Synaptic Dysregulation Drives Hyperexcitability in Pyramidal Neurons Surrounding Freeze-Induced Neocortical Malformations in Rats
Abstract
1. Introduction
2. Results
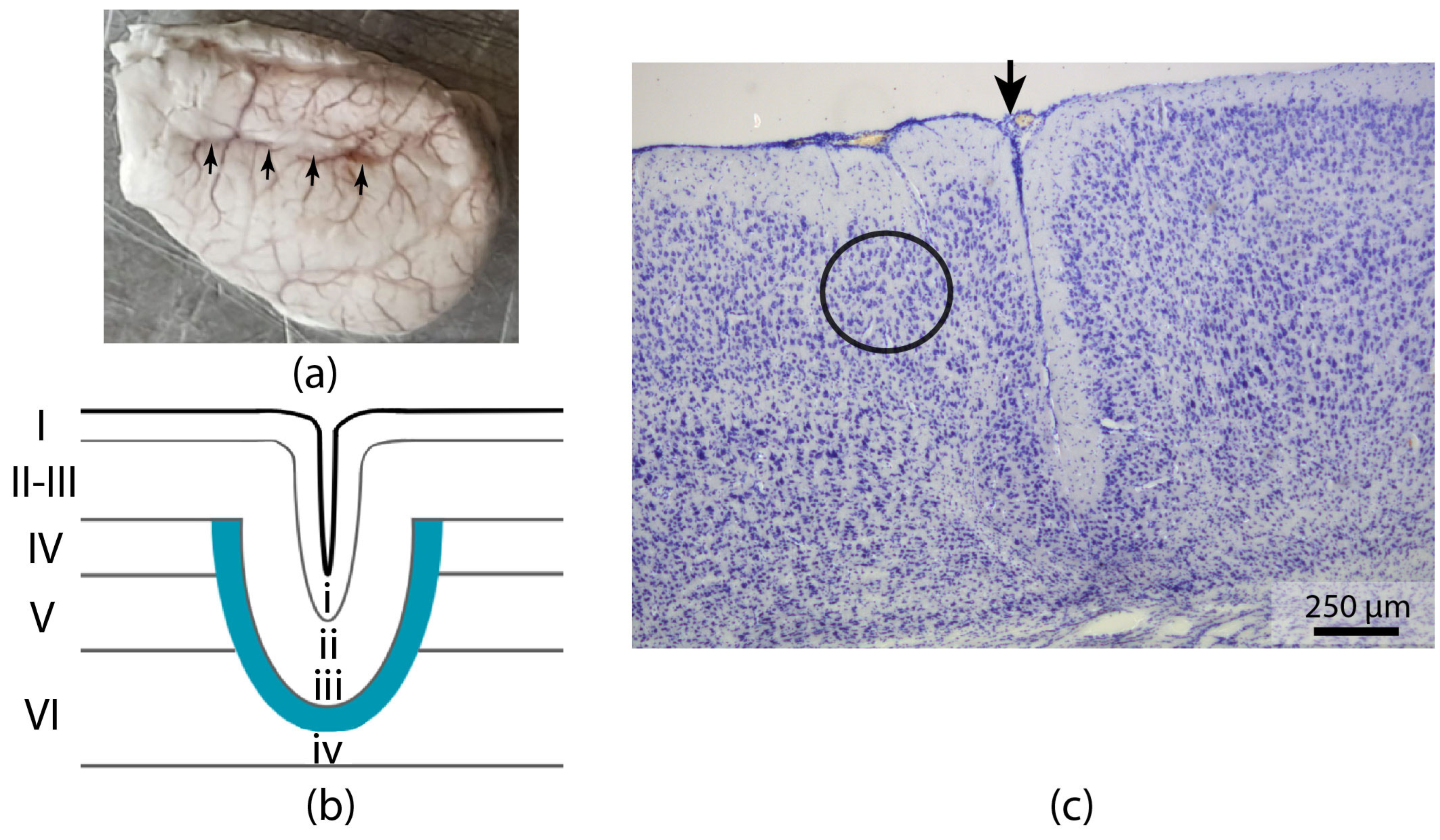
2.1. Morphology
2.2. Membrane Properties
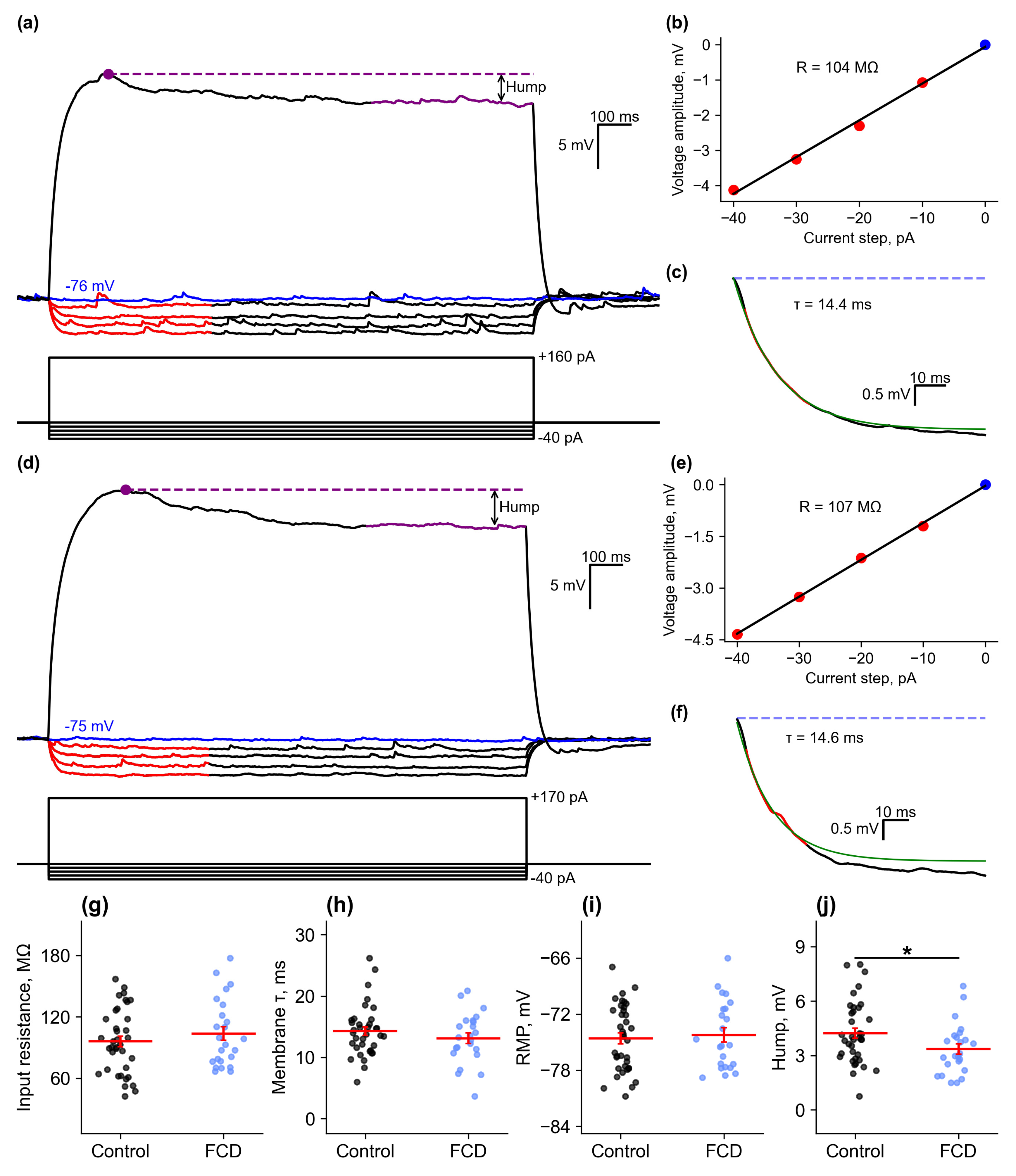
2.2.1. Passive Membrane Properties
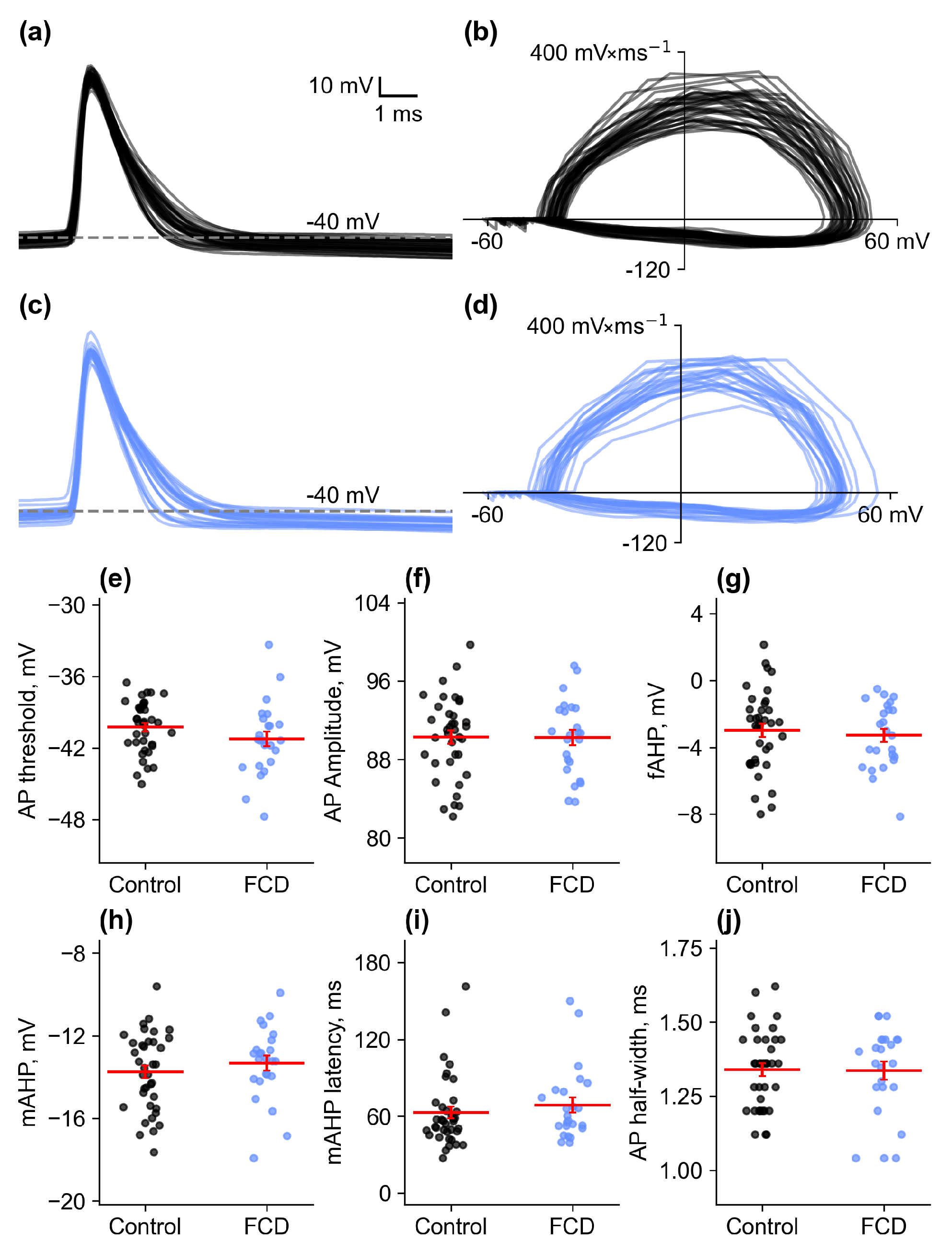
2.2.2. Action Potential Properties
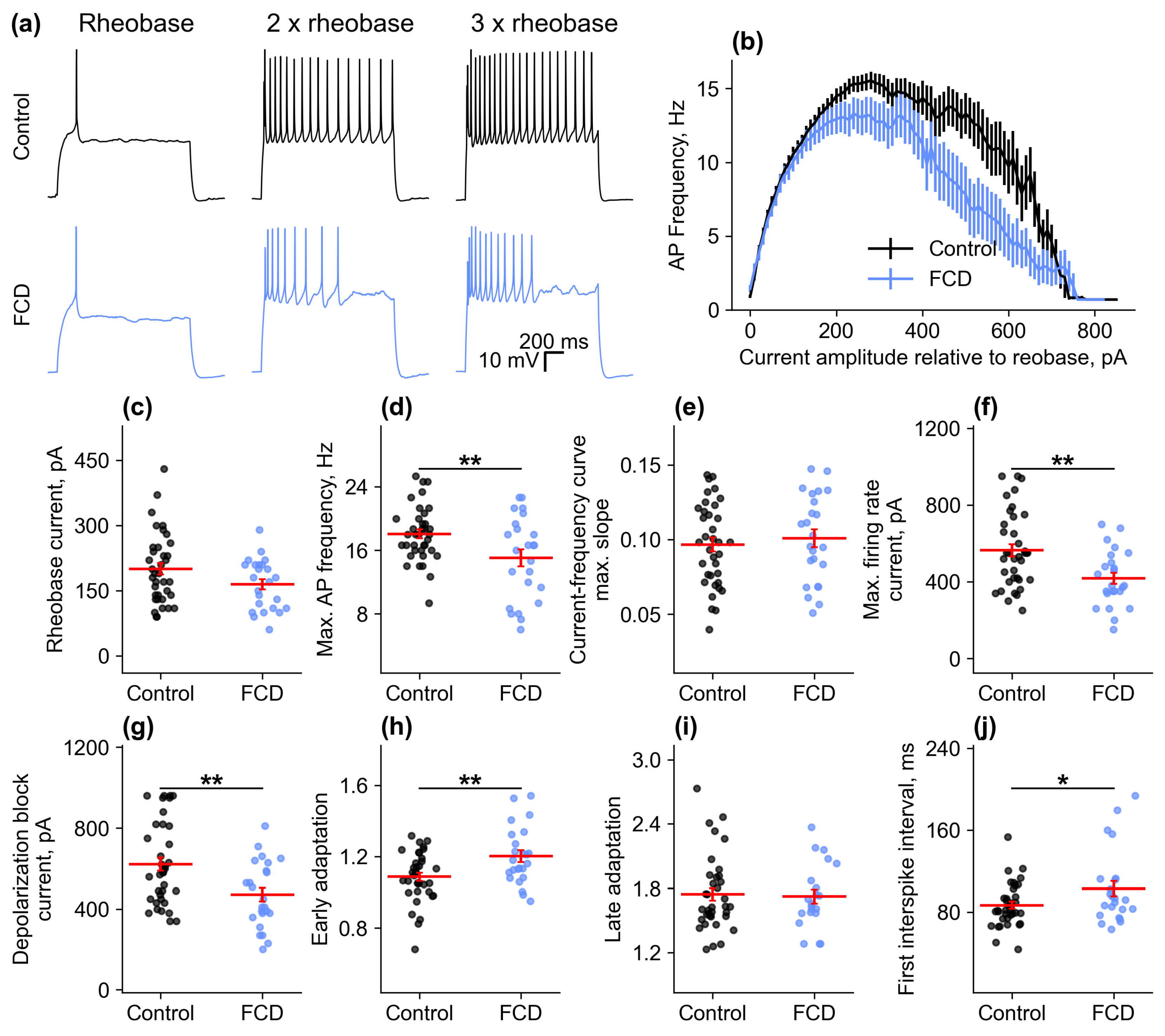
2.2.3. Firing Properties
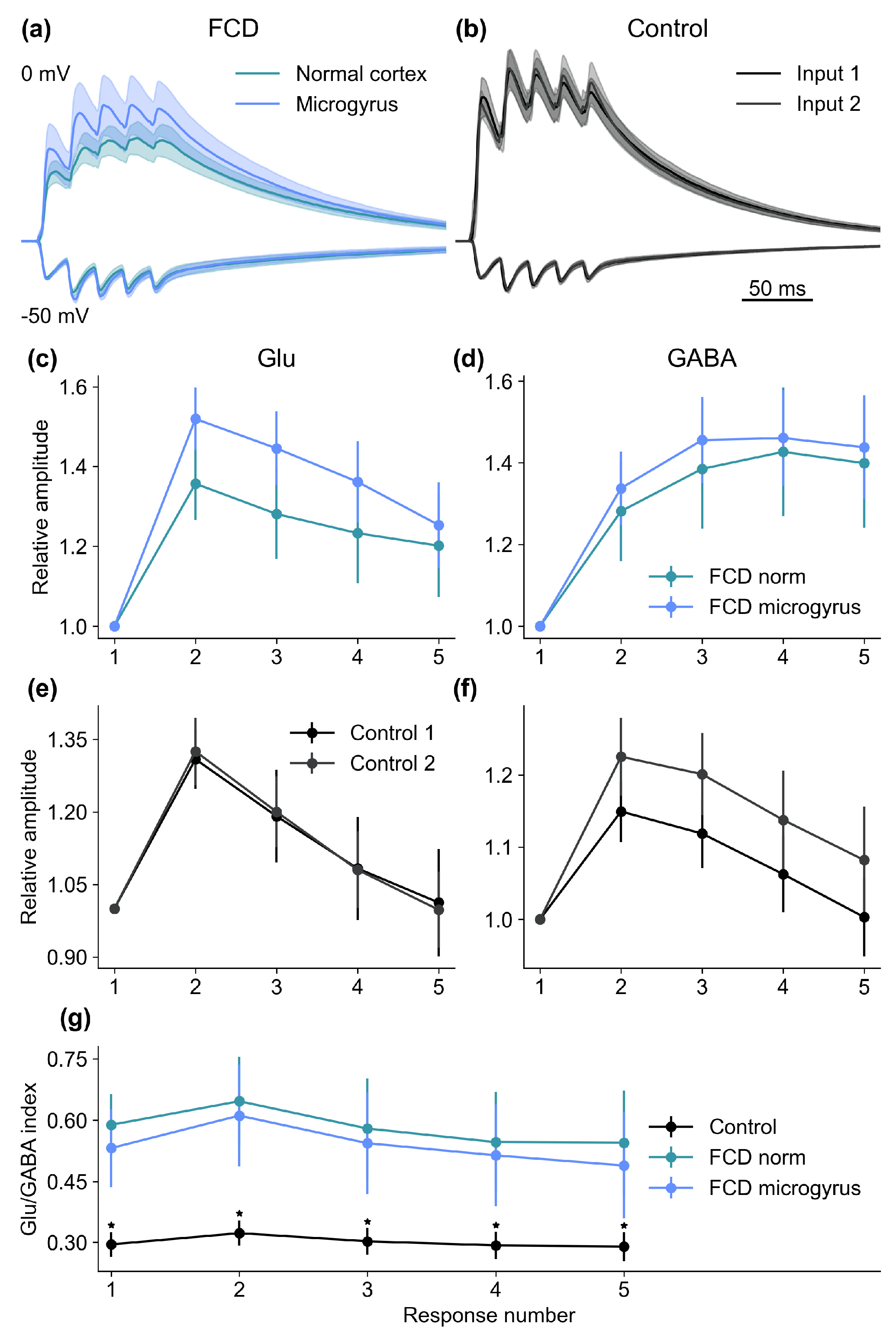
2.3. Alterations in Synaptic Properties
2.4. Characteristics of Epileptiform Activity in Animals with FCD
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Focal Freeze Lesion (FFL) Model
4.2. Slice Preparation
4.3. Electrophysiological Recordings
4.4. Morphology
4.5. Data Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luhmann, H.J. Malformations-Related Neocortical Circuits in Focal Seizures. Neurobiol. Dis. 2023, 178, 106018. [Google Scholar] [CrossRef]
- Cepeda, C.; Chen, J.Y.; Wu, J.Y.; Fisher, R.S.; Vinters, H.V.; Mathern, G.W.; Levine, M.S. Pacemaker GABA Synaptic Activity May Contribute to Network Synchronization in Pediatric Cortical Dysplasia. Neurobiol. Dis. 2014, 62, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.S.; Barkovich, A.J. Malformations of Cortical Development. Ann. Neurol. 2016, 80, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Luhmann, H.J. Models of Cortical Malformation—Chemical and Physical. J. Neurosci. Methods 2016, 260, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, K.; Feit, J.; Juránková, Z. Experimentally Induced Focal Microgyria and Status Verrucosus Deformis in Rats--Pathogenesis and Interrelation. Histological and Autoradiographical Study. Acta Neuropathol. 1978, 44, 121–129. [Google Scholar] [CrossRef]
