Review of Elevated Para-Cresol in Autism and Possible Impact on Symptoms
Abstract
1. Introduction
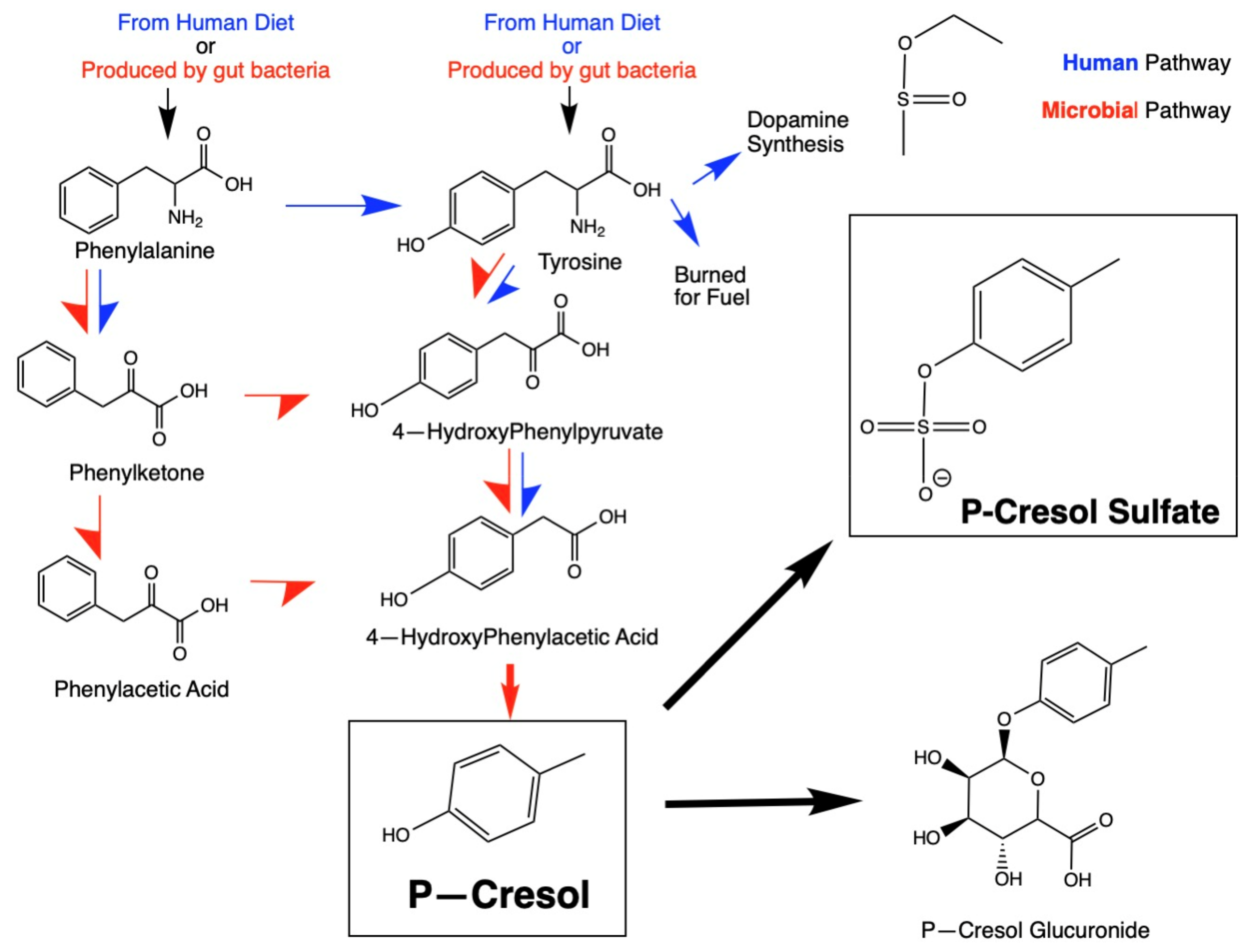
1.1. P-Cresol Production
Human Transformation of P-Cresol and Detoxification Pathways
2. Methods
3. Results
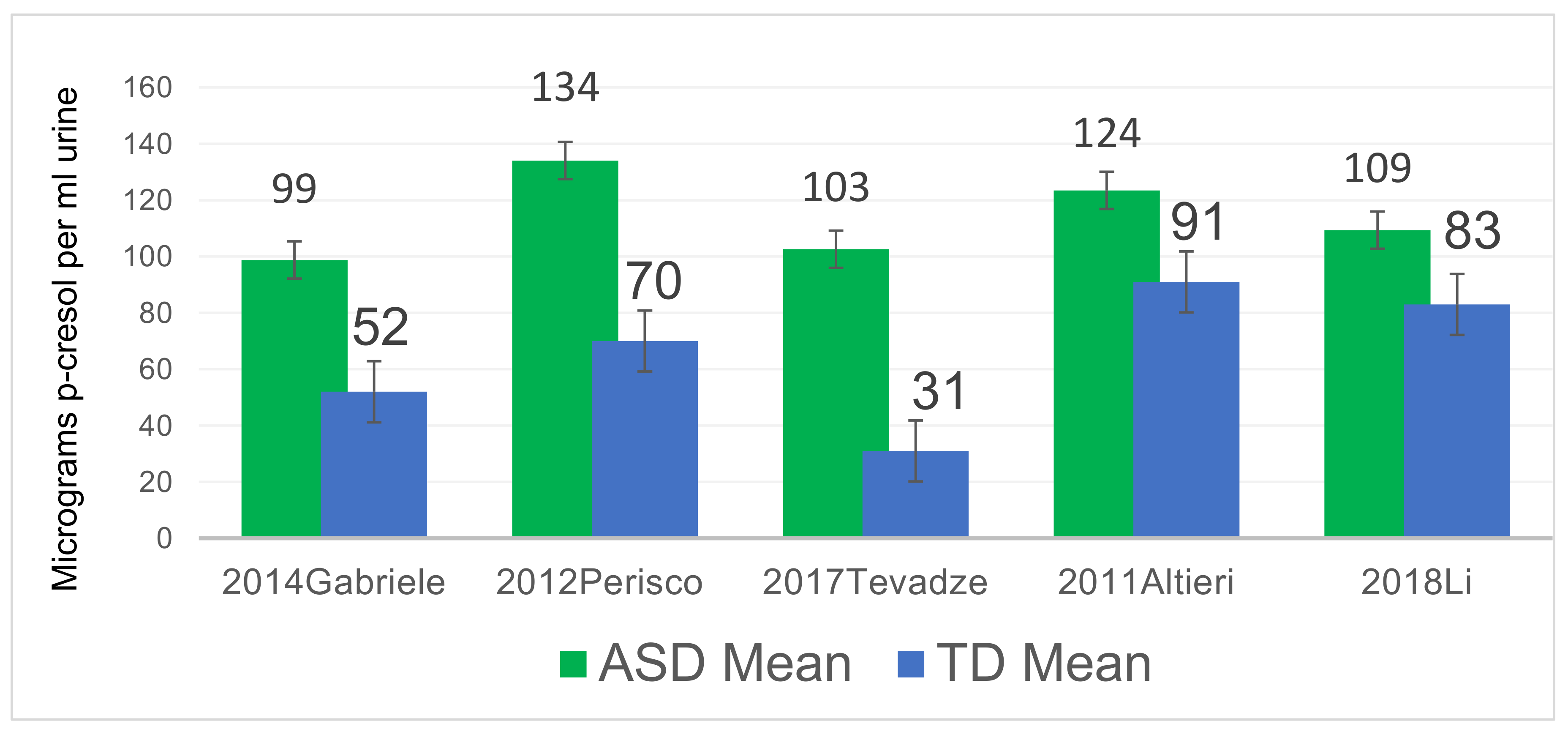
3.1. P-Cresol Is Significantly Higher in Autistic Individuals than Controls Across the World
3.2. Relationships of Age, ASD Severity, and Gender to P-Cresol and pCS Levels
3.3. Health Affects of P-Cresol and/or pCS
P-Cresol May Be Linked to Catecholamine Abnormalities in ASD
3.4. Possible Contribution of P-Cresol and pCS to Sulfation Abnormalities in ASD
