Cytotoxicity and Antibacterial Activity of Protonated Diallylammonium Polymers: Influence of End Groups and Molecular Weight
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characteristics of Polymers
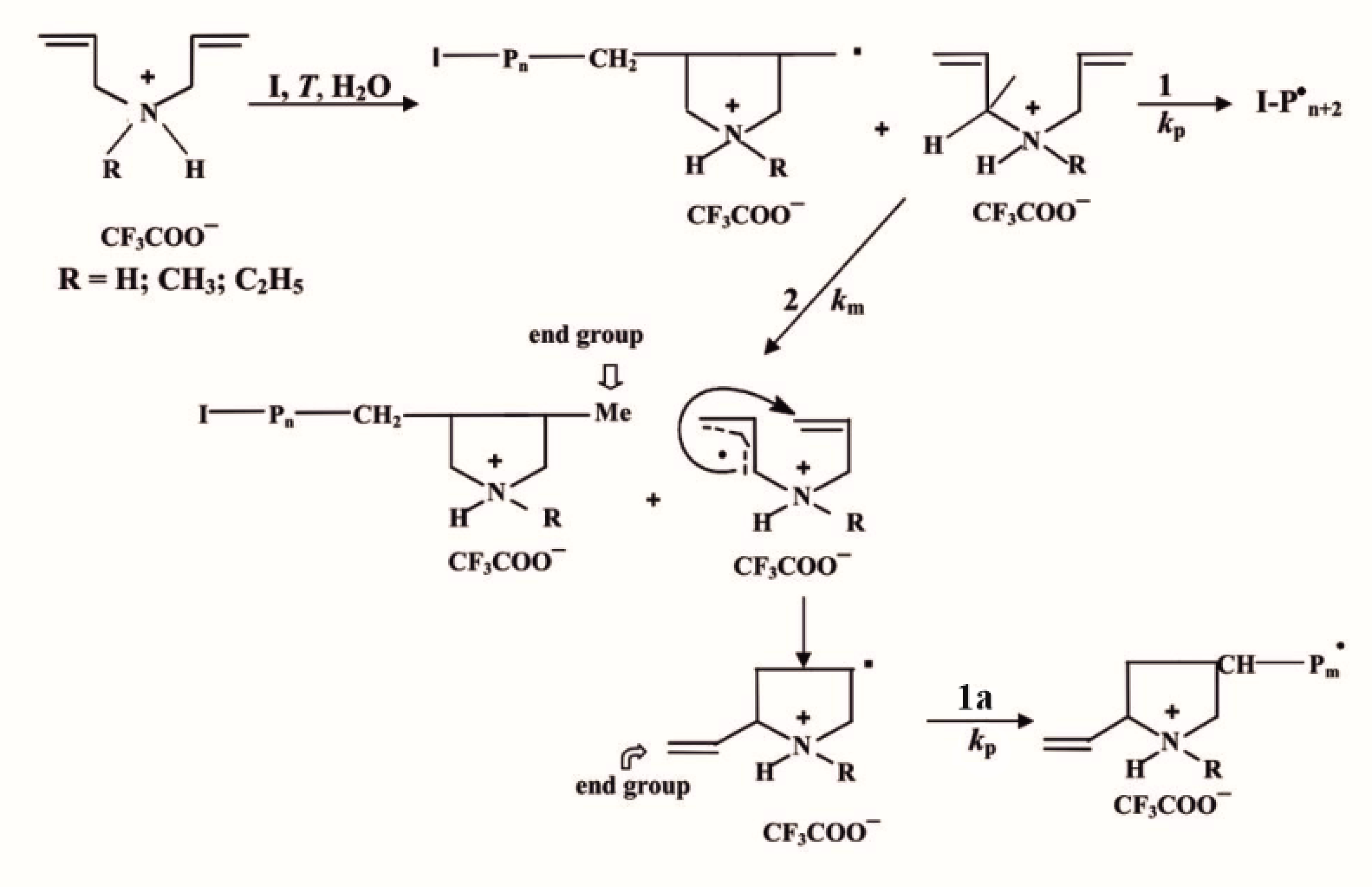


| Polymer Sample | Polymer | I | [I], mol·L−1 | T, °C | [η], cm·g−1 (k′) | MDη × 10−3, g·mol−1 (Based on A0) | Mη × 10−3, g·mol−1 (from M-K-H) |
|---|---|---|---|---|---|---|---|
| P0 | PDAATFA | APS | 2 × 10−2 | 50 | 13.0 ± 0.8 | 43 ± 1 | 42.8 ± 4.9 |
| P1 | PDAATFA | APS | 4 × 10−2 | 40 | 12.8 ± 0.8 | 40 ± 2 | 41.6 ± 4.8 |
| P2 | ″ | ″ | 10−1 | 50 | 8.1 ± 0.4 (k′ = 0.439) | 17.9 ± 0.9 | 17.8 ± 1.6 |
| P3 | ″ | ″ | 4 × 10−2 | 50 | 10.0 ± 0.6 (k′ = 0.398) | 27 ± 2 | 26.3 ± 2.9 |
| P4 | ″ | ″ | 10−1 | 40 | 10.0 ± 0.8 (k′ = 0.429) | 28 ± 1 | 26.3 ± 3.9 |
| P5 | ″ | ACVA | 10−1 | 70 | 9.3 ± 0.4 (k’ = 0.40) | - | 23.0 ± 1.8 |
| P6 | ″ | ″ | 4 × 10−2 | 70 | 11.8 ± 0.5 (k′ = 0.496) | - | 35.8 ± 2.8 |
| P8 a | ″ | ACVA | 5 × 10−3 | 70 | 5.1 ± 0.5 | 8.0 ± 0.5 | 7.6 ± 1.4 |
2.2. Toxicity of Tested Polymers
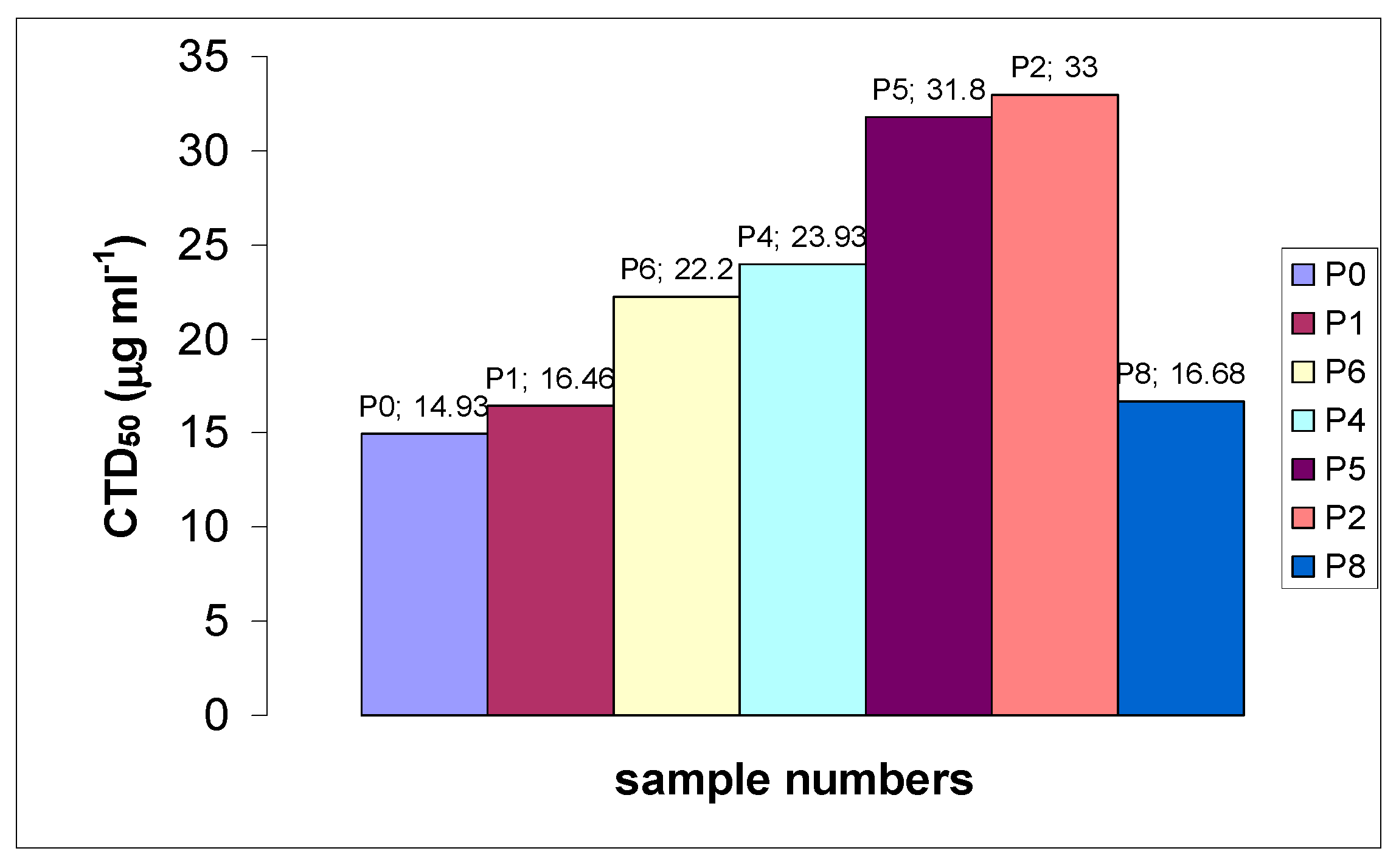
| Sample | MW × 10−3, g·mol−1 | End Group | CTD50, μg·mL−1 Relative to A-549 | CTD50, μg·mL−1 Relative to MA-104 |
|---|---|---|---|---|
| P0 | 43.0 | CH2=CH-; CH3 | 14.93 ± 0.36 | 25.44 ± 1.14 |
| P1 | 41.6 | CH2=CH-; CH3 | 16.46 ± 0.92 | 24.87 ± 0.10 |
| P2 | 17.8 | -O-S(=O)2-O¯ | 33.0 ± 2.97 | 43.65 ± 1.17 |
| P4 | 26.3 | -O-S(=O)2-O¯ | 23.93 ± 1.5 | 31.14 ± 1.81 |
| P5 | 23.0 | -C((C≡N)(CH3))-(CH2)2-COOH | 31.68 ± 1.75 | - |
| P6 | 35.8 | -C((C≡N)(CH3))-(CH2)2-COOH | 22.0 ± 3.41 | - |
| P8 | 8.0 | -S-C(=S)-O-CH2-CH3; -CH2-COOH | 16.68 ± 0.42 | 25.80 ± 1.08 |
2.3. Bactericidal Activity of Tested Polymers
| Sample | MW × 10−3, g·mol−1 | MBC Staphylococcus aureus, μg·mL−1 | MBC Pseudomonas aeruginosa, μg·mL−1 |
|---|---|---|---|
| P2 | 17.8 | 37.5 ± 7.5 | 60 ± 2 |
| P4 | 26.3 | 37.5 ± 7.5 | 52.5 ± 7.5 |
| P5 | 23.0 | 60 ± 2 | 60 ± 2 |
| P6 | 35.8 | 52.5 ± 7.5 | 52.5 ± 7.5 |
| P8 | 8.0 | 31 ± 3.1 | 31 ± 3.1 |

3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. DAATFA Polymerization
3.4. DAATFA RAFT Polymerization
3.5. Measurements
3.6. Determination of Molecular Characteristics of Polymers
3.7. Methodology of Toxicity Investigations
3.8. Mathematical/Statistical Analysis of the Results