- Dvorák, K.; Feit, J. Migration of Neuroblasts through Partial Necrosis of the Cerebral Cortex in Newborn Rats-Contribution to the Problems of Morphological Development and Developmental Period of Cerebral Microgyria. Histological and Autoradiographical Study. Acta Neuropathol. 1977, 38, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, K.M.; Gutnick, M.J.; Prince, D.A. Hyperexcitability in a Model of Cortical Maldevelopment. Cereb. Cortex 1996, 6, 514–523. [Google Scholar] [CrossRef]
- Luhmann, H.J.; Raabe, K. Characterization of Neuronal Migration Disorders in Neocortical Structures: I. Expression of Epileptiform Activity in an Animal Model. Epilepsy Res. 1996, 26, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Luhmann, H.J.; Raabe, K.; Qü, M.; Zilles, K. Characterization of Neuronal Migration Disorders in Neocortical Structures: Extracellular in Vitro Recordings. Eur. J. Neurosci. 1998, 10, 3085–3094. [Google Scholar] [CrossRef] [PubMed]
- Hablitz, J.J.; Defazio, T. Excitability Changes in Freeze-Induced Neocortical Microgyria. Epilepsy Res. 1998, 32, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Luhmann, H.J.; Karpuk, N.; Qü, M.; Zilles, K. Characterization of Neuronal Migration Disorders in Neocortical Structures. II. Intracellular in Vitro Recordings. J. Neurophysiol. 1998, 80, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, K.M.; Hwang, B.J.; Prince, D.A. Focal Epileptogenesis in a Rat Model of Polymicrogyria. J. Neurophysiol. 1999, 81, 159–173. [Google Scholar] [CrossRef]
- Zilles, K.; Qü, M.; Schleicher, A.; Luhmann, H.J. Characterization of Neuronal Migration Disorders in Neocortical Structures: Quantitative Receptor Autoradiography of Ionotropic Glutamate, GABAA and GABAB Receptors. Eur. J. Neurosci. 1998, 10, 3095–3106. [Google Scholar] [CrossRef]
