Aqueous Humor Cytokine Profiling Reveals Distinct Roles for Serum Amyloid A, Interleukin-8, and Endothelin-1 in Pseudoexfoliation Syndrome and Glaucoma
Abstract
1. Introduction
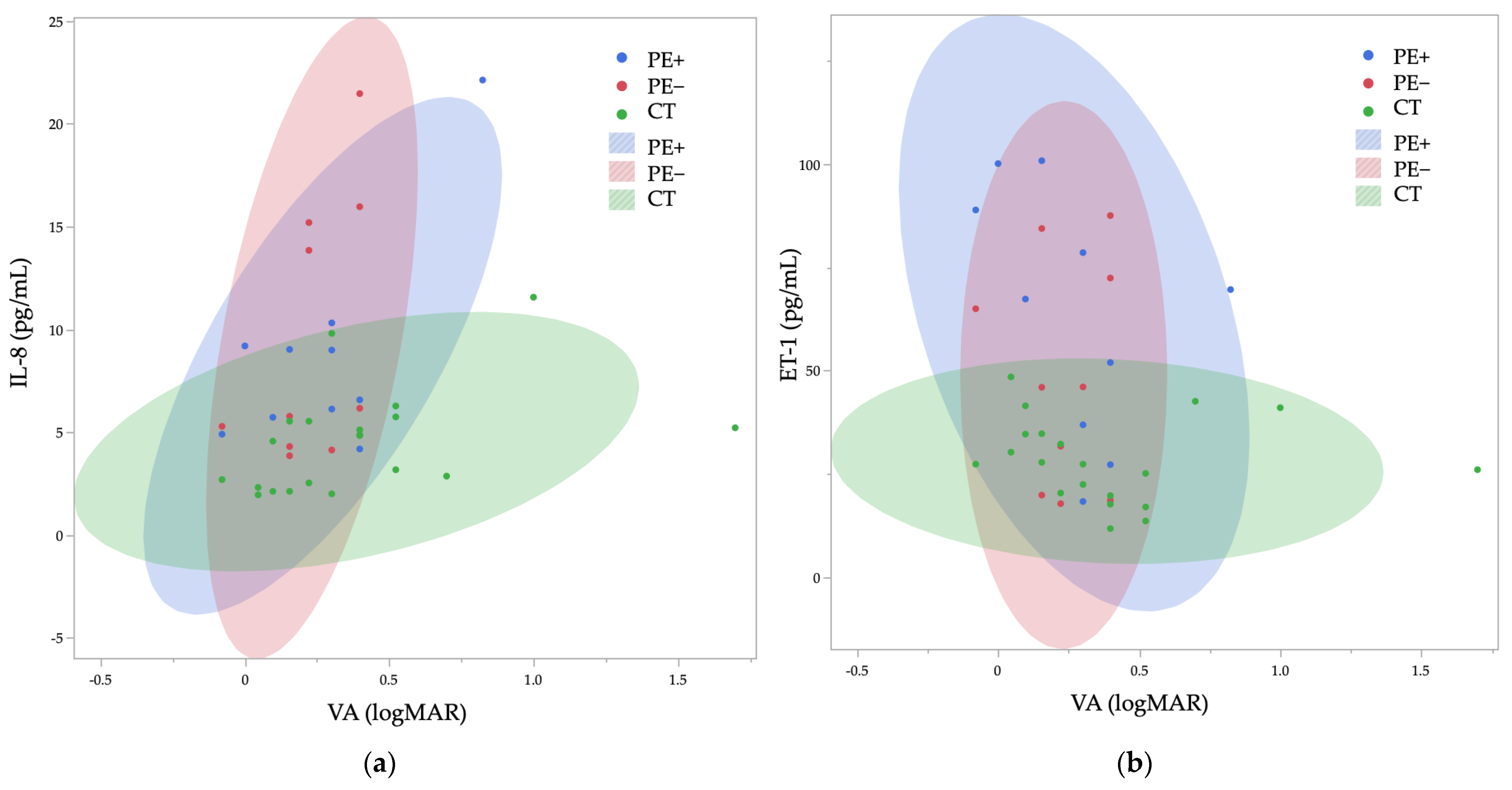
2. Results
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Measurement of Cytokine Levels
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourne, R.R.A.; Jonas, J.B.; Bron, A.M.; Cicinelli, M.V.; Das, A.; Flaxman, S.R.; Friedman, D.S.; Keeffe, J.E.; Kempen, J.H.; Leasher, J.; et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe in 2015: Magnitude, temporal trends and projections. Br. J. Ophthalmol. 2018, 102, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Ahrlich, K.G.; De Moraes, C.G.; Teng, C.C.; Prata, T.S.; Tello, C.; Ritch, R.; Liebmann, J.M. Visual field progression differences between normal-tension and exfoliative high-tension glaucoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Grødum, K.; Heijl, A.; Bengtsson, B. Risk of glaucoma in ocular hypertension with and without pseudoexfoliation. Ophthalmology 2005, 112, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Naumann, G.O.; Schlötzer-Schrehardt, U.; Küchle, M. Pseudoexfoliation syndrome for the comprehensive ophthalmologist. Intraocular and systemic manifestations. Ophthalmology 1998, 105, 951–968. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Naumann, G.O. Ocular and systemic pseudoexfoliation syndrome. Am. J. Ophthalmol. 2006, 141, 921–937. [Google Scholar] [CrossRef]
- Siordia, J.A.; Franco, J.; Golden, T.R.; Dar, B. Ocular Pseudoexfoliation Syndrome Linkage to Cardiovascular Disease. Curr. Cardiol. Rep. 2016, 18, 61. [Google Scholar] [CrossRef]
- Ozaki, M. Mechanisms of Glaucoma in Exfoliation Syndrome. J. Glaucoma. 2018, 27 (Suppl. S1), S83–S86. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Khor, C.C. Pseudoexfoliation syndrome and glaucoma: From genes to disease mechanisms. Curr. Opin. Ophthalmol. 2021, 32, 118–128. [Google Scholar] [CrossRef]
- Jeng, S.M.; Karger, R.A.; Hodge, D.O.; Burke, J.P.; Johnson, D.H.; Good, M.S. The risk of glaucoma in pseudoexfoliation syndrome. J. Glaucoma. 2007, 16, 117–121. [Google Scholar] [CrossRef]
