Decreasing β-Catenin Leads to Altered Endothelial Morphology, Increased Barrier Permeability and Cognitive Impairment During Chronic Methamphetamine Exposure
Abstract
1. Introduction
2. Results
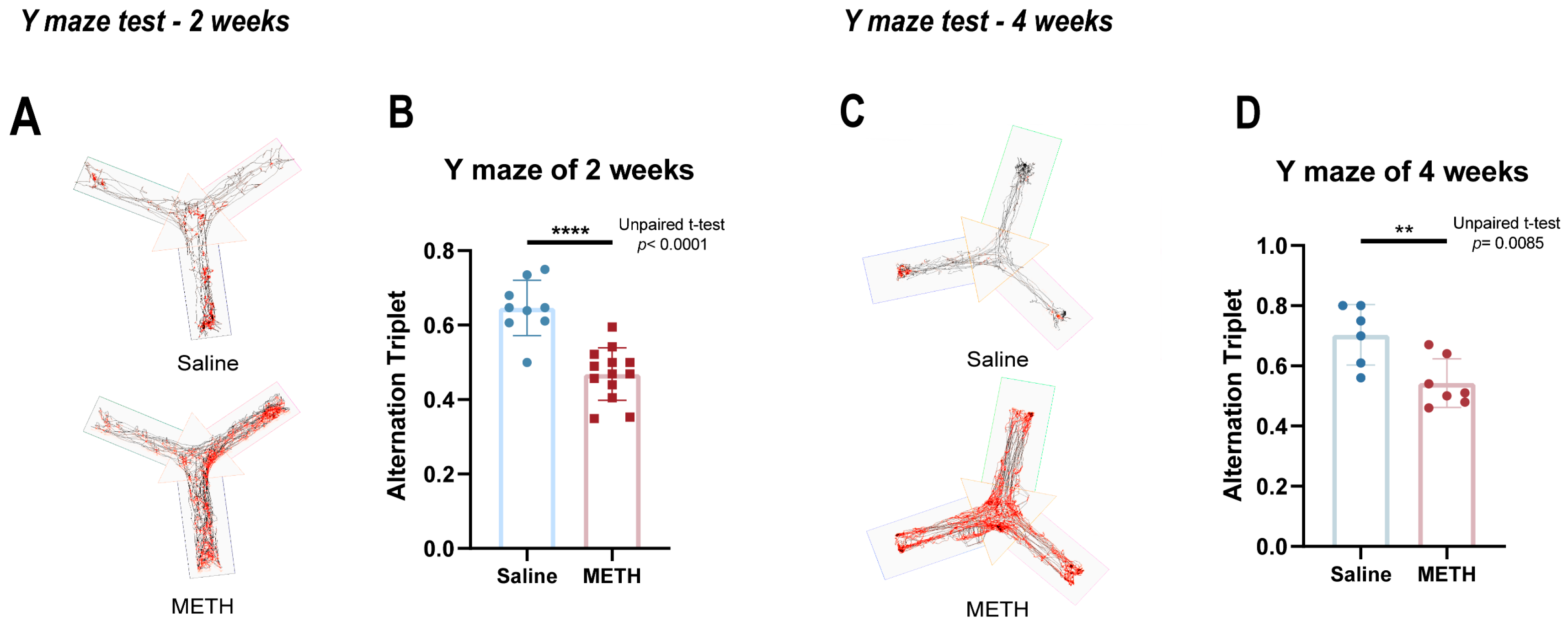
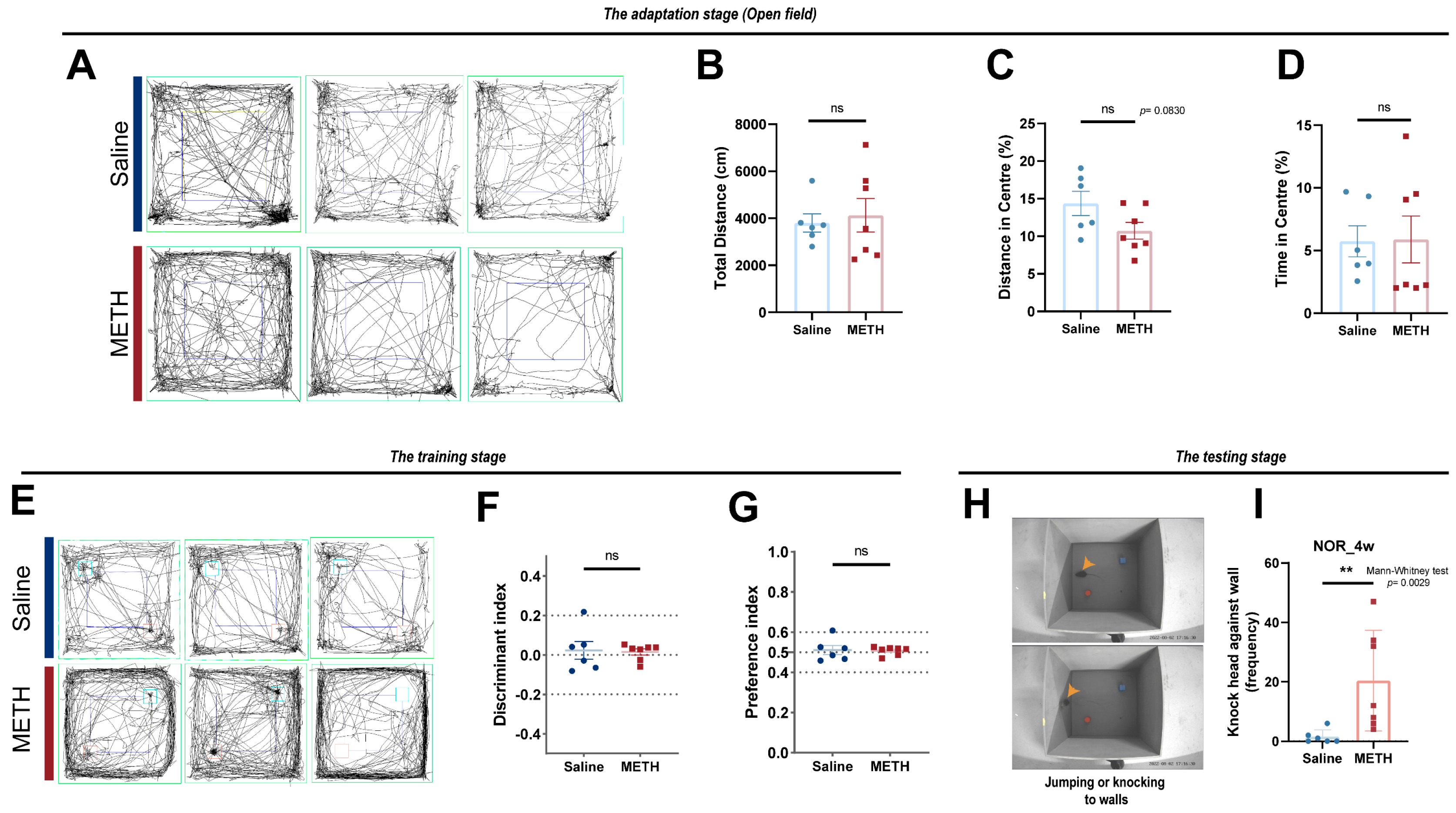
2.1. Chronic METH Exposure Resulted in Reduced Cognitive Function in Mice
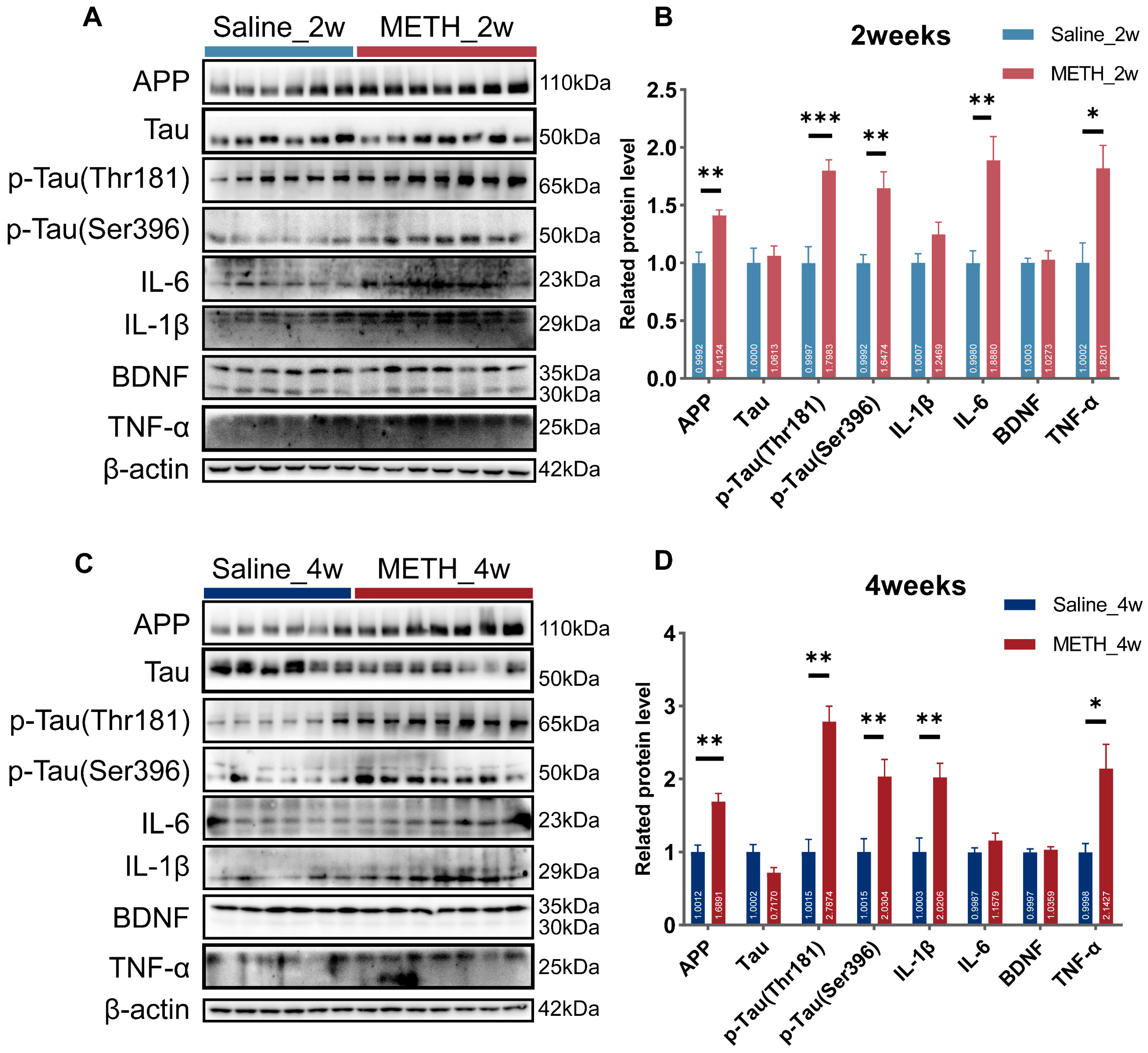
2.2. Chronic METH Exposure Induced Neural Injury and Neuroinflammation in the Hippocampus