- Jacobs, K.M.; Prince, D.A. Excitatory and Inhibitory Postsynaptic Currents in a Rat Model of Epileptogenic Microgyria. J. Neurophysiol. 2005, 93, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Zsombok, A.; Jacobs, K.M. Postsynaptic Currents Prior to Onset of Epileptiform Activity in Rat Microgyria. J. Neurophysiol. 2007, 98, 178–186. [Google Scholar] [CrossRef][Green Version]
- Sawant-Pokam, P.M.; Suryavanshi, P.; Mendez, J.M.; Dudek, F.E.; Brennan, K.C. Mechanisms of Neuronal Silencing After Cortical Spreading Depression. Cereb. Cortex 2017, 27, 1311–1325. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, M.; Kovac, S.; Shahabi, P.; Khaleghi Ghadiri, M.; Dreier, J.P.; Stummer, W.; Speckmann, E.-J.; Pape, H.-C.; Gorji, A. Spreading Depression Triggers Ictaform Activity in Partially Disinhibited Neuronal Tissues. Exp. Neurol. 2014, 253, 1–15. [Google Scholar] [CrossRef]
- Brill, J.; Huguenard, J.R. Enhanced Infragranular and Supragranular Synaptic Input onto Layer 5 Pyramidal Neurons in a Rat Model of Cortical Dysplasia. Cereb. Cortex 2010, 20, 2926–2938. [Google Scholar] [CrossRef] [PubMed]
- Beck, H.; Yaari, Y. Plasticity of Intrinsic Neuronal Properties in CNS Disorders. Nat. Rev. Neurosci. 2008, 9, 357–369. [Google Scholar] [CrossRef]
- Postnikova, T.Y.; Amakhin, D.V.; Trofimova, A.M.; Smolensky, I.V.; Zaitsev, A.V. Changes in Functional Properties of Rat Hippocampal Neurons Following Pentylenetetrazole-Induced Status Epilepticus. Neuroscience 2019, 399, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Griflyuk, A.V.; Postnikova, T.Y.; Zaitsev, A.V. Animal Models of Febrile Seizures: Limitations and Recent Advances in the Field. Cells 2024, 13, 1895. [Google Scholar] [CrossRef]