- Parekh, P.; Green, W.R.; Stark, W.J.; Akpek, E.K. Electron microscopic investigation of the lens capsule and conjunctival tissues in individuals with clinically unilateral pseudoexfoliation syndrome. Ophthalmology 2008, 115, 614–619.e2. [Google Scholar] [CrossRef]
- Wang, N.; Chintala, S.K.; Fini, M.E.; Schuman, J.S. Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype. Nat. Med. 2001, 7, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.E.; Pepple, K.L. Cytokines in uveitis. Curr. Opin. Ophthalmol. 2018, 29, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.; Iserovich, P. Pro-inflammatory cytokines in glaucomatous aqueous and encysted Molteno implant blebs and their relationship to pressure. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4851–4855. [Google Scholar] [CrossRef] [PubMed]
- Kuchtey, J.; Rezaei, K.A.; Jaru-Ampornpan, P.; Sternberg, P., Jr.; Kuchtey, R.W. Multiplex cytokine analysis reveals elevated concentration of interleukin-8 in glaucomatous aqueous humor. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6441–6447. [Google Scholar] [CrossRef]
- Takai, Y.; Tanito, M.; Ohira, A. Multiplex cytokine analysis of aqueous humor in eyes with primary open-angle glaucoma, exfoliation glaucoma, and cataract. Investig. Ophthalmol. Vis. Sci. 2012, 53, 241–247. [Google Scholar] [CrossRef]
- Takayanagi, Y.; Takai, Y.; Kaidzu, S.; Tanito, M. Association between Systemic Antioxidant Capacity and Retinal Vessel Diameters in Patients with Primary-Open Angle Glaucoma. Life 2020, 10, 364. [Google Scholar] [CrossRef]
- Hua, S.; Song, C.; Geczy, C.L.; Freedman, S.B.; Witting, P.K. A role for acute-phase serum amyloid A and high-density lipoprotein in oxidative stress, endothelial dysfunction and atherosclerosis. Redox Rep. 2009, 14, 187–196. [Google Scholar] [CrossRef]
- Feng, J.; Zhao, T.; Zhang, Y.; Ma, Y.; Jiang, Y. Differences in aqueous concentrations of cytokines in macular edema secondary to branch and central retinal vein occlusion. PLoS ONE 2013, 8, e68149. [Google Scholar] [CrossRef]
- Bauer, D.; Kasper, M.; Walscheid, K.; Koch, J.M.; Müther, P.S.; Kirchhof, B.; Heiligenhaus, A.; Heinz, C. Multiplex Cytokine Analysis of Aqueous Humor in Juvenile Idiopathic Arthritis-Associated Anterior Uveitis With or Without Secondary Glaucoma. Front. Immunol. 2018, 9, 708. [Google Scholar] [CrossRef]