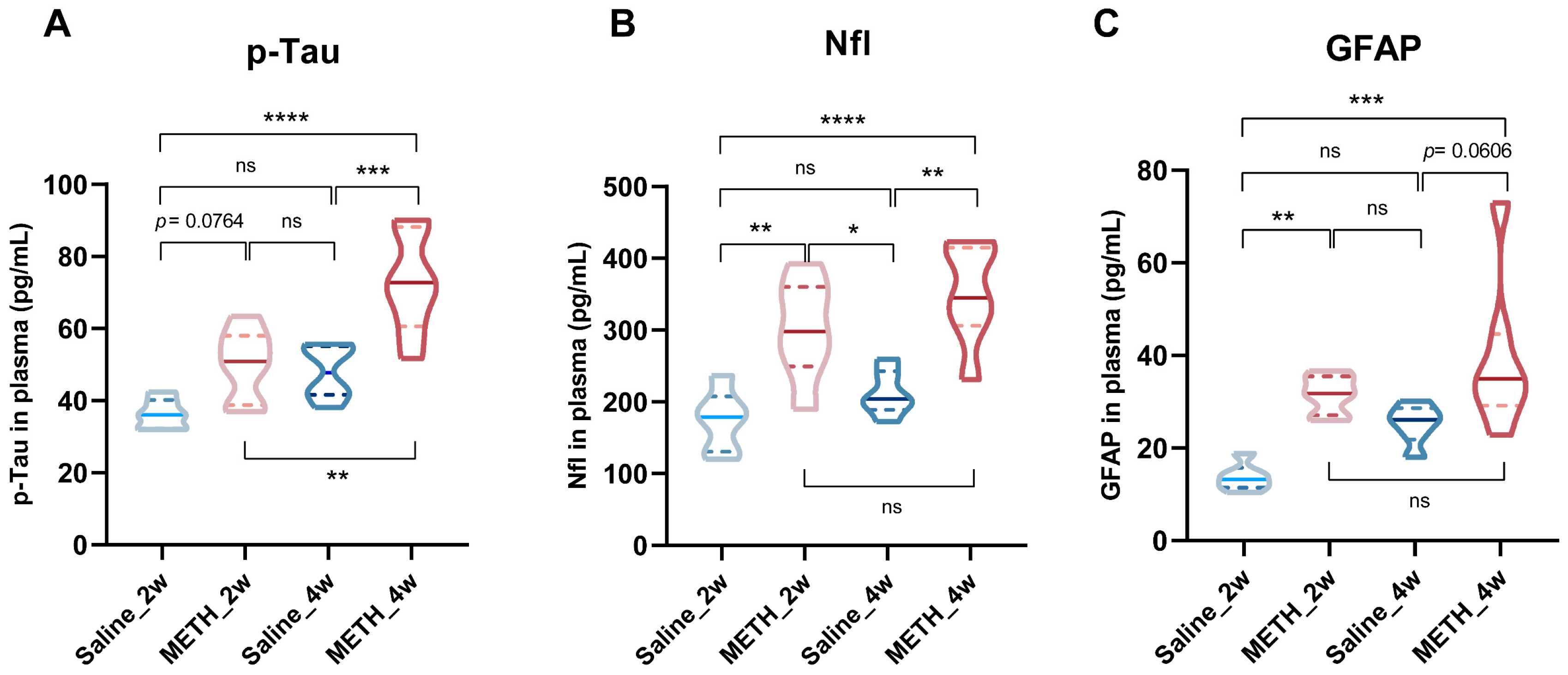
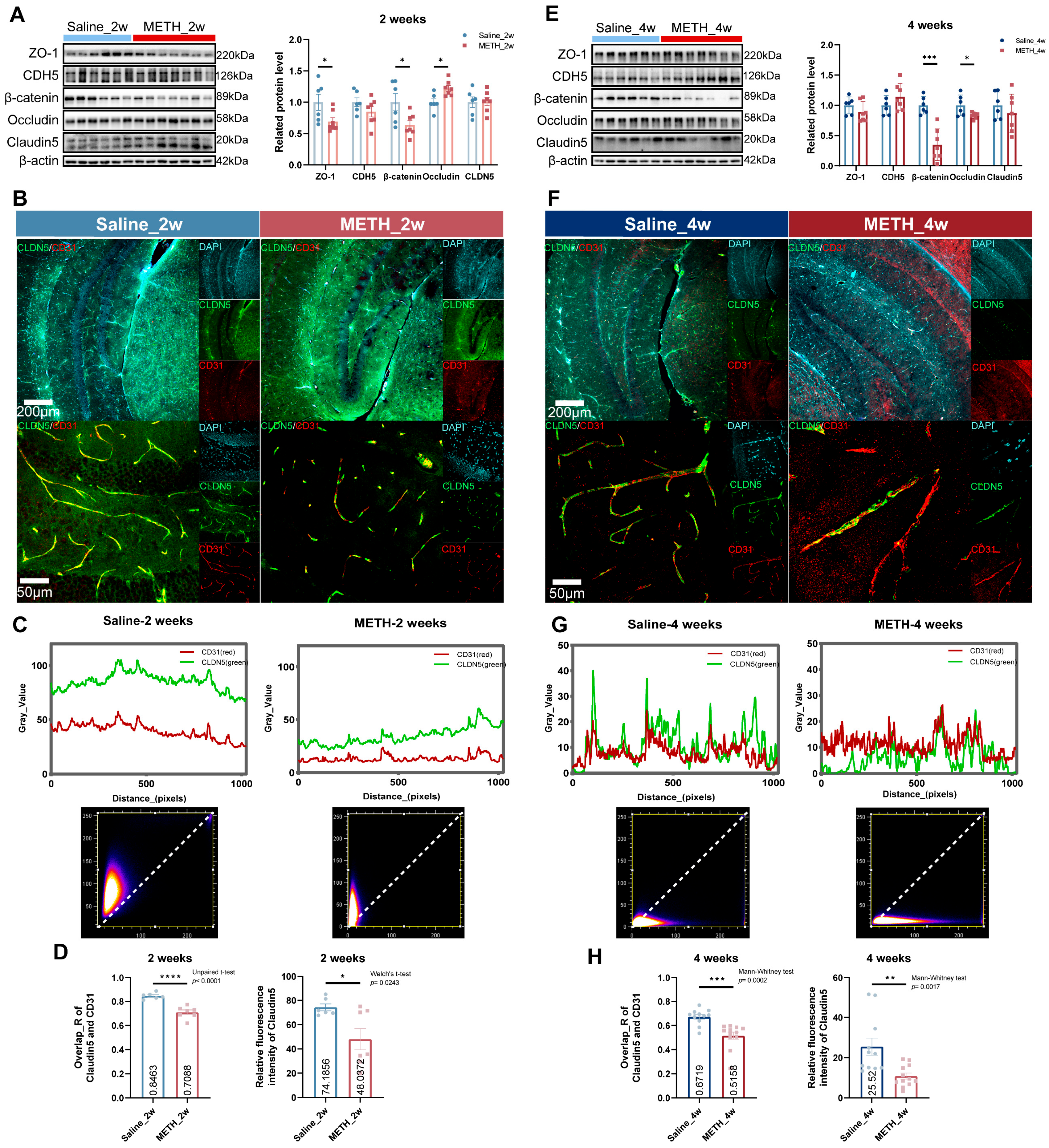
2.3. Chronic METH Exposure Leads to BBB Leakage and β-Catenin Decreasing in Hippocampus
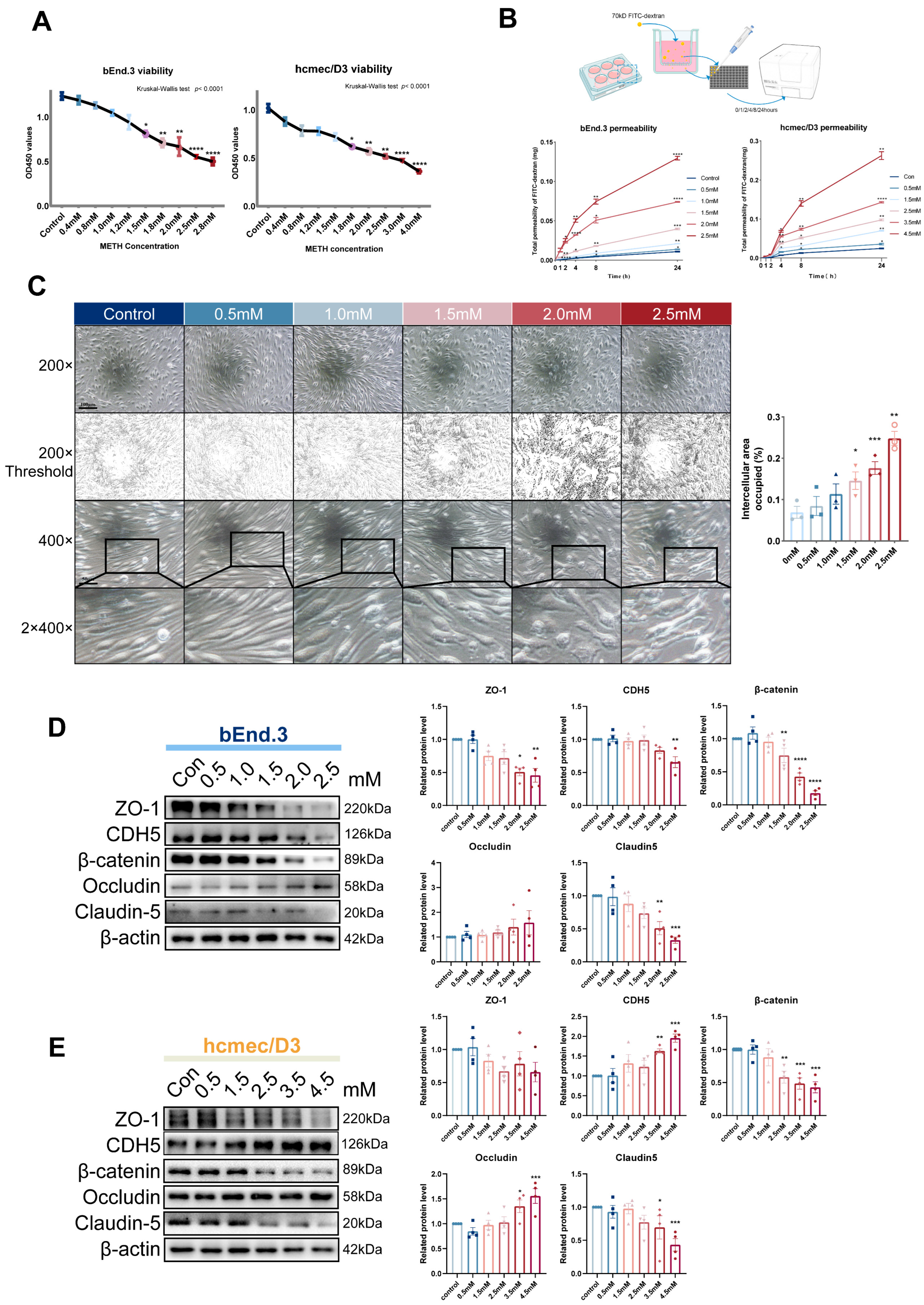
2.4. The Increase of METH Concentration Leads to Endothelial Injury and Decreased β-Catenin
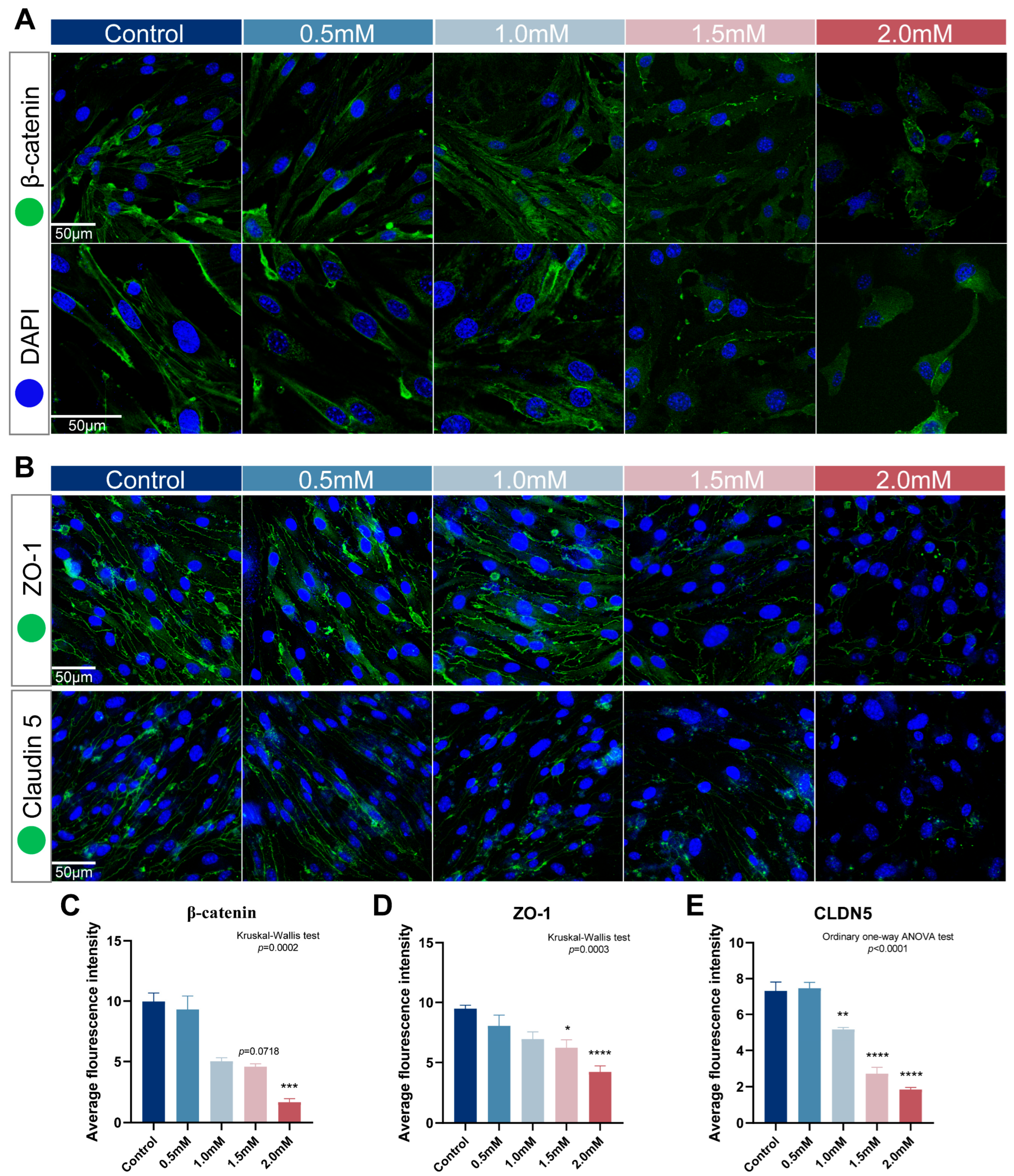
2.5. METH Treatment Duration Induced the Decrease of β-Catenin and Its Transfer from Cytomembrane to Nucleus
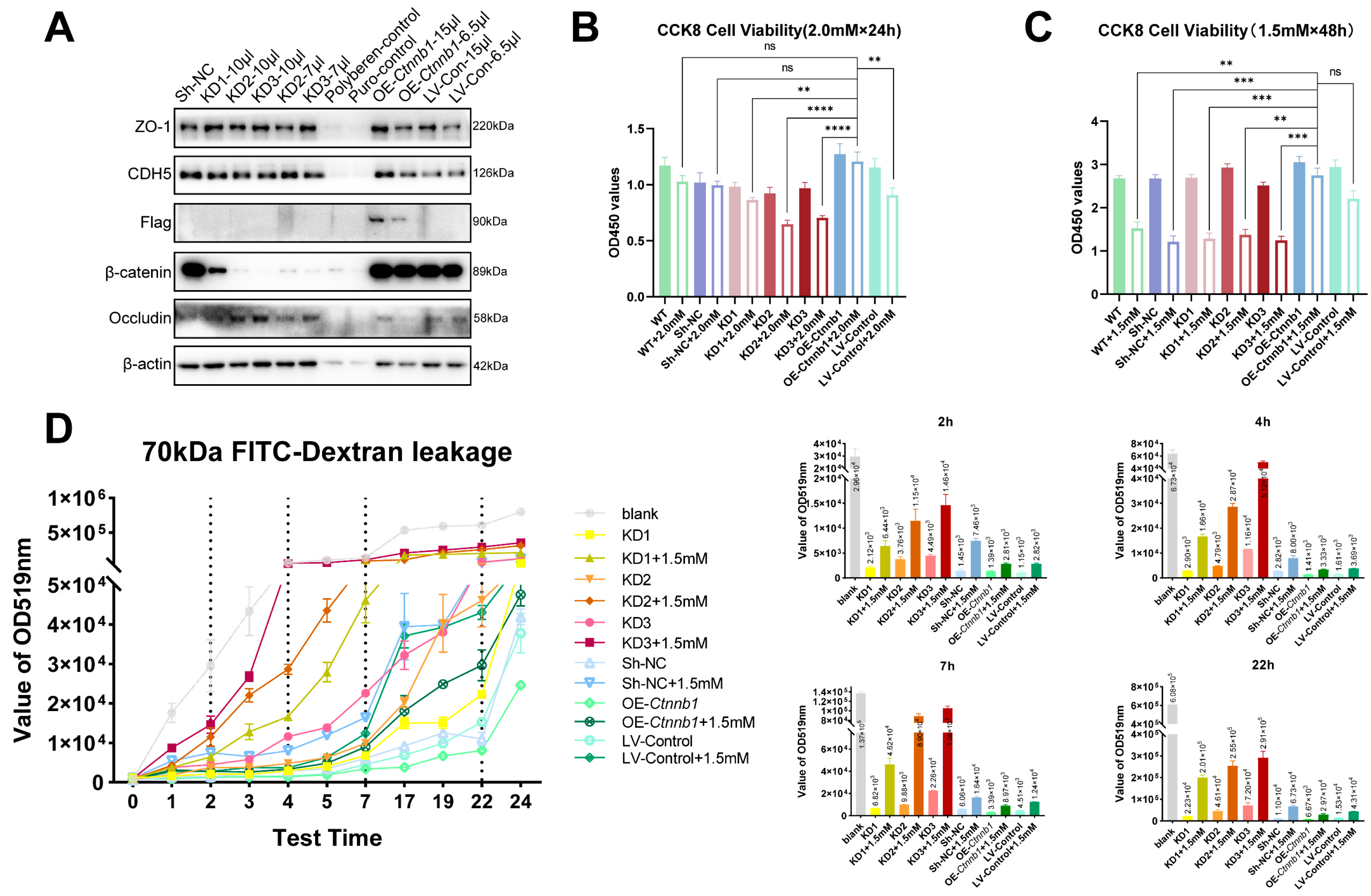