3.9. Procedure for Antibacterial Activity Research
3.10. Estimation of Bacterial Viability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyzioł, A.; Khan, W.; Sebastian, V.; Kyzioł, K. Tackling microbial infections and increasing resistance involving formulations based on antimicrobial polymers. Chem. Eng. J. 2020, 385, 123888–123941. [Google Scholar] [CrossRef]
- Siedenbiedel, F.; Tiller, J.S. Antimicrobial polymers in solution and on surfaces: Overview and functional principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef]
- Chen, A.; Peng, H.; Blakey, I.; Whittaker, A.K. Biocidal Polymers: A Mechanistic Overview. Polym. Rev. 2017, 57, 276–310. [Google Scholar] [CrossRef]
- Palermo, E.F.; Lienkamp, K.; Gilles, E.R.; Ragogna, P.J. Antibacterial activity of polymers: Discussions on the nature of amphiphilic balance. Angew. Chem. Int. Ed. 2019, 58, 3690–3693. [Google Scholar] [CrossRef]
- Ergene, C.; Yasuhara, K.; Palermo, E.F. Biomimetic antimicrobial polymers: Recent advances in molecular design. Polym. Chem. 2018, 9, 2407–2427. [Google Scholar]
- Sarapas, J.M.; Backlund, C.M.; deRonde, B.M.; Minter, L.M.; Tew, G.N. ROMP- and RAFT-Based Guanidinium-Containing Polymers as Scaffolds for Protein Mimic Synthesis. Chem.-Eur. J. 2017, 23, 6858–6863. [Google Scholar] [CrossRef]
- Boschert, D.; Schneider-Chaabane, A.; Himmelsbach, A.; Eickenscheidt, A.; Lienkamp, K. Synthesis and Bioactivity of Polymer-Based Synthetic Mimics of Antimicrobial Peptides (SMAMPs) Made from Asymmetrically Disubstituted Itaconates. Chem.-Eur. J. 2018, 24, 8217–8227. [Google Scholar] [CrossRef]
- Takahashi, H.; Palermo, E.F.; Yasuhara, K.; Caputo, G.A.; Kuroda, K. Molecular Design, Structures, and Activity of Antimicrobial Peptide-Mimetic Polymers. Macromol. Biosci. 2013, 13, 1285–1299. [Google Scholar]
- Oda, Y.; Yasuhara, K.; Kanaoka, S.; Sato, T.; Aoshima, S.; Kuroda, K. Aggregation of Cationic Amphiphilic Block and Random Copoly(vinyl ether)s with Antimicrobial Activity. Polymers 2018, 10, 93. [Google Scholar] [CrossRef]
- Krumm, C.; Trump, S.; Benski, L.; Wilken, J.; Oberhaus, F.; Kцller, M.; Tiller, J.C. Fast-Acting Antibacterial, Self-Deactivating Polyionene Esters. ACS Appl. Mater. Interfaces 2020, 12, 21201–21209. [Google Scholar] [CrossRef]
