toyohara, T.; et al. Deferasirox Targeting Ferroptosis Synergistically Ameliorates Myocardial Ischemia Reperfusion Injury in Conjunction with Cyclosporine A. J. Am. Heart Assoc. 2024, 13, e031219. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M.R.; Ford, S.J.; Horniblow, R.D.; Iqbal, T.H.; Tselepis, C. Iron chelation in the treatment of cancer: A new role for deferasirox? J. Clin. Pharmacol. 2013, 53, 885–891. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, J.S.; Won, Y.W.; Uhm, J.; Park, B.B.; Lee, Y.Y. The potential of deferasirox as a novel therapeutic modality in gastric cancer. World J. Surg. Oncol. 2016, 14, 77. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Zhu, Y.; Zhang, Y.; Zhu, H.; Liu, T. M1 Macrophage-Derived Exosomes Loaded with Gemcitabine and Deferasirox against Chemoresistant Pancreatic Cancer. Pharmaceutics 2021, 13, 1493. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, X.; Dong, Z.; Fu, Y.; Ma, Y.; Hailong, W. Research progress on anti-tumor mechanism of TAOK kinases. Cell Signal 2024, 124, 111385. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, T.A.; Salvagno, C.; Chae, C.S.; Awasthi, D.; Giovanelli, P.; Marin Falco, M.; Hwang, S.M.; Teran-Cabanillas, E.; Suominen, L.; Yamazaki, T.; et al. Iron Chelation Therapy Elicits Innate Immune Control of Metastatic Ovarian Cancer. Cancer Discov. 2024, 14, 1901–1921. [Google Scholar] [CrossRef] [PubMed]
- Buss, J.L.; Torti, F.M.; Torti, S.V. The role of iron chelation in cancer therapy. Curr. Med. Chem. 2003, 10, 1021–1034. [Google Scholar] [CrossRef]
- Bayanbold, K.; Singhania, M.; Fath, M.A.; Searby, C.C.; Stolwijk, J.M.; Henrich, J.B.; Pulliam, C.F.; Schoenfeld, J.D.; Mapuskar, K.A.; Sho, S.; et al. Depletion of Labile Iron Induces Replication Stress and Enhances Responses to Chemoradiation in Non-Small-Cell Lung Cancer. Antioxidants 2023, 12, 2005. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Han, X.; Zheng, R.; Yan, J.; Wu, X.; Wang, Y.; Zhang, H. Upregulation of MHC-I and downregulation of PD-L1 expression by doxorubicin and deferasirox codelivered liposomal nanoparticles for chemoimmunotherapy of melanoma. Int. J. Pharm. 2022, 624, 122002. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Liu, S.; Zhou, X.; Hou, D.; Jia, H.; Tang, R.; Zhang, Y.; Song, M. Deferasirox Targets TAOK1 to Induce p53-Mediated Apoptosis in Esophageal Squamous Cell Carcinoma. Int. J. Mol. Sci. 2025, 26, 1524. https://doi.org/10.3390/ijms26041524
Li B, Liu S, Zhou X, Hou D, Jia H, Tang R, Zhang Y, Song M. Deferasirox Targets TAOK1 to Induce p53-Mediated Apoptosis in Esophageal Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2025; 26(4):1524. https://doi.org/10.3390/ijms26041524
Chicago/Turabian StyleLi, Boyang, Shihui Liu, Xiaowan Zhou, Dongpu Hou, Huajie Jia, Rude Tang, Yunqing Zhang, and Mengqiu Song. 2025. "Deferasirox Targets TAOK1 to Induce p53-Mediated Apoptosis in Esophageal Squamous Cell Carcinoma" International Journal of Molecular Sciences 26, no. 4: 1524. https://doi.org/10.3390/ijms26041524
APA StyleLi, B., Liu, S., Zhou, X., Hou, D., Jia, H., Tang, R., Zhang, Y., & Song, M. (2025). Deferasirox Targets TAOK1 to Induce p53-Mediated Apoptosis in Esophageal Squamous Cell Carcinoma. International Journal of Molecular Sciences, 26(4), 1524. https://doi.org/10.3390/ijms26041524