- Wang, W.H.; McNatt, L.G.; Pang, I.H.; Hellberg, P.E.; Fingert, J.H.; McCartney, M.D.; Clark, A.F. Increased expression of serum amyloid A in glaucoma and its effect on intraocular pressure. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1916–1923. [Google Scholar] [CrossRef][Green Version]
- Liu, Q.; Sun, S.; Yang, Z.; Shao, Y.; Li, X. Serum Amyloid A 4 as a Common Marker of Persistent Inflammation in Patients with Neovascular Age-Related Macular Degeneration and Polypoidal Choroidal Vasculopathy. J. Inflamm. Res. 2023, 16, 3783–3797. [Google Scholar] [CrossRef] [PubMed]
- Amer, R.; Koriat, A. Aqueous humor perturbations in chronic smokers: A proteomic study. Sci. Rep. 2024, 14, 11279. [Google Scholar] [CrossRef] [PubMed]
- Zenkel, M.; Lewczuk, P.; Jünemann, A.; Kruse, F.E.; Naumann, G.O.; Schlötzer-Schrehardt, U. Proinflammatory cytokines are involved in the initiation of the abnormal matrix process in pseudoexfoliation syndrome/glaucoma. Am. J. Pathol. 2010, 176, 2868–2879. [Google Scholar] [CrossRef]
- Sugiyama, T.; Moriya, S.; Oku, H.; Azuma, I. Association of endothelin-1 with normal tension glaucoma: Clinical and fundamental studies. Surv. Ophthalmol. 1995, 39 (Suppl. S1), S49–S56. [Google Scholar] [CrossRef]
- Borazan, M.; Karalezli, A.; Kucukerdonmez, C.; Bayraktar, N.; Kulaksizoglu, S.; Akman, A.; Akova, Y.A. Aqueous humor and plasma levels of vascular endothelial growth factor and nitric oxide in patients with pseudoexfoliation syndrome and pseudoexfoliation glaucoma. J. Glaucoma. 2010, 19, 207–211. [Google Scholar] [CrossRef]
- Tsutsui, A.; Hamanaka, T.; Kaidzu, S.; Kobayashi, K.; Ishida, N.; Kumasaka, T.; Tanito, M. Comparison of Schlemm’s Canal Morphology Parameters Between Propensity Score-Matched Primary Open-Angle Glaucoma and Exfoliation Glaucoma. Investig. Ophthalmol. Vis. Sci. 2024, 65, 15. [Google Scholar] [CrossRef]
- Dismuke, W.M.; Liang, J.; Overby, D.R.; Stamer, W.D. Concentration-related effects of nitric oxide and endothelin-1 on human trabecular meshwork cell contractility. Exp. Eye Res. 2014, 120, 28–35. [Google Scholar] [CrossRef]
- Agilli, M.; Aydin, F.N.; Cayci, T.; Gulcan Kurt, Y. Evaluation of serum amyloid a as a marker of persistent inflammation in patients with rheumatoid arthritis. Mediat. Inflamm. 2015, 2015, 843152. [Google Scholar] [CrossRef]