3.5. pCS Was Elevated in Feces of ASD Individuals and Decreased After Microbiota Transplant Therapy (MTT)
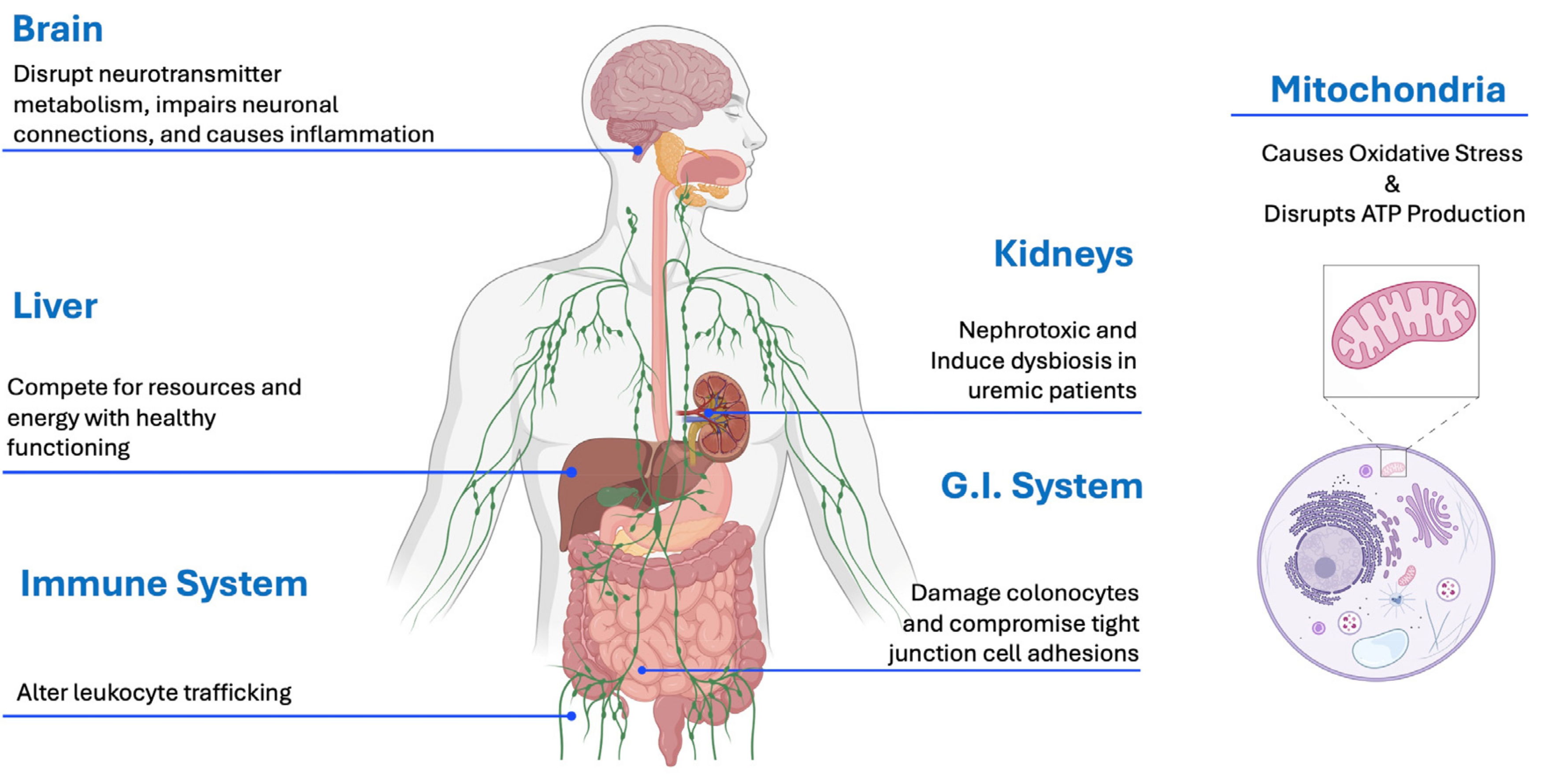
3.6. P-Cresol Toxicity to Various Organ Systems
3.6.1. P-Cresol Causes Mitochondrial Dysfunction and Oxidative Stress
3.6.2. Gastrointestinal Effects of P-Cresol
3.6.3. Neuronal Toxicity of P-Cresol
3.6.4. Nephrotoxicity of P-Cresol Sulfate
3.6.5. Hepatic Effects
3.6.6. Immunological Effects of P-Cresol
3.7. Other Diseases with Links to High Levels of P-Cresol and/or P-CS
3.7.1. Parkinson’s Disease
3.7.2. Epilepsy
3.8. Summary of Effects of P-Cresol and pCS on ASD Symptoms and the Body
4. Discussion
Why Is P-Cresol Elevated in Children with ASD?