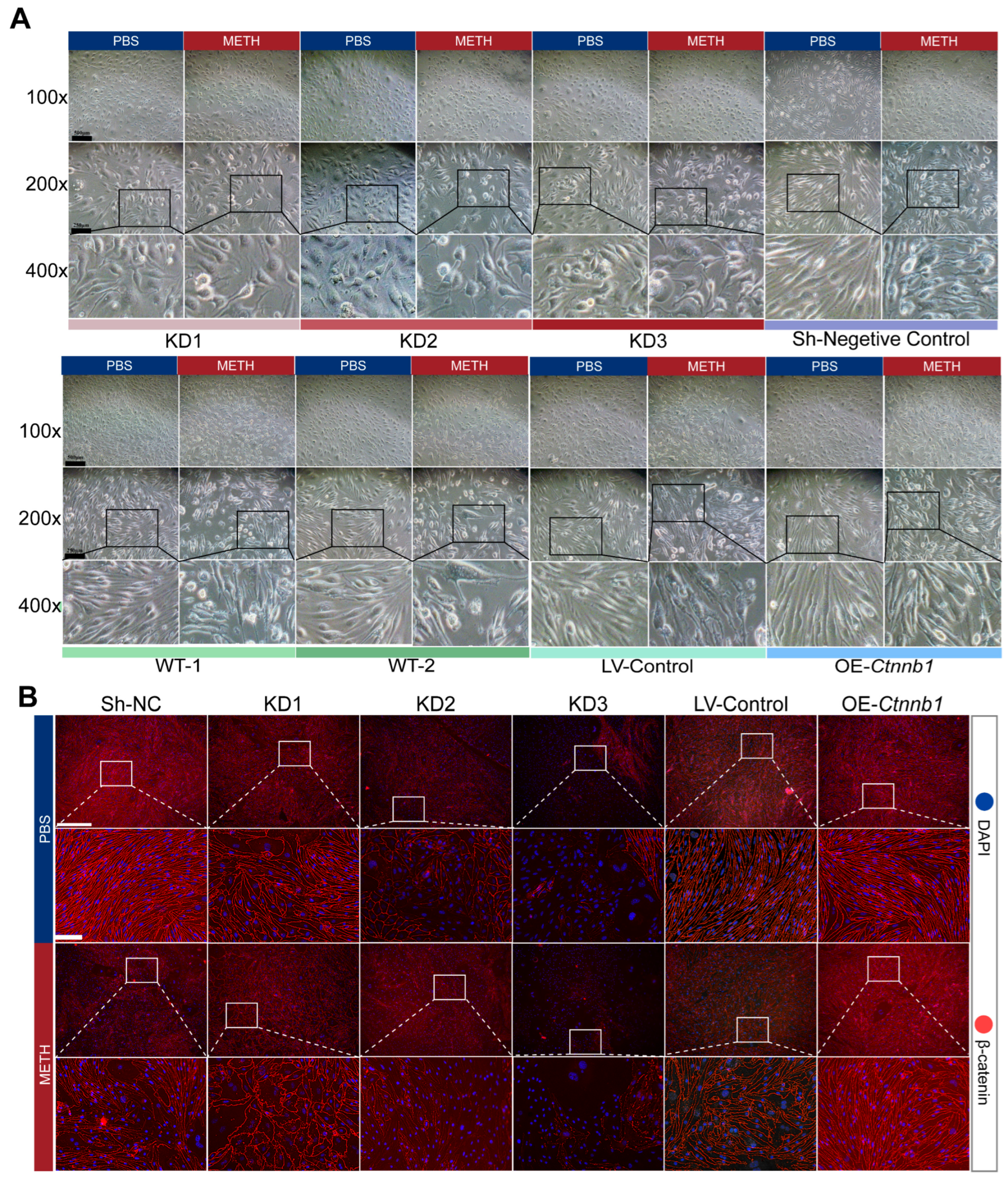
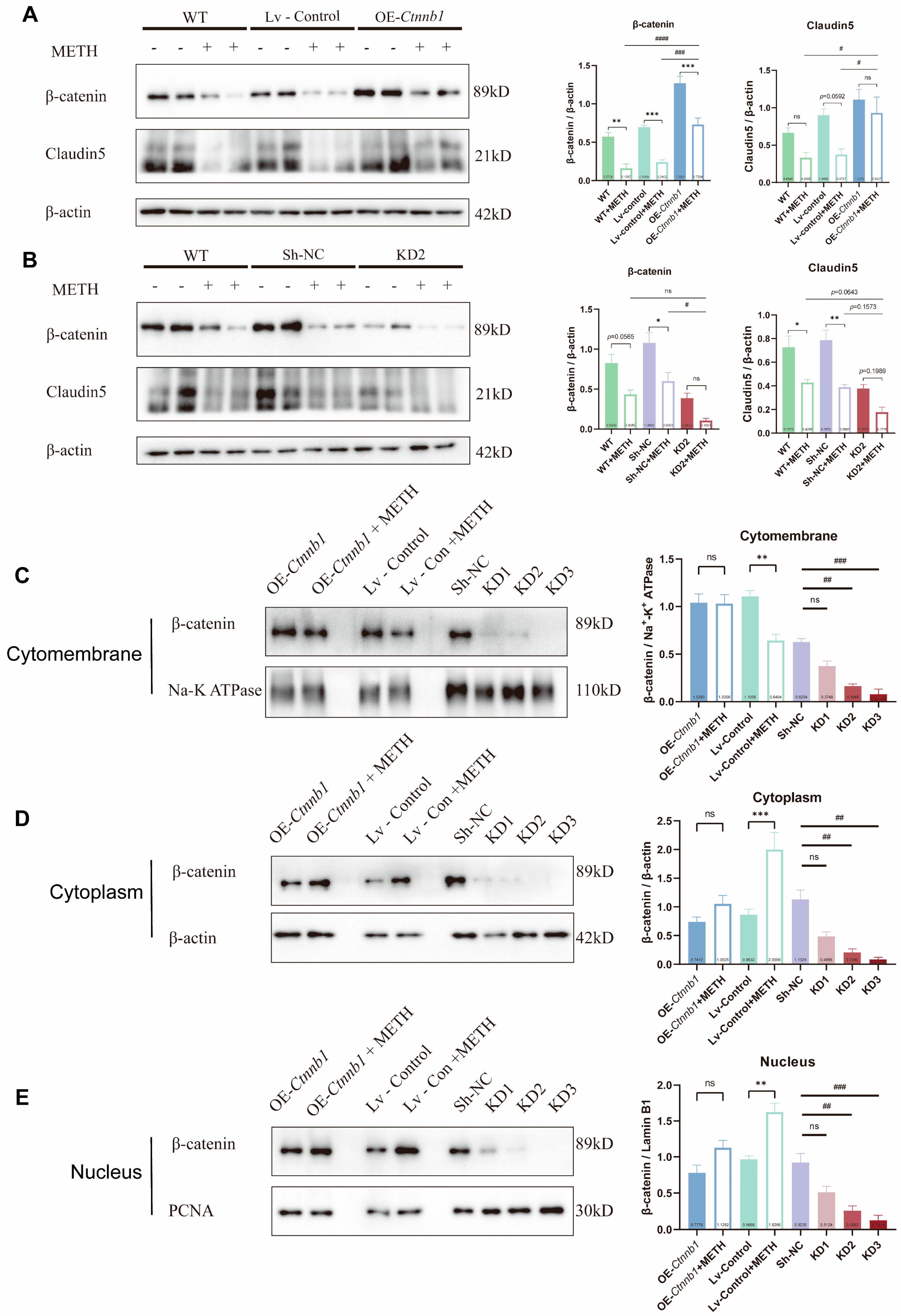
2.6. The Decrease of β-Catein Is Detrimental to Endothelial Cell Junction While Overexpress of β-Catein Can Reduce the Endothelial Injury Effect of METH
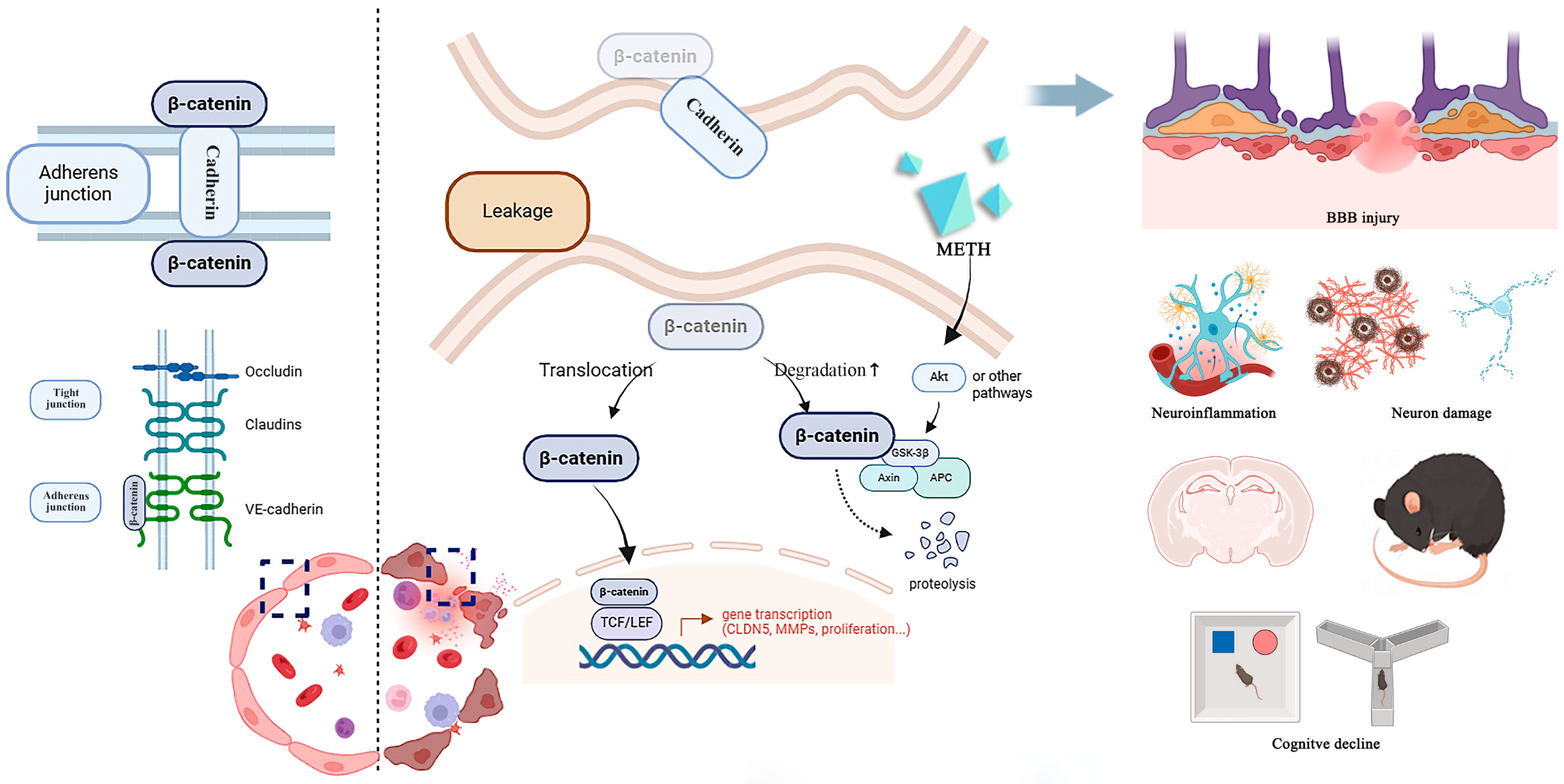
3. Discussion
4. Materials and Methods
4.1. Animal and Treatments
4.2. Animal Behavioral Tests
4.3. Magnetic Resonance Imaging (MRI) Scans
4.4. Immunohistochemistry Staining (IHC)
4.5. Immunofluorescence (IF) and Quantification of Claudin5 Levels
