Expanding the Genetic and Clinical Spectrum of SCN1A-Related Hemiplegic Migraine: Analysis of Mutations in Japanese
Abstract
1. Introduction
2. Results
2.1. The Whole Exome Sequence Analysis of SCN1A
2.2. Allele Frequency and In Silico Analysis
2.3. Three-Dimensional Structural Analysis
2.4. Five Variants Classification
2.5. Clinical Features of Patients
3. Discussion
4. Methods
4.1. Genome DNA Extraction from Peripheral Blood
4.2. The Whole Exome Sequence of SCN1A
4.3. Direct Sequencing of SCN1A
4.4. Allele Frequency and Variant Prediction Analysis (In Silico Analysis)
4.5. Three-Dimensional Structural Analysis
4.6. Variant Classification of ACMG Guidelines
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakai, F.; Igarashi, H. Prevalence of migraine in Japan: A nationwide survey. Cephalalgia 1997, 17, 15–22. [Google Scholar] [CrossRef]
- Robbins, M.S.; Lipton, R.B. The epidemiology of primary headache disorders. Semin. Neurol. 2010, 30, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS). Cephalalgia. In The International Classification of Headache Disorders, 3rd ed.; Headache Classification Committee of the International Headache Society: London, UK, 2018; Volume 38, pp. 1–211. [Google Scholar]
- Gotra, P.; Bhardwaj, N.; Ludhiadch, A.; Singh, G.; Munshi, A. Epilepsy and Migraine Shared Genetic and Molecular Mechanism: Focus on Therapeutic Strategies. Mol. Neurobiol. 2021, 58, 3874–3883. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.B.; Ducros, A. Sporadic and familial hemiplegic migraine: Pathophysiological mechanisms, clinical characteristics, diagnosis, and management. Lancet Neurol. 