- Timofeeva, L.M.; Vasilieva, Y.A.; Klescheva, N.A.; Gromova, G.L.; Timofeeva, G.I.; Rebrov, A.I.; Topchiev, D.A. Synthesis of high-molecular-weight polymers based on N, N-diallyl-N-methylamine. Macromol. Chem. Phys. 2002, 203, 2296–2304. [Google Scholar] [CrossRef]
- Timofeeva, L.M.; Klescheva, N.A.; Vasilieva, Y.A.; Gromova, G.L.; Timofeeva, G.I.; Filatova, M.P. Mechanism and kinetic features of producing new polymers based on monomers of diallylamine series. Polym. Sci. Ser. A 2005, 47, 551–565. [Google Scholar]
- Timofeeva, L.M.; Klescheva, N.A.; Moroz, A.F.; Didenko, L.V. Secondary and tertiary polydiallylammonium salts: Novel polymers with high antimicrobial activity. Biomacromolecules 2009, 10, 2976–2986. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, L.M.; Klescheva, N.A.; Shleeva, M.O.; Filatova, M.P.; Simonova, Y.A.; Ermakov, Y.A.; Kaprelyants, A.S. Nonquaternary poly(diallylammonium) polymers with different amine structure and their biocidal effect on Mycobacterium tuberculosis and Mycobacterium smegmatis. Appl. Microbiol. Biotechnol. 2015, 99, 2557–2571. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Disinfection, Sterilization, and Control of Hospital Waste. In Book Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Bennett, J.E., Dolin, R., Blaser, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3294–3309. [Google Scholar]
- Timofeeva, L.; Bondarenko, G.; Nikitushkin, V.; Simonova, Y.; Topchiy, M.; Eremenko, I.; Shleeva, M.; Mulyukin, A.; Kaprelyants, A. On the molecular mechanism of nonspecific antimicrobial action of protonated diallylammonium polymers on mycobacterial cells. Europ. Polym. J. 2022, 171, 111214–111229. [Google Scholar] [CrossRef]
- Li Petri, G.; Raimondi, M.V.; Spano, V.; Holl, R.; Barraja, P.; Montalbano, A. Pyrrolidine in Drug Discovery: A Versatile Scaffold for Novel Biologically Active Compounds. Top. Curr. Chem. 2021, 379, 34–79. [Google Scholar] [CrossRef]
- Raimondi, M.V.; Listro, R.; Cusimano, M.G.; La Franca, M.; Faddetta, T.; Gallo, G.; Schillaci, D.; Collina, S.; Leonchiks, A.; Barone, G. Pyrrolomycins as antimicrobial agents. Microwave-assisted organic synthesis and insights into their antimicrobial mechanism of action. Bioorg. Med. Chem. 2019, 27, 721–728. [Google Scholar] [CrossRef]