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Fluorescence Detection of Evans Blue to Evaluate BBB Leakage in Hippocampus
4.8. Cell Culture and Treatment
4.9. Assessment of Cell Viability
4.10. 70 kDa FITC (Fluorescein Isothiocyanate)-Dextran Permeability Test In Vitro
4.11. Western Blot Analysis
4.12. Knockdown and Overexpression of Ctnnb1
| Primer Name | Primer Sequence (5′-3′) | Primer Length |
| shNC-oligo-F | CCGGGCCACAACGTCTATATCATGGCTCGAGCCATGATATAGACGTTGTGGCTTTTTT | 58 bp |
| shNC-oligo-R | AATTCAAAAAAGCCACAACGTCTATATCATGGCTCGAGCCATGATATAGACGTTGTGG | 58 bp |
| Ctnnb1KD1- TRCN0000012690-F | CCGGGCTGATATTGACGGGCAGTATCTCGAGATACTGCCCGTCAATATCAGCTTTTTG | 58 bp |
| Ctnnb1KD1- TRCN0000012690-R | AATTCAAAAAGCTGATATTGACGGGCAGTATCTCGAGATACTGCCCGTCAATATCAGC | 58 bp |
| Ctnnb1KD2- TRCN0000012692-F | CCGGCCCAAGCCTTAGTAAACATAACTCGAGTTATGTTTACTAAGGCTTGGGTTTTTG | 58 bp |
| Ctnnb1KD2- TRCN0000012692-R | AATTCAAAAACCCAAGCCTTAGTAAACATAACTCGAGTTATGTTTACTAAGGCTTGGG | 58 bp |
| Ctnnb1KD3- TRCN0000321075-F | CCGGCGTGAAATTCTTGGCTATTACCTCGAGGTAATAGCCAAGAATTTCACGTTTTTG | 58 bp |
| Ctnnb1KD3- TRCN0000321075-R | AATTCAAAAACGTGAAATTCTTGGCTATTACCTCGAGGTAATAGCCAAGAATTTCACG | 58 bp |
4.13. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNODC United Nations Office on Drugs and Crime, T.U.N.O. World Drug Report 2023 (United Nations Publication, 2023). 2023. Available online: www.unodc.org/unodc/en/data-and-analysis/world-drug-report-2023.html (accessed on 15 March 2024).