2011, 10, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Hommersom, M.P.; van Prooije, T.H.; Pennings, M.; Schouten, M.I.; van Bokhoven, H.; Kamsteeg, E.J.; van de Warrenburg, B.P.C. The complexities of CACNA1A in clinical neurogenetics. J. Neurol. 2022, 269, 3094–3108. [Google Scholar] [CrossRef]
- Indelicato, E.; Boesch, S. From Genotype to Phenotype: Expanding the Clinical Spectrum of CACNA1A Variants in the Era of Next Generation Sequencing. Front. Neurol. 2021, 12, 639994. [Google Scholar] [CrossRef]
- Li, Y.; Tang, W.; Kang, L.; Kong, S.; Dong, Z.; Zhao, D.; Liu, R.; Yu, S. Functional correlation of ATP1A2 mutations with phenotypic spectrum: From pure hemiplegic migraine to its variant forms. J. Headache Pain. 2021, 22, 92. [Google Scholar] [CrossRef]
- Oda, I.; Danno, D.; Saigoh, K.; Wolf, J.; Kawashita, N.; Hirano, M.; Samukawa, M.; Kitamura, S.; Kikui, S.; Takeshima, T.; et al. Hemiplegic migraine type 2 with new mutation of the ATP1A2 gene in Japanese cases. Neurosci. Res. 2022, 180, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.; Haerian, B.S.; Ng, H.K.; Wong, V.C.; Ng, P.W.; Lui, C.H.; Sin, N.C.; Zhang, C.; Tomlinson, B.; Wong, G.W.K.; et al. Case-control association study of polymorphisms in the voltage-gated sodium channel genes SCN1A, SCN2A, SCN3A, SCN1B, and SCN2B and epilepsy. Hum. Genet. 2014, 133, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.; Nizzari, M.; Zanardi, I.; Pusch, M.; Gavazzo, P. Voltage-Gated Sodium Channel Dysfunctions in Neurological Disorders. Life 2023, 13, 1191. [Google Scholar] [CrossRef]