- Simonova, Y.A.; Eremenko, I.V.; Topchiy, M.A.; Kozobkova, N.V.; Shleeva, M.O.; Eropkin, M.Y.; Timofeeva, L.M. Antimicrobial protonated polydiallylamines: How to retain bactericidal efficiency at minimal toxicity. Mendeleev Commun. 2025. to be published. [Google Scholar]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living-Free radical polymerization by reversible addition–fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Destarac, M.; Bzducha, W.; Taton, D.; Gauthier-Gillaizeau, I.; Zard, S.Z. Xanthates as chain-transfer agents in controlled radical polymerization (MADIX): Structural effect of the O-alkyl group. Macromol. Rapid Commun. 2002, 23, 1049–1054. [Google Scholar] [CrossRef]
- Barner-Kowollik, C. (Ed.) Handbook of RAFT Polymerization; Wiley-VCHH Verlag Gmbh& Co.: Weinheim, Germany, 2008. [Google Scholar]
- Moad, G.; Chong, Y.K.; Postma, A.; Rizzardo, E.; Thang, S.H. Advances in RAFT polymerization: The synthesis of polymers with defined end-groups. Polymer 2005, 46, 8458–8468. [Google Scholar] [CrossRef]
- Michl, T.D.; Locock, K.E.S.; Stevens, N.E.; Hayball, J.D.; Vasilev, K.; Postma, A.; Qu, Y.; Traven, A.; Haeussler, M.; Meagher, L.; et al. RAFT-derived antimicrobial polymethacrylates: Elucidating the impact of end-groups on activity and cytotoxicity. Polym. Chem. 2014, 5, 5813–5822. [Google Scholar] [CrossRef]
- Simonova, Y.A.; Topchiy, M.A.; Filatova, M.P.; Yevlampieva, N.P.; Slyusarenko, M.A.; Bondarenko, G.N.; Asachenko, A.F.; Nechaev, M.S.; Timofeeva, L.M. Impact of the RAFT/MADIX agent on protonated diallylammonium monomer cyclopolymerization with efficient chain transfer to monomer. Eur. Polym. J. 2020, 122, 109363. [Google Scholar] [CrossRef]
- Timofeeva, L.M.; Vasilieva, Y.A.; Klescheva, N.A.; Gromova, G.L.; Topchiev, D.A. Radical polymerization of diallylamine compounds: From quantum chemical modeling to controllable synthesis of high-molecular-weight polymers. Int. J. Quantum. Chem. 2002, 88, 531–541. [Google Scholar] [CrossRef]
- Eremenko, I.V.; Simonova, Y.A.; Filatova, M.P.; Yevlampieva, N.P.; Bondarenko, G.N.; Kleshcheva, N.A.; Timofeeva, L.M. Optimization of methodology of protonated diallylammonium monomers free radical polymerization for the obtaining polymers with a low molecular weight. Russ. J. Appl. Chem. 2024, 97, 550–560. [Google Scholar] [CrossRef]