5. Future Research Directions
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | Autism Spectrum Disorder |
| TD | Typically Developed |
| pCS | p-Cresol Sulfate |
| G.I. | Gastrointestinal |
| CKD | Chronic Kidney Disease |
| OR | Odds Ratio |
| n.s. | Not Significant |
| ROS | Reactive Oxygen Species |
References
- Earle, W.J. DSM-5. Philos. Forum 2014, 45, 179–196. [Google Scholar] [CrossRef]
- Maenner, M.; Warren, Z.; Williams, A. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years-Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. In Mortality and Morbidity Weekly Report; CDC: Atlanta, GA, USA, 2023; Volume 72. [Google Scholar]
- World Health Organization. Autism Spectrum Disorders & Other Developmental Disorders from Raising Awareness to Building Capacity. Available online: https://iris.who.int/bitstream/handle/10665/103312/?sequence=1 (accessed on 15 November 2024).
- Adams, J.B.; Vargason, T.; Kang, D.W.; Krajmalnik-Brown, R.; Hahn, J. Multivariate analysis of plasma metabolites in children with autism spectrum disorder and gastrointestinal symptoms before and after microbiota transfer therapy. Processes 2019, 7, 806. [Google Scholar] [CrossRef]
- Nirmalkar, K.; Qureshi, F.; Kang, D.W.; Hahn, J.; Adams, J.B.; Krajmalnik-Brown, R. Shotgun Metagenomics Study Suggests Alteration in Sulfur Metabolism and Oxidative Stress in Children with Autism and Improvement after Microbiota Transfer Therapy. Int. J. Mol. Sci. 2022, 23, 13481. [Google Scholar] [CrossRef] [PubMed]
- Krajmalnik-Brown, R.; Lozupone, C.; Kang, D.-W.; Adams, J.B. Gut bacteria in children with autism spectrum disorders: Challenges and promise of studying how a complex community influences a complex disease. Microb. Ecol. Health Dis. 2015, 26, 26914. [Google Scholar] [CrossRef]
- Kang, D.-W.; Adams, J.B.; Vargason, T.; Santiago, M.; Hahn, J.; Krajmalnik-Brown, R. Distinct Fecal and Plasma Metabolites in Children with Autism Spectrum Disorders and Their Modulation after Microbiota Transfer Therapy. mSphere 2020, 5, e00314-20. [Google Scholar] [CrossRef]
- Nirmalkar, K.; Qureshi, F.; Kang, D.-W.; Hahn, J.; Adams, J.B.; Krajmalnik-Brown, R. Reanalysis of metabolomics data reveals that microbiota transfer therapy modulates important fecal and plasma metabolite profiles in children with autism spectrum disorders. bioRxiv 2024. [Google Scholar] [CrossRef]
- Lasheras, I.; Real-López, M.; Santabárbara, J. Prevalence of gastrointestinal symptoms in autism spectrum disorder: A meta-analysis. An. Pediatría (Engl. Ed.) 2023, 99, 102–110. [Google Scholar] [CrossRef]
- Phan, J.; Calvo, D.C.; Nair, D.; Jain, S.; Montagne, T.; Dietsche, S.; Blanchard, K.; Treadwell, S.; Adams, J.; Krajmalnik-Brown, R. Precision synbiotics increase gut microbiome diversity and improve gastrointestinal symptoms in a pilot open-label study for autism spectrum disorder. mSystems 2024, 9, e0050324. [Google Scholar] [CrossRef]
- Argou-Cardozo, I.; Zeidán-Chuliá, F. Clostridium Bacteria and Autism Spectrum Conditions: A Systematic Review and Hypothetical Contribution of Environmental Glyphosate Levels. Med. Sci. 2018, 6, 29. [Google Scholar] [CrossRef]
- Shaw, W. Clostridia bacteria in the GI tract affecting dopamine and norepinephrine metabolism. In Integrative Psychiatry for Depression: Redefining Models for Assessment, Treatment, and Prevention of Mood Disorders; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Zheng, Y.; Bek, M.K.; Prince, N.Z.; Marzal, L.N.P.; Garssen, J.; Pardo, P.P.; Kraneveld, A.D. The Role of Bacterial-Derived Aromatic Amino Acids Metabolites Relevant in Autism Spectrum Disorders: A Comprehensive Review. Front. Neurosci. 2021, 15, 738220. [Google Scholar] [CrossRef]
- Hill, Z.R.; Flynn, C.K.; Adams, J.B. Indoxyl Sulfate and Autism Spectrum Disorder: A Literature Review. Int. J. Mol. Sci. 2024, 25, 12973. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Sato, T.; Nomoto, K.; Tsuji, H. Identification of phenol- and p-cresol-producing intestinal bacteria by using media supplemented with tyrosine and its metabolites. FEMS Microbiol. Ecol. 2018, 94, fiy125. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Marzal, L.N.; Prince, N.; Bajic, D.; Roussin, L.; Naudon, L.; Rabot, S.; Garssen, J.; Kraneveld, A.D.; Perez-Pardo, P. The impact of gut microbiota-derived metabolites in autism spectrum disorders. Int. J. Mol. Sci. 2021, 22, 10052. [Google Scholar] [CrossRef] [PubMed]
- Al Hinai, E.A.; Kullamethee, P.; Rowland, I.R.; Swann, J.; Walton, G.E.; Commane, D.M. Modelling the role of microbial p-cresol in colorectal genotoxicity. Gut Microbes 2019, 10, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Curtius, H. Tyrosine Metabolism p-Cresol stoppage after antibiotic. J. Chromatogr. 1976, 126, 569–580. [Google Scholar] [CrossRef]
- Pascucci, T.; Colamartino, M.; Fiori, E.; Sacco, R.; Coviello, A.; Ventura, R.; Puglisi-Allegra, S.; Turriziani, L.; Persico, A.M. P-cresol Alters Brain Dopamine Metabolism and Exacerbates Autism-Like Behaviors in the BTBR Mouse. Brain Sci. 2020, 10, 233. [Google Scholar] [CrossRef]
- Rafehi, M.; Faltraco, F.; Matthaei, J.; Prukop, T.; Jensen, O.; Grytzmann, A.; Blome, F.G.; Berger, R.G.; Krings, U.; Vormfelde, S.V.; et al. Highly variable pharmacokinetics of tyramine in humans and polymorphisms in OCT1, CYP2D6, and MAO-A. Front. Pharmacol. 2019, 10, 1297. [Google Scholar] [CrossRef]
- Hand, A. Disorders of phenylalanine and tyrosine metabolism. Transl. Sci. Rare Dis. 2000, 5, 3–58. [Google Scholar]