- Matricardi, S.; Cestèle, S.; Trivisano, M.; Kassabian, B.; Leroudier, N.; Vittorini, R.; Nosadini, M.; Cesaroni, E.; Siliquini, S.; Marinaccio, C.; et al. Gain of function SCN1A disease-causing variants: Expanding the phenotypic spectrum and functional studies guiding the choice of effective antiseizure medication. Epilepsia 2023, 64, 1331–1347. [Google Scholar] [CrossRef] [PubMed]
- Marini, C.; Scheffr, I.E.; Nabbout, R.; Mei, D.; Cox, K.; Dibbens, L.M.; McMahon, J.M.; Iona, X.; Carpintero, R.S.; Elia, M.; et al. SCN1A duplications and deletions detected in Dravet syndrome: Implications for molecular diagnosis. Epilepsia 2009, 50, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, Y.; Zhao, X.; Li, B. Dravet syndrome: Advances in etiology, clinical presentation, and treatment. Epilepsy Res. 2022, 188, 107041. [Google Scholar] [CrossRef]
- Ding, J.; Li, X.; Tian, H.; Wang, L.; Guo, B.; Wang, Y.; Li, W.; Wang, F.; Sun, T. SCN1A Mutation-Beyond Dravet Syndrome: A Systematic Review and Narrative Synthesis. Front. Neurol. 2021, 12, 743726. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Nabbout, R. SCN1A-related phenotypes: Epilepsy and beyond. Epilepsia 2019, 60 (Suppl. S3), S17–S24. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, I.; Ouchida, M.; Kobayashi, K.; Jitsumori, Y.; Inoue, T.; Shimizu, K.; Matsui, H.; Ohtsuka, Y.; Maegaki, Y. Rasmussen encephalitis associated with SCN1A mutation. Epilepsia 2008, 49, 521–526. [Google Scholar] [CrossRef]
- Claes, L.; Del Favero, J.; Ceulemans, B.; Lagae, L.; Van Broeckhoven, C.; De Jonghe, P. De novo mutations in the sodium channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am. J. Hum. Genet. 2001, 68, 1327–1332. [Google Scholar] [CrossRef]