- Amakhin, D.V.; Soboleva, E.B.; Postnikova, T.Y.; Tumanova, N.L.; Dubrovskaya, N.M.; Kalinina, D.S.; Vasilev, D.S.; Zaitsev, A.V. Maternal Hypoxia Increases the Excitability of Neurons in the Entorhinal Cortex and Dorsal Hippocampus of Rat Offspring. Front. Neurosci. 2022, 16, 867120. [Google Scholar] [CrossRef]
- Hablitz, J.J.; Yang, J. Abnormal Pyramidal Cell Morphology and HCN Channel Expression in Cortical Dysplasia. Epilepsia 2010, 51, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Albertson, A.J.; Yang, J.; Hablitz, J.J. Decreased Hyperpolarization-Activated Currents in Layer 5 Pyramidal Neurons Enhances Excitability in Focal Cortical Dysplasia. J. Neurophysiol. 2011, 106, 2189–2200. [Google Scholar] [CrossRef]
- Jacobs, K.M. Experimental Microgyri Disrupt the Barrel Field Pattern in Rat Somatosensory Cortex. Cereb. Cortex 1999, 9, 733–744. [Google Scholar] [CrossRef]
- Rosen, G.D.; Burstein, D.; Galaburda, A.M. Changes in Efferent and Afferent Connectivity in Rats with Induced Cerebrocortical Microgyria. J. Comp. Neurol. 2000, 418, 423–440. [Google Scholar] [CrossRef]
- Jacobs, K.M.; Kharazia, V.N.; Prince, D.A. Mechanisms Underlying Epileptogenesis in Cortical Malformations. Epilepsy Res. 1999, 36, 165–188. [Google Scholar] [CrossRef]
- Jin, X.; Jiang, K.; Prince, D.A. Excitatory and Inhibitory Synaptic Connectivity to Layer V Fast-Spiking Interneurons in the Freeze Lesion Model of Cortical Microgyria. J. Neurophysiol. 2014, 112, 1703–1713. [Google Scholar] [CrossRef]
- Zucker, R.S.; Regehr, W.G. Short-Term Synaptic Plasticity. Annu. Rev. Physiol. 2002, 64, 355–405. [Google Scholar] [CrossRef] [PubMed]
- Jackman, S.L.; Regehr, W.G. The Mechanisms and Functions of Synaptic Facilitation. Neuron 2017, 94, 447–464. [Google Scholar] [CrossRef]
- Hanse, E.; Gustafsson, B. Paired-Pulse Plasticity at the Single Release Site Level: An Experimental and Computational Study. J. Neurosci. 2001, 21, 8362–8369. [Google Scholar] [CrossRef] [PubMed]