- Harrison, M.A.; Strahl, H.; Dawson, L.F. Regulation of para-cresol production in Clostridioides difficile. Curr. Opin. Microbiol. 2021, 65, 131–137. [Google Scholar] [CrossRef]
- Zhou, Y.; Bi, Z.; Hamilton, M.J.; Zhang, L.; Su, R.; Sadowsky, M.J.; Roy, S.; Khoruts, A.; Chen, C. p-Cresol Sulfate Is a Sensitive Urinary Marker of Fecal Microbiota Transplantation and Antibiotics Treatments in Human Patients and Mouse Models. Int. J. Mol. Sci. 2023, 24, 14621. [Google Scholar] [CrossRef]
- Clayton, T.A.; Baker, D.; Lindon, J.C.; Everett, J.R.; Nicholson, J.K. Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proc. Natl. Acad. Sci. USA 2009, 106, 14728–14733. [Google Scholar] [CrossRef] [PubMed]
- Salman, E.D.; Kadlubar, S.A.; Falany, C.N. Short communication expression and localization of cytosolic sulfotransferase (SULT) 1A1 and SULT1A3 in normal human brain. Drug Metab. Dispos. 2009, 37, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Schepers, E.; Meert, N.; Glorieux, G.; Goeman, J.; Van der Eycken, J.; Vanholder, R. P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol. Dial. Transplant. 2006, 22, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Veronese, M.E.; Burgess, W.; Zhu, X.; McManus, M.E. Functional characterization of two human sulphotransferase cDNAs that encode monoamine-and phenol-sulphating forms of phenol sulphotransferase: Substrate kinetics, thermal-stability and inhibitor-sensitivity studies. Biochem. J. 1994, 302, 497–502. [Google Scholar] [CrossRef]
- Natsuki, I. Organ distribution of endogenous p-cresol in hemodialysis patients. J. Investig. Med. 2019, 66, 81–86. [Google Scholar]
- Altieri, L.; Neri, C.; Sacco, R.; Curatolo, P.; Benvenuto, A.; Muratori, F.; Santocchi, E.; Bravaccio, C.; Lenti, C.; Saccani, M.; et al. Urinary p-cresol is elevated in small children with severe autism spectrum disorder. Biomarkers 2011, 16, 252–260. [Google Scholar] [CrossRef]
- Gabriele, S.; Sacco, R.; Cerullo, S.; Neri, C.; Urbani, A.; Tripi, G.; Malvy, J.; Barthelemy, C.; Bonnet-Brihault, F.; Persico, A.M. Urinary p-cresol is elevated in young French children with autism spectrum disorder: A replication study. Biomarkers 2014, 19, 463–470. [Google Scholar] [CrossRef]
- Diémé, B.; Mavel, S.; Blasco, H.; Tripi, G.; Bonnet-Brilhault, F.; Malvy, J.; Bocca, C.; Andres, C.R.; Nadal-Desbarats, L.; Emond, P. Metabolomics Study of Urine in Autism Spectrum Disorders Using a Multiplatform Analytical Methodology. J. Proteome Res. 2015, 14, 5273–5282. [Google Scholar] [CrossRef]
- Emond, P.; Mavel, S.; Aïdoud, N.; Nadal-Desbarats, L.; Montigny, F.; Bonnet-Brilhault, F.; Barthélémy, C.; Merten, M.; Sarda, P.; Laumonnier, F.; et al. GC-MS-based urine metabolic profiling of autism spectrum disorders. Anal. Bioanal. Chem. 2013, 405, 5291–5300. [Google Scholar] [CrossRef]
- Noto, A.; Fanos, V.; Barberini, L.; Grapov, D.; Fattuoni, C.; Zaffanello, M.; Casanova, A.; Fenu, G.; De Giacomo, A.; De Angelis, M.; et al. The urinary metabolomics profile of an Italian autistic children population and their unaffected siblings. J. Matern.-Fetal Neonatal Med. 2014, 27, 46–52. [Google Scholar] [CrossRef]
- Piras, C.; Mussap, M.; Noto, A.; De Giacomo, A.; Cristofori, F.; Spada, M.; Fanos, V.; Atzori, L.; Francavilla, R. Alterations of the Intestinal Permeability are Reflected by Changes in the Urine Metabolome of Young Autistic Children: Preliminary Results. Metabolites 2022, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Tevzadze, G.; Shanshiashvilli, L.; Mikeladze, D. Children with epilepsy and autistic spectrum disorders show similarly high levels of urinary p-cresol. J. Biol. Phys. Chem. 2017, 17, 77–80. [Google Scholar] [CrossRef]
- Li, C.; Shen, K.; Ning, Y.; Wang, S.; Song, Y.; Liu, P.; Han, Q.; Wei, L.; Kang, X. Improved Sample Preparation Using Packed-Fiber Solid Phase Extraction in the Determination of Urinary P-Cresol. Nanosci. Nanotechnol. Lett. 2018, 10, 1469–1475. [Google Scholar] [CrossRef]
- Persico, A.M.; Napolioni, V. Urinary p-cresol in autism spectrum disorder. Neurotoxicol. Teratol. 2013, 36, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Osredkar, J.; Baškovič, B.Ž.; Finderle, P.; Bobrowska-Korczak, B.; Gątarek, P.; Rosiak, A.; Giebułtowicz, J.; Vrhovšek, M.J.; Kałużna-Czaplińska, J. Relationship between Excreted Uremic Toxins and Degree of Disorder of Children with ASD. Int. J. Mol. Sci. 2023, 24, 7078. [Google Scholar] [CrossRef]
- Mussap, M.; Siracusano, M.; Noto, A.; Fattuoni, C.; Riccioni, A.; Rajula, H.S.R.; Fanos, V.; Curatolo, P.; Barberini, L.; Mazzone, L. The Urine Metabolome of Young Autistic Children Correlates with Their Clinical Profile Severity. Metabolites 2020, 10, 476. [Google Scholar] [CrossRef]
- Gevi, F.; Belardo, A.; Zolla, L. A metabolomics approach to investigate urine levels of neurotransmitters and related metabolites in autistic children. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165859. [Google Scholar] [CrossRef]
- Chen, Q.; Qiao, Y.; Xu, X.; You, X.; Tao, Y. Urine Organic Acids as Potential Biomarkers for Autism-Spectrum Disorder in Chinese Children. Front. Cell Neurosci. 2019, 13, 150. [Google Scholar] [CrossRef]
- Daneberga, Z.; Nakazawa-Miklasevica, M.; Berga-Svitina, E.; Murmane, D.; Isarova, D.; Cupane, L.; Masinska, M.; Nartisa, I.; Lazdane, A.; Miklasevics, E. Urinary organic acids spectra in children with altered gut microbiota composition and autistic spectrum disorder. Nord. J. Psychiatry 2022, 76, 523–529. [Google Scholar] [CrossRef]