- Ohmori, I.; Ouchida, M.; Ohtsuka, Y.; Oka, E.; Shimizu, K. Significant correlation of the SCN1A mutations and severe myoclonic epilepsy in infancy. Biochem. Biophys. Res. Commun. 2002, 295, 17–23. [Google Scholar] [CrossRef]
- Ohmori, I.; Kahlig, K.M.; Rhodes, T.H.; Wang, D.W.; George, A.L., Jr. Nonfunctional SCN1A is common in severe myoclonic epilepsy of infancy. Epilepsia 2006, 47, 1636–1642. [Google Scholar] [CrossRef]
- Yu, F.H.; Mantegazza, M.; Westenbroek, R.E.; Robbins, C.A.; Kalume, F.; Burton, K.A.; Spain, W.J.; McKnight, G.S.; Scheuer, T.; Catterall, W.A. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat. Neurosci. 2006, 9, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Hiz-Kurul, A.Y.Ş.E.; Gursoy, S.; Ayanoglu, M.; Yiş, U.; Erçal, D. Expanding spectrum of SCN1A-related phenotype with novel mutations. Turk. J. Pediatr. 2017, 59, 570–575. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brunklaus, A.; Brünger, T.; Feng, T.; Fons, C.; Lehikoinen, A.; Panagiotakaki, E.; Vintan, M.A.; Symonds, J.; Andrew, J.; Arzimanoglou, A.; et al. The gain of function SCN1A disorder spectrum: Novel epilepsy phenotypes and therapeutic implications. Brain 2022, 145, 3816–3831. [Google Scholar] [CrossRef]
- Fang, H.; Hu, W.; Kang, Q.; Kuang, X.; Wang, L.; Zhang, X.; Liao, H.; Yang, L.; Yang, H.; Jiang, Z.; et al. Clinical characteristics and genetic analysis of pediatric patients with sodium channel gene mutation-related childhood epilepsy: A review of 94 patients. Front. Neurol. 2023, 14, 1310419. [Google Scholar] [CrossRef] [PubMed]
- Ogiwara, I.; Miyamoto, H.; Morita, N.; Atapour, N.; Mazaki, E.; Inoue, I.; Takeuchi, T.; Itohara, S.; Yanagawa, Y.; Obata, K.; et al. Na(v)1.1 localizes to axons of parvalbumin positive inhibitory interneurons:a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J. Neurosci. 2007, 27, 5903–5914. [Google Scholar] [CrossRef] [PubMed]