- Kellinghaus, C.; Möddel, G.; Shigeto, H.; Ying, Z.; Jacobsson, B.; Gonzalez-Martinez, J.; Burrier, C.; Janigro, D.; Najm, I.M. Dissociation between in Vitro and in Vivo Epileptogenicity in a Rat Model of Cortical Dysplasia. Epileptic Disord. 2007, 9, 11–19. [Google Scholar] [CrossRef]
- Lee, M.C.; Shim, J.J.; Kim, J.H.; Kim, M.K.; Woo, Y.J.; Chung, W.K.; Suh, J.J.; Nam, S.C.; Lee, J.S.; Kim, Y.S.; et al. Upregulation of Glutamate Receptors in Rat Cerebral Cortex with Neuronal Migration Disorders. J. Korean Med. Sci. 2004, 19, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Scantlebury, M.H.; Ouellet, P.L.; Psarropoulou, C.; Carmant, L. Freeze Lesion-Induced Focal Cortical Dysplasia Predisposes to Atypical Hyperthermic Seizures in the Immature Rat. Epilepsia 2004, 45, 592–600. [Google Scholar] [CrossRef]
- Bell, A.; Jacobs, K.M. Early Susceptibility for Epileptiform Activity in Malformed Cortex. Epilepsy Res. 2014, 108, 241–250. [Google Scholar] [CrossRef]
- dos Santos Heringer, L.; Rios Carvalho, J.; Teixeira Oliveira, J.; Texeira Silva, B.; de Souza Aguiar dos Santos, D.M.; Martinez Martinez Toledo, A.L.; Borges Savoldi, L.M.; Magalhães Portela, D.; Adriani Marques, S.; Campello Costa Lopes, P.; et al. Altered Excitatory and Inhibitory Neocortical Circuitry Leads to Increased Convulsive Severity after Pentylenetetrazol Injection in an Animal Model of Schizencephaly, but Not of Microgyria. Epilepsia Open 2022, 7, 462–473. [Google Scholar] [CrossRef]
- Ragot, A.; Luhmann, H.J.; Dipper-Wawra, M.; Heinemann, U.; Holtkamp, M.; Fidzinski, P. Pathology-Selective Antiepileptic Effects in the Focal Freeze-Lesion Rat Model of Malformation of Cortical Development. Exp. Neurol. 2021, 343, 113776. [Google Scholar] [CrossRef]