- Gevi, F.; Zolla, L.; Gabriele, S.; Persico, A.M. Urinary metabolomics of young Italian autistic children supports abnormal tryptophan and purine metabolism. Mol. Autism 2016, 7, 47. [Google Scholar] [CrossRef]
- Timperio, A.M.; Gevi, F.; Cucinotta, F.; Ricciardello, A.; Turriziani, L.; Scattoni, M.L.; Persico, A.M. Urinary Untargeted Metabolic Profile Differentiates Children with Autism from Their Unaffected Siblings. Metabolites 2022, 12, 797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chu, Y.; Meng, Q.; Ding, R.; Shi, X.; Wang, Z.; He, Y.; Zhang, J.; Liu, J.; Zhang, J.; et al. A quasi-paired cohort strategy reveals the impaired detoxifying function of microbes in the gut of autistic children. Sci. Adv. 2020, 6, eaba3760. [Google Scholar] [CrossRef] [PubMed]
- Bossard, M.J.; Klinman, J.P. Mechanism-based Inhibition of Dopamine Beta Monooxygenase by Aldehydes Amides. J. Biol. Chem. 1986, 251, 16421–16427. [Google Scholar] [CrossRef]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Effect of a vitamin/mineral supplement on children and adults with autism. BMC Pediatr. 2011, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Alberti, A.; Pirrone, P.; Elia, M.; Waring, R.H.; Romano, C. Sulphation Deficit in ‘Low-Functioning’ Autistic Children: A Pilot Study. Biol. Psychiatry 1999, 46, 420–424. [Google Scholar] [CrossRef]
- Horvath, K.; Waring, R. Abnormal Sulfate Metabolism in Autism. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 335. [Google Scholar] [CrossRef]
- Waring, R.H.; Klovrza, L.V. Sulphur metabolism in autism. J. Nutr. Environ. Med. 2000, 10, 25–32. [Google Scholar] [CrossRef]
- Marto, N.; Morello, J.; Antunes, A.M.M.; Azeredo, S.; Monteiro, E.C.; Pereira, S.A. A simple method to measure sulfonation in man using paracetamol as probe drug. Sci. Rep. 2021, 11, 9036. [Google Scholar] [CrossRef]
- Geier, D.A.; Kern, J.K.; Garver, C.R.; Adams, J.B.; Audhya, T.; Geier, M.R. A Prospective Study of Transsulfuration Biomarkers in Autistic Disorders. Neurochem. Res. 2009, 34, 386–393. [Google Scholar] [CrossRef]
- Pagan, C. Decreased phenol sulfotransferase activities associated with hyperserotonemia in autism spectrum disorders. Transl. Psychiatry 2021, 11, 23. [Google Scholar] [CrossRef]
- Kang, D.-W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci. Rep. 2019, 9, 5821. [Google Scholar] [CrossRef] [PubMed]
- Holingue, C.; Pfeiffer, D.; Ludwig, N.N.; Reetzke, R.; Hong, J.S.; Kalb, L.G.; Landa, R. Prevalence of gastrointestinal symptoms among autistic individuals, with and without co-occurring intellectual disability. Autism Res. 2023, 16, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.-J.; Andersen, K.; Stecher, B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013, 83, 1010–1016. [Google Scholar] [CrossRef]
- Blachier, F.; Andriamihaja, M. Effects of the l-tyrosine-derived bacterial metabolite p-cresol on colonic and peripheral cells. Amino Acids 2022, 54, 325–338. [Google Scholar] [CrossRef]
- Gryp, T.; Vanholder, R.; Vaneechoutte, M.; Glorieux, G. p-Cresyl Sulfate. Toxins 2017, 9, 52. [Google Scholar] [CrossRef]
- Kitigawa, A. Effects of Cresols (O-, M-, AND P-Isomers) on the Bioenergetic System In Isolated Rat Liver Mitochondria. Drug Chem. Toxicol. 2001, 24, 39–47. [Google Scholar] [CrossRef]
- Tevzadze, G.; Barbakadze, T.; Kvergelidze, E.; Zhuravliova, E.; Shanshiashvili, L.; Mikeladze, D. Gut neurotoxin p-cresol induces brain-derived neurotrophic factor secretion and increases the expression of neurofilament subunits in PC-12 cells. AIMS Neurosci. 2021, 9, 12–23. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Yuan, J.; Nazertehrani, S.; Ni, Z.; Liu, S. Chronic Kidney Disease Causes Disruption of Gastric and Small Intestinal Epithelial Tight Junction. Am. J. Nephrol. 2013, 38, 99–103. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Mitochondrial dysfunction in autism spectrum disorders: A systematic review and meta-analysis. Mol. Psychiatry 2012, 17, 290–314. [Google Scholar] [CrossRef]
- Kozieł, R.; Pircher, H.; Kratochwil, M.; Lener, B.; Hermann, M.; Dencher, N.A.; Jansen-Dürr, P. Mitochondrial respiratory chain complex I is inactivated by NADPH oxidase Nox4. Biochem. J. 2013, 452, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Snell, E.E. Reversibility of the Tryptophanase Reaction: Synthesis of Tryptophan from Indole, Pyruvate, and Ammonia. Proc. Natl. Acad. Sci. USA 1972, 69, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Edamatsu, T.; Fujieda, A.; Itoh, Y. Phenyl sulfate, indoxyl sulfate and p-cresyl sulfate decrease glutathione level to render cells vulnerable to oxidative stress in renal tubular cells. PLoS ONE 2018, 13, e0193342. [Google Scholar] [CrossRef]
- Sun, C.Y.; Hsu, H.H.; Wu, M.S. P-Cresol sulfate and indoxyl sulfate induce similar cellular inflammatory gene expressions in cultured proximal renal tubular cells. Nephrol. Dial. Transplant. 2013, 28, 70–78. [Google Scholar] [CrossRef]
- Sun, C.Y.; Cheng, M.L.; Pan, H.C.; Lee, J.H.; Lee, C.C. Protein-bound uremic toxins impaired mitochondrial dynamics and functions. Oncotarget 2017, 8, 77722. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, H.M.; Maher, N.; Torres, R.; Leo, G.C.; Caldwell, G.W.; Huebert, N. Bioactivation of 4-Methylphenol (p-cresol) via CYP-mediated Aromatic Oxidation in Human Liver Microsomes. Drug Metab. Dispos. 2005, 33, 1867–1876. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Li, X.; Yuan, F.-H. Protein-Bound P-Cresol Inhibits Human Umbilical Vein Endothelial Cell Proliferation by Inducing Cell Cycle Arrest at G0/G1. 2017. Available online: www.ajtr.org (accessed on 23 January 2024).