- Ishii, A.; Watkins, J.C.; Chen, D.; Hirose, S.; Hammer, M.F. Clinical implications of SCN1A missense and truncation variants in a large Japanese cohort with Dravet syndrome. Epilepsia 2017, 58, 282–290. [Google Scholar] [CrossRef]
- Meng, H.; Xu, H.Q.; Yu, L.; Lin, G.W.; He, N.; Su, T.; Shi, Y.W.; Li, B.; Wang, J.; Liu, X.R.; et al. The SCN1A mutation database: Updating information and analysis of the relationships among genotype, functional alteration, and phenotype. Hum. Mutat. 2015, 36, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Wirrell, E.C.; Hood, V.; Knupp, K.G.; Meskis, M.A.; Nabbout, R.; Scheffer, I.E.; Wilmshurst, J.; Sullivan, J. International consensus on diagnosis and management of Dravet syndrome. Epilepsia 2022, 63, 1761–1777. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; D’Onofrio, G.; Amadori, E.; Tripodi, D.; Balagura, G.; Iurilli, V.; Vari, M.S.; Verrotti, A.; Striano, P. Current and promising therapeutic options for Dravet syndrome. Expert Opin. Pharmacother. 2022, 23, 1727–1736. [Google Scholar] [CrossRef]
- Miller, I.O.; Sotero de Menezes, M.A. SCN1A Seizure Disorders. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; National Institutes of Health: Bethesda, MD, USA, 2007. [Google Scholar]
- Rentzsch, P.; Witten, D.; Cooper, G.M.; Shendure, J.; Kircher, M. CADD: Predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019, 47, D886–D894. [Google Scholar] [CrossRef] [PubMed]