- Cai, D.; Huang, E.; Luo, B.; Yang, Y.; Zhang, F.; Liu, C.; Lin, Z.; Xie, W.B.; Wang, H. Nupr1/Chop signal axis is involved in mitochondrion-related endothelial cell apoptosis induced by methamphetamine. Cell Death Dis. 2016, 7, e2161. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Narasipura, S.D.; Richards, M.H.; Hu, X.T.; Yamamoto, B.; Al-Harthi, L. HIV and drug abuse mediate astrocyte senescence in a beta-catenin-dependent manner leading to neuronal toxicity. Aging Cell 2017, 16, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Melo, P.; Magalhaes, A.; Alves, C.J.; Tavares, M.A.; de Sousa, L.; Summavielle, T.; Moradas-Ferreira, P. Methamphetamine mimics the neurochemical profile of aging in rats and impairs recognition memory. Neurotoxicology 2012, 33, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.; Katila, N.; Lee, S.; Seo, J.H.; Jeong, J.H.; Yook, S. Methamphetamine induced neurotoxic diseases, molecular mechanism, and current treatment strategies. Biomed. Pharmacother. 2022, 154, 113591. [Google Scholar] [CrossRef]
- Panmak, P.; Nopparat, C.; Permpoonpattana, K.; Namyen, J.; Govitrapong, P. Melatonin protects against methamphetamine-induced Alzheimer’s disease-like pathological changes in rat hippocampus. Neurochem. Int. 2021, 148, 105121. [Google Scholar] [CrossRef]
- Pang, L.; Wang, Y. Overview of blood-brain barrier dysfunction in methamphetamine abuse. Biomed. Pharmacother. 2023, 161, 114478. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Northrop, N.A.; Yamamoto, B.K. Methamphetamine effects on blood-brain barrier structure and function. Front. Neurosci. 2015, 9, 69. [Google Scholar] [CrossRef]
- Abdul, M.P.; Alikunju, S.; Szlachetka, A.M.; Murrin, L.C.; Haorah, J. Impairment of brain endothelial glucose transporter by methamphetamine causes blood-brain barrier dysfunction. Mol. Neurodegener. 2011, 6, 23. [Google Scholar] [CrossRef]
- Park, M.; Kim, H.J.; Lim, B.; Wylegala, A.; Toborek, M. Methamphetamine-induced occludin endocytosis is mediated by the Arp2/3 complex-regulated actin rearrangement. J. Biol. Chem. 2013, 288, 33324–33334. [Google Scholar] [CrossRef]
- Chilunda, V.; Weiselberg, J.; Martinez-Meza, S.; Mhamilawa, L.E.; Cheney, L.; Berman, J.W. Methamphetamine induces transcriptional changes in cultured HIV-infected mature monocytes that may contribute to HIV neuropathogenesis. Front. Immunol. 2022, 13, 952183. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.; Baptista, S.; Goncalves, J.; Leal, E.; Milhazes, N.; Borges, F.; Ribeiro, C.F.; Quintela, O.; Lendoiro, E.; Lopez-Rivadulla, M.; et al. Methamphetamine transiently increases the blood-brain barrier permeability in the hippocampus: Role of tight junction proteins and matrix metalloproteinase-9. Brain Res. 2011, 1411, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Maoz, B.M.; Herland, A.; FitzGerald, E.A.; Grevesse, T.; Vidoudez, C.; Pacheco, A.R.; Sheehy, S.P.; Park, T.E.; Dauth, S.; Mannix, R.; et al. A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat. Biotechnol. 2018, 36, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Turowski, P.; Kenny, B.A. The blood-brain barrier and methamphetamine: Open sesame? Front. Neurosci. 2015, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Valenta, T.; Hausmann, G.; Basler, K. The many faces and functions of β-catenin. EMBO J. 2012, 31, 2714–2736. [Google Scholar] [CrossRef]
- Paulus, M.P.; Stewart, J.L. Neurobiology, Clinical Presentation, and Treatment of Methamphetamine Use Disorder: A Review. JAMA Psychiatry 2020, 77, 959–966. [Google Scholar] [CrossRef]
- Shukla, M.; Vincent, B. Methamphetamine abuse disturbs the dopaminergic system to impair hippocampal-based learning and memory: An overview of animal and human investigations. Neurosci. Biobehav. Rev. 2021, 131, 541–559. [Google Scholar] [CrossRef]
- Marshall, J.F.; O’Dell, S.J. Methamphetamine influences on brain and behavior: Unsafe at any speed? Trends Neurosci. 2012, 35, 536–545. [Google Scholar] [CrossRef]
- Luo, Y.L.; Bian, J.W.; Zheng, Z.J.; Zhao, L.; Han, S.; Sun, X.H.; Li, J.F.; Ni, G.X. Effects of methamphetamine abuse on spatial cognitive function. Sci. Rep. 2018, 8, 5502. [Google Scholar] [CrossRef]
- Potvin, S.; Pelletier, J.; Grot, S.; Hebert, C.; Barr, A.M.; Lecomte, T. Cognitive deficits in individuals with methamphetamine use disorder: A meta-analysis. Addict. Behav. 2018, 80, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Belcher, A.M.; Feinstein, E.M.; O’Dell, S.J.; Marshall, J.F. Methamphetamine influences on recognition memory: Comparison of escalating and single-day dosing regimens. Neuropsychopharmacology 2008, 33, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Lazarov, O.; Hollands, C. Hippocampal neurogenesis: Learning to remember. Prog. Neurobiol. 2016, 138–140, 1–18. [Google Scholar] [CrossRef]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Liebner, S.; Dijkhuizen, R.M.; Reiss, Y.; Plate, K.H.; Agalliu, D.; Constantin, G. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. 2018, 135, 311–336. [Google Scholar] [CrossRef]
- Yang, J.; Ran, M.; Li, H.; Lin, Y.; Ma, K.; Yang, Y.; Fu, X.; Yang, S. New insight into neurological degeneration: Inflammatory cytokines and blood-brain barrier. Front. Mol. Neurosci. 2022, 15, 1013933. [Google Scholar] [CrossRef]
- Hu, C.; Li, T.; Xu, Y.; Zhang, X.; Li, F.; Bai, J.; Chen, J.; Jiang, W.; Yang, K.; Ou, Q.; et al. CellMarker 2.0: An updated database of manually curated cell markers in human/mouse and web tools based on scRNA-seq data. Nucleic Acids Res. 2023, 51, D870–D876. [Google Scholar] [CrossRef]
- Montagne, A.; Zhao, Z.; Zlokovic, B.V. Alzheimer’s disease: A matter of blood-brain barrier dysfunction? J. Exp. Med. 2017, 214, 3151–3169. [Google Scholar] [CrossRef]
- Shigetomi, K.; Ikenouchi, J. Cell Adhesion Structures in Epithelial Cells Are Formed in Dynamic and Cooperative Ways. Bioessays 2019, 41, e1800227. [Google Scholar] [CrossRef]