- Morton, J. Multi-omic analysis along the gut-brain axis points to a functional architecture of autism Conflict of Interest. BioRxiv 2022. [Google Scholar] [CrossRef]
- Watanabe, H.; Miyamoto, Y.; Honda, D.; Tanaka, H.; Wu, Q.; Endo, M.; Noguchi, T.; Kadowaki, D.; Ishima, Y.; Kotani, S.; et al. p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase. Kidney Int. 2013, 83, 582–592. [Google Scholar] [CrossRef]
- Passmore, I.J.; Letertre, M.P.M.; Preston, M.D.; Bianconi, I.; Harrison, M.A.; Nasher, F.; Kaur, H.; Hong, H.A.; Baines, S.D.; Cutting, S.M.; et al. Para-cresol production by Clostridium difficile affects microbial diversity and membrane integrity of Gram-negative bacteria. PLoS Pathog. 2018, 14, e1007191. [Google Scholar] [CrossRef]
- Schmid, R.; Schmid, R.; Petras, D.; Petras, D.; Nothias, L.-F.; Nothias, L.-F.; Wang, M.; Wang, M.; Aron, A.T.; Aron, A.T.; et al. Ion identity molecular networking for mass spectrometry-based metabolomics in the GNPS environment. Nat. Commun. 2021, 12, 3832. [Google Scholar] [CrossRef]
- Liu, Q.; Yin, W.; Meijsen, J.; Reichenberg, A.; Gådin, J.; Schork, A.; Adami, H.-O.; Kolevzon, A.; Sandin, S.; Fang, F. Cancer risk in individuals with autism spectrum disorder. Ann. Oncol. 2022, 33, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, S.; Sacco, R.; Altieri, L.; Neri, C.; Urbani, A.; Bravaccio, C.; Riccio, M.P.; Iovene, M.R.; Bombace, F.; De Magistris, L.; et al. Slow intestinal transit contributes to elevate urinary p-cresol level in Italian autistic children. Autism Res. 2016, 9, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Martin, P.; Becker, J.A.J.; Caramello, N.; Fernandez, S.P.; Costa-Campos, R.; Canaguier, J.; Barbosa, S.; Martinez-Gili, L.; Myridakis, A.; Dumas, M.-E.; et al. The microbial metabolite p-Cresol induces autistic-like behaviors in mice by remodeling the gut microbiota. Microbiome 2021, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-C.; Tomino, Y.; Lu, K.-C. Impacts of Indoxyl Sulfate and p-Cresol Sulfate on Chronic Kidney Disease and Mitigating Effects of AST-120. Toxins 2018, 10, 367. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Z.; Liu, C.; Liu, T.; Gao, J.; Cai, Y.; Fan, X. Alteration of Gut Microbiota: New Strategy for Treating Autism Spectrum Disorder. Front. Cell Dev. Biol. 2022, 10, 792490. [Google Scholar] [CrossRef]
- Guzmán-Salas, S.; Weber, A.; Malci, A.; Lin, X.; Herrera-Molina, R.; Cerpa, W.; Dorador, C.; Signorelli, J.; Zamorano, P. The metabolite p-cresol impairs dendritic development, synaptogenesis, and synapse function in hippocampal neurons: Implications for autism spectrum disorder. J. Neurochem. 2022, 161, 335–349. [Google Scholar] [CrossRef]
- Sankowski, B.; Księżarczyk, K.; Raćkowska, E.; Szlufik, S.; Koziorowski, D.; Giebułtowicz, J. Higher cerebrospinal fluid to plasma ratio of p-cresol sulfate and indoxyl sulfate in patients with Parkinson’s disease. Clin. Chim. Acta 2020, 501, 165–173. [Google Scholar] [CrossRef]
- Redcay, E.; Courchesne, E. When Is the Brain Enlarged in Autism? A Meta-Analysis of All Brain Size Reports. Biol. Psychiatry 2005, 58, 1–9. [Google Scholar] [CrossRef]
- Miot, S.; Akbaraly, T.; Michelon, C.; Couderc, S.; Crepiat, S.; Loubersac, J.; Picot, M.-C.; Pernon, É.; Gonnier, V.; Jeandel, C.; et al. Comorbidity Burden in Adults With Autism Spectrum Disorders and Intellectual Disabilities—A Report From the EFAAR (Frailty Assessment in Ageing Adults With Autism Spectrum and Intellectual Disabilities) Study. Front. Psychiatry 2019, 10, 617. [Google Scholar] [CrossRef]
- Bjørklund, G.; Meguid, N.A.; El-Bana, M.A.; Tinkov, A.A.; Saad, K.; Dadar, M.; Hemimi, M.; Skalny, A.V.; Hosnedlová, B.; Kizek, R.; et al. Oxidative Stress in Autism Spectrum Disorder. Mol. Neurobiol. 2020, 57, 2314–2332. [Google Scholar] [CrossRef]
- Bammens, B.; Evenepoel, P.; Keuleers, H.; Verbeke, K.; Vanrenterghem, Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006, 69, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, R.; De, A.; Izhar, R.; Abate, M.; Zappavigna, S.; Capasso, A.; Perna, A.F.; La Russa, A.; Capasso, G.; Caraglia, M.; et al. Possible Effects of Uremic Toxins p-Cresol, Indoxyl Sulfate, p-Cresyl Sulfate on the Development and Progression of Colon Cancer in Patients with Chronic Renal Failure. Genes 2023, 14, 1257. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; De Smet, R.; Waterloos, M.-A.; Van Landschoot, N.; Vogeleere, P.; Hoste, E.; Ringoir, S. Mechanisms of uremic inhibition of phagocyte reactive species production: Characterization of the role of p-cresol. Kidney Int. 1995, 47, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Wratten, M.; Tetta, C.; De Smet, R.; Neri, R.; Sereni, L.; Camussi, G.; Vanholder, R. Uremic Ultrafiltrate Inhibits Platelet-Activating Factor Synthesis. Blood Purif. 1999, 17, 134–141. [Google Scholar] [CrossRef]
- Kawakami, K.; Makino, I.; Kato, I.; Uchida, K.; Onoue, M. p-Cresol inhibits IL-12 production by murine macrophages stimulated with bacterial immunostimulant. Immunopharmacol. Immunotoxicol. 2009, 31, 304–309. [Google Scholar] [CrossRef]