- Kircher, M.; Witten, D.M.; Jain, P.; O’Roak, B.J.; Cooper, G.M.; Shendure, J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014, 46, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesh, K.A.; Wenger, A.M.; Berger, M.J.; Guturu, H.; Stenson, P.D.; Cooper, D.N.; Bernstein, J.A.; Bejerano, G. M-CAP eliminates a majority of variants of uncertain significance in clinical exomes at high sensitivity. Nat. Genet. 2016, 48, 1581–1586. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
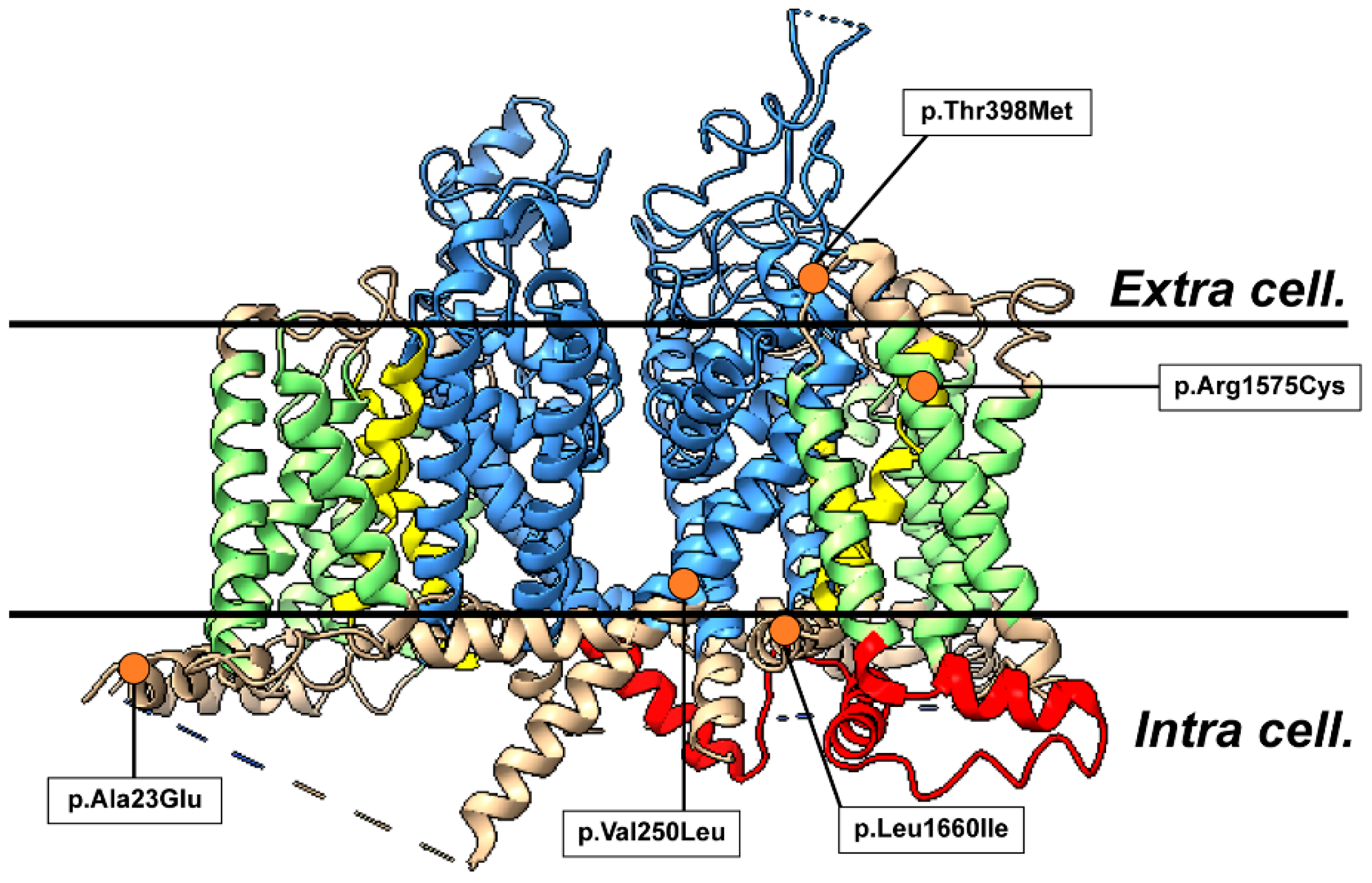
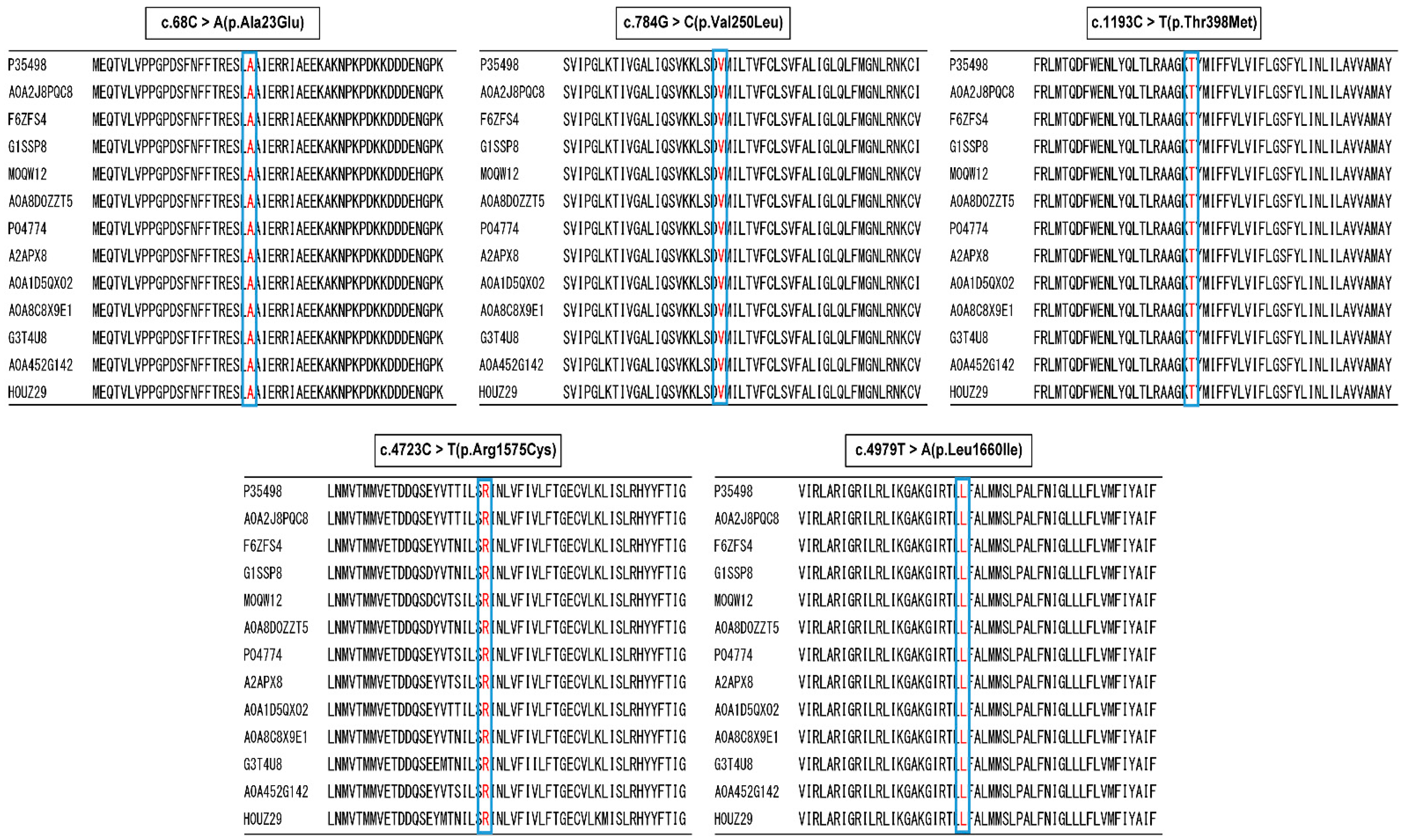
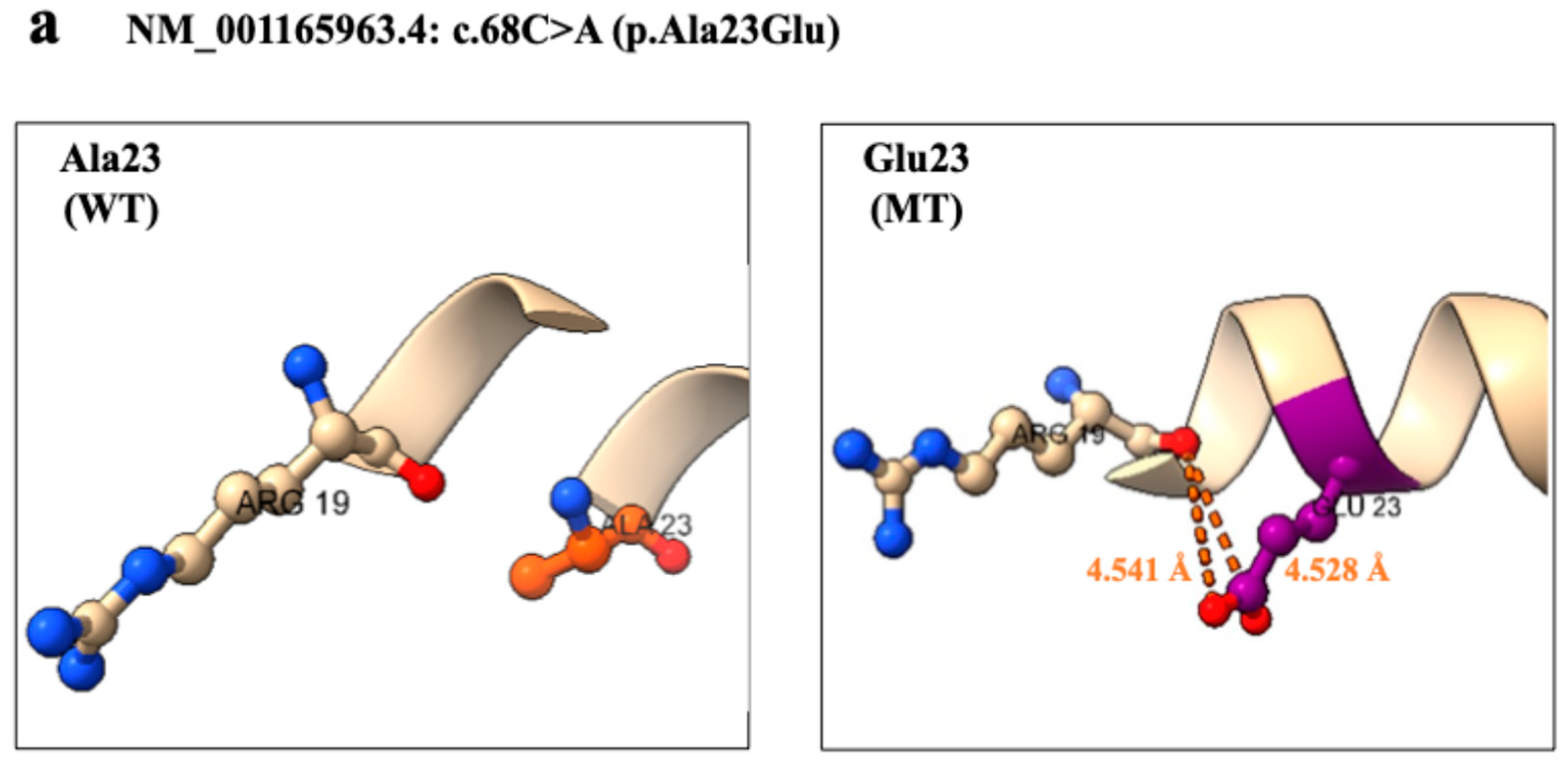
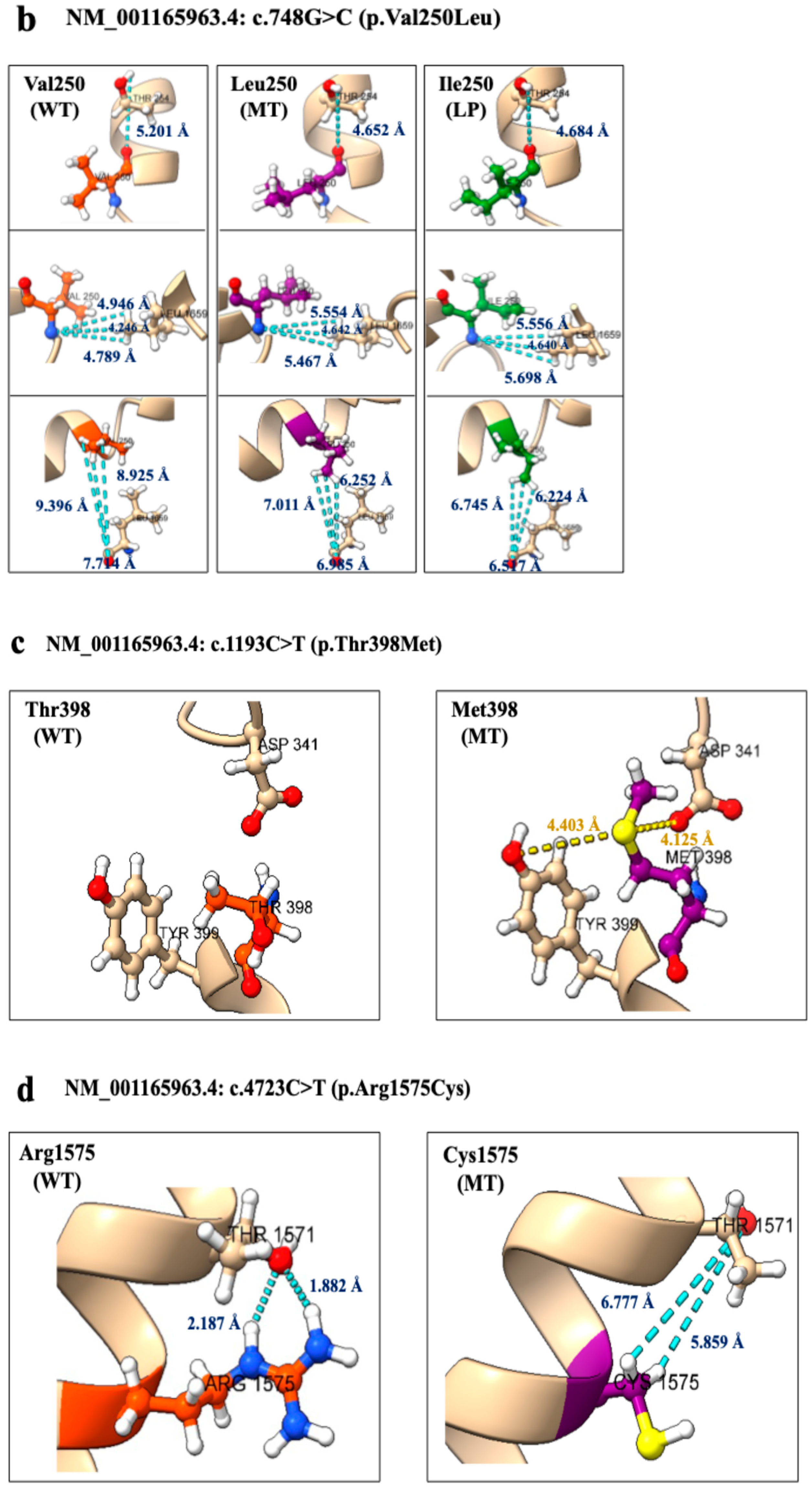

| c.68T>A (p.Ala23Glu) | c.748G>C (p.Val250Leu) | c.1193C>T (p.Thr398Met) | c.4723C>T (p.Arg1575Cys) | c.4979T>A (p.Leu1660Ile) | |
|---|---|---|---|---|---|
| Mutation Taster | Disease causing (107) | Disease causing (32) | Disease causing (81) | Polymorphism −180 | Disease causing (99) |
| PolyPhen-2 | Possibly damaging −0.744 | Possibly damaging −0.903 | Possibly damaging −0.989 | Possibly damaging −1 | Possibly damaging −1 |
| PROVEAN | Deleterious (−0.760) | Deleterious −2.931 | Deleterious (−4.215) | Neutral (−1.973) | Neutral (−1.742) |
| SIFT | Predict tolerated −0.28 | Predict not tolerated −0.01 | Predict tolerated −0.34 | Predict tolerated −0.18 | Predict not tolerated −0.01 |
| PANTHER | Probably damaging −0.74 | Probably damaging −0.85 | Probably damaging −0.74 | Probably damaging −0.5 | Probably damaging −0.85 |
| CADD | 24.5 | 24.9 | 24.8 | 24.3 | 28.4 |
| M-CAP | Possibly pathogenic −0.137 | Possibly pathogenic −0.8717 | Possibly pathogenic −0.789 | Possibly pathogenic −0.177 | Possibly pathogenic −0.9671 |
| No1 | No2 | No3 | No4 | No5 | ||