- Simonova, Y.A.; Filatova, M.P.; Timofeeva, L.M. Radical Polymerization of Protonated Diallylammonium Monomers in Bidistilled Aqueous Solution: Kinetic Study. Polym. Sci. Ser. B 2018, 60, 445–454. [Google Scholar] [CrossRef]
- Seibert, H.; Balls, M.; Fentem, J.H.; Bianchi, V.; Clothier, R.H.; Dierickx, P.J.; Ekwall, B.; Garle, M.J.; Gómez-Lechón, M.J.; Gribaldo, L.; et al. Acute Toxicity Testing in Vitro and the Classification and Labelling of Chemicals: The Report and Recommendations of ECVAM Workshop 16. Altern. Lab. Anim. 1996, 24, 499–510. [Google Scholar] [CrossRef]
- Clemedson, C.; Ekwall, B. Overview of the final MEIC results. I. The in vitro- in vitro evaluation. Toxicol. In Vitro 1999, 13, 657–663. [Google Scholar] [CrossRef]
- Balls, M.; Worth, A.P. The History of Alternative Test Methods in Toxicology; Balls, M., Combes, R., Worth, A.P., Eds.; Academic Press: San Diego, CA, USA, 2019; pp. 307–314. [Google Scholar]
- Borenfreund, E.; Babich, H.; Martin-Alguicil, N. Comparison of two in vitro cytotoxicity assays—The neutral red (NR) and tetrazolium (MTT) tests. Toxicol. In Vitro 1988, 2, 1–6. [Google Scholar] [CrossRef]
- Kwon, S.; Yang, W.; Moon, D.; Kim, K.S. Comparison of Cancer Cell Elasticity by Cell Type. J. Cancer 2020, 11, 5403–5412. [Google Scholar] [CrossRef]
- Ikeda, T.; Hirayama, H.; Yamaguchi, H.; Tazuke, S.; Watanabe, M. Polycationic Biocides with Pendant Active Groups: Molecular Weight Dependence of Antibacterial Activity. Antimicrob. Agents. Chemother. 1986, 30, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.-R.; Worley, S.D.; Broughton, R. The Chemistry and Applications of Antimicrobial Polymers: A State-of-the-Art Review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial polymers: Mechanism of action, factors of activity and applications. Appl. Microbiol. Biotechnol. 2011, 89, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial polymers. Adv. Healthc. Mater. 2014, 3, 1969–1985. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Franklin, T.J.; Snow, G.A. Biochemistry and Molecular Biology of Antimicrobial Drug Action; Springer: New York, NY, USA, 2005. [Google Scholar]
- Yevlampieva, N.; Vezo, O.; Simonova, Y.; Timofeeva, L. Protonated Member of Poly(diallylammonium) Family: Hydrodynamic and Conformational Properties. Int. J. Polym. Anal. Character 2018, 23, 403–414. [Google Scholar] [CrossRef]