- Dou, L.; Cerini, C.; Brunet, P.; Guilianelli, C.; Moal, V.; Grau, G.; De Smet, R.; Vanholder, R.; Sampol, J.; Berland, Y. P-cresol, a uremic toxin, decreases endothelial cell response to inflammatory cytokines. Kidney Int. 2002, 62, 1999–2009. [Google Scholar] [CrossRef]
- Faure, V.; Cerini, C.; Paul, P.; Berland, Y.; Dignat-George, F.; Brunet, P. The uremic solute p-cresol decreases leukocyte transendothelial migration in vitro. Int. Immunol. 2006, 18, 1453–1459. [Google Scholar] [CrossRef][Green Version]
- Cerini, C.; Dou, L.; Anfosso, F.; Sabatier, F.; Moal, V.; Glorieux, G.; De Smet, R.; Vanholder, R.; Dignat-George, F.; Sampol, J.; et al. P-cresol, a uremic retention solute, alters the endothelial barrier function in vitro. Thromb. Haemost. 2004, 92, 140–150. [Google Scholar] [CrossRef]
- Vanholder, R.; Bammens, B.; de Loor, H.; Glorieux, G.; Meijers, B.; Schepers, E.; Massy, Z.; Evenepoel, P. Warning: The unfortunate end of p-cresol as a uraemic toxin. Nephrol. Dial. Transplant. 2011, 26, 1464–1467. [Google Scholar] [CrossRef]
- Akchurin, O.M.; Kaskel, F. Update on Inflammation in Chronic Kidney Disease. Blood Purif. 2015, 39, 84–92. [Google Scholar] [CrossRef]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Yuan, J.; Rahimi, A.; Ni, Z.; Said, H.; Subramanian, V.S. Disintegration of colonic epithelial tight junction in uremia: A likely cause of CKD-associated inflammation. Nephrol. Dial. Transplant. 2012, 27, 2686–2693. [Google Scholar] [CrossRef] [PubMed]
- Wong, X.; Carrasco-Pozo, C.; Escobar, E.; Navarrete, P.; Blachier, F.; Andriamihaja, M.; Lan, A.; Tomé, D.; Cires, M.J.; Pastene, E.; et al. Deleterious Effect of p-Cresol on Human Colonic Epithelial Cells Prevented by Proanthocyanidin-Containing Polyphenol Extracts from Fruits and Proanthocyanidin Bacterial Metabolites. J. Agric. Food Chem. 2016, 64, 3574–3583. [Google Scholar] [CrossRef] [PubMed]
- Meijers, B.K.I.; Van Kerckhoven, S.; Verbeke, K.; Dehaen, W.; Vanrenterghem, Y.; Hoylaerts, M.F.; Evenepoel, P. The Uremic Retention Solute p-Cresyl Sulfate and Markers of Endothelial Damage. Am. J. Kidney Dis. 2009, 54, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Pletinck, A.; Glorieux, G.; Schepers, E.; Cohen, G.; Gondouin, B.; Van Landschoot, M.; Eloot, S.; Rops, A.; Van de Voorde, J.; De Vriese, A.; et al. Protein-Bound Uremic Toxins Stimulate Crosstalk between Leukocytes and Vessel Wall. J. Am. Soc. Nephrol. 2013, 24, 1981–1994. [Google Scholar] [CrossRef]
- Shiba, T.; Kawakami, K.; Sasaki, T.; Makino, I.; Kato, I.; Kobayashi, T.; Uchida, K.; Kaneko, K. Effects of intestinal bacteria-derived p-cresyl sulfate on Th1-type immune response in vivo and in vitro. Toxicol. Appl. Pharmacol. 2014, 274, 191–199. [Google Scholar] [CrossRef]
- Azevedo, M.; Bonan, N.; Dias, G.; Brehm, F.; Steiner, T.; Souza, W.; Stinghen, A.; Barreto, F.; Elifio-Esposito, S.; Pecoits-Filho, R.; et al. p-Cresyl sulfate affects the oxidative burst, phagocytosis process, and antigen presentation of monocyte-derived macrophages. Toxicol. Lett. 2016, 263, 1–5. [Google Scholar] [CrossRef]
- Zheng, Y.; Prince, N.Z.; Marzal, L.N.P.; Ahmed, S.; Garssen, J.; Pardo, P.P.; Kraneveld, A.D. The Autism Spectrum Disorder-Associated Bacterial Metabolite p-Cresol Derails the Neuroimmune Response of Microglial Cells Partially via Reduction of ADAM17 and ADAM10. Int. J. Mol. Sci. 2022, 23, 11013. [Google Scholar] [CrossRef]
- Jorissen, E.; Prox, J.; Bernreuther, C.; Weber, S.; Schwanbeck, R.; Serneels, L.; Snellinx, A.; Craessaerts, K.; Thathiah, A.; Tesseur, I.; et al. The Disintegrin/Metalloproteinase ADAM10 Is Essential for the Establishment of the Brain Cortex. J. Neurosci. 2010, 30, 4833–4844. [Google Scholar] [CrossRef]
- Zhuang, J.; Wei, Q.; Lin, Z.; Zhou, C. Effects of ADAM10 deletion on Notch-1 signaling pathway and neuronal maintenance in adult mouse brain. Gene 2015, 555, 150–158. [Google Scholar] [CrossRef]
- Mai, A.S.; Yau, C.E.; Tseng, F.S.; Foo, Q.X.J.; Wang, D.Q.; Tan, E.-K. Linking autism spectrum disorders and parkinsonism: Clinical and genetic association. Ann. Clin. Transl. Neurol. 2023, 10, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.C.; Zhang, K.; Walker, D.I.; Sinsheimer, J.; Yu, Y.; Kusters, C.; Del Rosario, I.; Folle, A.D.; Keener, A.M.; Bronstein, J.; et al. Untargeted serum metabolomics reveals novel metabolite associations and disruptions in amino acid and lipid metabolism in Parkinson’s disease. Mol. Neurodegener. 2023, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Li, T.; Liu, Z.; Wang, X.; Xu, X.; Li, S.; Xu, G.; Le, W. Comprehensive metabolic profiling of Parkinson’s disease by liquid chromatography-mass spectrometry. Mol. Neurodegener. 2021, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Liu, L.-F.; Tang, Z.; Zhang, M.; Chua, K.-K.; Song, J.-X.; Mok, V.C.; Li, M.; Cai, Z. Comprehensive urinary metabolomic profiling and identification of potential noninvasive marker for idiopathic Parkinson s disease. Sci. Rep. 2015, 5, srep13888. [Google Scholar] [CrossRef]
- Yao, L.; Liang, W.; Chen, J.; Wang, Q.; Huang, X. Constipation in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Eur. Neurol. 2023, 86, 34–44. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Constipation in Parkinson’s Disease. Semin. Neurol. 2023, 43, 562–571. [Google Scholar] [CrossRef]