|---|---|---|---|---|---|---|
| Bacic characteristics | Mutation | p.A23E | p.V250L | p.T398M | p.R1575C | p.L1660I |
| Age (y)/Sex | 29 man | 60 woman | 23 woman | 24 women | 54 women | |
| HM onset (y) | 4 | 20 | 23 | 24 | 14 | |
| Past history | convulsion in 5y | motion sickness in childhood | motion sickness in childhood | motion sickness in childhood | - | |
| Family history | mother | - | father | - | doughter | |
| Dominant hand | right | right | right | right | N/A | |
| Aura symptoms during hemiplegic attacks | Hemiplegia | + | + | + | + | + |
| Hemiplegia Side | birateral | left dominant | birateral | N/A | left dominat | |
| sensory disturbance | + | + | + | + | + | |
| visual disturbance | Light like a tunnel | Fortification | Flickering light, blurred | Scintillation | Jagged light | |
| aphasia | + | + | + | + | + | |
| Triggering factors for hemiplegic attacks | stress lack of sleep | atmospheric pressure stress | - | stress, fatigure | crowd of human | |
| Others | vertigo | vertigo | - | - | vertigo | |
| Duration time and Frequency of attacks | Duration time | intermittent and continuous | 5–30 min | 10~15 min | 30 min | 20–30 min |
| Frequency | intermittent and continuous | 2 times > per year | 50 times > per year | 5 times > per year | - | |
| Headache attack characteristics | NRS score (0–10) | 8–10 | 2–8 | 4–6 | 3–5 | 2–10 |
| Duration time | intermittent and continuous | 30 min > | 2 h > | 30 min > | 2 h > | |
| headache day/month | 30 | 20 | 7–15 | 10–15 | 2–10 | |
| Temporal relation to hemiplegic attacks | + | + | + | + | + | |
| Anatomical relation to hemiplegic attacks | - | - | - | - | - | |
| Nausea/vomiting | + | + | + | + | + | |