- Redecker, C.; Luhmann, H.J.; Hagemann, G.; Fritschy, J.M.; Witte, O.W. Differential Downregulation of GABAA Receptor Subunits in Widespread Brain Regions in the Freeze-Lesion Model of Focal Cortical Malformations. J. Neurosci. 2000, 20, 5045–5053. [Google Scholar] [CrossRef] [PubMed]
- Rosen, G.D.; Jacobs, K.M.; Prince, D.A. Effects of Neonatal Freeze Lesions on Expression of Parvalbumin in Rat Neocortex. Cereb. Cortex 1998, 8, 753–761. [Google Scholar] [CrossRef][Green Version]
- Wang, T.; Kumada, T.; Morishima, T.; Iwata, S.; Kaneko, T.; Yanagawa, Y.; Yoshida, S.; Fukuda, A. Accumulation of GABAergic Neurons, Causing a Focal Ambient GABA Gradient, and Downregulation of KCC2 Are Induced during Microgyrus Formation in a Mouse Model of Polymicrogyria. Cereb. Cortex 2014, 24, 1088–1101. [Google Scholar] [CrossRef]
- Aronica, E.; Boer, K.; Redeker, S.; Spliet, W.G.M.; van Rijen, P.C.; Troost, D.; Gorter, J.A. Differential Expression Patterns of Chloride Transporters, Na+-K+-2Cl--Cotransporter and K+-Cl--Cotransporter, in Epilepsy-Associated Malformations of Cortical Development. Neuroscience 2007, 145, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Shimizu-Okabe, C.; Okabe, A.; Kilb, W.; Sato, K.; Luhmann, H.J.; Fukuda, A. Changes in the Expression of Cation-Cl- Cotransporters, NKCC1 and KCC2, during Cortical Malformation Induced by Neonatal Freeze-Lesion. Neurosci. Res. 2007, 59, 288–295. [Google Scholar] [CrossRef]
- Shimizu-Okabe, C.; Tanaka, M.; Matsuda, K.; Mihara, T.; Okabe, A.; Sato, K.; Inoue, Y.; Fujiwara, T.; Yagi, K.; Fukuda, A. KCC2 Was Downregulated in Small Neurons Localized in Epileptogenic Human Focal Cortical Dysplasia. Epilepsy Res. 2011, 93, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Munakata, M.; Watanabe, M.; Otsuki, T.; Nakama, H.; Arima, K.; Itoh, M.; Nabekura, J.; Iinuma, K.; Tsuchiya, S. Altered Distribution of KCC2 in Cortical Dysplasia in Patients with Intractable Epilepsy. Epilepsia 2007, 48, 837–844. [Google Scholar] [CrossRef]