- Gremese, E.; Tolusso, B.; Bruno, D.; Perniola, S.; Ferraccioli, G.; Alivernini, S. The forgotten key players in rheumatoid arthritis: IL-8 and IL-17—Unmet needs and therapeutic perspectives. Front. Med. 2023, 10, 956127. [Google Scholar] [CrossRef]
- Park, D.Y.; Kim, M.; Cha, S.C. Cytokine and Growth Factor Analysis in Exfoliation Syndrome and Glaucoma. Investig. Ophthalmol. Vis. Sci. 2021, 62, 6. [Google Scholar] [CrossRef]
- Emre, M.; Orgül, S.; Haufschild, T.; Shaw, S.G.; Flammer, J. Increased plasma endothelin-1 levels in patients with progressive open angle glaucoma. Br. J. Ophthalmol. 2005, 89, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Koukoula, S.C.; Katsanos, A.; Tentes, I.K.; Labiris, G.; Kozobolis, V.P. Retrobulbar hemodynamics and aqueous humor levels of endothelin-1 in exfoliation syndrome and exfoliation glaucoma. Clin. Ophthalmol. 2018, 12, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
| Parameters | PE+ | PE− | CT-R | CT-L | p-Value |
|---|---|---|---|---|---|
| N | 10 | 10 | 10 | 10 | - |
| Age (years) | 78.7 ± 6.1 | 78.8 ± 6.0 | 78.7 ± 4.9 | 78.7 ± 4.9 | >0.99 |
| Mean ± SD | 78.7 ± 6.1 | 78.8 ± 6.0 | 78.7 ± 4.9 | 78.7 ± 4.9 | >0.99 |
| Men, n (%) | 3 (30.0%) | 3 (30.0%) | 3 (30.0%) | 3 (30.0%) | 1.00 |
| Right eye, n (%) | 5 (50.0%) | 5 (50.0%) | 10 (100.0%) | 0 (0.0%) | <0.0001 ** |
| VA (logMAR) | 0.27 ± 0.25 | 0.23 ± 0.15 | 0.39 ± 0.49 | 0.38 ± 0.31 | 0.82 |
| IOP (mmHg) | 16.3 ± 4.3 | 15.7 ± 4.9 | 13.8 ± 1.5 | 14.1 ± 1.8 | 0.30 |
| Medication number | 2.9 ± 0.4 | 1.4 ± 0.4 | - | - | 0.0139 * |
| Parameters | PE+ | PE− | p-Value |
|---|---|---|---|
| SAA (μg/mL) | 2.54 ± 3.15 | 0.39 ± 0.73 | 0.044 * |
| IL-8 (pg/mL) | 8.71 ± 5.14 | 9.59 ± 6.38 | 0.40 |
| ET-1 (pg/mL) | 64.0 ± 29.5 | 48.9 ± 27.1 | 0.23 |
| VEGF (pg/mL) | 255.6 ± 112.4 | 253.4 ± 62.3 | 0.51 |
| Parameters | CT-R | CT-L | p-Value |
|---|---|---|---|
| SAA (μg/mL) | 0.53 ± 1.00 | 0.60 ± 1.22 | 0.44 |
| IL-8 (pg/mL) | 4.18 ± 3.08 | 4.90 ± 2.07 | 0.12 |
| ET-1 (pg/mL) | 29.8 ± 12.1 | 26.4 ± 8.0 | 0.50 |
| VEGF (pg/mL) | 206.5 ± 74.7 | 256.0 ± 93.5 | 0.07 |
| Parameters | PE+ | PE− | CT | p-Value |
|---|---|---|---|---|
| SAA (μg/mL) | 2.54 ± 3.15 | 0.39 ± 0.73 | 0.57 ± 1.09 | 0.07 |