| photophobia | + | + | + | - | + | |
| phonophobia | + | - | + | - | 5 | |
| Other types of attacks | vertigo, fatigue | vertigo, fatigue | vertigo | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danno, D.; Tada, H.; Oda, I.; Kawashita, N.; Hirano, M.; Kitamura, S.; Kikui, S.; Samukawa, M.; Yoshikawa, K.; Mitsui, Y.; et al. Expanding the Genetic and Clinical Spectrum of SCN1A-Related Hemiplegic Migraine: Analysis of Mutations in Japanese. Int. J. Mol. Sci. 2025, 26, 1426. https://doi.org/10.3390/ijms26041426
Danno D, Tada H, Oda I, Kawashita N, Hirano M, Kitamura S, Kikui S, Samukawa M, Yoshikawa K, Mitsui Y, et al. Expanding the Genetic and Clinical Spectrum of SCN1A-Related Hemiplegic Migraine: Analysis of Mutations in Japanese. International Journal of Molecular Sciences. 2025; 26(4):1426. https://doi.org/10.3390/ijms26041426
Chicago/Turabian StyleDanno, Daisuke, Haruka Tada, Itsuki Oda, Norihito Kawashita, Makito Hirano, Shigekazu Kitamura, Shoji Kikui, Makoto Samukawa, Keisuke Yoshikawa, Yoshiyuki Mitsui, and et al. 2025. "Expanding the Genetic and Clinical Spectrum of SCN1A-Related Hemiplegic Migraine: Analysis of Mutations in Japanese" International Journal of Molecular Sciences 26, no. 4: 1426. https://doi.org/10.3390/ijms26041426
APA StyleDanno, D., Tada, H., Oda, I., Kawashita, N., Hirano, M., Kitamura, S., Kikui, S., Samukawa, M., Yoshikawa, K., Mitsui, Y., Nagai, Y., Takeshima, T., & Saigoh, K. (2025). Expanding the Genetic and Clinical Spectrum of SCN1A-Related Hemiplegic Migraine: Analysis of Mutations in Japanese. International Journal of Molecular Sciences, 26(4), 1426. https://doi.org/10.3390/ijms26041426