- Tsvetkov, V.N. Rigid-Chain Polymers; Plenum Press: NewYork, NY, USA, 1989. [Google Scholar]
- GOST MU 1.2.2635-10; Rospotrebnadzor: 2010; 122p. Available online: https://www.russiangost.com/p-146565-mu-122635-10.aspx (accessed on 12 November 2024).
- Fay, J.M.; Kabanov, A.V. Interpolyelectrolyte Complexes as an Emerging Technology for Pharmaceutical Delivery of Polypeptides. Rev. Adv. Chem. 2022, 12, 137–162. [Google Scholar] [CrossRef]
- Sinelnikova, D.G.; Novoskoltseva, O.A.; Loiko, N.G.; Nikolaev, Y.A.; Yaroslavov, A.A. Hybrid polycomplexes of anionic alginate with a synthetic cationic polymer: Attractive and poisonous for microorganisms. Mendeleev Commun. 2024, 34, 31–33. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timofeeva, L.M.; Simonova, Y.A.; Eremenko, I.V.; Filatova, M.P.; Topchiy, M.A.; Kozobkova, N.V.; Shleeva, M.O.; Eropkin, M.Y. Cytotoxicity and Antibacterial Activity of Protonated Diallylammonium Polymers: Influence of End Groups and Molecular Weight. Int. J. Mol. Sci. 2025, 26, 1501. https://doi.org/10.3390/ijms26041501
Timofeeva LM, Simonova YA, Eremenko IV, Filatova MP, Topchiy MA, Kozobkova NV, Shleeva MO, Eropkin MY. Cytotoxicity and Antibacterial Activity of Protonated Diallylammonium Polymers: Influence of End Groups and Molecular Weight. International Journal of Molecular Sciences. 2025; 26(4):1501. https://doi.org/10.3390/ijms26041501
Chicago/Turabian StyleTimofeeva, Larisa M., Yulia A. Simonova, Ivan V. Eremenko, Marina P. Filatova, Maxim A. Topchiy, Nataliya V. Kozobkova, Margarita O. Shleeva, and Mikhail Yu. Eropkin. 2025. "Cytotoxicity and Antibacterial Activity of Protonated Diallylammonium Polymers: Influence of End Groups and Molecular Weight" International Journal of Molecular Sciences 26, no. 4: 1501. https://doi.org/10.3390/ijms26041501
APA StyleTimofeeva, L. M., Simonova, Y. A., Eremenko, I. V., Filatova, M. P., Topchiy, M. A., Kozobkova, N. V., Shleeva, M. O., & Eropkin, M. Y. (2025). Cytotoxicity and Antibacterial Activity of Protonated Diallylammonium Polymers: Influence of End Groups and Molecular Weight. International Journal of Molecular Sciences, 26(4), 1501. https://doi.org/10.3390/ijms26041501