| IL-8 (pg/mL) | 8.71 ± 5.14 | 9.59 ± 6.38 | 4.54 ± 2.58 | 0.0049 ** |
| vs. PE+ p = 0.96 | vs. PE+ p = 0.0126 * | |||
| vs. PE− p = 0.0435 * | ||||
| ET-1 (pg/mL) | 64.0 ± 29.5 | 48.9 ± 27.1 | 28.0 ± 10.2 | 0.0052 ** |
| vs. PE+ p = 0.47 | vs. PE+ p = 0.0073 ** | |||
| vs. PE− p = 0.1347 | ||||
| VEGF (pg/mL) | 255.6 ± 112.4 | 253.4 ± 62.3 | 231.3 ± 86.2 | 0.51 |
| p\ρ | SAA | IL-8 | ET-1 | VEGF |
|---|---|---|---|---|
| SAA | 0.31 | 0.22 | −0.04 | |
| IL-8 | 0.12 | 0.38 | 0.22 | |
| ET-1 | 0.62 | 0.14 | 0.04 | |
| VEGF | 0.54 | 0.12 | 0.88 |
| Parameters | Age (/y) | VA (/logMAR) | IOP (/mmHg) | |||
|---|---|---|---|---|---|---|
| p/ρ | ρ | p | ρ | p | ρ | p |
| SAA | −0.05 | 0.29 | 0.15 | 0.21 | 0.09 | 0.99 |
| IL-8 | 0.14 | 0.43 | 0.24 | 0.046 * | 0.15 | 0.21 |
| ET-1 | 0.12 | 0.94 | −0.22 | 0.026 * | 0.18 | 0.10 |
| VEGF | 0.26 | 0.06 | −0.02 | 0.76 | 0.03 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadoh, Y.; Takayanagi, Y.; Sugihara, K.; Kaidzu, S.; Takai, Y.; Tanito, M. Aqueous Humor Cytokine Profiling Reveals Distinct Roles for Serum Amyloid A, Interleukin-8, and Endothelin-1 in Pseudoexfoliation Syndrome and Glaucoma. Int. J. Mol. Sci. 2025, 26, 1461. https://doi.org/10.3390/ijms26041461
Kadoh Y, Takayanagi Y, Sugihara K, Kaidzu S, Takai Y, Tanito M. Aqueous Humor Cytokine Profiling Reveals Distinct Roles for Serum Amyloid A, Interleukin-8, and Endothelin-1 in Pseudoexfoliation Syndrome and Glaucoma. International Journal of Molecular Sciences. 2025; 26(4):1461. https://doi.org/10.3390/ijms26041461
Chicago/Turabian StyleKadoh, Yoichi, Yuji Takayanagi, Kazunobu Sugihara, Sachiko Kaidzu, Yasuyuki Takai, and Masaki Tanito. 2025. "Aqueous Humor Cytokine Profiling Reveals Distinct Roles for Serum Amyloid A, Interleukin-8, and Endothelin-1 in Pseudoexfoliation Syndrome and Glaucoma" International Journal of Molecular Sciences 26, no. 4: 1461. https://doi.org/10.3390/ijms26041461
APA StyleKadoh, Y., Takayanagi, Y., Sugihara, K., Kaidzu, S., Takai, Y., & Tanito, M. (2025). Aqueous Humor Cytokine Profiling Reveals Distinct Roles for Serum Amyloid A, Interleukin-8, and Endothelin-1 in Pseudoexfoliation Syndrome and Glaucoma. International Journal of Molecular Sciences, 26(4), 1461. https://doi.org/10.3390/ijms26041461