- Bazzoni, G.; Dejana, E. Endothelial cell-to-cell junctions: Molecular organization and role in vascular homeostasis. Physiol. Rev. 2004, 84, 869–901. [Google Scholar] [CrossRef]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/beta-catenin signaling in cancers and targeted therapies. Signal Transduct. Target. Ther. 2021, 6, 307. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Ji, Y.B.; Huang, X.W.; Ma, Y.Z.; Fang, C.; Qiu, L.H.; Tan, X.X.; Chen, Y.M.; Wang, S.N.; Chang, J.; et al. Lithium attenuates blood-brain barrier damage and brain edema following intracerebral hemorrhage via an endothelial Wnt/beta-catenin signaling-dependent mechanism in mice. CNS Neurosci. Ther. 2022, 28, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Boye, K.; Geraldo, L.H.; Furtado, J.; Pibouin-Fragner, L.; Poulet, M.; Kim, D.; Nelson, B.; Xu, Y.; Jacob, L.; Maissa, N.; et al. Endothelial Unc5B controls blood-brain barrier integrity. Nat. Commun. 2022, 13, 1169. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Lee, S.J.; Vlahos, L.; Yuki, K.; Rada, C.C.; van Unen, V.; Vuppalapaty, M.; Chen, H.; Sura, A.; McCormick, A.K.; et al. Therapeutic blood-brain barrier modulation and stroke treatment by a bioengineered FZD(4)-selective WNT surrogate in mice. Nat. Commun. 2023, 14, 2947. [Google Scholar] [CrossRef] [PubMed]
- Manukjan, N.; Chau, S.; Caiment, F.; van Herwijnen, M.; Smeets, H.J.; Fulton, D.; Ahmed, Z.; Blankesteijn, W.M.; Foulquier, S. Wnt7a Decreases Brain Endothelial Barrier Function Via beta-Catenin Activation. Mol. Neurobiol. 2023, 61, 4854–4867. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, X.; Su, Y.; Yin, G.; Wang, S.; Yu, B.; Li, H.; Qi, J.; Chen, H.; Zeng, W.; et al. Activation of Wnt/beta-catenin pathway mitigates blood-brain barrier dysfunction in Alzheimer’s disease. Brain 2022, 145, 4474–4488. [Google Scholar] [CrossRef]
- Gao, Z.X.; Zhang, C.; Lu, J.C.; Zhao, X.; Qiu, H.; Wang, H.J. Pathological methamphetamine exposure triggers the accumulation of neuropathic protein amyloid-beta by inhibiting UCHL1. Neurotoxicology 2021, 86, 19–25. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, C.; Zhao, X.; Lu, J.; Zhang, M.; Qiu, H.; Yue, X.; Wang, H. New insight into methamphetamine-associated heart failure revealed by transcriptomic analyses: Circadian rhythm disorder. Toxicol. Appl. Pharmacol. 2022, 451, 116172. [Google Scholar] [CrossRef]
- Chen, R.; Huang, P.; Wei, S.; Zhang, C.; Lai, X.; Wang, H.; Tang, J. Methamphetamine exposure increases cardiac microvascular permeability by activating the VEGF-PI3K-Akt-eNOS signaling pathway, reversed by Bevacizumab. Hum. Exp. Toxicol. 2022, 41, 774827261. [Google Scholar] [CrossRef]
- Li, B.; Chen, R.; Chen, L.; Qiu, P.; Ai, X.; Huang, E.; Huang, W.; Chen, C.; Liu, C.; Lin, Z.; et al. Effects of DDIT4 in Methamphetamine-Induced Autophagy and Apoptosis in Dopaminergic Neurons. Mol. Neurobiol. 2017, 54, 1642–1660. [Google Scholar] [CrossRef]
- Dean, A.C.; Groman, S.M.; Morales, A.M.; London, E.D. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology 2013, 38, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Lester, D.G.L.A. Some effects of drugs on spontaneous alternation in the Y-maze. J. Comp. Physiol. Psychol. 1950, 6, 482–488. [Google Scholar]
- Riva, M.; Moriceau, S.; Morabito, A.; Dossi, E.; Sanchez-Bellot, C.; Azzam, P.; Navas-Olive, A.; Gal, B.; Dori, F.; Cid, E.; et al. Aberrant survival of hippocampal Cajal-Retzius cells leads to memory deficits, gamma rhythmopathies and susceptibility to seizures in adult mice. Nat. Commun. 2023, 14, 1531. [Google Scholar] [CrossRef] [PubMed]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Menard, C.; Pfau, M.L.; Hodes, G.E.; Kana, V.; Wang, V.X.; Bouchard, S.; Takahashi, A.; Flanigan, M.E.; Aleyasin, H.; LeClair, K.B.; et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 2017, 20, 1752–1760. [Google Scholar] [CrossRef]
- Young, K.; Morrison, H. Quantifying Microglia Morphology from Photomicrographs of Immunohistochemistry Prepared Tissue Using ImageJ. J. Vis. Exp. 2018, 57648. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Franklin, G.P.K.B. The Mouse Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Sun, J.; Ou, W.; Han, D.; Paganini-Hill, A.; Fisher, M.J.; Sumbria, R.K. Comparative studies between the murine immortalized brain endothelial cell line (bEnd.3) and induced pluripotent stem cell-derived human brain endothelial cells for paracellular transport. PLoS ONE 2022, 17, e268860. [Google Scholar] [CrossRef]
- Puscas, I.; Bernard-Patrzynski, F.; Jutras, M.; Lecuyer, M.A.; Bourbonniere, L.; Prat, A.; Leclair, G.; Roullin, V.G. IVIVC Assessment of Two Mouse Brain Endothelial Cell Models for Drug Screening. Pharmaceutics 2019, 11, 587. [Google Scholar] [CrossRef]
- Eigenmann, D.E.; Xue, G.; Kim, K.S.; Moses, A.V.; Hamburger, M.; Oufir, M. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids Barriers CNS 2013, 10, 33. [Google Scholar] [CrossRef]
- Available online: http://rnaidesigner.thermofisher.com/rnaiexpress/ (accessed on 19 September 2023).








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, H.; Zhang, M.; Chen, C.; Wang, H.; Yue, X. Decreasing β-Catenin Leads to Altered Endothelial Morphology, Increased Barrier Permeability and Cognitive Impairment During Chronic Methamphetamine Exposure. Int. J. Mol. Sci. 2025, 26, 1514. https://doi.org/10.3390/ijms26041514
Qiu H, Zhang M, Chen C, Wang H, Yue X. Decreasing β-Catenin Leads to Altered Endothelial Morphology, Increased Barrier Permeability and Cognitive Impairment During Chronic Methamphetamine Exposure. International Journal of Molecular Sciences. 2025; 26(4):1514. https://doi.org/10.3390/ijms26041514
Chicago/Turabian StyleQiu, Hai, Manting Zhang, Chuanxiang Chen, Huijun Wang, and Xia Yue. 2025. "Decreasing β-Catenin Leads to Altered Endothelial Morphology, Increased Barrier Permeability and Cognitive Impairment During Chronic Methamphetamine Exposure" International Journal of Molecular Sciences 26, no. 4: 1514. https://doi.org/10.3390/ijms26041514
APA StyleQiu, H., Zhang, M., Chen, C., Wang, H., & Yue, X. (2025). Decreasing β-Catenin Leads to Altered Endothelial Morphology, Increased Barrier Permeability and Cognitive Impairment During Chronic Methamphetamine Exposure. International Journal of Molecular Sciences, 26(4), 1514. https://doi.org/10.3390/ijms26041514