- Avoli, M.; Barbarosle, M.; Lücke, A.; Nagao, T.; Lopantsev, V.; Köhling, R. Synchronous GABA-Mediated Potentials and Epileptiform Discharges in the Rat Limbic System in Vitro. J. Neurosci. 1996, 16, 3912–3924. [Google Scholar] [CrossRef]
- Hagemann, G.; Kluska, M.M.; Redecker, C.; Luhmann, H.J.; Witte, O.W. Distribution of Glutamate Receptor Subunits in Experimentally Induced Cortical Malformations. Neuroscience 2003, 117, 991–1002. [Google Scholar] [CrossRef]
- Postnikova, T.Y.; Diespirov, G.P.; Amakhin, D.V.; Vylekzhanina, E.N.; Soboleva, E.B.; Zaitsev, A.V. Impairments of Long-Term Synaptic Plasticity in the Hippocampus of Young Rats during the Latent Phase of the Lithium-Pilocarpine Model of Temporal Lobe Epilepsy. Int. J. Mol. Sci. 2021, 22, 13355. [Google Scholar] [CrossRef]
- Smirnova, E.Y.; Amakhin, D.V.; Malkin, S.L.; Chizhov, A.V.; Zaitsev, A.V. Acute Changes in Electrophysiological Properties of Cortical Regular-Spiking Cells Following Seizures in a Rat Lithium–Pilocarpine Model. Neuroscience 2018, 379, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, A.V.; Povysheva, N.V.; Gonzalez-Burgos, G.; Lewis, D.A. Electrophysiological Classes of Layer 2/3 Pyramidal Cells in Monkey Prefrontal Cortex. J. Neurophysiol. 2012, 108, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Ciganok-Hückels, N.; Jehasse, K.; Kricsfalussy-Hrabár, L.; Ritter, M.; Rüland, T.; Kampa, B.M. Postnatal Development of Electrophysiological and Morphological Properties in Layer 2/3 and Layer 5 Pyramidal Neurons in the Mouse Primary Visual Cortex. Cereb. Cortex 2023, 33, 5875–5884. [Google Scholar] [CrossRef]