- Adams-Carr, K.L.; Bestwick, J.P.; Shribman, S.; Lees, A.; Schrag, A.; Noyce, A.J. Constipation preceding Parkinson’s disease: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 710–716. [Google Scholar] [CrossRef]
- Desai, P.R. Mechanistic and Structural Studies of Dopamine Beta Hydroxylase; The Pennsylvania State University: State College, PA, USA, 1987. [Google Scholar]
- Lewine, J.; Andrews, R.; Che, M. Magnetoencephalographic Patterns of Epileptiform Activity in Children with Regressive Autism Spectrum Disorders. Pediatrics 1999, 104, 405–418. [Google Scholar] [CrossRef]
- Tevzadze, G.; Zhuravliova, E.; Barbakadze, T.; Shanshiashvili, L.; Dzneladze, D.; Nanobashvili, Z.; Lordkipanidze, T.; Mikeladze, D. Gut neurotoxin p-cresol induces differential expression of GLUN2B and GLUN2A subunits of the NMDA receptor in the hippocampus and nucleus accumbens in healthy and audiogenic seizure-prone rats. AIMS Neurosci. 2020, 7, 30–42. [Google Scholar] [CrossRef]
- Williams, R.J. Sulfate Deficiency as a Risk Factor for Autism. J. Autism Dev. Disord. 2020, 50, 153–161. [Google Scholar] [CrossRef]

| Study | ASD Participants | TD Participants | Country of Origin | Findings |
|---|---|---|---|---|
| Altieri, 2011 [29] | 59 | 59 | Italy | Significantly ↑ |
| Chen, 2013 [41] | 156 | 64 | China | Significantly ↑ |
| Daneberga, 2021 [42] | 44 | ** | Latvia | ** |
| Diémé, 2015 [31] | 30 | 32 | France | Significantly ↑ |
| Emond, 2013 [32] | 26 | 24 | France | Significantly ↑ |
| Gabriele, 2014 [30] | 33 | 33 | France | Significantly ↑ |
| Gevi, 2016 [43] | 30 | 30 | Italy | ↑ |
| Gevi, 2020 [40] | 40 | 40 | Italy | Significantly ↑ |
| Li, 2018 [36] | 33 | 44 | China | Significantly ↑ |
| Mussap, 2020 [39] | 31 | 26 | Italy | Significantly ↑ |
| Noto, 2014 [33] | 30 | 28 | France | ↑ |
| Osredkar, 2023 [38] | 143 | 48 | Slovenia | Significantly ↑ |
| Perisco, 2012 [37] | 59 | 59 | Italy | Significantly ↑ |
| Piras, 2022 [34] | 13 | 14 | Italy | Significantly ↑ |
| Timperio, 2022 [44] | 14 | 14 | Italy | Significantly ↑ |
| Tevzadze, 2017 [35] | 14 | 14 | Georgia | Significantly ↑ |
| Zhang, 2020 [45] | 39 | 40 | China | ↑ |
| System | Effects |
|---|---|
| ASD (General) | Causally induces ASD-like behaviors and social deficits in animal experiments |
| Urinary levels of P-cresol or pCS sulfate in children with ASD correlated with severity of ASD and ASD-related symptoms | |
| Mitochondria | P-cresol and pCS disrupts mitochondrial function |
| Increased reactive oxygen species and oxidative stress | |
| Depleted glutathione (primary antioxidant) | |
| Decreased production of ATP (major fuel for body and brain) | |
| Gut | P-cresol is an antibiotic to many gut bacteria species, altering the gut microbiome |
| Weakens the intestinal barrier, allowing greater amounts of microbial metabolites to leak into the body | |
| Brain | Competes with neurotransmitter synthesis metabolism as they share a parent compound, phenylalanine |
| Impairs dendritic growth and arborization of neurons | |
| Modulates early brain growth and affects NMDAR and D1 Dopamine receptor development | |
| Irreversibly inhibits Dopamine-Beta-Hydroxylase, an enzyme vital to catecholamine metabolism | |
| Liver | Competes with bilirubin and other microbial metabolites as it is bound to albumin |
| Liver converts p-cresol into p-cresol sulfate, which reduces the sulfate pool for detoxification of other substances, including many neurotransmitters | |
| Cytochrome P450 enzymes in liver microsomes convert p-cresol into quinone methides, which are highly reactive oxygen species | |
| Kidneys | pCS is highly toxic to the kidneys (nephrotoxin) |
| Induces renal tubular cell necrosis in a dose-dependent manner | |
| Creates oxidative stress and inflammatory responses | |
| May interfere with filtration efficiency | |
| Immune System | Depresses phagocytic activity of macrophages |
| Activates nuclear factor kappa beta signaling | |
| Reduces Th1 cytokine production | |
| Alters leukocyte trafficking |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flynn, C.K.; Adams, J.B.; Krajmalnik-Brown, R.; Khoruts, A.; Sadowsky, M.J.; Nirmalkar, K.; Takyi, E.; Whiteley, P. Review of Elevated Para-Cresol in Autism and Possible Impact on Symptoms. Int. J. Mol. Sci. 2025, 26, 1513. https://doi.org/10.3390/ijms26041513
Flynn CK, Adams JB, Krajmalnik-Brown R, Khoruts A, Sadowsky MJ, Nirmalkar K, Takyi E, Whiteley P. Review of Elevated Para-Cresol in Autism and Possible Impact on Symptoms. International Journal of Molecular Sciences. 2025; 26(4):1513. https://doi.org/10.3390/ijms26041513
Chicago/Turabian StyleFlynn, Christina K., James B. Adams, Rosa Krajmalnik-Brown, Alexander Khoruts, Michael J. Sadowsky, Khemlal Nirmalkar, Evelyn Takyi, and Paul Whiteley. 2025. "Review of Elevated Para-Cresol in Autism and Possible Impact on Symptoms" International Journal of Molecular Sciences 26, no. 4: 1513. https://doi.org/10.3390/ijms26041513
APA StyleFlynn, C. K., Adams, J. B., Krajmalnik-Brown, R., Khoruts, A., Sadowsky, M. J., Nirmalkar, K., Takyi, E., & Whiteley, P. (2025). Review of Elevated Para-Cresol in Autism and Possible Impact on Symptoms. International Journal of Molecular Sciences, 26(4), 1513. https://doi.org/10.3390/ijms26041513