- Postnikova, T.Y.; Griflyuk, A.V.; Amakhin, D.V.; Kovalenko, A.A.; Soboleva, E.B.; Zubareva, O.E.; Zaitsev, A.V. Early Life Febrile Seizures Impair Hippocampal Synaptic Plasticity in Young Rats. Int. J. Mol. Sci. 2021, 22, 8218. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array Programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Campagnola, L.; Kratz, M.B.; Manis, P.B. ACQ4: An Open-Source Software Platform for Data Acquisition and Analysis in Neurophysiology Research. Front. Neuroinform. 2014, 8, 3. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malkin, S.L.; Amakhin, D.V.; Soboleva, E.B.; Postnikova, T.Y.; Zaitsev, A.V. Synaptic Dysregulation Drives Hyperexcitability in Pyramidal Neurons Surrounding Freeze-Induced Neocortical Malformations in Rats. Int. J. Mol. Sci. 2025, 26, 1423. https://doi.org/10.3390/ijms26041423
Malkin SL, Amakhin DV, Soboleva EB, Postnikova TY, Zaitsev AV. Synaptic Dysregulation Drives Hyperexcitability in Pyramidal Neurons Surrounding Freeze-Induced Neocortical Malformations in Rats. International Journal of Molecular Sciences. 2025; 26(4):1423. https://doi.org/10.3390/ijms26041423
Chicago/Turabian StyleMalkin, Sergey L., Dmitry V. Amakhin, Elena B. Soboleva, Tatiana Y. Postnikova, and Aleksey V. Zaitsev. 2025. "Synaptic Dysregulation Drives Hyperexcitability in Pyramidal Neurons Surrounding Freeze-Induced Neocortical Malformations in Rats" International Journal of Molecular Sciences 26, no. 4: 1423. https://doi.org/10.3390/ijms26041423
APA StyleMalkin, S. L., Amakhin, D. V., Soboleva, E. B., Postnikova, T. Y., & Zaitsev, A. V. (2025). Synaptic Dysregulation Drives Hyperexcitability in Pyramidal Neurons Surrounding Freeze-Induced Neocortical Malformations in Rats. International Journal of Molecular Sciences, 26(4), 1423. https://doi.org/10.3390/ijms26041423




